Abstract
OBJECTIVE
The mean amplitude of glycemic excursions (MAGE) is a significant determinant of overall metabolic control as well as increased risk for diabetes complications. Older individuals with type 2 diabetes are more likely to have moderate cognitive deficits and structural changes in brain tissue. Considering that poor metabolic control is considered a deranging factor for cognitive performance in diabetic patients, we evaluated whether the contributions of MAGE to cognitive status in older patients with type 2 diabetes were independent from the main markers of glycemic control, such as sustained chronic hyperglycemia (A1C), postprandial glycemia (PPG), and fasting plasma glucose (FPG).
RESEARCH DESIGN AND METHODS
In 121 older patients with type 2 diabetes, 48-h continuous subcutaneous glucose monitoring (CSGM) were assessed. MAGE and PPG were evaluated during CSGM. The relationship of MAGE to performance on cognitive tests was assessed, with adjustment for age, glycemic control markers, and other determinants of cognitive status. The cognitive tests were a composite score of executive and attention functioning and the Mini Mental Status Examination (MMSE).
RESULTS
MAGE was significantly correlated with MMSE (r = 0.83; P < 0.001) and with cognition composite score (r = 0.68; P < 0.001). Moreover, MAGE was associated with the MMSE (P < 0.001) and cognition composite score (P < 0.001) independently of age, sex, BMI, waist-to-hip (WHR) ratio, drug intake, physical activity, mean arterial blood pressure, FPG, PPG, and A1C.
CONCLUSIONS
MAGE during a daily period was associated with an impairment of cognitive functioning independent of A1C, FPG, and PPG. The present data suggest that interventional trials in older patients with type 2 diabetes should target not only A1C, PPG, and FPG but also daily acute glucose swings.
It is widely known that older individuals with type 2 diabetes are more likely to experience cognitive decline than those without type 2 diabetes (1). The underlying mechanisms, however, are still unclear, but emerging evidence suggests that a relationship between measures of glucose control and cognitive function exists (2). For instance, a cross-sectional analysis in 378 high-functioning individuals with diabetes showed that higher A1C but not fasting plasma glucose (FPG) levels were consistently associated with lower scores on two cognitive tests (3). Further evidence comes from studies using other indexes of dysglycemia, such as postprandial glycemia (PPG) and acute hyperglycemia (4,5). Therefore, despite the fact that several studies have investigated and compared the roles of the different glycemic indexes participating in diabetic cognitive disorders, an accurate assessment of their respective contributions is still difficult. Considering that the brain is dependent on an appropriate supply of glucose as its principal energy source, one cannot rule out the possibility that plasma glucose instability over 24 h may affect cognitive functioning. From a more practical point of view, exposure to glycemic disorders can be described as a function of two components: 1) the duration and magnitude of chronic sustained hyperglycemia and 2) the acute fluctuations of glucose over a daily period (6,7). The first component was integrated by A1C, which depends on both interprandial and postprandial hyperglycemia, the percentage of each contributor being modulated by the degree of diabetic control (8). The acute fluctuations of glucose around a mean value is more difficult to assess, but the recent development of devices that allow continuous glucose monitoring on an ambulatory basis certainly represents a new approach for studying the influence of acute blood glucose fluctuations in real life (9). By applying this technology, we have attempted to gain further insight into the respective role of both sustained chronic hyperglycemia and acute glucose fluctuations over a daily period on global cognitive functioning as well as executive and attention functioning neuropsychological tests.
RESEARCH DESIGN AND METHODS
A total of 121 older outpatients with type 2 diabetes in Naples, Italy, were included from 2007 to 2009. The patients were entered consecutively without any selection based on A1C levels. Eligibility for the study was based on a diagnosis over a minimum 1-year period. The main clinical and laboratory characteristics of the patients are given in Table 1. Frequency and duration of walking and other leisure-time physical activities were assessed by interview. Exclusion criteria, assessed with self-report and medical records, included treatment with steroids or nonsteroidal anti-inflammatory drugs, acute concurrent illness during the 3-month period preceding the investigation, and cerebrovascular diseases. The study was conducted after each patient had given oral informed consent.
