Abstract
OBJECTIVE
Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are considered pre-diabetes states. There are limited data in pediatrics in regard to their pathophysiology. We investigated differences in insulin sensitivity and secretion among youth with IFG, IGT, and coexistent IFG/IGT compared with those with normal glucose tolerance (NGT) and type 2 diabetes.
RESEARCH DESIGN AND METHODS
A total of 24 obese adolescents with NGT, 13 with IFG, 29 with IGT, 11 with combined IFG/IGT, and 30 with type 2 diabetes underwent evaluation of hepatic glucose production ([6,6-2H2]glucose), insulin-stimulated glucose disposal (Rd, euglycemic clamp), first- and second-phase insulin secretion (hyperglycemic clamp), body composition (dual-energy X-ray absorptiometry), abdominal adiposity (computed tomography), and substrate oxidation (indirect calorimetry).
RESULTS
Adolescents with NGT, pre-diabetes, and type 2 diabetes had similar body composition and abdominal fat distribution. Rd was lower (P = 0.009) in adolescents with type 2 diabetes than in those with NGT. Compared with adolescents with NGT, first-phase insulin was lower in those with IFG, IGT, and IFG/IGT with further deterioration in those with type 2 diabetes (P < 0.001), and β-cell function relative to insulin sensitivity (glucose disposition index [GDI]) was also lower in those with IFG, IGT, and IFG/IGT (40, 47, and 47%, respectively), with a further decrease (80%) in those with type 2 diabetes (P < 0.001). GDI was the major determinant of fasting and 2-h glucose levels.
CONCLUSIONS
Obese adolescents who show signs of glucose dysregulation, including abnormal fasting glucose, glucose intolerance or both, are more likely to have impaired insulin secretion rather than reduced insulin sensitivity. Given the impairment in insulin secretion, they are at high risk for progression to type 2 diabetes. Further deterioration in insulin sensitivity or secretion may enhance the risk for this progression.
Pre-diabetes, defined as the presence of elevated fasting glucose, abnormal glucose tolerance, or both, is associated with an enhanced risk for development of type 2 diabetes in adults (1), but there are limited data to define the significance in children. A recent change in the definition of the abnormal fasting glucose to a lower level (100–125 mg/dl) has increased the prevalence of pre-diabetes in both adults and youth (2–4). It is unclear from the literature what role a defect in insulin secretion or an abnormality of insulin sensitivity might play in the impairment of glucose regulation, leading to glucose intolerance or elevated fasting plasma glucose.
Epidemiological studies suggest that subjects with impaired fasting glucose (IFG) have lower insulin sensitivity and higher insulin secretion (5,6) based largely on fasting indexes of insulin sensitivity and an oral glucose tolerance (OGTT)–derived single index of insulin secretion (5). Adult studies reveal similar or lower insulin sensitivity in subjects with impaired glucose tolerance (IGT) compared with those with IFG who have lower insulin secretion (7,8). These studies are contrasted with clamp studies in Pima Indians showing similar insulin sensitivity in subjects with IFG and IGT but lower insulin secretion in those with fasting dysglycemia (9).
Pediatric data are limited. In overweight Latino children with a family history of type 2 diabetes (10), children with impaired versus normal fasting glucose had no significant differences in insulin sensitivity or acute insulin response. However, the glucose disposition index (GDI), or insulin secretion relative to insulin sensitivity, was significantly reduced (15% lower) in children with IFG. A more recent study in obese adolescents revealed that subjects with IFG had decreased glucose sensitivity of first-phase insulin secretion and liver insulin sensitivity, whereas those with IGT had more severe degrees of peripheral insulin resistance compared with subjects with normal glucose tolerance (NGT) (11). We recently demonstrated that insulin secretion relative to insulin sensitivity shows a significantly declining pattern: highest in youth with NGT, intermediate in youth with IGT, and lowest in youth with type 2 diabetes (12).
