Abstract
OBJECTIVE
Proinflammatory advanced glycation end products (AGEs) found in thermally processed foods correlate with serum AGEs (sAGEs) and promote type 1 and type 2 diabetes in mice. Herein we assess the relationship of maternal blood and food AGEs to circulating glycoxidants, inflammatory markers, and insulin levels in infants up to age 1 year.
RESEARCH DESIGN AND METHODS
AGEs (Nε-carboxymethyllysine [CML] and methylglyoxal derivatives) were tested in sera of healthy mothers in labor (n = 60), their infants, and infant foods. Plasma 8-isoprostane, fasting glucose, insulin, leptin, and adiponectin levels were assessed in 12-month-old infants.
RESULTS
Significant correlations were found between newborn and maternal serum CML (sCML) (r = 0.734, P = 0.001) serum methylglyoxal derivatives (sMGs) (r = 0.593, P = 0.001), and 8-isoprostanes (r = 0.644, P = 0.001). Infant adiponectin at 12 months negatively correlated with maternal sCML (r = −0.467, P = 0.011), whereas high maternal sMGs predicted higher infant insulin or homeostasis model assessment (P = 0.027). Infant sAGEs significantly increased with the initiation of processed infant food intake, raising daily AGE consumption by ∼7.5-fold in year 1.
CONCLUSIONS
Maternal blood and food-derived AGEs prematurely raise AGEs in children to adult norms, preconditioning them to abnormally high oxidant stress and inflammation and thus possibly to early onset of disease, such as diabetes.
Diabetes is a major chronic inflammatory disease, the incidence of which is currently exploding across the world. Especially disturbing has been the rising incidence of childhood diabetes (1,2). The current pathogenesis of both diabetes type 1 and type 2 includes a strong inflammatory component and is characterized by an earlier onset than before and an escalating incidence (3).
The causes of the underlying inflammation and oxidant stress are still being debated. Beyond genetic considerations, maternal transmission of disease risk has recently been raised as a serious contributing factor to diabetes, which increasingly affects younger adults and children (2). Children of obese, of diabetic, and of pre-diabetic mothers are more susceptible to diabetes, pointing to maternal transmission of pro-diabetic risk factor(s) (1,2). Changes in the modern diet have been implicated in both type 1 and type 2 diabetes (4).
Among the recently proposed exogenous factors that might predispose individuals to an abnormal increase in oxidant stress and inflammation are diet-derived pro-oxidant advanced glycation end products (AGEs) (5). These compounds have been shown to be orally absorbed and/or to increase circulating AGEs in the absence of diabetes (6). AGE compounds common in vivo and in foods, such as Nε-carboxymethyllysine (CML) and methylglyoxal derivatives, have been shown to promote type 1 diabetes in NOD mice, via early autoimmune cytotoxic T-cell activation (7), as well as type 2 diabetes in db/db+/+ mice (8), high-fat–fed (9), or aging C57BL6 mice (10) via increased oxidant stress and inflammation. Moreover, lowering the concentration of AGEs in food prevented diabetes in these animal models (7–10). Clinical studies in healthy and diabetic subjects have supported the view that AGEs inherent in thermally processed westernized foods predispose individuals to increased oxidant stress and inflammation, independent of caloric or nutrient consumption and that an AGE-restricted diet can improve their inflammation and oxidant stress (11,12).
Because serum AGEs (sAGEs) can be elevated in healthy individuals consuming AGE-rich foods (13), were this to occur in pregnancy, an excess of maternal oxidants could adversely influence fetal development, infancy, or adult health, as previously shown for high glucose (2), cigarette smoke, or alcohol (14,15). Specific to diabetes, AGEs can impair insulin production (16) or function (17). The contribution of maternal and/or of food-derived AGEs to the circulating AGEs of newborn and infant children or their implications, however, has not been addressed.
The clinical study presented herein addresses the following relationships: 1) maternal to infant sAGEs and oxidants, 2) infant sAGEs and select metabolic and inflammatory markers, and 3) food AGEs and infant sAGEs during the 1st year of life. We report that at birth infant sAGEs closely correlate with maternal AGEs and later with food AGEs. High AGE levels in mothers predict lower adiponectin and higher plasma insulin levels or homeostasis model assessment (HOMA). We postulate that prematurely elevated infant glycoxidant burden from maternal and dietary AGEs may contribute to the increased incidence of diabetes in the young.