Table 1.
Clinical characteristics of patients
| Type 2 diabetic patients | |
|---|---|
| n | 121 |
| Variables | |
| Age (years) | 78 ± 6.7 |
| Male/female sex | 46/76 |
| BMI (kg/m2) | 27.1 ± 0.8 |
| Systolic blood pressure (mmHg) | 145 ± 6.1 |
| Diastolic blood pressure (mmHg) | 85 ± 3.8 |
| Diabetes duration (years) | 7.8 ± 3.1 |
| Risk factors | |
| Hypertension | 30 (25) |
| Hypercholesterolemia | 13 (11) |
| Smokers | 13 (11) |
| Previous CVD | 38 (24) |
| Metabolic profile | |
| Fasting glycemia (mg/dl) | 153 ± 10.3 |
| 2-h PPG (mg/dl) | 198 ± 27.4 |
| A1C (%) | 7.9 ± 0.3 |
| MAGE (mg/dl glucose) | 71 ± 19 |
| Fasting insulin (pmol/l) | 170 ± 55 |
| Postmeal insulin (pmol/l) | 398 ± 109 |
| Cognitive function | |
| MMSE | 26.1 ± 1.3 |
| TMT-A (s) | 83 ± 34 |
| TMT-B (s) | 187 ± 85 |
| DIFF B-A (s) | 103 ± 35 |
| DSP-Backward | 7.06 ± 1.76 |
| DSP-Forward | 5.83 ± 0.74 |
| Verbal fluency | 25.7 ± 4.49 |
| Intimal-media thickness (mm) | 0.77 ± 0.2 |
Data are means ± SD, n, or n (%).
Subcutaneous interstitial glucose levels were monitored on an ambulatory basis over a period of 3 consecutive days by using continuous subcutaneous glucose monitoring (CSGM) (GlucoDay; A. Menarini Diagnostics, Florence, Italy) as described previously (10). The sensor was inserted on day 0 and removed on day 3 at mid-morning. The data were downloaded to a computer for evaluation of glucose variations, but calculations of glucose variations were limited to data obtained on days 1 and 2 to avoid bias due to both insertion and removal of the sensor and, thus, to insufficient stabilization of the monitoring system. The characteristic glucose pattern of each patient was calculated by averaging the profiles obtained on study days 1 and 2. Standardized meal tests with 24-h sampling comprising three mixed meals were performed on days 1, 2, and 3. After an overnight fast, patients received medications at 0700 h and consumed breakfast 30 min after treatment. Lunch and dinner were provided 5 and 10 h after the beginning of breakfast, respectively. The standardized breakfast contained 419 kcal (57% carbohydrate, 17% protein, and 26% fat), lunch contained 692 kcal (66% carbohydrate, 16% protein, and 18% fat), and dinner contained 507 kcal (41% carbohydrate, 26% protein, and 32% fat). Before enrollment, all patients underwent carotid ultrasound examination and magnetic resonance imaging (MRI) for the screening of carotid atherosclerosis and white matter lesions, significant signs of cortical or subcortical atrophy. Patients with alterations in MRI scans such as white matter lesions or cortical or subcortical atrophy were considered lost to follow-up analysis because morphological brain lesions as evidenced by MRI might affect cognitive functioning independently of the changes in metabolic control. All MRI evaluations were made by physicians not involved in the study and blinded to the study design. In brief, all patients were investigated in the supine position with the head slightly turned from the sonographer. The carotid arteries were carefully examined for wall changes from different longitudinal and transverse views. The common carotid artery, the carotid bulb, and the internal and external carotid arteries were examined in all subjects. A region ∼1.5 cm proximal to the carotid bifurcation was identified, and the intimal-media thickness of the far wall was evaluated as the distance between the luminal-intimal interface and the medial-adventitial interface. One transversal and two longitudinal measurements of intimal-media thickness were obtained from 10 contiguous sites at 1-mm intervals, and the average of the 10 measurements were used for the analysis. All ultrasound measurements were performed before enrollment by two trained investigators who were unaware of the study protocol.