In an attempt to clarify the controversy concerning the metabolic derangements in the different categories of the pre-diabetes state, the aims of the present study were to 1) to investigate the metabolic characteristics of insulin sensitivity and secretion in obese youth, with IFG versus IGT, of similar body composition and abdominal adiposity and 2) to compare them not only with those with NGT but also with children with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Twenty-four obese adolescents with NGT, 13 with IFG, 29 with IGT, 11 with combined IFG/IGT, and 30 with type 2 diabetes (African American n = 45 and American white n = 62) adolescents were studied. IFG was defined according to the 2003 American Diabetes Association (ADA) guidelines as fasting plasma glucose (FPG) of ≥100–125 mg/dl (13), based on the average of two fasting glucose measurements at the time of the OGTT (at −15 and 0 min) or the average of seven fasting glucose measurements obtained during the two clamp procedures (three samples every 15 min at the baseline of the hyperglycemic clamp and four samples every 10 min at the baseline of the euglycemic clamp) and NGT with 2-h post-OGTT glucose of <140 mg/dl. IGT was defined as normal FPG <100 mg/dl and 2-h post-OGTT glucose of ≥140–199 mg/dl according to ADA criteria (13). Those with combined IFG/IGT had FPG ≥100–125 mg/dl and 2-h glucose between ≥140 and 199 mg/dl (13). All subjects were pubertal and had exogenous obesity with no clinical evidence of endocrinopathy associated with obesity. They were not involved in any regular physical activity or weight reduction programs. Type 2 diabetes in the adolescents was clinically diagnosed according to ADA and World Health Organization criteria (14). Type 2 diabetic adolescents were negative for GAD and insulinoma-associated protein-2 autoantibody. They were being treated with lifestyle alone (n = 7), metformin (n = 11), metformin + insulin (n = 10), or insulin alone (n = 2). All other participants were not taking any medications that affect glucose metabolism. In type 2 diabetic subjects, metformin and long-acting insulin were discontinued 48 h before the clamp studies. Some of the participants (12 with NGT, 19 with IGT, and 17 with type 2 diabetes) have been reported before (12). All studies were approved by the institutional review board of the University of Pittsburgh. Informed consent was obtained. Clinical characteristics of the study subjects are summarized in Table 1.
Table 1.
Phenotypic and metabolic characteristics of obese adolescents with NGT, IFG, IGT, IFG/IGT, and type 2 diabetes
| NGT | IFG | IGT | IFG/IGT | Type 2 diabetes | P value* | |
|---|---|---|---|---|---|---|
| 24 | 13 | 29 | 11 | 30 | ||
| Age (years) | 13.9 ± 1.9 | 14.9 ± 1.9 | 14.5 ± 2.0 | 14.3 ± 2.1 | 15.3 ± 1.7 | NS |
| Sex (male/female)† | 9/15 | 7/6 | 6/23 | 5/6 | 13/17 | NS |
| Ethnicity† | ||||||
| African American | 10 | 7 | 6 | 6 | 16 | NS |
| American white | 14 | 6 | 23 | 5 | 14 | |
| Tanner stage† | ||||||
| II–III | 6 | 1 | 5 | 3 | 2 | NS |
| IV–V | 18 | 12 | 24 | 8 | 28 | |
| BMI (kg/m2) | 36.2 ± 4.1 | 33.5 ± 6.9 | 37.3 ± 7.3 | 36.0 ± 6.5 | 36.8 ± 5.3 | NS |
| Waist circumference (cm) | 108.2 ± 14.6 | 100.0 ± 14.9 | 106.0 ± 14.9 | 109.3 ± 13.1 | 108.6 ± 13.6 | NS |
| Body fat (%) | 46.5 ± 5.5 | 41.3 ± 7.4 | 45.5 ± 5.1 | 44.7 ± 5.3 | 41.7 ± 6.3 | NS |
| Subcutaneous abdominal fat (cm2) | 551.4 ± 138.6 | 452.1 ± 192.0 | 563.4 ± 167.4 | 511.3 ± 147.2 | 542.1 ± 136.1 | NS |
