Abstract
OBJECTIVE
Weight loss through lifestyle changes is recommended for nonalcoholic fatty liver disease (NAFLD). However, its efficacy in patients with type 2 diabetes is unproven.
RESEARCH DESIGN AND METHODS
Look AHEAD (Action for Health in Diabetes) is a 16-center clinical trial with 5,145 overweight or obese adults with type 2 diabetes, who were randomly assigned to an intensive lifestyle intervention (ILI) to induce a minimum weight loss of 7% or a control group who received diabetes support and education (DSE). In the Fatty Liver Ancillary Study, 96 participants completed proton magnetic resonance spectroscopy to quantify hepatic steatosis and tests to exclude other causes of liver disease at baseline and 12 months. We defined steatosis >5.5% as NAFLD.
RESULTS
Participants were 49% women and 68% white. The mean age was 61 years, mean BMI was 35 kg/m2, mean steatosis was 8.0%, and mean aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 20.5 and 24.2 units/l, respectively. After 12 months, participants assigned to ILI (n = 46) lost more weight (−8.5 vs. −0.05%; P < 0.01) than those assigned to DSE and had a greater decline in steatosis (−50.8 vs. −22.8%; P = 0.04) and in A1C (−0.7 vs. −0.2%; P = 0.04). There were no significant 12-month changes in AST or ALT levels. At 12 months, 26% of DSE participants and 3% (1 of 31) of ILI participants without NAFLD at baseline developed NAFLD (P < 0.05).
CONCLUSIONS
A 12-month intensive lifestyle intervention in patients with type 2 diabetes reduces steatosis and incident NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases in the general population with up to 20–30% of adults having hepatic steatosis. Furthermore, NAFLD is known to lead to serious liver-related complications and cardiovascular disease, especially in individuals with type 2 diabetes (1,2). Obesity, diabetes, and insulin resistance are the main risk factors for more advanced forms of the disease; up to 70–80% of individuals with NAFLD have insulin resistance or metabolic syndrome (3). Currently, there is no approved therapy, and identifying an effective treatment remains a priority area for research. In their last Medical Position Statement in 2002, the American Gastroenterology Association and American Association for the Study of Liver Diseases stated that “Weight loss should be considered in overweight patients with NAFLD” (4). However, both organizations acknowledged that this recommendation was based on clinical impressions rather than on objective evidence.
Although a number of clinical studies have been conducted since 1990 to assess the effect of lifestyle change and/or weight loss on hepatic steatosis, these studies have differed in treatment intensity and have been limited by small study size, short duration, and the presence of confounding by other factors related to weight changes and NAFLD. In addition, most studies have relied on nonspecific (liver enzymes) or semiquantitative (ultrasound) outcomes to assess changes in hepatic steatosis. Finally, large controlled trials focused on patients with type 2 diabetes are lacking.
To fill this gap, we conducted an ancillary study within the Look AHEAD (Action for Health in Diabetes) trial, a National Institutes of Health–funded, randomized controlled trial investigating the long-term health impact of an intensive lifestyle intervention (ILI) in overweight or obese adults with type 2 diabetes. We hypothesized that the ILI would reduce hepatic steatosis and incident NAFLD compared with those of individuals in the comparison group who received diabetes support and education (DSE).
RESEARCH DESIGN AND METHODS
This study was conducted at one of the 16 Look AHEAD clinical sites (https://www.lookaheadtrial.org/public/home.cfm). The design of the Look AHEAD trial has been published previously (5). In brief, participants were eligible for the study if they were aged between 45 and 76 years, had type 2 diabetes, had a BMI of at least 25 kg/m2, and were able to complete a maximal exercise test. For the main Look AHEAD study, participants were excluded if they had known chronic liver disease, cirrhosis, or inflammatory bowel disease requiring treatment in the past year, consumed >14 alcoholic drinks per week, had prior bariatric surgery, were currently using weight loss medications (e.g., sibutramine, phentermine, and orlistat), or had uncontrolled medical conditions (e.g., A1C >11% or blood pressure ≥160/100 mmHg), chronic use of systemic corticosteroids, or known conditions that would limit their adherence to the study protocol (e.g., inability to engage in moderate exercise) or their life span (e.g., cancer). All participants were required to have a regular source of medical care outside the study.
