Abstract
OBJECTIVE
New clinical practice recommendations include A1C as an alternative to fasting glucose as a diagnostic test for identifying pre-diabetes. The impact of these new recommendations on the diagnosis of pre-diabetes is unknown.
RESEARCH DESIGN AND METHODS
Data from the National Health and Nutrition Examination Survey 1999–2006 (n = 7,029) were analyzed to determine the percentage and number of U.S. adults without diabetes classified as having pre-diabetes by the elevated A1C (5.7–6.4%) and by the impaired fasting glucose (IFG) (fasting glucose 100–125 mg/dl) criterion separately. Test characteristics (sensitivity, specificity, and positive and negative predictive values) using IFG as the reference standard were calculated.
RESULTS
The prevalence of pre-diabetes among U.S. adults was 12.6% by the A1C criterion and 28.2% by the fasting glucose criterion. Only 7.7% of U.S. adults, reflecting 61 and 27% of those with pre-diabetes by A1C and fasting glucose, respectively, had pre-diabetes according to both definitions. A1C used alone would reclassify 37.6 million Americans with IFG to not having pre-diabetes and 8.9 million without IFG to having pre-diabetes (46.5 million reclassified). Using IFG as the reference standard, pre-diabetes by the A1C criterion has 27% sensitivity, 93% specificity, 61% positive predictive value, and 77% negative predictive value.
CONCLUSIONS
Using A1C as the pre-diabetes criterion would reclassify the pre-diabetes diagnosis of nearly 50 million Americans. It is imperative that clinicians and health systems understand the differences and similarities in using A1C or IFG in diagnosis of pre-diabetes.
Diabetes, a condition associated with high morbidity and mortality, has reached epidemic status among Americans (1). At present, 24 million Americans have diabetes with indirect and direct costs exceeding 174 billion dollars annually (1,2). The high burden and prognostic implications of diabetes have led to increasing attempts to prevent its development. Interventions for individuals and populations at high risk for diabetes or with pre-diabetes are a major focus for these prevention efforts (3).
Until recently, clinical practice guidelines have defined pre-diabetes as either impaired fasting glucose (IFG) (fasting plasma glucose [FPG] of 100–125 mg/dl) or impaired glucose tolerance (IGT) (glucose of 140–199 mg/dl on a 2-h oral glucose tolerance test [OGTT]). Using these definitions, ∼57 million Americans have pre-diabetes (1). In clinical practice, FPG is a more commonly used test, compared with an OGTT, because of its logistical advantages (4). However, IFG still requires individuals to fast for at least 8 h before testing.
The American Diabetes Association (ADA) recently updated its screening recommendation for pre-diabetes to include A1C, a nonfasting test, as another diagnostic testing option (5). Specific to pre-diabetes, these recommendations state that A1C from 5.7 to 6.4% identifies individuals at high risk for diabetes and that the label of pre-diabetes can be applied (5). The revised recommendations imply that the practical advantages of A1C over FPG and OGTT will make screening more widespread and help clinicians identify and intervene in this high-risk population. The guidelines do not comment on whether clinicians should use each test in isolation or in combination, but logistical advantages may make A1C a clinician's preferred test.
In this study, we used data from the National Health and Nutrition Examination Survey (NHANES) 1999–2006 to evaluate the impact of this new recommendation on the proportion of Americans in the general population who would be classified as having pre-diabetes. Specifically, we examined the concordance in the number of U.S. adults identified as having pre-diabetes via FPG and A1C. In addition, we determined differences and similarities among the populations identified as having pre-diabetes by the A1C criterion and also by the IFG screening criterion.
RESEARCH DESIGN AND METHODS
NHANES 1999–2000, 2001–2002, 2003–2004, and 2005–2006 are serial cross-sectional surveys including nationally representative samples of the noninstitutionalized civilian U.S. population identified through a stratified, multistage probability sampling design. Methods for pooling these datasets are published on the NHANES website (6). NHANES 1999–2006 included 7,975 adults, aged ≥20 years, who attended the morning examination and had fasted for 9–24 h at the time of their blood collection. Participants missing FPG or A1C measurements (n = 37) with a prior self-reported diagnosis of diabetes (n = 537) and those meeting the criteria for diabetes (FPG ≥126 mg/dl or A1C ≥6.5%) were excluded (n = 372) from the current analyses. After these exclusions, the current analyses were based on data from 7,029 participants without diabetes.