| Visceral fat (cm2) | 72.4 (46.8–93.4) | 67.8 (46.8–91.0) | 82.0 (55.6–104.0) | 50.7 (40.6–92.6) | 78.3 (62.4–88.6) | NS |
| A1C (%) | 5.3 ± 0.4a | 5.6 ± 0.4b | 5.4 ± 0.4c | 5.2 ± 0.5d | 6.6 ± 0.8a,b,c,d | <0.001 |
| Fasting glucose (mg/dl) | 92.0 (88.3–96.1) | 102.6 (100.17–104.75) | 92.15 (89.0–94.5) | 104.5 (101.8–108.7) | 118.4 (103.1–138.5) | <0.001 |
| Fasting insulin (μU/ml) | 37.4 (29.8–44.2) | 26.3 (21.7–56.6) | 39.1 (27.4–55.6) | 36.8 (28.4–56.1) | 40.4 (33.9–57.2) | NS |
| Fasting glucose–to-insulin ratio | 2.5 (2.0–3.3) | 3.9 (1.9–4.8) | 2.2 (1.7–3.5) | 2.8 (2.0–3.8) | 3.1 (2.4–4.1) | NS |
| Proinsulin-to-insulin ratio | 0.17 (0.10–0.2) | 0.14 (0.12–0.16) | 0.13 (0.09–0.15) | 0.18 (0.09–0.25) | 0.20 (0.09–0.35) | 0.002 |
| Postabsorptive hepatic glucose production (mg/kg/min) | 1.9 (1.7–2.3) | 2.1 (2.0–2.7) | 2.1 (1.7–2.6) | 2.5 (1.9–2.9) | 2.4 (2.1–3.2) | 0.007 |
| Postabsorptive hepatic insulin resistance (mg/kg/min · μU/ml) | 75.1 (53.3–106.6) | 83.7 (48.3–116.3) | 83.9 (58.6–130.9) | 98.2 (60.2–169.7) | 102.4 (71.4–180.1) | 0.05 |
| Cholesterol (mg/dl) | 170.1 ± 36.6 | 157.2 ± 36.9 | 171.3 ± 35.9 | 175.1 ± 39.8 | 158.1 ± 29.4 | NS |
| HDL (mg/dl) | 39.1 (34.4–49.6) | 36.9 (30.6–45.3) | 38.0 (32.4–44.6) | 38.1 (33.5–45.6) | 38.7 (33.1–41.8) | NS |
| LDL (mg/dl) | 104.0 ± 34.4 | 94.1 ± 30.7 | 103.3 ± 32.5 | 110.1 ± 32.0 | 94.0 ± 27.3 | NS |
| Triglycerides (mg/dl) | 110.0 (92.0–161.0) | 84.0 (75.5–157.5) | 108.0 (92.0–189.0) | 109.0 (102.5–142.8) | 108.0 (87.0–148.8) | NS |
| Triglycerides-to-HDL ratio | 2.8 (1.8–3.8) | 2.0 (1.7–5.0) | 2.9 (2.1–5.3) | 3.0 (2.7–3.8) | 3.1 (2.2–4.4) | NS |
Data are means ± SD or medians (25th percentile–75th percentile). Body composition data was missing for 2 subjects with NGT, 2 with IGT, and 5 with type 2 diabetes who exceeded the weight limit of 250 pounds of the dual-energy X-ray absorptiometry machine.
*P value from ANOVA for continuous variables with means presented and Kruskal-Wallis test with medians presented.
†P value from χ2 test for categorical variables. Superscript letters are significant post hoc analysis (Bonferroni correction, P < 0.05):
atype 2 diabetes vs. NGT;
btype 2 diabetes vs. IFG;
ctype 2 diabetes vs. IGT;
dtype 2 diabetes vs. IFG/IGT. NS, not significant.
Clamp studies
Participants were admitted twice within a 1- to 3-week period to the Pediatric Clinical and Translational Research Center on the day before the clamp studies, once for a hyperinsulinemic-euglycemic clamp and the other time for a hyperglycemic clamp in random order. The 2-h OGTT (1.75 g/kg Glucola [maximum 75 g]) was performed on the day before the first Pediatric Clinical and Translational Research Center admission.
In vivo insulin-stimulated glucose disposal
A fasting blood sample was obtained for determination of cholesterol, LDL, HDL, VLDL, triglycerides, A1C, proinsulin, and C-peptide. Fasting endogenous glucose production was measured with a primed constant rate infusion of [6,6-2H2] glucose (0.306 ± 0.009 μmol/kg/min; Isotech, Miamisburg, OH) (12). Insulin-mediated glucose metabolism (Rd) and insulin sensitivity were evaluated during a 3-h hyperinsulinemic-euglycemic clamp (12). Continuous indirect calorimetry by a ventilated hood (Deltatrac Metabolic Monitor, Sensormedics, Anaheim, CA) was used to measure CO2 production, O2 consumption, and respiratory quotient. Measurements were made for 30 min at baseline and at the end of the euglycemic clamp (12).
