Abstract
OBJECTIVE
To study mortality in relation to fasting plasma glucose (FPG) and 2-h plasma glucose levels within the normoglycemic range.
RESEARCH DESIGN AND METHODS
Data from 19 European cohorts comprising 12,566 men and 10,874 women who had FPG <6.1 mmol/l and 2-h plasma glucose <7.8 mmol/l at baseline examination were analyzed. Multivariate-adjusted hazard ratios (HRs) and 95% CIs for deaths from cardiovascular disease (CVD), non-CVD, and all causes were estimated for individuals whose 2-h plasma glucose > FPG (group II) compared with those whose 2-h plasma glucose ≤ FPG (group I).
RESULTS
A total of 827 (246) CVD and 611 (351) non-CVD and 1,438 (597) all-cause deaths occurred in men (women). Group II was older and had higher BMI, blood pressure, and fasting insulin than group I. The multivariate-adjusted HRs (95% CIs) for CVD, non-CVD, and all-cause mortality were 1.22 (1.05–1.41), 1.09 (0.92–1.29), and 1.16 (1.04–1.30) in men and 1.40 (1.03–1.89), 0.99 (0.79–1.25), and 1.13 (0.94–1.35) in women, respectively, for group II as compared with group I. HRs were 1.25 (1.05–1.50), 1.09 (0.89–1.34), and 1.18 (1.03–1.35) in men and 1.60 (1.03–2.48), 1.05 (0.78–1.42), and 1.18 (0.93–1.51) in women, respectively, after additional adjustment for fasting insulin in a subgroup of individuals.
CONCLUSIONS
In individuals with both FPG and 2-h plasma glucose within the normoglycemic range, high 2-h plasma glucose was associated with insulin resistance and increased CVD mortality.
It is well known that type 2 diabetes (1,2) and nondiabetic hyperglycemia such as impaired glucose tolerance are risk factors for cardiovascular disease (CVD) mortality (3–5). The relations of fasting plasma glucose (FPG) and 2-h plasma glucose with CVD mortality and morbidity have been extensively investigated during the last few decades (6–9). Evidence has shown that 2-h plasma glucose is a stronger risk predictor than FPG for incident coronary heart disease (6) and CVD mortality (7), but little is known about the impact of FPG versus 2-h plasma glucose in the normoglycemic range. It has been suggested that individuals with normoglycemia, whose 2-h plasma glucose did not return to the FPG levels during an oral glucose tolerance test (OGTT) had a significantly higher risk of developing type 2 diabetes (10) and a worse cardiovascular risk factor profile (11) than individuals whose 2-h plasma glucose returned to the FPG levels. In the current study, based on the data of the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) study, we compared CVD mortality in individuals whose 2-h plasma glucose was higher than FPG with those whose 2-h plasma glucose was equal to or lower than FPG.
RESEARCH DESIGN AND METHODS
The methods to recruit participants for the DECODE cohorts have been described previously (6,12–14). In brief, the database was collected from researchers who had performed epidemiological studies using a standard OGTT in Europe. Data of individuals from participating study centers were sent to the Diabetes Prevention Unit, Department of Chronic Disease Prevention of the National Institute for Health and Welfare in Helsinki, Finland, for analyses. Each study had been approved by the local ethics committees, and the ethics committee of the National Institute for Health and Welfare approved the data analysis. In this article, only the cohorts with prospective data on cause-specific mortality and with all required covariates of BMI, blood pressure, total cholesterol, and smoking status were included.
Subjects with known diabetes and those classified as having newly diagnosed diabetes and pre-diabetes according to the World Health Organization/International Diabetes Federation 2006 criteria (15) were excluded from the current study. Thus, the current data analysis is restricted to normoglycemic individuals whose FPG <6.1 mmol/l and 2-h plasma glucose <7.8 mmol/l (15), comprising 12,566 (53.6% of all participants) men and 10,874 (46.4%) women aged 25–90 years from 19 European cohorts. The maximum duration of follow-up ranged from 4.8 to 36.8 years among different cohorts with a median follow-up of 9.0 years. According to FPG and 2-h plasma glucose levels, these individuals were further divided into two groups. In group I, an individual's 2-h plasma glucose concentration was equal to or less than his or her FPG, whereas in group II, 2-h plasma glucose was greater than FPG.
BMI was defined as the individual's body weight in kilograms divided by the square of height in meters. An individual with a history of hypertension diagnosed by a physician or having systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg was classified as having hypertension (16). Smoking status was classified as current smoker, former smoker, or nonsmoker.