NHANES data were collected through questionnaires, a physical examination, and blood collection. Details of the phlebotomy process in NHANES 1999–2006 are provided elsewhere (6). Of particular relevance to the current report, serum glucose was measured using a modified hexokinase enzymatic method, and A1C was measured using a high-performance liquid chromatography system by the Diabetes Diagnostic Laboratory at the University of Missouri–Columbia and by the Fairview Medical Center Laboratory at the University of Minnesota for 2005–2006. A1C and glucose values were recalibrated for NHANES 2005–2006 to account for the differences in the assays used among laboratories (7,8). Both assays have been validated and are Diabetes Complications and Control Trial–aligned (9). The coefficient of variation was <3% in each 2-year period for glucose and <2% for A1C.
In 2005–2006, a subset of 1,680 participants completed an OGTT after an overnight fast of 9–24 h. Of these participants, the current analysis was conducted among 1,382 participants without diabetes (i.e., no self-report, A1C <6.5%, FPG <126 mg/dl, and OGTT <200 mg/dl). After the initial venipuncture, participants were asked to drink a calibrated dose, ∼75 g of glucose, and had a second venipuncture 2 h (±15 min) later.
Statistical analysis
Participants were categorized into one of four mutually exclusive groups by the presence or absence of pre-diabetes according to IFG and A1C criteria. Characteristics of the study population were calculated for each of these four categories as mean ± SE, median (25th, 75th percentiles), or percentages, as appropriate. The prevalence of pre-diabetes was then calculated for IFG and various cut points of A1C. The sensitivity, specificity, and positive and negative predictive values for having pre-diabetes by IFG were then calculated for each A1C level. In addition, the number of U.S. adults with discordant pre-diabetes results based on IFG and A1C values was determined. Two sensitivity analyses were conducted. First, the test characteristics of pre-diabetes by A1C versus IFG defined as FPG levels of 110–125 mg/dl, the previous fasting glucose range for defining pre-diabetes, were determined. Second, using the subset of participants with OGTT results, the sensitivity, specificity, and positive and negative predictive values were determined for pre-diabetes by IFG and A1C, separately, and for the presence of either or both of these test results using IGT as the reference standard. All analyses were weighted to represent the U.S. population and conducted using SUDAAN (version 9; Research Triangle Institute, Research Triangle Park, NC) to account for the complex survey design of NHANES. NHANES sampling weights were adjusted to account for missing values. Adjustment of the sampling weights corrects for differences in missing data across age, sex, and race/ethnicity strata but assumes that data within strata are missing, randomly (10).
RESULTS
Participants with pre-diabetes by A1C but not by IFG were more likely to be women, non-Hispanic blacks, and hypertensive and to have hypercholesterolemia, chronic kidney disease, microalbuminuria, elevated C-reactive protein, and lower triglycerides than those with pre-diabetes by IFG but normal A1C (Table 1). Overall, participants with pre-diabetes by both criteria had higher levels of the cardiovascular risk factors investigated.
Table 1.