In vivo insulin secretion
First- and second-phase insulin and C-peptide secretion were evaluated during a 2-h hyperglycemic clamp (12.5 mmol/l) as before (12).
Body composition
Body composition was determined by dual-energy X-ray absorptiometry and subcutaneous abdominal adipose tissue and visceral adipose tissue by a single-slice computed tomography scan at L4–L5 (12).
Biochemical measurements
Plasma glucose was measured with a glucose analyzer (YSI, Yellow Springs, OH); insulin and C-peptide were measured by radioimmunoassay as before (12). A1C was measured by high-performance liquid chromatography (Tosoh Medics), and lipids were measured using the standards of the Centers for Disease Control and Prevention (12). Deuterium enrichment of glucose in the plasma was determined on a Hewlett-Packard (Palo Alto, CA) 5973 mass spectrometer coupled to a 6890 gas chromatograph (12). Pancreatic autoantibodies were determined in the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington (Seattle, WA) using the National Institute of Diabetes and Digestive and Kidney Diseases–sponsored standardization assay.
Calculations
Fasting hepatic glucose production (HGP) was calculated during the last 30 min of the 2-h isotope infusion according to steady-state tracer dilution equations (12). In the fasting state, an index of hepatic insulin resistance was calculated as the product of HGP and fasting insulin levels (14). Insulin-stimulated glucose disposal rate (Rd) was calculated during the last 30 min of the euglycemic clamp to be equal to the rate of exogenous glucose infusion and expressed per fat free mass (FFM) (milligrams per minute per kilogram FFM). Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin level and expressed per FFM (milligrams per minute per FFM per microunits per milliliter) (12). Insulin-stimulated carbohydrate oxidation rates were calculated according to the formulas of Frayn (12).
During the hyperglycemic clamp, the first- and second-phase insulin and C-peptide concentrations were calculated as described previously (12). The GDI was calculated as the product of insulin sensitivity × first-phase insulin and expressed as milligrams per minute per kilogram FFM.
Statistics
Statistical analyses were performed using ANOVA followed by a post hoc Bonferroni correction for five group comparisons. A Kruskal-Wallis test was used for multiple group comparison of nonparametric variables and a χ2 test to evaluate categorical variables. Spearman's correlation and multiple regression analyses were used to evaluate bivariate and multivariate relationships, respectively. Nonparametric variables were log-transformed for the regression analyses. Data are presented as means ± SD. Two-tailed P ≤ 0.05 was considered statistically significant.
RESULTS
Study subjects and fasting metabolic profile
Table 1 depicts characteristics of the five groups of obese adolescents with NGT, IFG, IGT, combined IFG/IGT, and type 2 diabetes. There were no significant differences in age, sex, Tanner stage, or ethnic distribution among the five groups. All subjects were pubertal. There were no significant differences in BMI, percent body fat, or abdominal visceral or subcutaneous fat among the five groups.
Fasting glucose was different among the groups as expected on the basis of predefined categorization. There was no difference in fasting insulin levels among the five groups. Fasting endogenous glucose production (HGP) was significantly higher in the type 2 diabetic group compared with the NGT group (post hoc P = 0.004) with no difference among the pre-diabetic groups. Postabsorptive hepatic insulin resistance tended to be higher in the type 2 diabetic group versus the NGT group (post hoc P = 0.07) with no difference among the other pre-diabetic groups. The proinsulin-to-insulin ratio was higher in the type 2 diabetic group but not significantly higher in the pre-diabetic groups compared with the NGT group. The fasting lipid profile was not different among the groups (Table 1).