Laboratory measurements
Blood samples for the assay of insulin were collected in EDTA tubes, and plasma was immediately aliquoted in Eppendorf vials and stored at −80°C until thawed for assays. Plasma insulin was determined by a commercial double-antibody, solid-phase radioimmunoassay (Sorin Biomedica, Milan, Italy) (intra-assay coefficient of variation 3.1 + 0.3%; cross-reactivity vs. proinsulin 0.9%). Serum glucose, serum lipid, and serum lipoprotein were quantified from fresh samples drawn after participants had been fasting for at least 12 h. The serum glucose level was determined by an enzymatic colorimetric assay using a modified glucose oxidase-peroxidase method (Roche Diagnostics, Mannheim, Germany) and a Roche-Hitachi 917 analyzer. Commercial enzymatic tests were used for determining serum total and HDL cholesterol and triglyceride (Roche Diagnostics) levels.
Assessment of glycemic instability
The mean amplitude of glycemic excursions (MAGE), which has been described by Service et al. (11), was used in the present study for assessing glucose fluctuations during 24 h. In particular, we used the glucose profiles obtained from continuous glucose monitoring system data on study days 1 and 2, i.e., from continuous monitoring for 48 h. This parameter was designed to quantify major swings of glycemia and to exclude minor ones. For this reason, only increases of >1 SD of the mean glycemic values were taken into account. Calculation of the MAGE was obtained by measuring the arithmetic mean of the differences between consecutive peaks and nadirs; measurement in the peak-to-nadir or nadir-to-peak direction was determined by the first qualifying excursion. The measurement of this parameter, which has been proved to be independent of mean glycemia, is of particular interest because the greater the MAGE, the higher the glycemic instability (12).
Assessment of cognitive functions
Twenty-four to 48 h before CSGM, global cognitive function was assessed with the Mini-Mental State Examination (MMSE) corrected for educational levels of patients (13). This cognitive test covers many cognitive skills, and scores range from 0 to 30. The Trail Making Test (TMT) is a visuomotor speeded task that consists of two parts: TMT-A and TMT-B. TMT-A, a visual scanning test, requires one to draw a line connecting consecutive numbers from 1 to 25. TMT-B adds cognitive flexibility to TMT-A and requires one to draw a line connecting numbers and letters in alternating sequence (14). Although time-to-completion scores are typically used to examine aspects of attention and executive function, the difference between the two scores (TMT-B – TMT-A [DIFF B-A]) provides a measure of cognitive efficiency (15). The Wechsler Adult Intelligence Scale–Revised Digit Span is a measure of mental tracking as well as of brief storage and mental manipulation (15). The Backward Digit Span (DSP–Backward) requires the participant listen to increasingly longer lists of digits presented for immediate recall in the reverse order presented, whereas the Forward Digit Span (DSP–Forward) requires immediate recall in the exact order presented. The Verbal Fluency Test requires participants to generate as many words as possible in 1 min for a given letter (F, A, S) excluding nouns and variations of the same word (16). Dementia was assessed using a two-stage screening method. The first-stage screening was based on the MMSE. According to Italian standards (17), participants with an age- and education-adjusted MMSE score of >26 were considered to not have dementia and those with a score of 26 were directed to the second-stage screening that was performed by a geriatrician and a psychologist with long-term experience in the evaluation of older individuals with cognitive problems. The diagnosis of “dementia syndrome,” independent of etiology was established using a standard evaluation protocol based on DSM-IV criteria (18). All participants with diagnosed dementia were excluded from the protocol.