RESEARCH DESIGN AND METHODS
This observational study was approved by the institutional review board at the University of Chile School of Medicine, Santiago, Chile. All participating mothers provided informed consent. All women were admitted for delivery at the Hospital San Borja Arriaran, Santiago, Chile, during the period between May and September 2006. Exclusion criteria included any chronic disease, chronic use of medications, abnormal pregnancy, or inability to attend the clinic visits at 6 and 12 months postpartum. All women had normal fasting glucose (<100 mg/dl) and a normal oral glucose tolerance test (oral intake of 75 g of glucose) with 2-h blood glucose ≤140 mg/dl during pregnancy.
Fasting blood samples were obtained from all mothers and from the umbilical cord of all newborns at the time of delivery. A full clinical assessment of each newborn was performed including weight, height, and circumference of head, abdomen (at the level of the umbilicus), and hip. During two follow-up visits, at 6 and 12 months, a full clinical and anthropometric assessment was performed, which included questions about feeding patterns or intercurrent illness, and a sample of blood was obtained.
Blood samples were centrifuged, and aliquots of plasma or serum were used for glucose and insulin measurements by the hospital clinical laboratory or frozen (at −80°C) until assessed by the Laboratory of Experimental Diabetes and Aging, The Mount Sinai School of Medicine (New York, NY). Serum samples were tested for CML (4G9 monoclonal antibody; Alteon, Northvale, NJ) and for methylglyoxal derivatives, such as MG-H1 (MG3D11 monoclonal antibody) by enzyme-linked immunosorbent assays (ELISAs), based on standards characterized by high-performance liquid chromatography and gas-liquid chromatography-mass spectrometry, as described (18). Plasma 8-isoprostane, adiponectin, leptin, and insulin levels were measured using commercially available ELISA kits. Insulin resistance was calculated according to the HOMA index as fasting insulin × fasting glucose (millimoles per liter)/22.5 (13).
All newborns were provided with powdered fortified cow milk (whole bovine milk homogenized and enriched with vitamin C, iron, zinc, and copper) (Purita fortificada; CALO [Central de Abastecimiento del Ministerio de Salud de Chile], Osorno, Chile) according to Chilean government policy, which applies until a child reaches 6 years of age.
Statistical analysis
Data are presented as means ± SD, except in figures, where they are presented as mean ± SEM. Differences of mean values between groups were tested by an unpaired Student t test or ANOVA (followed by a Bonferroni correction for multiple comparisons), depending on the number of groups. Correlation analyses were evaluated by Pearson correlation coefficient. Significant differences were defined as P < 0.05 and are based on two-sided tests. Data were analyzed using the SPSS statistical program (SPSS 16.0 for Windows; SPSS, Chicago, IL).
RESULTS
General results
All women admitted in labor (n = 60) were apparently healthy, with an average ± SD age of 23 ± 6 years. The BMI was 23.9 kg/m2 at the beginning and 29.9 kg/m2 at the end of pregnancy, and average weight gain during pregnancy was 15 ± 6 kg. The infants were all born by vaginal delivery and had an average gestational age of 39 ± 0.8 weeks (range 38–41 weeks), and their APGAR score at 1 and 5 min was >8. Anthropometric parameters of infants during their 1st year are described in Table 1.
Table 1.
Anthropometric characteristics of children during the study
| Newborn | 6 months | 12 months | |
|---|---|---|---|
| Weight (g) | 3,477 ± 375 | 8,264 ± 962 | 10,252 ± 955 |
| Height (cm) | 50 ± 3 | 67 ± 2 | 76 ± 2 |
| Heart rate (bpm) | 147 ± 12 | 128 ± 13 | 128 ± 13 |
| Mean arterial pressure (mmHg) | NA | 66.8 ± 8.9 | 72.8 ± 11.3 |
| Head circumference (cm) | 34.6 ± 1.2 | 43.5 ± 1.7 | 46.3 ± 1.2 |
| Chest circumference (cm) | NA | 44.9 ± 2.2 | 47.3 ± 1.7 |
| Abdominal circumference (cm) | 30 ± 1.6 | 42.9 ± 3 | 44 ± 2 |
| Hip circumference (cm) | 28 ± 1.4 | 41 ± 3 | 43 ± 2 |
Data are means ± SD. As expected, the difference of all these parameters over time is statistically significant (ANOVA). NA, not available.
Maternal-infant relationship of circulating AGEs and 8-isoprostanes
Average levels of maternal sAGEs, serum CML (sCML) (4.9 ± 3 units/ml), and serum methylglyoxal derivatives (sMGs) (1.09 ± 0.60 nmol/ml) at labor were within the normal range. There were significant intraindividual correlations between levels of sCML and corresponding levels of sMG (r = 0.634, P = 0.001) and 8-isoprostane (r = 0.744, P = 0.001). Maternal sAGEs were not correlated with BMI or biochemical parameters.