After random assignment, all 318 participants at The Johns Hopkins University site were invited to participate in the Fatty Liver Ancillary Study; 244 participants in the ILI (n = 124) and DSE (n = 120) groups agreed. A representative sample of them (n = 185) also agreed to undergo proton magnetic resonance spectroscopy (1H MRS) and, of these, 151 had successful 1H MRS at baseline. Of the 151 participants who completed a baseline 1H MRS, 102 successfully underwent a 12-month 1H MRS and were eligible for the current analyses. After exclusion for alcohol consumption (>1 drink/day for women and >2 drinks/day for men) or other potential causes of liver disease (see below) (total n = 6), a total of 96 participants were included in the current analyses (Fig. S1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0856/DC1).
The study was reviewed and approved by local institutional review board. All participants gave written informed consent.
Measurements
As a part of both the parent Look AHEAD trial and our ancillary study, participants underwent extensive data collection at baseline and 12 months after the intervention. Age, sex, race/ethnicity, and medication use were obtained by questionnaire. Lifetime alcohol use was estimated using the Skinner Lifetime Drinking History (6). Weight, height, and waist circumference were directly measured by trained data collectors using standardized techniques. Blood samples were obtained from all patients after an overnight fast; analyses included serum aminotransferases, A1C (NGSP-certified autoanalyzer [G7 Tosoh] with interassay coefficients of variation [CVs]) of 0.9 and 0.6% for the low- and high-quality control samples, respectively), creatinine, and lipid levels.
For those who completed the 1H MRS, blood samples were also tested for hepatitis B surface antigen, hepatitis C antibody, α-1-antitrypsin phenotype, iron, transferrin saturation, iron-binding capacity, antinuclear antibodies, antimitochondrial antibodies, and anti–smooth muscle antibodies. After centrifugation, serum for insulin, adipokines, cytokines, and inflammatory markers were frozen at −70°F and subsequently hand transported on dry ice to the Core Laboratory at The Johns Hopkins General Clinical Research Center. After a single thaw, the following assays were performed: interleukin IL-8 (R&D Systems) (interassay CV 10.32% and intra-assay CV 2.33%), IL-10 (R&D Systems) (10.17% and 3.38%), tumor necrosis factor-α (TNF-α) (R&D Systems) (8.79% and 7.0%), insulin (Linco) (8.77% and 5.78%), ghrelin (Linco) (5.72% and 5.17%), resistin (ALPCO Diagnostics) (3.16% and 2.11%), and adiponectin (Linco) (3.5% and 3.6%).
1H MRS was performed on a 1.5-T whole-body scanner (Philips Gyroscan ACS-NT; Philips Medical Systems, Best, the Netherlands). Percent hepatic steatosis was calculated as fat/fat + water as determined from proton magnetic resonance spectra by integration of the respective signals. In our center, the reproducibility of steatosis measurement by 1H MRS was excellent with intra- and interrater intraclass correlation coefficients of 0.99.
Hepatic steatosis was defined as ≥5.5% hepatic fat by 1H-MRS and NAFLD as hepatic steatosis plus alcohol consumption <1 drink/day for women or <2 drinks/day for men and negative serology for hepatitis B and hepatitis C.
To estimate intra-abdominal fat volume, eight axial magnetic resonance T1-weighted spin echo images were also acquired at vertebral bodies L2–L3 during a single breath-hold and estimated using “NIH Image” (http://rsb.info.nih.gov/nih-image/Default.html). These measurements were also highly reliable (intraclass correlation coefficients 0.96–0.99) (7).
Look AHEAD interventions
A description of the Look AHEAD intervention has been published previously (8). In brief, participants assigned to the ILI were encouraged to lose at least 10% of initial weight at 12 months through a combination of moderate caloric restriction (1,200–1,500 kcal/day for those individuals weighing <114 kg and 1,500–1,800 kcal/day for those weighing >114 kg, with <30% calories from fat and <10% from saturated fat) and increased physical activity with a goal of 175 min of moderate intensity physical activity per week. During the first 6 months, participants attended weekly meetings, including one individual and three group sessions per month. During months 7–12, participants attended monthly individual session and the group sessions.
Participants assigned to the DSE group attended three group sessions per year, which provided general information on nutrition, physical activity, and social support. DSE participants were given no individual goals, were not weighed during the sessions, and received no counseling in behavioral strategies for changing diet and physical activity.
Statistical analysis
Twelve-month changes in measures of adiposity, biochemical and metabolic parameters, adipokines and cytokines, and medication use by group were assessed using ANCOVA. Despite the randomized design of the parent study, our study groups were not comparable in all respects. To address these imbalances, we adjusted all of our analyses by differences in sex, baseline weight, and baseline hepatic steatosis.