In vivo insulin-stimulated glucose disposal and insulin secretion
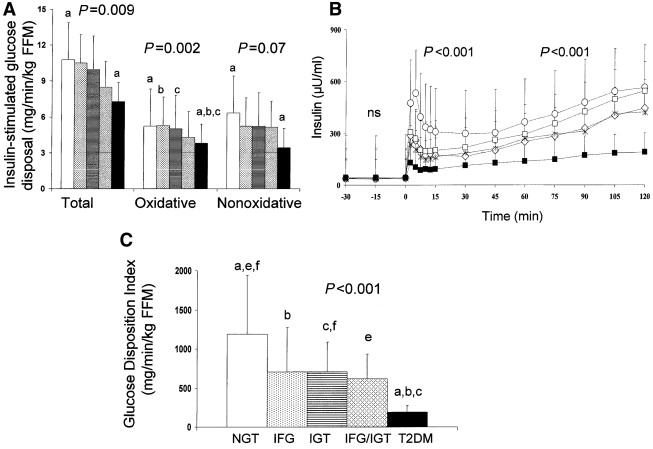
Total, oxidative, and nonoxidative glucose disposal were lower in the type 2 diabetic group compared with the NGT group. Oxidative glucose disposal was lower in the type 2 diabetic group compared with the NGT (P = 0.016), IFG (P = 0.003), and IGT (P = 0.023) groups in post hoc analysis but was not different from that in the IFG/IGT group (Fig. 1A). First-phase insulin levels were significantly lower in the IFG (post hoc P = 0.02), IGT (P = 0.009), and IFG/IGT (P = 0.011) groups and lowest in the type 2 diabetic group (P < 0.001) compared with the NGT group (Fig. 1B). Similarly, first-phase C-peptide levels were lowest in the type 2 diabetic group and significantly different between the type 2 diabetic and NGT groups (P < 0.001). Second-phase insulin (Fig. 1B) levels were significantly reduced in the type 2 diabetic group compared with the NGT (P < 0.001) and IGT (P = 0.001) but not the IFG (P = 0.3) or IFG/IGT groups. GDI, which represents insulin secretion relative to insulin sensitivity, was significantly impaired in all categories of pre-diabetes, was lowest in type 2 diabetes, and was significantly different from IFG and IGT but not IFG/IGT (Fig. 1C). Youth with type 2 diabetes receiving different treatment modalities did not differ with respect to their peripheral glucose disposal, insulin secretion, or GDI (data not shown).
Figure 1.
A: Insulin-stimulated total, oxidative, and nonoxidative glucose disposal in subjects with NGT (open bars), IFG (dotted bars), IGT (striped bar), IFG/IGT (diamond bars), and type 2 diabetes (T2DM) (filled bars). P values are for trend (ANOVA P values). B: First- and second-phase insulin levels during the hyperglycemic clamp in NGT (○), IFG (♢), IGT (□), IFG/IGT (*), and type 2 diabetes (■). C: GDI in subjects with NGT (open bar), IFG (dotted bar), IGT (striped bar), IFG/IGT (diamond bar), and type 2 diabetes (filled bar). In A and C, letters are significant post hoc analysis (Bonferroni correction): P < 0.05 (a, type 2 diabetes versus NGT; b, type 2 diabetes versus IFG; c, type 2 diabetes versus IGT; e, NGT versus IFG/IGT; f, NGT versus IGT). Data are means ± SD.
Determinants of fasting glucose and oral glucose tolerance
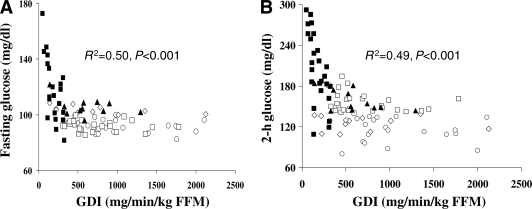
Fasting glucose correlated with hepatic insulin resistance (r = 0.30, P = 0.004), with first-phase (r = −0.58, P < 0.001) and second-phase (r = −0.47, P < 0.001) insulin, and with GDI (r = −0.57, P < 0.001) but not with peripheral insulin sensitivity. Similarly, 2-h OGTT glucose correlated with first-phase (r = −0.48, P < 0.001) and second-phase (r = −0.40, P < 0.001) insulin and with GDI (r = −0.63, P < 0.001) but not with insulin sensitivity. In a multiple regression analysis with age, sex, ethnicity, BMI, hepatic insulin resistance, and GDI as independent variables and 2-h OGTT glucose or fasting glucose as the dependent variable, GDI was the significant determinant of the variance in the 2-h glucose (β = −0.47, P < 0.001) and the fasting glucose (β = −0.32, P = 0.009). With visceral adipose tissue or fat mass instead of BMI in the regression model, GDI remains the significant determinant of the variance in mean fasting glucose (β = −0.4, P < 0.001) and in 2-h glucose (β = −0.5, P < 0.001). The relationship between GDI and 2-h OGTT glucose or fasting glucose is depicted in Fig. 2.