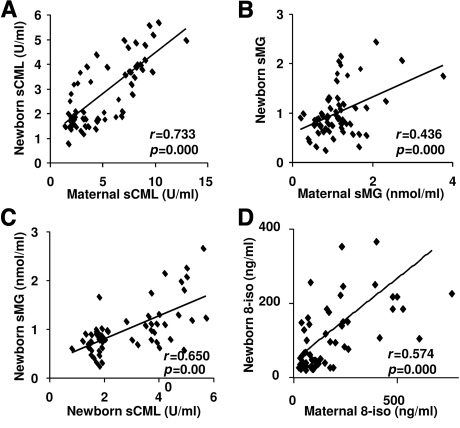
Maternal sCML levels correlated with newborn sCML levels (r = 0.734, P = 0.001) (Fig. 1A), maternal sMG with infant sMG levels (r = 0.593, P = 0.001) (Fig. 1B), and maternal 8-isoprostanes with those of their infants (r = 0.644, P = 0.001) (Fig. 1D), but these correlations were no longer evident by 12 months of age (data not shown). In addition, infant sCML levels correlated with the corresponding infant sMG levels (r = 0.525, P = 0.001) (Fig. 1C). sCML levels at birth also correlated with the respective sCML at 6 months of age (r = 0.648, P = 0.001), although not with sMGs. These findings suggested maternal transmission of sAGEs to the newborn child.
Figure 1.
Association between circulating AGE and 8-isoprostane levels in mothers and newborns. Correlations between maternal and newborn sCML (A), maternal and newborn sMGs (B), sCML and sMGs at birth (C), and maternal and newborn 8-isoprostane (8-iso) (D). Correlations were estimated by Pearson correlation coefficients.
Circulating AGEs in infants increase markedly within the 1st year
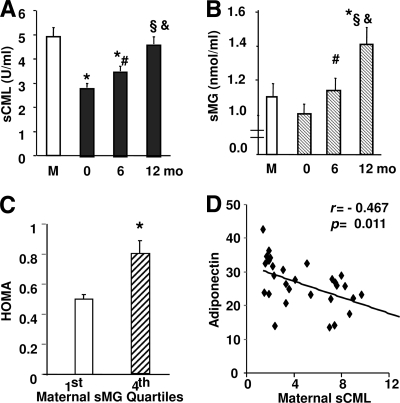
Infant sCML levels at birth were on average ∼50% less than the maternal sCML levels, but increased progressively with time in the absence of hyperglycemia, evident stress (i.e., infection), or other adverse events (Fig. 2A). At 12 months, infant sCML levels were nearly twofold above those at birth and were similar to maternal or adult values. sMG levels in the infants, although similar at birth to maternal levels, significantly exceeded maternal sMGs by 12 months (Fig. 2B). Both sCML and sMGs retained a positive correlation with 8-isoprostanes from birth throughout the 1st year of life (r = 0.744, P = 0.001 for sCML and r = 0.575, P = 0.001 for sMGs at birth; r = 0.822, P = 0.001 for sCML and r = 0.390, P = 0.021 for sMGs at 6 months; and r = 0.621, P = 0.001 for sCML and r = 0.400, P = 0.031 for sMGs at 12 months).
Figure 2.
A and B: Circulating levels of AGEs in infants increase with age. Serum levels of CML (A) and methylglyoxal derivatives (B) were measured by ELISAs in maternal (M) and umbilical cord blood from newborns at delivery (0) and children at age 6 and 12 months. Data shown are means ± SEM. Statistically significant differences are shown between mother and infants at any age (*), between newborn and 6 months (#), newborn and 12 months (§), and 6 and 12 months (&). C and D: At 12 months of age, lower adiponectin levels (D) and higher HOMA levels (C) in infants correlate with higher maternal sAGE levels. HOMA levels were analyzed at 12 months after separation of infants in groups by extreme quartiles of maternal sMGs. *P < 0.05. D: Plasma adiponectin levels in 12-month-old infants were correlated (Pearson correlation coefficient) with maternal sCML levels. Data are means ± SEM.
No relationship was observed between infant sCML or sMG levels and anthropometric parameters. No sex-based differences were observed in any clinical and biochemical parameters at any age, except for body weight at 6 months (boys 8,576 ± 970 vs. girls 7,887 ± 834, P = 0.034) and at 12 months (boys 10,526 ± 1,113 vs. girls 9,830 ± 490, P = 0.030).