Characteristics of NHANES 1999–2006 study participants by A1C and fasting glucose
| A1C <5.7% |
A1C ≥5.7% |
|||
|---|---|---|---|---|
| FPG <100 mg/dl | FPG ≥100 mg/dl | FPG <100 mg/dl | FPG ≥100 mg/dl | |
| n | 4,439 | 1,430 | 446 | 714 |
| Age (years) | 40.8 ± 0.4 | 49.1 ± 0.6 | 54.0 ± 0.9 | 58.1 ± 0.6 |
| Women (%) | 57.8 | 36.0 | 54.2 | 51.3 |
| Race/ethnicity | ||||
| Non-Hispanic white (%) | 76.2 | 80.8 | 62.5 | 71.7 |
| Non-Hispanic black (%) | 11.2 | 5.6 | 25.5 | 15.2 |
| Hispanic (%) | 7.5 | 8.3 | 8.2 | 7.0 |
| Current smoker (%) | 25.8 | 22.2 | 27.1 | 20.7 |
| Systolic blood pressure (mmHg) | 118.3 ± 0.4 | 125.9 ± 0.6 | 129.9 ± 1.1 | 130.7 ± 0.8 |
| Diastolic blood pressure (mmHg) | 70.5 ± 0.3 | 72.5 ± 0.4 | 72.2 ± 0.7 | 71.8 ± 0.7 |
| Hypertension (%) | 18.1 | 34.2 | 40.8 | 53.4 |
| BMI (kg/m2) | 26.8 ± 0.1 | 29.4 ± 0.3 | 29.9 ± 0.4 | 31.4 ± 0.4 |
| Waist circumference (cm) | 92.1 ± 0.3 | 101.0 ± 0.6 | 101.2 ± 1.0 | 105.4 ± 0.7 |
| Total cholesterol (mg/dl) | 198.0 ± 0.9 | 205.1 ± 1.4 | 211.7 ± 2.5 | 207.6 ± 2.1 |
| HDL cholesterol (mg/dl) | 55.1 ± 0.3 | 50.0 ± 0.6 | 51.1 ± 0.9 | 49.5 ± 0.7 |
| Triglycerides (mg/dl) | 102 (73–149) | 134 (91–192) | 129 (90–188) | 136 (101–195) |
| eGFR <60 ml/min per 1.73m2 (%) | 6.1 | 8.7 | 12.3 | 15.5 |
| Microalbuminuria (%) | 5.6 | 8.3 | 13.1 | 13.3 |
| Hemoglobin (g/dl) | 14.4 ± 0.1 | 15.0 ± 0.1 | 14.3 ± 0.1 | 14.6 ± 0.1 |
| Serum albumin (g/dl) | 4.30 ± 0.01 | 4.32 ± 0.01 | 4.17 ± 0.03 | 4.18 ± 0.02 |
| Ferritin (ng/ml) | 56 (28–111) | 104 (51–193) | 81 (32–159) | 93 (45–173) |
| Aspartate aminotransferase (units/l) | 24.2 ± 0.3 | 26.5 ± 0.5 | 27.1 ± 1.7 | 25.6 ± 0.4 |
| Alanine aminotransferase (units/l) | 24.3 ± 0.3 | 30.3 ± 1.8 | 28.7 ± 2.1 | 27.9 ± 0.8 |
| C-reactive protein ≥2 mg/l (%) | 45.4 | 55.2 | 65.0 | 69.5 |
| Fasting plasma glucose (mg/dl) | 90.2 ± 0.2 | 106.1 ± 0.2 | 93.3 ± 0.3 | 109.6 ± 0.4 |
| A1C (%) | 5.2 (5.0–5.3) | 5.3 (5.1–5.5) | 5.8 (5.7–5.9) | 5.9 (5.8–6.0) |
Data are ± SE, %, or median (25th–75th percentiles). eGFR, estimated glomerular filtration rate.
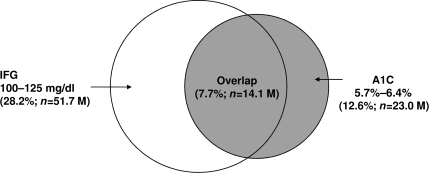
Table 2 depicts how various A1C cut points affect the potential distribution of U.S. adults categorized as having pre-diabetes by the A1C and/or IFG criteria. The recommended pre-diabetes A1C range between 5.7 and 6.4% provides the highest agreement with IFG. Nonetheless, the overlap is low; among U.S. adults without diabetes, 7.7% have pre-diabetes according to both the IFG and A1C criteria, whereas 4.9% have pre-diabetes by the A1C but not the IFG criterion and 20.5% by the IFG but not the A1C criterion (Fig. 1).
Table 2.