Definition of fatal events
Vital status was recorded for each of the subjects attending the baseline examination in all of the studies. Subjects who had emigrated and for whom vital status could not be confirmed were treated as censored at the time of emigration. Causes of death were coded according to ICD-9 (ICD-10). Total CVD death was defined as ICD codes 401–448 (I10–I79); all other deaths were classified as non-CVD.
Statistical methods
A general linear model of univariate ANOVA was used to estimate means adjusted for age and cohort. The χ2 test was used to test the difference in proportions between groups. Considering the difference in laboratory assays of fasting insulin between cohorts, we determined a z score (z = [χ − μ]/σ) transformation for fasting insulin for each cohort before the data were pooled together. The assumption of the proportionality was examined using log minus log survival plots for each categorical variable. None of these indicated departure from the assumption of the proportionality of hazards. Cox proportional hazards analysis was used to calculate hazard ratios (HRs) and their 95% CIs for CVD, non-CVD, and all-cause mortality. The models were adjusted for age, cohort, FPG, BMI, total cholesterol, smoking status, hypertension status, and fasting insulin. The difference between the 2-h plasma glucose and the FPG (2-h plasma glucose − FPG) as a continuous variable was also fitted in a separate multivariable model to examine whether the relationship was linear. In addition, to check whether the “return of the 2-h plasma glucose to the FPG level” was determined by the FPG levels, the comparison of group II versus group I was further made in two FPG subgroups: FPG ≤5.6 mmol/l and 5.6 mmol/l < FPG <6.1 mmol/l. Cumulative survival curves were derived from the same multivariate Cox proportional hazards analysis. Statistical analyses were performed using SPSS for Windows (version 15.0; SPSS Inc. Chicago, IL). P < 0.05 (two-tailed) was considered statistically significant.
RESULTS
A total of 1,438 deaths in men and 597 in women occurred during the follow-up. Among these, 827 men and 246 women died of CVD (Table 1). Mortality from CVD and all causes was higher in group II than in group I in both men and women (P < 0.001 for all comparisons) (Table 2). People in group II were older and had significantly higher levels of BMI, blood pressure, and fasting insulin than individuals in group I for both men and women (P < 0.001 for all comparisons) (Table 2).
Table 1.
Baseline characteristics of study cohorts and number of deaths from CVD, non-CVD, and all causes in individuals with FPG <6.1 mmol/l and 2-h plasma glucose <7.8 mmol/l
| Countries and studies | n (men/women) | Age (years) | FPG (mmol/l)* | 2-h PG (mmol/l)* | No. of deaths (men/women) |
Follow-up years | ||
|---|---|---|---|---|---|---|---|---|
| CVD | Non-CVD | All cause | ||||||
| Demark | ||||||||
| Glostrup | 461/544 | 54 (39–70) | 5.4 (5.4–5.5) | 5.9 (5.8–5.9) | 98/61 | 68/47 | 166/108 | 17.1 (15.6, 19.1) |
| Finland | ||||||||
| East-West | 187/— | 76 (70–90) | 5.2 (5.1–5.3) | 5.6 (5.5–5.8) | 69/— | 34/— | 103/— | 8.8 (4.6, 14.8) |
| FINRISK 1987 | 985/1,100 | 53 (44–64) | 5.1 (5.1–5.1) | 5.6 (5.5–5.6) | 125/58 | 84/60 | 209/118 | 19.8 (19.8, 19.9) |
| FINRISK 1992 | 566/814 | 54 (44–64) | 5.3 (5.2–5.3) | 5.5 (5.4–5.5) | 28/11 | 16/36 | 44/47 | 14.9 (14.8, 14.9) |
| FINRISK 2002 | 842/1,323 | 57 (45–74) | 5.5 (5.5–5.5) | 5.5 (5.5–5.6) | 5/3 | 9/7 | 14/10 | 4.8 (4.8, 4.9) |