We analyzed changes in steatosis using two approaches: first as the absolute difference between 12 months and baseline (change steatosis = steatosis [percent]) at 12 months minus steatosis [percent] at baseline) and then as relative difference (percent change steatosis = [(steatosis [percent] at 12 months minus steatosis [percent] at baseline)/steatosis [percent] at baseline] × 100). Because the distribution of percent change in steatosis was skewed, we used quintile regression methods to model the median percent change steatosis from baseline to 12 months in hepatic steatosis in the ILI compared with the DSE group, adjusting for other covariates.
We used multivariate regression analyses to assess the influence of potential mediators of the intervention including changes in weight and other measures of adiposity, metabolic parameters, and adipokines and cytokines. First, we assessed the role of changes in other adiposity deposits. Second, we evaluated changes in metabolic parameters and, third, we determined changes in adipokines and cytokines. All models were also adjusted for sex, baseline weight, and baseline steatosis.
To assess the correlations between hepatic steatosis and other parameters we used partial Spearman rank coefficients to account for the nonnormal distribution of liver fat while adjusting for treatment group and sex. The odds of incident NAFLD was assessed using logistic regression and included only individuals with baseline steatosis <5.5%.
RESULTS
Baseline characteristics
We included 96 participants randomly assigned to ILI (n = 46) and DSE (n = 50). The sample had a mean ± SD age of 61.6 ± 6.7 years and BMI of 34.9 ± 5.0 kg/m2; 60% were white, 32% were African American, 5% were other, and 2% were Hispanic. Overall 49% were women, with slightly more women in the ILI group than in the DSE group (59 vs. 40% P = 0.06). A1C was 7.2 ± 1.0%, and 87% of participants were using any diabetes medication, including 12% taking insulin and 50% taking metformin. Although this study was nested in the main Look AHEAD trial, the final sample included a subset of the randomly assigned participants, and there were significant differences in baseline weight, steatosis, waist circumference, and use of lipid-lowering medications between the groups (Table 1). Hepatic steatosis (≥5.5%) was present in 44% including 15 (36%) in the ILI group and 27 (64%) in the DSE group (P = 0.04). All analyses were adjusted for these baseline differences.
Table 1.
Baseline and 1-year characteristics of Look AHEAD participants
| Variable | ILI (intervention) |
DSE (control) |
P value deltas (ILI vs. DSE) 1 year | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 year | Absolute change | Relative change | Baseline | 1 year | Absolute change | Relative change | ||
| n | 46 | 50 | |||||||
| Measures of adiposity | |||||||||
| Hepatic fat | 4.2 (2.3−7.2)* | 2.9 (1.3−3.9) | −2.3 (−4.3 to −0.4) | −50.8 (−66.9 to −27.8) | 6.3 (2.7−12.7) | 4.9 (1.9−8.6) | −1.1 (−3.1 to 1.2) | −22.8 (−51.2 to 32.2) | 0.04 |