Distribution of U.S. adults without diagnosed diabetes by the cross-classification of A1C and fasting glucose, using different A1C cut points
| A1C cut point | < A1C cut point |
≥ A1C cut point |
||
|---|---|---|---|---|
| FPG <100 mg/dl | FPG ≥100 mg/dl | FPG <100 mg/dl | FPG ≥100 mg/dl | |
| 5.3% | 39.8 ± 1.3 | 7.3 ± 0.5 | 32.0 ± 0.9 | 20.9 ± 0.8 |
| 5.4% | 48.2 ± 1.3 | 10.3 ± 0.6 | 23.6 ± 0.9 | 17.9 ± 0.7 |
| 5.5% | 57.8 ± 1.3 | 13.8 ± 0.7 | 14.0 ± 0.7 | 14.4 ± 0.6 |
| 5.6% | 63.2 ± 1.2 | 17.5 ± 0.8 | 8.6 ± 0.5 | 10.8 ± 0.5 |
| 5.7% | 66.9 ± 1.1 | 20.5 ± 0.9 | 4.9 ± 0.3 | 7.7 ± 0.4 |
| 5.8% | 69.2 ± 1.1 | 23.0 ± 1.0 | 2.5 ± 0.2 | 5.3 ± 0.4 |
| 5.9% | 70.2 ± 1.1 | 24.6 ± 1.0 | 1.6 ± 0.2 | 3.7 ± 0.3 |
| 6.0% | 71.1 ± 1.1 | 25.8 ± 1.1 | 0.7 ± 0.1 | 2.5 ± 0.2 |
| 6.1% | 71.4 ± 1.1 | 26.7 ± 1.1 | 0.4 ± 0.1 | 1.6 ± 0.2 |
| 6.2% | 71.6 ± 1.1 | 27.3 ± 1.1 | 0.2 ± 0.1 | 0.9 ± 0.1 |
| 6.3% | 71.7 ± 1.1 | 27.8 ± 1.1 | 0.1 ± 0.1 | 0.4 ± 0.1 |
| 6.4% | 71.8 ± 1.1 | 28.1 ± 1.1 | 0 ± 0 | 0.1 ± 0.004 |
Data are prevalence estimates ± SE.
Figure 1.
Overlap of pre-diabetes by IFG and A1C among U.S. adults without diabetes. %, percentage of U.S. adults. IFG, FPG of 100–125 mg/dl; M, million.
The prevalence of pre-diabetes by the IFG criterion was 28.2%. Using IFG as the reference standard for pre-diabetes, A1C levels between 5.7% and 6.4% would reclassify 37.6 million Americans with IFG as not having pre-diabetes and 8.9 million without IFG as having pre-diabetes for a total of 46.5 million reclassified (Table 3). At higher A1C cut points for defining pre-diabetes, the number of U.S. adults reclassified as having pre-diabetes by A1C but not IFG is lower. This modification decreases the sensitivity and negative predictive value of diagnosing pre-diabetes but at the same time increases the specificity and positive predictive value. The total number of U.S. adults reclassified, using IFG as the reference standard for pre-diabetes, is lowest at an A1C level of 5.7–6.4%.
Table 3.
Sensitivity, specificity, positive predictive value, negative predictive value, and number reclassified according to different A1C cut points
| A1C cut point | Test characteristics (%) |
Number reclassified (millions) |
|||||
|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | IFG | No IFG | Total reclassified | |
| n | 51.7 | 131.4 | |||||
| 5.3% | 74 | 55 | 40 | 84 | 13.4 | 58.6 | 72.0 |
| 5.4% | 63 | 67 | 43 | 82 | 18.9 | 43.2 | 62.1 |
| 5.5% | 51 | 81 | 51 | 81 | 25.3 | 25.6 | 50.9 |
| 5.6% | 38 | 88 | 56 | 78 | 32.0 | 15.8 | 47.8 |
| 5.7% | 27 | 93 | 61 | 77 | 37.6 | 8.9 | 46.5 |
| 5.8% | 19 | 96 | 68 | 75 | 42.0 | 4.6 | 46.6 |
| 5.9% | 13 | 98 | 70 | 74 | 45.0 | 2.9 | 47.9 |
| 6.0% | 9 | 99 | 77 | 73 | 47.2 | 1.3 | 48.5 |
| 6.1% | 6 | 99 | 82 | 73 | 48.8 | 0.6 | 49.4 |
| 6.2% | 3 | 100 | 82 | 72 | 49.9 | 0.3 | 50.2 |
| 6.3% | 1 | 100 | 87 | 72 | 51.0 | 0.1 | 51.1 |
| 6.4% | 0 | 100 | 98 | 72 | 51.5 | 0.0 | 51.5 |
For test characteristics, values higher than the A1C cut point are considered positive test results, and fasting plasma glucose of 100–125 mg/dl is the reference standard for pre-diabetes. In calculating the number reclassified, NHANES sampling weights were adjusted to account for missing values. Specifically, sampling weights were calibrated based on the proportion of NHANES 1999–2004 participants missing data by age-group (<40, 40–59, 60–74, and ≥75 years), sex, and race/ethnicity. Adjusting the sampling weights correct for differences in missing data across age, sex, and race/ethnicity strata but assumes that data within strata are missing randomly (10).