Statistical analysis
All data are expressed as means ± SD. Plasma insulin and triglycerides were log transformed for data analyses and back transformed for data presentation. We calculated the number of patients required for the study to reject the null hypothesis 99% of the time (i.e., with a one-tailed type II error rate of 0.01) when r was ≥0.80 with a two-tailed type I error at the 0.05 level of significance. Because this calculation led to a sample size of at least 110, the number of required patients was set at 121. A cluster analysis, using the squared sum of z scores, showed whether an overall value obtained by clustering attention and executive function test results was associated with MAGE. To create such a cluster analysis, we created a cognition composite score of attention and executive functions, as sum of the z scores of TMT-A, TMT-B, DIFF B-A, DSP–Forward, DSP–Backward, and Verbal Fluency. A z score indicates the position of an individual value of a variable in the total distribution of the variable in the population and is calculated as follows: (individual value – mean value)/SD. The analysis transforms the individual test scores to z scores, summing these and restandardizing this sum.
Multiple regression models were used to explore the relationship between MAGE and cognition (cognition composite score and MMSE score) independently of several covariates. The effect of therapy in patients categorized for number of antidiabetes therapies was assessed by ANOVA, and Ptrend was calculated. P < 0.05 was used of levels of significance in all tests performed.
RESULTS
The study group had mean ± SD age of 78 ± 6.7 years (range 88–65 years), A1C of 7.9 ± 0.3%, and FPG of 153 ± 10.3 mg/dl. A slightly higher number of patients were women, with modest overweight. According to age most of them were affected by comorbidity (CVD that was not stroke-related; 13 reported hypercholesterolemia and 30 reported previous hypertension) (Table 1). No patients examined had carotid plaque >70% (four patients had carotid plaque at 20–25% without significant alterations in blood flow) on a carotid ultrasound examination or white matter lesions or significant signs of cortical or subcortical atrophy with an MRI scan (data not show). All patients were treated with oral antidiabetes agents: glyburide alone (10–15 mg/day in 19 patients) or metformin alone (1,700 mg/day in 38 patients) or a combination of metformin (1,700 mg/day) and glyburide (15 mg/day) (in 51 patients) or a combination of metformin (1,700 mg/day) and thiazolidinediones (4 mg/day) (in 13 patients). The MAGE over 24 h obtained from the continuous glucose monitoring system in the 121 patients was 71 ± 19 mg/dl and the 24-h glycemic value was 176 ± 45 mg/dl.
Relationships among the cognition composite score, MMSE score, and markers of diabetic control
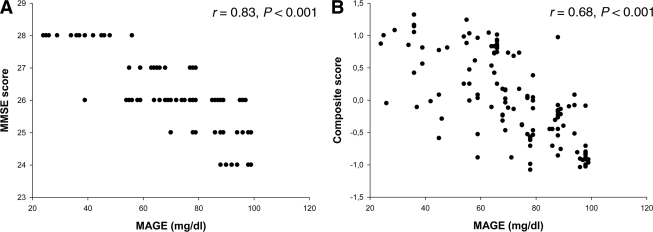
MAGE was significantly correlated with MMSE (r = 0.83 P < 0.001) and with the cognition composite score (r = 0.68, P < 0.001) (Fig. 1). This relationship persisted after adjustment for the main anthropometric (BMI and waist-to-hip ratio [WHR]), metabolic (FPG and 2-h PPG and A1C), and/or vascular (mean arterial blood pressure) covariables (data not shown). No correlation between MAGE and fasting insulin was observed (r = 0.02, P = 0.1).
Figure 1.
A: Relationship between MMSE and MAGE. B: Relationship between cognitive composite score and MAGE.