| 0−1 (%) | 7 (15.2) | 8 (17.4) | 5 (10.0) | 6 (12.2) | |||||
| 1.1−5.49 (%) | 24 (52.2) | 29 (63.0) | 18 (36.0) | 20 (40.8) | |||||
| 5.5−10 (%) | 5 (10.9) | 5 (10.9) | 9 (18.0) | 13 (26.5) | |||||
| 10.1−20 (%) | 8 (17.4) | 3 (6.5) | 14 (28.0) | 6 (12.2) | |||||
| >20 (%) | 2 (4.4) | 1 (2.2) | 4 (8.0) | 4 (8.2) | |||||
| BMI | 34.7 ± 5.4 | 32.1 ± 5.2 | −2.6 ± 2.6 | −7.3 ± 6.8 | 35.3 ± 4.7 | 35.3 ± 4.8 | −0.02 ± 2.0 | 0.03 ± 5.7 | <0.001 |
| 25−29.9 kg/m2 | 8 (17.4) | 15 (68.2) | 3 (6.0) | 7 (14.0) | |||||
| 30−34.9 kg/m2 | 21 (44.7) | 20 (43.5) | 26 (52.0) | 19 (38.0) | |||||
| 35−39.9 kg/m2 | 12 (26.1) | 9 (19.6) | 14 (28.0) | 15 (30.0) | |||||
| ≥40 kg/m2 | 5 (10.9) | 2 (4.4) | 7 (14.0) | 9 (18.0) | |||||
| Weight (kg) | 98.1 ± 16.6* | 90.6 ± 14.9 | −8.5 ± 8.3 | −8.3 ± 6.9 | 104.8 ± 16.7 | 104.7 ± 16.9 | −0.05 ± 5.7 | −0.02 ± 5.2 | <0.001 |
| Waist circumference (cm) | 112.0 ± 11.7* | 102.4 ± 11.7 | −9.9 ± 11.1 | −8.4 ± 8.9 | 115.0 ± 11.8 | 113.5 ± 12.4 | −1.8 ± 6.5 | −1.4 ± 5.5 | <0.001 |
| Total fat (per 10 cm2) | 51.3 ± 15.4 | 46.4 ± 14.8 | −5.3 ± 11.0 | −8.8 ± 17.2 | 56.0 ± 14.5 | 56.0 ± 15.6 | −0.03 ± 7.8 | 0.5 ± 14.1 | 0.002 |
| Subcutaneous (per 10 cm2) | 28.8 ± 13.2 | 26.6 ± 11.8 | −2.9 ± 7.2 | −6.7 ± 19.2 | 29.1 ± 10.3 | 28.4 ± 10.7 | −0.8 ± 4.16 | −1.9 ± 16.8 | 0.02 |
| Intraperitoneal (per 10 cm2) | 15.5 ± 6.6 | 13.0 ± 5.5 | −2.5 ± 4.4 | −12.7 ± 28.5 | 18.7 ± 7.7 | 18.2 ± 7.3 | −0.4 ± 4.4 | 1.8 ± 24.4 | 0.02 |
| Retroperitoneal (per 10 cm2) | 5.8 ± 2.7 | 5.9 ± 3.1 | 0.2 ± 1.6 | 5.3 ± 27.4 | 7.1 ± 3.2 | 8.2 ± 4.1 | 1.1 ± 2.0 | 15.4 ± 26.6 | 0.05 |
| Biochemical and metabolic parameters | |||||||||
| ALT (units/l) | 17.5 (14−28) | 20 (16−27) | 1 (−3 to 7) | 20 (17−26) | 19 (14−24) | −2 (−6 to 1) | 0.31 | ||
| AST (units/l) | 18 (15−24) | 21 (18−25) | 3 (−2 to 5) | 19 (15−24) | 17 (15−22) | 0 (−3 to 2) | 0.45 | ||
| ALT−to−AST ratio | 1 (0.8−1.1) | 1 (0.8−1.2) | 0.1 (−0.2 to 0.2) | 0.8 (0.7−1) | 0.9 (0.8−1.1) | 0.1 (−0.04 to 0.2) | 0.19 | ||
| GGT (units/l) | 24 (18−36) | 22 (17.5−32.0) | −3 (−6 to 1) | 25 (22−45) | 23 (18−35) | −4 (−11 to 2) | 0.27 | ||
| A1C (%) | 7.1 ± 1.0 | 6.5 ± 0.9 | −0.7 ± 1.1 | 7.3 ± 1.0 | 7.1 ± 1.0 | −0.2 ± 0.8 | 0.04 | ||
| HDL cholesterol (mg/dl) | 47.9 ± 11.7 | 52.7 ± 12.0 | 4.1 ± 7.0 | 42.9 ± 12.0 | 44.2 ± 11.4 | 2 ± 6.5 | 0.11 | ||
| Triglycerides (mg/dl) | 111.5 (88−169) | 107 (66−139) | −5 (−46 to 18) | 122.5 (91−194) | 121 (87−190) | −5 (−31 to 20) | 0.23 | ||
| LDL cholesterol (mg/dl) | 118.0 ± 34.5 | 107.3 ± 30.7 | −9.3 ± 23.3 | 109.8 ± 29.9 | 98.1 ± 27.7 | −12.3 ± 25.0 | 0.53 | ||
| HDL−to−triglyceride ratio | 2.3 (1.6−3.9) | 2.1 (1.31−3.1) | −0.2 (−1.1 to 0.3) | 3.2 (2.1−5.4) | 2.6 (1.9−4.7) | −0.2 (−0.9 to 0.4) | 0.4 | ||
| Adipokines and cytokines | |||||||||
| IL−8 (pg/ml) | 31.7 (24.3−38.2) | 23.0 (17.0−29.6) | −9.3 (−17.3 to 5.9) | 31.9 (21.1−41.7) | 21.0 (15.9−26.3) | −9.3 (−21.1 to −0.1) | 0.65 | ||