Figure 2.
Relationship of GDI to FPG (A) and 2-h OGTT glucose level (B) in NGT (○), IFG (♢), IGT (□), IFG/IGT (▲), and type 2 diabetes (■).
CONCLUSIONS
In this study, we hypothesized that for similar degrees of adiposity insulin sensitivity will not differ among the different pre-diabetic groups compared with youth with NGT but will be lower in youth with type 2 diabetes, whereas insulin secretion will be impaired in all categories of glucose dysregulation. Consistent with our hypothesis, the current findings demonstrate that all pre-diabetes states in obese youth of similar BMI, percent body fat, and abdominal adiposity are characterized by reductions in β-cell function relative to insulin sensitivity, with no difference in insulin sensitivity. In youth with IFG, insulin-stimulated glucose disposal is preserved compared with that in those with NGT, whereas first- and second-phase insulin secretion is ∼50 and 30% impaired. In youth with IGT, first-phase insulin is ∼40% lower compared with that in those with NGT with preservation of second-phase insulin. When both defects, IFG and IGT, coexist, the impairment in insulin secretion is a mixture of both with ∼55% lower first-phase insulin and 30% lower second-phase insulin. In the full-blown diabetic state, insulin-stimulated glucose disposal is impaired by ∼30%, first-phase insulin is impaired by ∼75%, and second-phase insulin is impaired by ∼65% compared with those in youth with NGT. Such cross-sectional observations are consistent with longitudinal studies showing a higher risk of progression to type 2 diabetes in the subjects with combined IFG/IGT compared with those with isolated IFG or IGT (15).
The present study confirms the results in some of the existing adult literature but contradicts others. Our findings are consistent with observations in adults demonstrating greater impairment in insulin secretion in individuals with IFG (9,14,16,17) compared with those with IGT, in that the defect in insulin secretion involves both first-phase and second-phase insulin in IFG, whereas second-phase insulin is preserved in IGT. Moreover, adult studies indicate that the loss of β-cell function may start at levels of FPG on the higher end of the conventional normal range (18). A recent longitudinal study suggests that a defect in insulin secretion (evaluated by an OGTT-derived index) is present in subjects with IFG and apparent 5 years before the development of fasting hyperglycemia (19). On the other hand, other investigations in adults show greater insulin resistance in IGT groups compared with IFG or NGT groups unlike our findings (17,18). However, a major difference between our study and the adult studies, besides the age factor, is that almost invariably, the reported adults with IGT (9,15,17,18,20) or IFG (9,15,18,20) have higher BMI and/or abdominal fat compared with the NGT groups, which could contribute to the observed differences in insulin action between IGT, IFG, and NGT categories. This observation is supported by the fact that when subjects have similar anthropometric measures (21), investigators did not find significant differences in peripheral glucose uptake in the IFG or IFG/IGT groups compared with the NGT group (21). In addition, controlling for body composition (BMI and waist-to-hip ratio) eliminated differences in insulin sensitivity among NGT, IFG, and IGT subgroups in one study (22) and between IGT and NGT in another study (23). In that same study, lower insulin sensitivity is evident in the type 2 diabetic group compared with that in the NGT group and with that in the pre-diabetic groups after controlling for overweight (23), consistent with our current and previous findings (12).