The independent effects of MAGE on both the cognition composite and MMSE scores were tested in three different multiple linear regression models (Table 2) having, respectively, age, sex, BMI, WHR, drug intake, physical activity, and MAGE (model 1), model 1 + systolic and diastolic blood pressure (model 2), and model 1+ model 2 + A1C, fasting and postprandial glucose (model 3) as covariates. In the more complex model 3 (Table 2), MAGE was associated with MMSE and cognition composite score (independently of age, sex, BMI, WHR, medication, physical activity, mean arterial blood pressure, FPG, PPG, and A1C). This last model explained 77 and 44% variability of MMSE and cognition composite score, respectively, whereas MAGE per se explained 24 and 26% variability of MMSE and cognition, respectively. Among FPG, PPG, and A1C, only PPG has been found to be independently associated with cognitive function (Table 2, model 3). All of the analyses performed gave the same results, even after adjustment for education level (data not shown).
Table 2.
Linear multivariate analyses with MMSE and composite score as dependent variable
| MMSE |
Composite score |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SEM | β | t | P value | B | SEM | β | t | P value | |
| Model 1 | ||||||||||
| Age | −0.014 | 0.005 | −0.144 | −2.68 | 0.009 | −0.021 | 0.014 | 0.125 | 1.572 | 0.119 |
| Sex | 0.106 | 0.130 | 0.045 | 0.812 | 0.418 | 0.583 | 0.345 | 0.137 | 1.692 | 0.094 |
| BMI | −0.047 | 0.020 | −0.128 | −2.31 | 0.023 | −0.056 | 0.034 | −0.138 | −2.68 | 0.031 |
| WHR | −0.474 | 0.789 | −0.032 | −0.60 | 0.549 | −1.24 | 2.09 | −0.047 | −0.597 | 0.552 |
| Drug intake | −0.069 | 0.127 | −0.029 | −0.543 | 0.588 | −0.165 | 0.336 | −0.038 | −0.491 | 0.625 |
| Physical activity | −0.012 | 0.066 | −0.010 | −0.183 | 0.855 | 0.242 | 0.175 | 0.107 | 1.38 | 0.168 |
| MAGE | −0.044 | 0.003 | −0.746 | −13.04 | 0.000 | −0.059 | 0.009 | −0.556 | −6.57 | 0.000 |
| Model 2 | ||||||||||
| Age | −0.014 | 0.005 | −0.142 | −2.77 | 0.007 | −0.022 | 0.014 | −0.129 | −1.60 | 0.112 |
| Sex | 0.161 | 0.124 | 0.068 | 1.30 | 0.195 | 0.57 | 0.348 | 0.135 | 1.64 | 0.104 |
| BMI | −0.056 | 0.020 | −0.152 | −2.84 | 0.005 | −0.047 | 0.025 | −0.111 | −2.36 | 0.004 |
| WHR | −1.00 | 0.764 | −0.068 | −1.31 | 0.193 | −1.34 | 2.114 | −0.051 | −0.635 | 0.527 |
| Drug intake | −0.053 | 0.121 | −0.022 | −0.44 | 0.660 | −0.159 | 0.340 | −0.037 | −0.468 | 0.641 |
| Physical activity | −0.006 | 0.064 | −0.005 | −0.09 | 0.921 | 0.238 | 0.177 | 0.105 | 1.344 | 0.182 |
| MAGE | −0.037 | 0.004 | −0.628 | −10.28 | 0.000 | −0.058 | 0.009 | −0.548 | −6.341 | 0.000 |
| SBP | −0.013 | 0.006 | −0.189 | −2.11 | 0.030 | −0.025 | 0.010 | −0.234 | −2.51 | 0.038 |
| DBP | −0.008 | 0.007 | −0.058 | −1.16 | 0.249 | −0.011 | 0.019 | −0.045 | −0.584 | 0.560 |
| Model 3 | ||||||||||
| Age | −0.013 | 0.005 | −0.140 | −2.73 | 0.008 | −0.021 | 0.014 | −0.122 | −1.52 | 0.131 |
| Sex | 0.190 | 0.127 | 0.080 | 1.49 | 0.139 | 0.774 | 0.357 | 0.182 | 2.16 | 0.033 |
| BMI | −0.059 | 0.020 | −0.160 | −2.98 | 0.004 | −0.025 | 0.056 | −0.038 | −0.454 | 0.651 |
| WHR | −1.23 | 0.789 | −0.083 | −1.56 | 0.121 | −2.69 | 2.217 | −0.102 | −1.216 | 0.227 |
| Drug intake | −0.056 | 0.122 | −0.023 | −0.463 | 0.644 | −0.176 | 0.342 | −0.041 | −0.513 | 0.609 |
| Physical activity | 0.005 | 0.065 | 0.004 | 0.075 | 0.940 | 0.212 | 0.182 | 0.093 | 1.161 | 0.248 |
| MAGE | −0.037 | 0.004 | −0.635 | −10.31 | 0.000 | −0.050 | 0.010 | −0.472 | −4.88 | 0.000 |
| SBP | −0.005 | 0.003 | −0.071 | −1.424 | 0.158 | −0.004 | 0.010 | −0.031 | −0.394 | 0.695 |
| DBP | −0.008 | 0.007 | −0.058 | −1.168 | 0.246 | −0.017 | 0.019 | −0.067 | −0.858 | 0.393 |