| IL−10 (pg/ml) | 5.5 (4.8−5.9) | 6.8 (5.5−7.8) | 0.9 (−0.2 to 3.0) | 5.3 (4.8−6.4) | 7.1 (5.9−8.7) | 1.4 (0.04−3.4) | 0.08 | ||
| TNF−α (pg/ml) | 1.7 (1.2−2.6) | 1.7 (1.4−2.2) | 0.2 (−0.7 to 0.6) | 1.8 (1.4−2.8) | 1.9 (1.6−2.5) | 0.03 (−0.7 to 0.7) | 0.82 | ||
| Adiponectin (μg/ml) | 5.9 (4.5−7.2) | 11.5 (7.67−21.21) | 6.7 (2.4 to 10.8) | 5.4 (4.3−6.3) | 9.3 (6.8−15.2) | 3.8 (1.7 to 8.9) | 0.1 | ||
| Ghrelin (ng/ml) | 1.2 (0.9−2.0) | 1.4 (0.9−1.8) | −0.03 (−0.9 to 0.7) | 1.2 (0.9−2.1) | 0.9 (0.8−1.2) | −0.3 (−1.1 to 0.2) | 0.35 | ||
| Resistin (ng/ml) | 4.1 (2.9−6.6) | 6.8 (4.7−8.8) | 1.9 (0.5 to 2.6) | 4.5 (3.5−5.9) | 6.2 (4.8−8.2) | 1.5 (0.1 to 3.0) | 0.84 | ||
| Medications | |||||||||
| No. of diabetes medications | 1.3 ± 0.8 | 1.2 ± 0.9 | −0.1 ± 0.5 | 1.4 ± 0.8 | 1.5 ± 0.8 | 0.1 ± 0.6 | 0.06 | ||
| 0−1 | 29 (63.0) | 30 (65.2) | 27 (44.0) | 22 (44.0) | |||||
| 2 | 12 (26.1) | 11 (23.9) | 19 (38.0) | 23 (46.0) | |||||
| 3 | 5 (10.9) | 5 (10.9) | 4 (8.0) | 5 (10.0) | |||||
| Use of insulin (%) | 13 | 11 | −2 | 10 | 8 | −2 | 0.94 | ||
| Use of metformin (%) | 52.20 | 45.70 | −6.5 | 48 | 54.20 | 6.2 | 0.38 | ||
| Use of thiazolidinedione (%) | 28.30 | 23.90 | −4.4 | 34 | 30.00 | −4 | 0.95 | ||
| Use of lipid-lowering drug (%) | 41.3* | 45.70 | 4.4 | 70 | 70.80 | 0.8 | 0.82 | ||
Data are means ± SEM, median (interquartile range), or frequency (%).
*ILI vs. DSE baseline difference P > 0.05, adjusted for sex and baseline weight.
Baseline levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT) were 23.9, 20.4, and 45.1 units/l, respectively, and did not differ by group (all P ≥ 0.05). Overall, 6 participants (6%) had elevated ALT levels (≥40 units/l) and 43 (45%) had an AST-to-ALT ratio ≥1.
12-Month changes in steatosis, adiposity, and other metabolic parameters
As shown in Table 1, the ILI was effective and resulted in significant decreases in BMI (−2.6 vs. 0.03 kg/m2; P < 0.001), weight (−8.5 vs. −0.05 kg; P < 0.001), percent weight (−8.3 vs. −0.03%; P < 0.001), waist circumference (−9.5 vs. −1.8 cm; P < 0.001), percent total fat (−8.8 vs. 0.53%; P = 0.001), percent subcutaneous fat (−6.7 vs. −1.9%; P = 0.02), and percent intraperitoneal fat (−12.7 vs. 1.8%; P = 0.02) compared with the DSE. These findings are similar to the 1-year results of the main Look AHEAD trial (9).
After adjustment for sex, baseline weight, and baseline steatosis, the 12-month median absolute change in steatosis in the ILI group was more than double that in the DSE group (−2.3 vs. −1.1; P = 0.04). The median percent decrease in steatosis was −50.8% in the ILI group and −22.8% in the DSE group (P = 0.04).
Participants in the ILI group also had significant decreases in A1C (−0.7 vs. −0.2%; P = 0.04). This difference in glucose control occurred despite the fact that the proportion of individuals using any diabetes medicine decreased from baseline in the ILI group compared with that in the DSE group. No statistically significant differences were observed in liver enzymes or lipids at 12 months.