In this study, use of the hyperglycemic clamp allowed us to examine second-phase insulin secretion, information on which is not widely available in the published literature. The defect in first-phase insulin secretion in our pre-diabetic groups is consistent with the findings of Cali' et al. (11) of decreased glucose sensitivity of first-phase insulin secretion in the pre-diabetic state. In their study, absolute values of first- and second-phase insulin levels were not significantly different in the pre-diabetic groups compared with those in the NGT group, and glucose sensitivity of second-phase insulin was not affected except in the combined IFG/IGT group. Their study, however, did not include subjects with type 2 diabetes to allow them to evaluate the magnitude of impairment across the spectrum of glucose tolerance. In our study, inclusion of adolescents with type 2 diabetes allowed us to assess not only deviations from normal but also differences from the extreme abnormal. Although absolute levels of second-phase insulin were significantly lower in the type 2 diabetic versus IGT group, there was no difference between the type 2 diabetic and IFG or coexisting IFG/IGT groups. Such an observation suggests that in IFG the impairment in insulin secretion may play a more critical role in the progression to type 2 diabetes than is the case with IGT. Another contrast between the two studies is the study population. Although our participants were limited to a balanced representation of African Americans and whites, their study included subjects of multiple ethnicities with a significant number of Hispanics who may differ in their metabolic response to perturbations in glucose homeostasis. In studies limited to Latino adolescents, investigators did not find significant differences in the acute insulin response between those with IFG and NGT (10) or between those with IGT and NGT (24), although GDI was reduced in the IFG and IGT groups compared with that in the NGT group, indicating an impairment in β-cell function relative to insulin sensitivity.
Several adult studies suggested that the IFG state is characterized by hepatic insulin resistance measured during the euglycemic clamp (9,15,25). However, the population in those studies consisted of Mexican American adults in one (15) and Native Americans (9) in another. In addition, the pre-diabetic subjects had higher BMI and waist circumference (15) compared with those in the NGT group, which could have contributed to their hepatic insulin resistance. In a study by Bock et al. (25), mild hepatic insulin resistance was found in white subjects with IFG compared with those with NGT, which was attributed to increased gluconeogenesis. However, again the subjects with IFG were significantly more obese and had higher visceral fat (25). Our study participants in the five different groups had comparable degrees of total and abdominal adiposity, and thus it is possible that with similar degrees of obesity, the earliest detected abnormality is in β-cell function and insulin secretion, and hepatic insulin resistance develops later and becomes more marked in individuals of certain ethnic backgrounds. Therefore, we propose that the defect in insulin secretion in the IFG group in combination with hepatic insulin resistance (which we did not measure during the clamp) may be responsible for the mild fasting hyperglycemia. On the other hand, the interplay between impaired insulin secretion and peripheral insulin resistance in subjects with coexisting IFG/IGT may prevent maintenance of plasma glucose within a normal range after a glucose load.
One limitation in our study is the relatively smaller sample size of the IFG and combined IFG/IGT groups. However, the use of the clamp, a sensitive method for assessing insulin sensitivity and secretion, allowed us to demonstrate significant differences in a five-group comparison.
In summary, all pre-diabetic states in obese youth have impaired insulin secretion relative to insulin sensitivity, although the magnitude of impairment in β-cell function may be variable. Such differences potentially translate to a differential in the risk of progression to type 2 diabetes. Further investigations into the underlying mechanisms/reasons are needed. The ultimate objective from such scientific advances is to individualize the therapeutic/preventive approach to the specific underlying metabolic dysfunction, leading to type 2 diabetes at a young age.
Acknowledgments
This work was supported by the U.S. Public Health Service (grants R01-HD-27503 and K24-HD-01357 to S.A.A.), Richard L. Day Endowed Chair (S.A.A.), Department of Defense (F.B., S.L., and S.A.A.), Thrasher Research Fund (F.B. and N.G.), General Clinical Research Center (grant M01-RR-00084), and Clinical and Translational Science Award (UL1-RR-024153).
No potential conflicts of interest relevant to this article were reported.
F.B. conducted the study, obtained funding, acquired data, analyzed data, and wrote the manuscript. S.L. contributed analytical tools for body composition. N.G. initiated the project and acquired data. S.A.A. contributed the study concept and design, acquired data, obtained funding, provided administrative technical and material support, supervised the study, and reviewed/edited the manuscript.