| Helsinki policemen | 687/— | 45 (31–69) | 5.6 (5.5–5.6) | 5.4 (5.3–5.5) | 199/— | 108/— | 307/— | 32.9 (21.8, 36.2) |
| Oulu | 93/172 | 55 (55–55) | 5.4 (5.4–5.5) | 6.1 (5.9–6.2) | 1/2 | 9/6 | 10/8 | 10.0 (10.0, 10.1) |
| Vantaa | 147/188 | 65 (64–66) | 5.3 (5.2–5.3) | 6.2 (6.1–6.3) | 16/6 | 3/3 | 19/9 | 13.3 (13.1, 13.6) |
| Italy | ||||||||
| Cremona Study | 618/794 | 57 (40–88) | 5.0 (5.0–5.0) | 4.9 (4.9–5.0) | 54/47 | 66/42 | 120/89 | 15.1 (14.6, 15.6) |
| Poland | ||||||||
| MONICA | 98/116 | 57 (44–73) | 5.3 (5.2–5.3) | 5.7 (5.5–5.8) | 9/1 | 3/2 | 12/3 | 6.5 (6.4, 6.5) |
| Sweden | ||||||||
| Malmö | —/834 | 54 (48–57) | 5.6 (5.6–5.7) | 6.6 (6.5–6.7) | —/11 | —/31 | —/42 | 14.6 (13.7, 17.8) |
| MONICA | 1,315/1,365 | 46 (25–74) | 5.1 (5.1–5.1) | 5.2 (5.2–5.3) | 31/11 | 22/21 | 53/32 | 12.6 (2.6, 16.6) |
| ULSAM | 651/— | 71 (70–74) | 5.1 (5.1–5.1) | 5.8 (5.7–5.9) | 62/— | 65/— | 127/— | 10.2 (9.1, 11.1) |
| The Netherlands | ||||||||
| Hoorn Study | 798/975 | 61 (49–77) | 5.3 (5.2–5.3) | 5.0 (4.9–5.0) | 41/19 | 41/31 | 82/50 | 8.9 (8.3, 9.3) |
| Zutphen Study | 289/— | 76 (70–90) | 5.3 (5.2–5.3) | 5.1 (5.0–5.7) | 29/— | 13/— | 42/— | 4.7 (4.6, 4.8) |
| U.K. | ||||||||
| ELY | 262/411 | 53 (40–67) | 5.5 (5.5–5.5) | 5.6 (5.5–5.7) | 11/3 | 13/17 | 24/20 | 14.6 (13.9, 15.4) |
| Goodinge Study | 214/346 | 52 (39–76) | 5.7 (5.6–5.7) | 5.5 (5.4–5.6) | 10/7 | 7/14 | 17/21 | 8.7 (8.4, 9.0) |
| Newcastle Heart Project | 224/254 | 53 (30–76) | 5.5 (5.5–5.5) | 5.5 (5.4–5.6) | 13/2 | 11/12 | 24/14 | 8.9 (8.5, 9.3) |
| Whitehall II study | 4,129/1,638 | 49 (39–62) | 5.1 (5.1–5.2) | 5.2 (5.2–5.3) | 26/4 | 39/22 | 65/26 | 5.9 (5.6, 6.1) |
| Total | 12,566/10,874 | 54 (25–90) | 5.3 (5.2–5.3) | 5.4 (5.4–5.4) | 827/246 | 611/351 | 1,438/597 | 9.0 (5.8, 14.9) |
Data are n, mean (range), or median (25th, 75th percentiles) unless otherwise indicated.
*Age-adjusted means (95% CIs). MONICA, Monitoring of Trends and Determinants in Cardiovascular Disease; PG, plasma glucose; ULSAM, Uppsala Longitudinal Study of Adult Men.
Table 2.
Baseline characteristics of participants and mortality from CVD, non-CVD, and all causes according to FPG and 2-h plasma glucose categories
| Men |
Women |
|||
|---|---|---|---|---|
| Group I: 2-h PG ≤ FPG | Group II: 2-h PG > FPG | Group I: 2-h PG ≤ FPG | Group II: 2-h PG > FPG | |
| n (%) | 6,663 (53.0) | 5,903 (47.0) | 4,096 (37.7) | 6,778 (62.3) |
| Age (years) | 52 (52–53)† | 55 (55–55) | 52 (52–53)† | 54 (54–54) |
| BMI (kg/m2) | 25.5 (25.4–25.6)† | 25.9 (25.8–26.0) | 25.4 (25.3–25.6)† | 26.0 (25.9–26.1) |
| FPG (mmol/l) | 5.3 (5.3–5.3) | 5.2 (5.2–5.2) | 5.2 (5.2–5.3) | 5.2 (5.2–5.2) |
| 2-h PG (mmol/l) | 4.3 (4.3–4.4)† | 6.2 (6.2–6.3) | 4.5 (4.5–4.5)† | 6.3 (6.3–6.3) |
| Fasting insulin (pmol/l)* | −0.03 (−0.06 to 0)† | 0.08 (0.05–0.11) | −0.11 (−0.14 to −0.08)† | 0.04 (0.01–0.07) |
| Total cholesterol (mmol/l) | 6.2 (6.2–6.3) | 6.2 (6.2–6.2) | 6.2 (6.2–6.3) | 6.3 (6.2–6.3) |
| Blood pressure (mmHg) | ||||
| Systolic | 131 (130–131) | 133 (133–134) | 128 (128–129) | 132 (132–133) |
| Diastolic | 81 (81–82) | 83 (83–83) | 78 (77–78) | 80 (80–80) |
| Current smoking (%) | 23.4† | 20.2 | 23.9† | 17.9 |
| Hypertension (%) | 35.6† | 47.1 | 34.3† | 45.2 |
| Mortality per 1,000 person years (n) | ||||
| CVD | 5.4 (378)† | 7.4 (449) | 1.4 (59)† | 2.3 (187) |
| Non-CVD | 4.3 (299) | 5.1 (312) | 2.8 (122) | 2.9 (229) |
| All-cause | 9.7 (677)† | 12.5 (761) | 4.2 (181)† | 5.2 (416) |
Data are n (%) or age- and study-adjusted means (95% CIs).