Sensitivity analyses
When a more restrictive IFG definition (i.e., FPG of 110–125 mg/dl) is applied, the prevalence of pre-diabetes decreases from 28.2–7.7%. Of the 20.5% of U.S. adults (n = 37.6 million) with pre-diabetes by IFG but not A1C, 79.0% (n = 29.8 million) have FPG between 100 and 109 mg/dl. Overall, 9.1% of U.S. adults have pre-diabetes by A1C but a FPG <110 mg/dl, 4.9% have A1C in the normal range but FPG of 110–125 mg/dl, and 3.4% have both pre-diabetes level A1C and FPG of 110–125 mg/dl. The test characteristics for pre-diabetes defined by A1C using FPG levels of 110–125 mg/dl rather than 100–125 mg/dl as the reference criterion, produces a lower sensitivity (45%) and specificity (90%). Further, because the prevalence of pre-diabetes is lower, as expected, the positive predictive value (27%) is lower and the negative predictive value (95%) is higher.
The prevalence of pre-diabetes by IGT was 16.5%, whereas 5.7 and 11.6% of U.S. adults have pre-diabetes via IGT despite normal FPG and A1C, respectively. However, using IGT alone would classify 24.3 and 9.4% of U.S. adults as not having pre-diabetes despite pre-diabetes by IFG and A1C, respectively. With IGT as the reference standard, the sensitivity, specificity, and positive and negative predictive values for pre-diabetes were 30, 89, 33, and 87% by A1C; 59, 79, 34, and 91% by IFG; 22, 95, 43, and 87% by A1C and IFG; and 66, 73, 31, and 92% by A1C or IFG, respectively.
CONCLUSIONS
Data from the current study indicate that the 2010 ADA recommendations for inclusion of A1C as an acceptable pre-diabetes diagnostic criterion will have a substantial impact on the number of U.S. adults identified as having pre-diabetes with the classification being different for nearly 50 million Americans. If providers use A1C alone, they will classify 8.9 million people who would have been considered normal by fasting glucose as having pre-diabetes. However, they will also reclassify 37.6 million people as not having pre-diabetes by A1C who would have been labeled as having pre-diabetes by the IFG criteria. This discordance is in contrast to a relatively good agreement between A1C and fasting glucose when applied to the diagnosis of diabetes (11).
The inclusion of A1C is designed to increase the feasibility and dissemination of diabetes screening because it eliminates the need for fasting before testing. This practical advantage is likely to be well received by primary care providers working in environments with increasing constraints. Because A1C, FPG, and OGTT are all considered acceptable diagnostic tests for pre-diabetes by the ADA, there may be a strong shift toward using A1C alone to identify patients with pre-diabetes and diabetes. Recent data demonstrating A1C as an independent predictor of incident cardiovascular disease and death in addition to diabetes will only reinforce this shift (12). The current data suggest that further discussion is needed because using A1C alone will lead to a reclassification as normal of many patients who previously (i.e., using IFG) were considered to have pre-diabetes. This reclassified group includes a large number of individuals (n = 8 million) with fasting glucose in the range of 110–125 mg/dl. Fasting glucose in this range is associated with a substantially higher risk for diabetes incidence (5–7 times greater than that with fasting glucose of 100–109 mg/dl) (13). Whereas combined use of either IFG or A1C as the criteria of pre-diabetes would be more sensitive, it would eliminate the practical advantages of using A1C alone.