Variables associated with changes in hepatic steatosis
As shown in Table 2, after adjustment for intervention group and sex, absolute changes in steatosis were significantly correlated with changes in weight (r = 0.231, P = 0.03), A1C (r = 0.311, P = 0.004), glucose (r = 0.291, P = 0.007), and ALT, AST, and GGT (r = 0.294, r = 0.348 and r = 0.277, all P ≤ 0.001). No significant correlations were found between absolute changes in steatosis and changes in any measured cytokine or adipokine. On a relative scale, the percent change in liver fat showed significant and strong correlation coefficients with changes in all of the above parameters. In addition, percent change in steatosis was significantly correlated with changes in BMI (r = 0.259, P = 0.02), insulin levels (r = −0.302, P = 0.02), triglycerides (r = 0.336, P = 0.002), and the HDL-to-triglyceride ratio (r = 0.276, P = 0.011).
Table 2.
Correlation of changes in liver fat with changes in other parameters, adjusted for sex and intervention group
| Absolute Δ liver fat | P value | Percent Δ liver fat | P value | |
|---|---|---|---|---|
| Measures of adiposity | ||||
| Δ weight | 0.231 | 0.030 | 0.349 | 0.001 |
| Δ waist | 0.022 | 0.835 | 0.116 | 0.281 |
| Δ BMI | 0.194 | 0.070 | 0.259 | 0.015 |
| Δ total fat | 0.095 | 0.378 | 0.097 | 0.367 |
| Δ subcutaneous fat | 0.135 | 0.209 | 0.111 | 0.304 |
| Δ intraperitoneal fat | 0.056 | 0.604 | 0.045 | 0.676 |
| Δ retroperitoneal fat | 0.080 | 0.456 | 0.145 | 0.179 |
| Metabolic parameters | ||||
| Δ A1C | 0.311 | 0.004 | 0.419 | <0.0001 |
| Δ glucose | 0.291 | 0.007 | 0.373 | 0.001 |
| Δ insulin* | −0.186 | 0.137 | −0.302 | 0.015 |
| Δ total cholesterol | 0.050 | 0.654 | 0.042 | 0.701 |
| Δ HDL | 0.072 | 0.517 | 0.005 | 0.964 |
| Δ triglycerides | 0.187 | 0.089 | 0.336 | 0.002 |
| Δ LDL | −0.084 | 0.447 | −0.105 | 0.342 |
| Δ HDL-to-triglyceride ratio | 0.128 | 0.246 | 0.276 | 0.011 |
| Liver tests | ||||
| Δ ALT | 0.294 | 0.005 | 0.211 | 0.047 |
| Δ AST | 0.348 | 0.001 | 0.280 | 0.008 |
| Δ GGT | 0.277 | 0.009 | 0.287 | 0.006 |
| Δ AST-to-ALT ratio | −0.030 | 0.781 | −0.033 | 0.762 |
| Inflammatory markers | ||||
| Δ IL-8 | 0.014 | 0.904 | −0.085 | 0.466 |
| Δ IL-10 | 0.136 | 0.245 | −0.010 | 0.934 |
| Δ TNF-α | −0.024 | 0.840 | −0.002 | 0.986 |
| Δ adiponectin | 0.062 | 0.600 | −0.124 | 0.290 |
| Δ ghrelin | 0.195 | 0.094 | 0.214 | 0.065 |
| Δ resistin | −0.122 | 0.297 | −0.079 | 0.498 |
*Among non–insulin users.
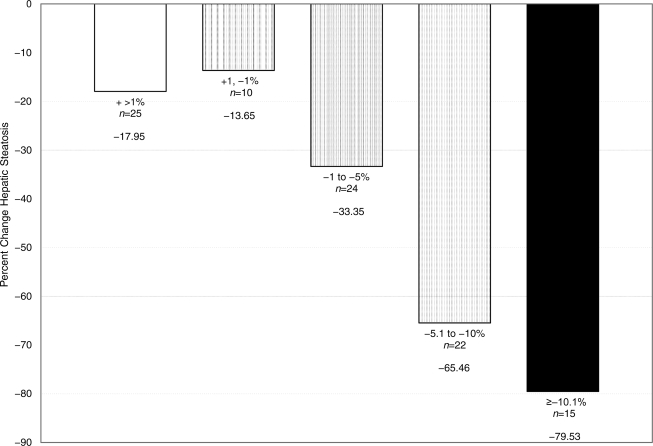
Greater weight loss was associated with the largest decreases in steatosis. Compared with those with little weight change (±1%), those with the largest weight loss (≥10%) had a significantly higher median percent reduction in steatosis of −79.5 vs. −13.7% (Fig. 1). We used multivariate models to assess whether these correlations were independently associated with changes in steatosis. After adjustment for any of the following: changes in weight, changes in BMI, changes in retroperitoneal fat, changes in A1C, changes in triglycerides, and changes in the HDL-to-triglyceride ratio as well as sex, treatment group, and baseline weight and hepatic fat, the effect of the intervention was no longer significant. Changes in adipokines or cytokines did not attenuate the effect of the intervention.