*9,978 men and 7,350 women with z score transformation.
†P < 0.001 for different between groups in men and women.
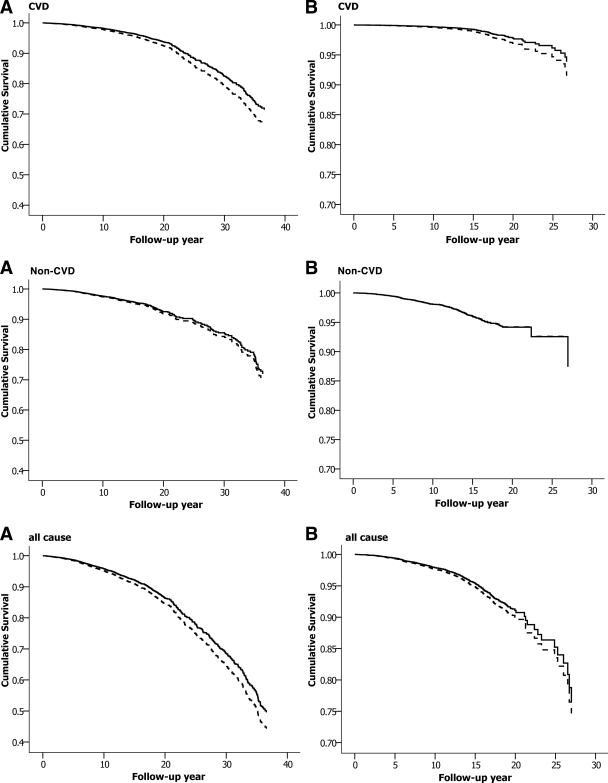
The multivariate adjusted HR for CVD death was significantly higher for group II than for group I for both sexes but did not differ between the two groups for non-CVD deaths (Table 3). The results were not altered after adjustment for fasting insulin in a subgroup of the study population (9,978 men and 7,350 women) (Table 3). Further analysis in the two FPG subgroups did not change the results either (supplementary Table, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-2328/DC1). HRs (95% CI) for the CVD mortality were 1.27 (1.08–1.48) in individuals with FPG ≤5.6 mmol/l and 1.26 (1.00–1.59) in those with FPG 5.6–6.1 mmol/l, for group II compared with group I. The cumulative survival profile was better in group I than in group II for CVD mortality, but the difference for non-CVD mortality was not statistically significant (Fig. 1).
Table 3.
HRs (95% CIs) for death from CVD, non-CVD, and all causes for group II compared with group I
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Men | |||
| n | 12,566 | 12,566 | 9,978 |
| CVD | 1.16 (1.01–1.34) | 1.22 (1.05–1.41) | 1.25 (1.05–1.50) |
| Non-CVD | 1.01 (0.86–1.19) | 1.09 (0.92–1.29) | 1.09 (0.89–1.34) |
| All causes | 1.10 (0.99–1.22) | 1.16 (1.04–1.30) | 1.18 (1.03–1.35) |
| Women | |||
| n | 10,874 | 10,874 | 7,350 |
| CVD | 1.23 (0.91–1.65) | 1.40 (1.03–1.89) | 1.60 (1.03–2.48) |
| Non-CVD | 0.92 (0.73–1.15) | 0.99 (0.79–1.25) | 1.05 (0.78–1.42) |
| All causes | 1.03 (0.86–1.23) | 1.13 (0.94–1.35) | 1.18 (0.93–1.51) |
Model 1: adjusted for age and cohort; model 2: model 1 plus fasting plasma glucose, BMI, total cholesterol, smoking and hypertension status; model 3: model 2 plus fasting insulin. Group I, 2-h plasma glucose ≤ FPG; group II, 2-h plasma glucose > FPG.