Plasma insulin and adiponectin levels in infants correlate with maternal AGEs
Insulin, leptin, and adiponectin levels were tested as indicators of oxidant stress. Infant plasma insulin and glucose (or HOMA) levels were within the normal range at birth and remained stable during the 1st year of life (insulin 5.4 ± 9, 3.2 ± 1.6, and 4.0 ± 3 units/ml; glucose 82 ± 22, 85 ± 10, and 84 ± 8 mg/dl; and HOMA 1.12 ± 2.1, 0.69 ± 0.4, and 0.83 ± 0.6 at birth, 6 months, and 12 months, respectively). However, 12-month-old infants born to mothers in the upper quartile for sMGs had insulin and HOMA levels significantly higher than those of infants born to mothers in the lowest sMG quartile (Fig. 2C). A similar trend was observed after analysis on the basis of maternal sCML extreme quartiles (NS).
A negative association was observed between maternal sCML and infant adiponectin (Fig. 2D), although values of plasma adiponectin were higher than those in mothers (26 ± 7 vs. 14.5 ± 6 μg/ml; P < 0.001). Negative correlations were also found between infant plasma adiponectin and the respective sCML (r = −0.527, P = 0.003), sMG (r = −0.404, P = 0.030), and 8-isoprostane (r = −0.474, P = 0.09) levels at 12 months, whereas leptin levels showed no distinct patterns (data not shown), except for lower values in infants than in mothers (6.4 ± 2 vs. 20 ± 1 ng/ml; P < 0.001).
Infant circulating AGE levels correlate with food AGE intake
The AGE content of several common infant foods was tested to determine the potential contribution of external AGEs from food to circulating AGEs in infants. The levels of AGEs in human and bovine milk were quite similar (Table 2). However, compared with milk, measured AGE levels in a common infant formula, such as Enfamil, were nearly 100-fold higher (Table 2) and estimates of AGE levels in common infant foods indicated a significant increase in AGE content (Table 2), associated with higher AGE delivery to the infants, compared with that of milk alone. Namely, for the first 6 months the infant diet consisted mostly of maternal breast milk (AGE intake ∼15 kU/kg/day, based on ∼1,000 ml of breast milk/day). This feeding mode was gradually replaced by nonmaternal milk and by infant nonmilk food. At age 6 months, all infants were receiving one solid food per day and additional feedings of maternal and nonmaternal milk (by bottle) (AGE intake ∼76 kU/kg/day). By age 12 months, 90% of the infants were receiving on average two solid meals per day in addition to milk (AGE intake ∼111 kU/kg/day) (Table 2). Therefore, by 6 months the infant daily AGE consumption had increased by fivefold and by 12 months it had increased by ∼7.5-fold relative to that in the first 3–6 months.
Table 2.
AGE content in common infant foods and estimated daily AGE intake by infants between birth and 12 months of age
| Infant food (AGE concentration) | Estimated AGE intake (kU/kg/day) |
||
|---|---|---|---|
| Newborn | 6 months | 12 months | |
| Milk | |||
| Human milk, whole, fresh (52 units/ml) | 15 | 7 | 0 |
| Cow milk whole, 4% fat (48 units/ml) | — | — | — |
| Cow milk, powder reconstituted at 10% (14 units/ml) | 0 | 2 | 3 |
| Formula | |||
| Enfamil (4,861 units/ml) | — | — | — |
| Solid food | |||
| Vegetables/chicken infant meal (2,210 units/ml)* | 0 | 67 | 108 |
| Vegetables/beef infant meal (2,812 units/ml)* | — | — | — |
| Vegetables/legume infant meal (1,781 units/ml) | — | — | — |
| Vegetables/egg infant meal (1,774 units/ml)* | — | — | — |
| Total estimated AGE intake (kU/kg/day)† | 15 | 76 | 111‡ |
*Puree prepared at home with the following ingredients: ∼15 g chicken, beef, legumes, or egg, 1 medium size white potato, 2 leaves of spinach or other green vegetables, 1 tablespoon of carrot or squash, and 1 teaspoon of white rice or noodles. All of these ingredients are added to water to a final volume of ∼250 ml and boiled. After the addition of 1 teaspoon of vegetable oil, the mix is placed in the blender.
†The estimated AGE intake above is representative of most of the infants in this study who went from breast-feeding to the use of some reconstituted cow milk and eventually solid foods during their 1st year of life. Infants predominantly fed with formulas (e.g., Enfamil) will have a much greater daily AGE intake.
‡Note that adult dietary AGE intake is ∼200 kU/kg/day, assuming an average adult weight of 70 kg (6).