| A1C | −0.037 | 0.080 | −0.027 | −0.458 | 0.648 | −0.084 | 0.225 | −0.034 | −0.372 | 0.711 |
| PPG | −0.006 | 0.002 | −0.167 | −2.581 | 0.011 | −0.028 | 0.009 | −0.205 | −2.37 | 0.025 |
| Glucose | −0.003 | 0.003 | −0.078 | −1.302 | 0.196 | 0.010 | 0.007 | 0.129 | 1.375 | 0.172 |
For MMSE: R2 = 0.73 (model 1); R2 = 0.73 (model 2); R2 = 0.77 (model 3). For composite score: R2 = 0.40 (model 1); R2 = 0.41 (model 2); R2 = 0.44 (model 3). SBP, systolic blood pressure; DBP, diastolic blood pressure.
Relationship between glucose fluctuation (MAGE) and therapy for diabetes
To assess the impact of antidiabetes therapy, all patients were categorized in three groups according to number of antidiabetes drugs used. No differences were observed in the mean values of MAGE according to antidiabetes treatment: glyburide, 70.2 ± 29 mg/dl; metformin, 70.1 ± 18 mg/dl; metformin plus glyburide, 71.7 ± 16; or metformin plus thiazolidinediones, 73.8 ± 23. No differences in PPG, A1C, cognitive composite score, and MMSE were found among the study groups, thus showing a null impact of therapy on MMSE score and cognition composite score (data not shown).
Conclusions
Our study shows that impairment of cognitive performance in older type 2 diabetic patients may be associated with daily acute glucose fluctuations. In particular, MAGE excursions were strongly correlated with cognitive functioning, and this relationship was independent of the main markers of sustained hyperglycemia (A1C, PPG, and FPG). Even though a number of studies have investigated and compared the roles of the different glycemic indexes participating in diabetic cognitive disorders (2–4), accurate assessment of their respective contributions is still being debated. By using different methods, including epidemiological (2), interventional (19), or pathophysiological studies (5), several authors have demonstrated that A1C is certainly an independent risk factor of decline in cognitive performance in type 2 diabetes. For instance, it has been established that chronic hyperglycemia induces an overproduction of superoxide which, after reacting with nitric oxide, produces subsequent nitrosative stress with generation of metabolic derivatives such as peroxynitrite and nitrotyrosine (20). The toxicity of these substances can lead to neuronal damages and, furthermore, to a decline in cognitive performance. In this context, daily glucose fluctuations, more generally, glucose swings such as peaks and troughs, exhibited a more specific triggering effect on oxidative stress than chronic sustained hyperglycemia (21). Thus, peaks, usually corresponding to maximum values after meals, and troughs, observed over interprandial periods, could lead to continuous production of oxidative stress, impairing cognitive functioning. Oxidative stress caused by free radicals damages the endothelial cells in the blood vessels, promotes lipid peroxidation, and plays a central role in pathogenesis of cerebral complications of type 2 diabetes. Various studies have shown that increased oxidative stress can lead to microvascular cerebral diseases, e.g., stroke, cerebral hemorrhage, and brain infarction. The reason for high risk of microvascular cerebral diseases is that brain consumes 20% of the oxygen consumed by the body and has a low concentration of antioxidants and high content of unsaturated fatty acids and catecholamines that are easily oxidized, making it more vulnerable to oxidative damage than any other organ in the body, and this may predispose diabetic patients to development of cognitive impairment (22). Because