Figure 1.
Median percent change in hepatic steatosis by percent weight change.
NAFLD incidence
Finally, during the 12-months of follow-up, 6 of 23 (26%) DSE participants and 1 of 31 (3%) ILI participants without NAFLD at baseline developed NAFLD at 12 months (odds ratio 0.07 [95% CI 0.007–0.71]).
CONCLUSIONS
Among adults with type 2 diabetes, 12 months of an intensive lifestyle intervention leading to 8% loss of body weight was successful in both reducing hepatic steatosis and decreasing the risk of incident NAFLD, compared with those in a control group Furthermore, a dose-response relationship was observed with weight loss, with the greatest reduction observed in those with the greatest weight loss (≥10%). Our findings therefore support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with NAFLD, including patients with type 2 diabetes and for those at risk for NAFLD.
We found that the decrease in steatosis was nearly double in the ILI group compared with that in the DSE group. In addition, as in the main Look AHEAD 1-year results (9), the use of overall and specific medications such as thiazolidinedione and metformin tended to decrease more among the intervention arm. We would anticipate that these differences would then favor the control arm, leading to a more conservative estimate. These findings are important because NAFLD not only disproportionately affects individuals with type 2 diabetes but also because once NAFLD is present, the risk of developing more advanced forms of NAFLD, such as nonalcoholic steatohepatitis and hepatocellular carcinoma is higher in this group than in the general population (3,10–13).
Our results are consistent and extend previous trials of weight loss for patients with NAFLD, suggesting improvement in hepatic steatosis. To our knowledge there have been a total of nine clinical studies of lifestyle intervention on hepatic steatosis measured by 1H MRS (14–22), and, of these, only two have been conducted among individuals with type 2 diabetes (17,18). Petersen et al. (17) treated eight individuals with obesity and diabetes with a 1,200-calorie liquid diet for 3–12 weeks to achieve 8% weight loss. Steatosis decreased on average 81% (from 12 to 2.2%). Tamura et al. (17) randomly assigned 14 subjects to a controlled diet only (25–30 kcal/kg ideal body weight) or exercise and diet (same diet plus two or three 30-min sessions of walking 5–6 days/week) for 2 weeks. In this inpatient study, the mean decreases in hepatic steatosis were 25 and 28% for the diet only and diet plus exercise group, respectively (18). Our study extends these findings in patients with type 2 diabetes by including a larger and diverse sample and a longer intervention.
Because most participants had normal liver enzymes at baseline, it is understandable that there was no significant change with weight loss and decrease in steatosis. However, consistent with other studies, our data show that normal liver test results are not good indicators of the presence or absence of hepatic steatosis.
Cytokines and adipokines have been posited to play an important role as mediators of improved hepatic insulin sensitivity with weight loss. In our study, changes in steatosis and adiposity were not associated with changes in IL-8, IL-10, TNF-α, adiponectin, ghrelin, or resistin. These results are consistent with two other previous studies (16,17) and suggest that among individuals with type 2 diabetes these may not play a major role in changing insulin sensitivity in the liver.
Although a clinically meaningful change in steatosis remains to be defined, our results suggest that among patients with type 2 diabetes, reduction in hepatic steatosis is significantly associated with levels of A1C and triglycerides, both of which are important markers of disease risk and control (23). Longer studies are needed to identify meaningful changes in liver fat, with respect to liver outcomes.