It is important to recognize that either criterion (fasting glucose or A1C) is a diagnostic test, not a traditional screening test in that it is not compared with a true criterion standard. Astute clinicians may recognize the shortcomings of each test; however, many providers will probably choose only one or the other for use in practice. The clinical guidelines do not comment on the need for follow-up testing for pre-diabetes. This situation increases the likelihood of higher degrees of variation in screening practices with a possibility of more confusion among providers and patients. Educational interventions may need to be developed to help primary care providers and patients understand the advantages and shortcomings of each test used alone or in combination.
Pre-diabetes is a label developed to identify those at highest risk for incident diabetes in the near future. Data from observational studies suggest that 25–40% of individuals with pre-diabetes will develop diabetes over the next 3–8 years (14–16). Guidelines suggest targeting individuals identified as having pre-diabetes for early interventions. Currently, the most effective intervention for the prevention or delay of diabetes is intensive lifestyle behavior change with metformin therapy a less potent alternative (14). Whereas intensive lifestyle interventions are recommended for individuals identified via IFG, A1C, or IGT, current guidelines recommend that metformin be reserved for those with both combined IFG and IGT plus other risk factors such as A1C >6%, hypertension, low HDL cholesterol, elevated triglycerides, or a family history of diabetes in a first-degree relative who are obese and aged >60 years (5). This recommendation has led some investigators to call for follow-up IGT testing in all individuals identified as having pre-diabetes based on their fasting glucose (17). This is in part due to the observation that the odds for incident diabetes are fourfold higher among individuals with both IFG and IGT than either alone (odds ratio 39.5 vs. 10.0 and 10.9, respectively) (18). With the use of A1C as a legitimate alternative diagnostic criterion for pre-diabetes, the need for a follow-up OGTT before prescribing metformin may become a point of contention. If an OGTT is not performed, combined criteria of A1C with follow-up IFG testing could be used because this group would also be very high risk, and the data could be combined with other metabolic abnormalities using one of the established diabetes risk calculators to generate a more precise risk estimate (19,20). Which criterion is more accurate and clinically relevant for predicting diabetes and tailoring interventions will need to be examined in future prospective studies.
The low concordance among A1C-, IFG-, and IGT-based diagnosis of pre-diabetes highlights the multifactorial pathophysiology of glucose dysfunction. IFG is predominately a dysfunction in hepatic insulin resistance, whereas IGT is dominated by muscle insulin resistance (21). Each is also associated with dysfunctional insulin secretion but with different patterns (21). In contrast to the daily glucose snapshot offered by IFG and IGT, A1C represents chronic exposure (over 2–3 months) to basal and postprandial hyperglycemia and reflects a combination of these mechanisms (22). Together, the different mechanisms underlying each test help explain the discordant diagnoses of pre-diabetes using IFG, A1C, and OGTT. As diabetes itself develops, each underlying mechanism plays a role, which may help explain the reasonable concordance between A1C and IFG in diabetes (11). Nevertheless, clinicians need to be aware that each test will classify as normal a substantial proportion of those found to be pre-diabetic by one of the other tests.
The current study needs to be interpreted in the context of certain potential limitations. Most notably, each test (FPG, A1C, and OGTT) was only performed once in NHANES 1999–2006, and the test results may change over time. Even though the coefficient of variation for A1C is quite small, the clustering of pre-diabetes around 5.8% suggests that small differences could have a disproportionate impact on classification of pre-diabetes. However, unpublished sensitivity analyses (and Table 2) that vary the pre-diabetes A1C criteria to 5.6% showed insignificant differences. In addition, hemolytic anemias are known to artificially lower A1C levels although this concern is minimal, considering the relative rarity of these conditions compared with pre-diabetes. Despite these limitations, the current study has many strengths. Most notably, NHANES 1999–2006 uses a complex stratified sampling design to recruit participants, which allows estimates to be made for the U.S. population. In addition, NHANES 1999–2006 includes a broad range of demographic, medical, and biochemical data collected by trained staff, following a standardized protocol.