the glycemic fluctuations as estimated from MAGE indexes reflect both upward and downward glucose changes, whereas A1C, PPG, and FPG values are only markers of upward variations, there is a reason to hypothesize that MAGE indexes are wider integrators of glycemic variations than the A1C, PPG, and FPG values. Our results, showing that MAGE values were associated with cognitive functioning independently of the main markers of glycemic control, seem to support such a hypothesis. We therefore suggest that acute glycemic excursions should be integrated into glycemic disorders that are larger than chronic hyperglycemia, i.e., into rapid glucose swings including declines from relatively high glucose levels during postprandial periods to low values or even to asymptomatic hypoglycemia, as observed over interprandial periods. As a consequence, low glycemic levels in type 2 diabetes might also evoke a decline in cognitive performance. Indeed, because the appropriate concentration of glucose may have a pivotal role on metabolic activity in the brain, the rapid glucose swings from relatively high glucose levels during postprandial periods to low values or even to asymptomatic hypoglycemia and its associated neuroglycopenia may contribute largely to a more rapid decline of metabolic activity in the brain (23) Thus, glucose variations over time, fluctuating from hyperglycemic peaks to glucose nadirs, may affect cognitive function in older individuals, increasing oxidative damage as well as altering the appropriate brain supply of glucose. These observations provide a possible explanation for the independent role of MAGE on cognitive function with respect to PPG, FPG, and A1C.
In summary, the present study demonstrates a significant relationship between acute glucose swings and cognitive performance impairment. A weakness in the present study is its cross-sectional, observational nature, and it is therefore difficult to draw causal relationships. However, because the glycemic disorders are risk factors for mild cognitive impairment and both vascular dementia (1–5) and Alzheimer disease, the present data open the field for conducting interventional studies with the aim of treating glycemic disorders not only by reducing A1C and mean hyperglycemia (24) but also by flattening acute glucose fluctuations.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
M.R.R. and R.M. designed the study, analyzed and interpreted data, wrote the manuscript, and revised/edited the manuscript. M.B., V.B., and F.V. analyzed and interpreted data. B.L. designed the study and revised/edited the manuscript. S.C. designed the study, wrote the manuscript, and revised/edited the manuscript. G.P. designed the study, analyzed and interpreted data, and revised/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Fontbonne A, Berr C, Ducimetière P, Alpérovitch A: Changes in cognitive abilities over a 4-year period are unfavourably affected in elderly diabetic subjects: results of the epidemiology of vascular aging study. Diabetes Care 2001; 24: 366– 370 [DOI] [PubMed] [Google Scholar]
- 2.Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, Launer LJ: Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care 2009; 32: 221– 226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shorr RI, de Rekeneire N, Resnick HE, Yaffe K, Somes GW, Kanaya AM, Simonsick EM, Newman AB, Harris TB: Glycemia and cognitive function in older adults using glucose-lowering drugs. J Nutr Health Aging 2006; 10: 297– 301 [PubMed] [Google Scholar]