These studies would not have been possible without the nurses and staff of the Pediatric Clinical and Translational Research Center, the efforts of the past fellows who participated in performing clamp experiments, the research team (Sabrina Kadri, Lori Bednarz, RN, CDE, and Nancy Guerra, CRNP), the laboratory expertise of Theresa Stauffer and Katie McDowell, and most importantly the commitment of the study volunteers and their parents.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.American Diabetes Association Screening for type 2 diabetes. Diabetes Care 2004;27:(Suppl. 1)S11–S14 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin S, Cadwell B, Geiss L, Engelgau M, Vinicor F: A change in definition results in an increased number of adults with prediabetes in the United States. Arch Intern Med 2004;164:2386. [DOI] [PubMed] [Google Scholar]
- 3.Williams DE, Cadwell BL, Cheng YJ, Cowie CC, Gregg EW, Geiss LS, Engelgau MM, Narayan KM, Imperatore G: Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics 2005;116:1122–1126 [DOI] [PubMed] [Google Scholar]
- 4.Li C, Ford ES, Zhao G, Mokdad AH: Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care 2009;32:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC: Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 2000;49:975–980 [DOI] [PubMed] [Google Scholar]
- 6.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T: Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 2003;26:868–874 [DOI] [PubMed] [Google Scholar]
- 7.Davies MJ, Raymond NT, Day JL, Hales CN, Burden AC: Impaired glucose tolerance and fasting hyperglycaemia have different characteristics. Diabet Med 2000;17:433–440 [DOI] [PubMed] [Google Scholar]
- 8.Carnevale Schianca GP, Rossi A, Sainaghi PP, Maduli E, Bartoli E: The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes Care 2003;26:1333–1337 [DOI] [PubMed] [Google Scholar]
- 9.Weyer C, Bogardus C, Pratley RE: Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999;48:2197–2203 [DOI] [PubMed] [Google Scholar]
- 10.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI: Decreased β-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care 2005;28:2519–2524 [DOI] [PubMed] [Google Scholar]
- 11.Cali' AM, Bonadonna RC, Trombetta M, Weiss R, Caprio S: Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab 2008;93:1767–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacha F, Gungor N, Lee S, Arslanian SA: In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–316714578255 [Google Scholar]
- 14.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 15.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R: The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003;52:1475–1484 [DOI] [PubMed] [Google Scholar]
- 16.Festa A, D'Agostino R, Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM: Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 2004;53:1549–1555 [DOI] [PubMed] [Google Scholar]
- 17.Laakso M, Zilinskaite J, Hansen T, Boesgaard TW, Vänttinen M, Stancáková A, Jansson PA, Pellmé F, Holst JJ, Kuulasmaa T, Hribal ML, Sesti G, Stefan N, Fritsche A, Häring H, Pedersen O, Smith U: EUGENE2 Consortium Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008;51:502–511 [DOI] [PubMed] [Google Scholar]
- 18.Godsland IF, Jeffs JA, Johnston DG: Loss of β cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–1166 [DOI] [PubMed] [Google Scholar]
- 19.Faerch K, Vaag A, Holst JJ, Hansen T, Jørgensen T, Borch-Johnsen K: Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 Study. Diabetes Care 2009;32:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R: Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006;55:3536–3549 [DOI] [PubMed] [Google Scholar]
- 21.Perreault L, Bergman BC, Playdon MC, Dalla Man C, Cobelli C, Eckel RH: Impaired fasting glucose with or without impaired glucose tolerance: progressive or parallel states of prediabetes? Am J Physiol Endocrinol Metab 2008;295:E428–E435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J: Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006;29:1909–1914 [DOI] [PubMed] [Google Scholar]
- 23.van Haeften TW, Pimenta W, Mitrakou A, Korytkowski M, Jenssen T, Yki-Jarvinen H, Gerich JE: Disturbances in β-cell function in impaired fasting glycemia. Diabetes 2002;51(Suppl. 1):S265–S270 [DOI] [PubMed] [Google Scholar]
- 24.Goran MI, Bergman RN, Avila Q, Watkins M, Ball GD, Shaibi GQ, Weigensberg MJ, Cruz ML: Impaired glucose tolerance and reduced β-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab 2004;89:207–212 [DOI] [PubMed] [Google Scholar]
- 25.Bock G, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Chandramouli V, Landau BR, Rizza RA: Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 2007;56:1703–1711 [DOI] [PubMed] [Google Scholar]