In summary, the revised 2010 ADA Standards of Medical Care in Diabetes includes three options for identifying patients at high risk for diabetes: FPG, A1C, and OGTT. Previously, IFG has been far preferred over IGT owing to its logistical advantages. Because A1C has significant practical advantages over IFG, it is likely to become the preferred test among primary care providers for diagnosing pre-diabetes. The clinical impact of this change is not yet known, but the current analysis suggests that it will substantially alter the population identified as having pre-diabetes with tens of millions of Americans who would have been considered to have pre-diabetes previously being classified as not having pre-diabetes. This subgroup of individuals includes a substantial number with fasting glucose levels of 110–125 mg/dl and a burden of cardiovascular risk factors similar to that of their counterparts with pre-diabetes by A1C and IFG. The impact of not identifying these individuals is unclear. Policy makers and clinicians will need to consider the tradeoffs between performing both FPG and A1C testing alone or in combination.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
D.M.M. contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript. A.P.C., D.S., V.F., and C.S.F. contributed to discussion and reviewed/edited the manuscript. P.M. researched data, contributed to discussion, and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Centers for Disease Control and Prevention National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008 [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008; 31: 596– 615 [DOI] [PubMed] [Google Scholar]
- 3.Narayan KM, Williamson DF: Prevention of type 2 diabetes: risk status, clinic, and community. J Gen Intern Med 2010; 25: 154– 157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanath A, Pereira O, Philip S, Mchardy K: Diagnosing diabetes mellitus: contemporary use of the oral glucose tolerance test in a regional diabetes centre. Pract Diabetes Int 2006; 23: 287– 290 [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010; 33: S11– S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, National Center for Health Statistics NHANES: Questionnaires, datasets, and related documentation [article online], 2009. Available from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm Accessed March, 2010
- 7.Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Survey 2005–2006: Documentation, codebook, and frequencies. Plasma fasting glucose and insulin [article online], 2009. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/glu_d.pdf Accessed March, 2010
- 8.Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Survey 2005–2006: Documentation, codebook, and frequencies. Glycohemoglobin [article online], 2010. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/ghb_d.pdf Accessed March, 2010
- 9.Kalyani RR, Saudek CD, Brancati FL, Selvin E: Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Diabetes Care 2010; 33: 1055– 1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1– 12 [DOI] [PubMed] [Google Scholar]
- 11.Carson AP, Reynolds K, Fonseca VA, Muntner P: Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 2010; 33: 95– 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL: Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010; 362: 800– 811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, Fox CS: Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol 2008; 51: 264– 270 [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M: Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002; 359: 2072– 2077 [DOI] [PubMed] [Google Scholar]
- 16.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR: Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006; 368: 1096– 1105 [DOI] [PubMed] [Google Scholar]
- 17.Rhee MK, Herrick K, Ziemer DC, Vaccarino V, Weintraub WS, Narayan KM, Kolm P, Twombly JG, Phillips LS: Many Americans have pre-diabetes and should be considered for metformin therapy. Diabetes Care 2010; 33: 49– 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vegt F, Dekker JM, Jager A, Hienkens E, Kostense PJ, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ: Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: the Hoorn Study. JAMA 2001; 285: 2109– 2113 [DOI] [PubMed] [Google Scholar]
- 19.Droumaguet C, Balkau B, Simon D, Caces E, Tichet J, Charles MA, Eschwege E: Use of HbA1c in predicting progression to diabetes in French men and women: data from an Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2006; 29: 1619– 1625 [DOI] [PubMed] [Google Scholar]
- 20.Mann DM, Bertoni AG, Shimbo D, Carnethon MR, Chen H, Jenny NS, Muntner P: Comparative validity of 3 diabetes mellitus risk prediction scoring models in a multi-ethnic US cohort: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010; 171: 980– 988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B: Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007; 30: 753– 759 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB: Tests of glycemia in diabetes. Diabetes Care 2004; 27: 1761– 1773 [DOI] [PubMed] [Google Scholar]