- 4.Abbatecola AM, Rizzo MR, Barbieri M, Grella R, Arciello A, Laieta MT, Acampora R, Passariello N, Cacciapuoti F, Paolisso G: Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 2006; 67: 235– 240 [DOI] [PubMed] [Google Scholar]
- 5.Cox DJ, Kovatchev BP, Gonder-Frederick LA, Summers KH, McCall A, Grimm KJ, Clarke WL: Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 2005; 28: 71– 77 [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Control and Complications Trial Research Group The relationship of a glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995; 44: 968– 983 [PubMed] [Google Scholar]
- 7.Ceriello A, Hanefeld M, Leiter L, Monnier L, Moses A, Owens D, Tajima N, Tuomilehto J: Postprandial glucose regulation and diabetic complications. Arch Intern Med 2004; 164: 2090– 2095 [DOI] [PubMed] [Google Scholar]
- 8.Monnier L, Lapinski H, Colette C: Contribution of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care 2003; 26: 881– 885 [DOI] [PubMed] [Google Scholar]
- 9.Klonoff DC: Continuous glucose monitoring: roadmap for 21st century therapy. Diabetes Care 2005; 28: 1231– 1239 [DOI] [PubMed] [Google Scholar]
- 10.Marfella R, Barbieri M, Ruggiero R, Rizzo MR, Grella R, Mozzillo AL, Docimo L, Paolisso G: Bariatric surgery reduces oxidative stress by blunting 24-h acute glucose fluctuations in type 2 diabetic obese patients. Diabetes Care 2010; 33: 287– 289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF: Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970; 19: 644– 655 [DOI] [PubMed] [Google Scholar]
- 12.Service FJ, O'Brien PC, Rizza RA: Measurements of glucose control. Diabetes Care 1987; 10: 225– 237 [DOI] [PubMed] [Google Scholar]
- 13.Lezak M, Howieson D, Loring D: Neuropsychological Assessment. 4th ed Oxford, U.K., Oxford University Press, 1999 [Google Scholar]
- 14.Reiten R, Wolfson D: eds. The Halstead–Reitan Neuropsychologic Test Battery: Theory and Clinical Interpretation. Tucson, AZ, Neuropsychology Press, 1993 [Google Scholar]
- 15.Heaton RK, Nelson LM, Thompson DS, Burks JS, Franklin GM: Neuropsychologic findings in relapsing-remitting and chronic-progressive multiple sclerosis. J Consult Clin Psychol 1985; 53: 103– 110 [DOI] [PubMed] [Google Scholar]
- 16.Carlesimo GA, Caltagirone C, Gainotti G: The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 1996; 36: 378– 384 [DOI] [PubMed] [Google Scholar]
- 17.Grigoletto F, Zappalà G, Anderson DW, Lebowitz BD: Norms for the Mini-Mental State Examination in a healthy population. Neurology 1999; 53: 315– 320 [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th ed Washington, DC, American Psychiatric Press, 1994 [Google Scholar]
- 19.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW: Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care 2006; 29: 345– 351 [DOI] [PubMed] [Google Scholar]
- 20.Brownlee M:: The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615– 1625 [DOI] [PubMed] [Google Scholar]
- 21.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C: Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681– 1687 [DOI] [PubMed] [Google Scholar]
- 22.Dickinson PJ, Carrington AL, Frost GS, Boulton AJ: Neurovascular disease, antioxidants and glycation in diabetes. Diabetes Metab Res Rev 2002; 18: 260– 272 [DOI] [PubMed] [Google Scholar]
- 23.Ratcliff G, Dodge H, Birzescu M, Ganguli M: Tracking cognitive functioning over time: ten-year longitudinal data from a community-based study. Appl Neuropsychol 2003; 2: 76– 88 [DOI] [PubMed] [Google Scholar]
- 24.Mussell M, Hewer W, Kulzer B, Bergis K, Rist F: Effects of improved glycaemic control maintained for 3 months on cognitive function in patients with type 2 diabetes. Diabet Med 2004; 21: 1253– 1256 [DOI] [PubMed] [Google Scholar]