Figure 1.
Cumulative survival probability from CVD, non-CVD, and all-cause deaths derived from Cox regression analysis for group I (——) and group II (– – –) in men (A) and in women (B). The analyses are adjusted for age, cohort, FPG, BMI, total cholesterol, smoking, and hypertension status.
HRs (95% CIs) corresponding to a 1-unit increase in the difference between the 2-h plasma glucose and the FPG concentrations (2-h plasma glucose-FPG) were 1.09 (1.03–1.16) for the CVD deaths, 1.04 (0.98–1.12) for non-CVD deaths, and 1.07 (1.02–1.12) for all-cause deaths in men and 1.09 (0.97–1.23), 1.00 (0.90–1.10), and 1.04 (0.96–1.12) in women, respectively. The analysis was adjusted for age, cohort, FPG, BMI, total cholesterol, smoking, and hypertension status.
CONCLUSIONS
In individuals with both FPG and 2-h plasma glucose within the normoglycemic range, elevated 2-h plasma glucose conveyed increased mortality risk from CVD but not from non-CVD.
Previous studies have shown that elevated 2-h plasma glucose was a stronger CVD risk predictor than elevated FPG when both were compared with individuals with normoglycemia (6,7). Thus far, none of the studies have restricted the comparison to individuals with normoglycemia, a category being considered as having a relatively low risk for either diabetes (10,17) or CVD (11). Compared with the individuals whose 2-h plasma glucose returned to their FPG levels or lower (group I), those whose 2-h plasma glucose remained higher than their FPG (group II) were older, more obese, and hypertensive, whereas the former had lower fasting insulin concentrations and comprised more smokers. These confounding factors did not, however, explain the difference in CVD mortality between the two groups. Continuous systolic blood pressure instead of hypertension (yes versus no) did not change the results at all. The higher CVD risk associated with group II compared with group I was consistently observed in the stratified analysis in the two FPG subgroups, suggesting that the effect was independent of the FPG levels. Moreover, the excess risk was also observed for the difference between the FPG and the 2-h plasma glucose expressed as a continuous variable. Our study lends support to previous reports that the increase in CVD risk is graded with increasing 2-h plasma glucose concentration and may extend to the 2-h plasma glucose levels below the current definition for impaired glucose tolerance (4,18,19). At the low end of the FPG distribution, the CVD risk did not increase when the FPG concentration increased (4,19,20).
The time that is required for the 2-h plasma glucose concentration to return to, or drop below, the FPG level depends on the insulin response during the OGTT and peripheral/hepatic insulin sensitivity (10). Fasting hyperglycemia is predominantly associated with hepatic insulin resistance and decreased first-phase insulin secretion (21), whereas an elevated postprandial glucose level is associated with peripheral insulin resistance and impairment of both early- and late-phase insulin responses (22). In the current study the 2-h plasma glucose concentration was higher in group II than in group I regardless of the elevated fasting insulin levels in group II, suggesting that insulin resistance already occurred among people with normoglycemia by the current definition. Insulin resistance and the clustering of the insulin resistance with other metabolic disorders such as obesity and hypertension might be associated with increased CVD mortality observed in group II (23,24). Because insulin levels during the OGTT were not available in the current study, further exploration of the issue is warranted.
The collaborative data analysis contains some strengths. First, it provides a large sample size and statistical power, which could not be achieved by a single study alone. Second, a standard OGTT was performed in all studies to enable a further classification of people based on both FPG and 2-h plasma glucose. To reduce the discrepancies caused by differences in study design and methods among these studies, we have considered study cohort as a covariate in the Cox model and calculated a cohort-specific z score for fasting insulin, which was used in the data analysis. Another potential limitation of this analysis was the relatively low number of CVD events in women. A1C was only available in two studies: the East-West Men Study (men = 161) in Finland and the Hoorn Study (men = 798, women = 974) in the Netherlands, accounting for only 8% of the study population. The number of CVD events was also low (98 in men and 19 in women), which did not enable reasonable data analysis to check the effect of the A1C. In addition, some lifestyle-related factors such as dietary and physical activity and baseline CVD history that may have a potential contribution to the CVD were not available for all cohorts. Further studies with first-ever CVD events are required to confirm the findings.