Our study has some limitations. First, we had no histopathological data to assess the effect of the intervention. Although 1H MRS is an excellent method to quantify changes in steatosis because it is noninvasive and reliable, it cannot assess inflammation or fibrosis. Recently, Promrat et al. (24) reported the results of an smaller trial of lifestyle intervention for 31 overweight patients with biopsy-proven nonalcoholic steatohepatitis, and their results are in agreement with our findings. In addition, our study included older individuals with type 2 diabetes and mostly with normal liver enzyme levels, probably reflecting a different spectrum of the disease. Second, even though this trial is by far is the largest of its kind, the study sample was not large enough to study participant subgroups (i.e., sex and race or to assess sex-treatment or race-treatment interactions). Third, we studied participants in a large randomized clinical trial who are likely to represent a very motivated group; however, although limiting generalizability, this setting is ideal to assess the efficacy of this intervention. Future studies will be needed to assess the effectiveness of this approach. Fourth, even though the parent study had a randomized design, our study groups were not comparable in all respects, probably because enrollment into this ancillary study occurred after randomization and by random chance. To address these imbalances we adjusted all our analyses by these baseline differences. Finally, because obesity hinders the successful acquisition of 1H MRS, the results may be conservative.
In summary, in patients with type 2 diabetes, an intensive lifestyle intervention that produced 8% weight loss resulted in a significant, 25% greater reduction in hepatic steatosis and a substantially lower incidence of NAFLD compared with that of a comparison group after 12 months of the intervention. The long-term efficacy as well as the effectiveness of an intensive lifestyle intervention needs to be further established.
Supplementary Material
Acknowledgments
The study was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (grants R01-DK-060427 and U01-DK-57149), The Johns Hopkins University School of Medicine General Clinical Research Center (grant M01-RR-00052), and the Department of Veterans Affairs.
No potential conflicts of interest relevant to this article were reported.
M.L. analyzed and interpreted data, performed statistical analysis, wrote the manuscript, and reviewed/edited the manuscript. S.F.S., A.M.D., and F.L.B. provided the study concept and design, analyzed and interpreted data, and reviewed/edited the manuscript. A.H. and S.B., acquired data and reviewed/edited the manuscript. L.E.W., F.X.P.-S., and S.E.K. analyzed and interpreted data and reviewed/edited the manuscript. J.M.C. provided the study concept and design, analyzed and interpreted data, wrote the manuscript, and reviewed/edited the manuscript.
Parts of this study were presented in abstract form at the Liver Meeting 2008, San Francisco, California, 31 October–4 November 2008.
Footnotes
Clinical trial registry no. NCT00017953, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G: Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–1218 [DOI] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri BA, Caldwell SH: Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202–1219 [DOI] [PubMed] [Google Scholar]
- 3.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M: Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–923 [DOI] [PubMed] [Google Scholar]
- 4.American Gastroenterological Association Medical Position Statement: nonalcoholic fatty liver disease. Gastroenterology 2002;123:1702–1704 [DOI] [PubMed] [Google Scholar]
- 5.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ: Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 6.Skinner HA, Sheu WJ: Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol 1982;43:1157–1170 [DOI] [PubMed] [Google Scholar]
- 7.Bonekamp S, Ghosh P, Crawford S, Solga SF, Horska A, Brancati FL, Diehl AM, Smith S, Clark JM: Quantitative comparison and evaluation of software packages for assessment of abdominal adipose tissue distribution by magnetic resonance imaging. Int J Obes (Lond) 2008;32:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S: The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ: Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P: The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121 [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Sanderson S, Lindor KD, Angulo P: The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132–138 [DOI] [PubMed] [Google Scholar]
- 12.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, Rizzetto M: Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134–140 [DOI] [PubMed] [Google Scholar]
- 13.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ: Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29:664–669 [DOI] [PubMed] [Google Scholar]
- 14.Cowin GJ, Jonsson JR, Bauer JD, Ash S, Ali A, Osland EJ, Purdie DM, Clouston AD, Powell EE, Galloway GJ: Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging 2008;28:937–945 [DOI] [PubMed] [Google Scholar]
- 15.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E: Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006;29:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E: Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity 2008;16:1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI: Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R: Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005;90:3191–3196 [DOI] [PubMed] [Google Scholar]
- 19.Thamer C, Machann J, Stefan N, Haap M, Schafer S, Brenner S, Kantartzis K, Claussen C, Schick F, Haring H, Fritsche A: High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 2007;15:531–538 [DOI] [PubMed] [Google Scholar]
- 20.Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD: Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol 2006;12:5813–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Häkkinen AM, Tamminen M, Teramo K, Yki-Järvinen H: Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes 2003;52:701–707 [DOI] [PubMed] [Google Scholar]
- 22.Viljanen AP, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, Järvisalo M, Parkkola R, Rönnemaa T, Guiducci L, Lehtimäki T, Raitakari OT, Mari A, Nuutila P: Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab 2009;94:50–55 [DOI] [PubMed] [Google Scholar]
- 23.Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR: Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.