In summary, in individuals with both FPG and 2-h plasma glucose within the normoglycemic range, individuals whose 2-h plasma glucose concentration did not return to their FPG level after a 75-g oral glucose load were more insulin resistant and had worse CVD outcomes than those whose 2-h plasma glucose returned to the FPG level.
Supplementary Material
Acknowledgments
This analysis was supported by the Academy of Finland (grants 118492 and 129197) and the Centre for International Mobility Fellowship in Finland (CIMO TM-08-5694).
No potential conflicts of interest relevant to this article were reported.
F.N. and Q.Q. researched data, contributed to discussion, wrote the manuscript and reviewed/edited data. J.T. researched data and reviewed/edited data. K.P. researched data, contributed to discussion, and reviewed/edited data. A.O. contributed to discussion. S.S. contributed to discussion and reviewed/edited data.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Stamler J, Vaccaro O, Neaton JD, Wentworth D: Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–444 [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, McGee DL: Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 3.Shahab A: Why does diabetes mellitus increase the risk of cardiovascular disease? Acta Med Indones 2006;38:33–41 [PubMed] [Google Scholar]
- 4.Barr EL, Boyko EJ, Zimmet PZ, Wolfe R, Tonkin AM, Shaw JE: Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009;52:415–424 [DOI] [PubMed] [Google Scholar]
- 5.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. DECODE Study Group. The European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe. Lancet 1999;354:617–621 [PubMed] [Google Scholar]
- 6.DECODE Study Group, the European Diabetes Epidemiology Group The European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405 [DOI] [PubMed] [Google Scholar]
- 7.Qiao Q, Pyörälä K, Pyörälä M, Nissinen A, Lindström J, Tilvis R, Tuomilehto J: Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur Heart J 2002;23:1267–1275 [DOI] [PubMed] [Google Scholar]
- 8.Nakagami T: DECODA Study Group Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004;47:385–394 [DOI] [PubMed] [Google Scholar]
- 9.Hyvärinen M, Qiao Q, Tuomilehto J, Laatikainen T, Heine RJ, Stehouwer CD, Alberti KG, Pyörälä K, Zethelius B, Stegmayr B: DECODE Study Group Hyperglycemia and stroke mortality: comparison between fasting and 2-h glucose criteria. Diabetes Care 2009;32:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Williams K, DeFronzo R, Stern M: Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006;29:1613–1618 [DOI] [PubMed] [Google Scholar]
- 11.Succurro E, Marini MA, Grembiale A, Lugarà M, Andreozzi F, Sciacqua A, Hribal ML, Lauro R, Perticone F, Sesti G: Differences in cardiovascular risk profile based on relationship between post-load plasma glucose and fasting plasma levels. Diabetes Metab Res Rev 2009;25:351–356 [DOI] [PubMed] [Google Scholar]
- 12.Qiao Q: DECODE Study Group Comparison of different definitions of the metabolic syndrome in relation to cardiovascular mortality in European men and women. Diabetologia 2006;49:2837–2846 [DOI] [PubMed] [Google Scholar]
- 13.DECODE Study Group Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–69 [DOI] [PubMed] [Google Scholar]
- 14.Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. BMJ 1998;317:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia. Report of a World Health Organization/International Diabetes Federation Consultation. Geneva, World Health Organization, 2006 [Google Scholar]
- 16.1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension. Guidelines subcommittee. J Hypertens 1999;17:151–183 [PubMed] [Google Scholar]
- 17.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 18.Coutinho M, Gerstein HC, Wang Y, Yusuf S: The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233–240 [DOI] [PubMed] [Google Scholar]
- 19.DECODE Study Group, European Diabetes Epidemiology Group Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 2003;26:688–696 [DOI] [PubMed] [Google Scholar]
- 20.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Stern MP, Blair SN: Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation 2000;101:2047–2052 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Ferrannini E, Simonson DC: Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism 1989;38:387–395 [DOI] [PubMed] [Google Scholar]
- 22.Bogardus C, Lillioja S, Howard BV, Reaven G, Mott D: Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest 1984;74:1238–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGee DL: Diverse Populations Collaboration. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol 2005;15:87–97 [DOI] [PubMed] [Google Scholar]
- 24.Henry P, Thomas F, Benetos A, Guize L: Impaired fasting glucose, blood pressure and cardiovascular disease mortality. Hypertension 2002;40:458–463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.