CONCLUSIONS
These studies demonstrate the appearance of proinflammatory AGEs at adult levels in the neonate circulation, first in a direct relationship to maternal blood AGE levels and later to AGEs derived from thermally processed infant foods. Infants of mothers with elevated sAGEs had higher plasma insulin and HOMA and lower adiponectin levels than infants born to mothers with low AGE levels. Because AGEs are inflammatory, an excess of AGEs transferred via the placenta or fed to infants could potentially initiate changes promoting disease, namely diabetes, at a later time. These hypothesis-generating clinical findings are supported by previous findings in mice (7) and warrant further inquiry.
The close relationships of circulating AGEs in both mothers and infants and their pro-oxidant potential were supported by highly significant correlations found in two sAGEs (sCML and sMG) and a marker of oxidant stress (8-isoprostane). Circulating AGE levels in healthy mothers were likely to reflect AGEs consumed with their diet (13), as pregnancy has no apparent effect on maternal sAGE levels (19). Maternal AGEs could be transferred from the mother to the fetus or influence the generation of fetal AGEs. The highly significant correlation between maternal and infant sAGEs before the introduction of exogenous foods to the infant supports a strong maternal influence. Levels of CML were lower in newborns than in mothers, a finding similar to that reported for another stable AGE, pentosidine (20), whereas levels of more reactive adducts, such as methylglyoxal derivatives were as high in newborns as in their mothers.
There was a continued escalation in sAGE levels over the ensuing months with near doubling of sCML levels the first 12 months. In addition, infant sMG levels significantly exceeded maternal sMG levels within the same period. These events were unrelated to blood glucose levels or to other adverse events that might promote new AGE formation or restrict their degradation and/or clearance by the kidneys. Instead, the rapid buildup of sAGEs coincided with the introduction of newly imported nutrients, which gradually replaced breast milk as the primary food source, and raised daily AGE consumption by 5- to 7.5-fold within the 1st year of life, in line with previous estimates (of ∼10-fold) (21).
AGE levels in human breast milk, as in bovine milk, are well below those in different commercially produced dairy products, which can vary by more than 10-fold, depending on the thermal processing and packaging procedures (22). Nutrient-enriched commercial infant formulas contain a far greater excess of AGEs (100- to 400-fold above human milk) (21,23), a fact that may explain the reported difference in sCML between breast-fed and formula-fed infants (22). Thus, widely used AGE-rich formulas, by raising body AGEs from this young age, expose infants to an elevated oxidant stress state, which, although not evident in the immediate time frame, can become manifest later in life.
Many of the 6- and 12-month-old infants of this cohort had sMG levels seen in individuals with diabetes or chronic kidney disease (12). It is possible that these levels decrease, as the infant's antioxidant and anti-AGE defenses, including glyoxalase I and II enzymatic activity or renal excretory function, attain maturity. However, given the prominence of glycoxidants in the modern food environment, sMG levels may increase further with age. It can be speculated that because AGEs promote inflammation, early build-up of AGE stores may start undermining innate defenses from childhood. This is a testable hypothesis that may explain the rising incidence of certain diseases in the young.
Among the multiple tissues and cells affected by AGEs, insulin-producing β-cells are also vulnerable to AGE-induced toxicity in vitro and in mice (10,16). The standard mouse diet, although normally of low fat content and with adequate antioxidants, contains sufficient levels of AGEs to promote diabetes, associated with pancreatic islet inflammatory cell infiltration and β-cell destruction, irrespective of genetic predisposition or type of diabetes (7–10). This evidence strongly points to the maternal and the external pro-oxidant environment as potential causes. Also, human studies indicate that insulin resistance is in part determined by the in utero environment (2) and the maternal eating behavior. Thus, exposure to high levels of AGEs during fetal development and early childhood could be a decisive event that turns on the innate immune response to a proinflammatory mode. Once started, such a course is likely to result in disease, often diabetes, at a young age (1). This speculation is herein supported by the relationship between high maternal sAGEs and higher plasma insulin levels (or HOMA) in children as young as 1 year and provides a strong rationale for longer follow-up studies.
These initial and limited-in-scope clinical observations are strongly supported by long-term mouse studies (7). NOD mice fed a low AGE diet for three generations had a significantly reduced incidence of type 1 diabetes and a marked delay in the onset of diabetes with each successive generation (7). Of note, sAGE levels in each generation of these mice were notably lower than in the preceding progeny, illustrating in the reverse the cumulative influence of maternal and external AGEs (7). A recent study showing that a maternal “junk food” diet in pregnancy and lactation promotes insulin resistance in rat offspring (24) supports our postulate.
Among the limitations of this clinical study were the small sample size, restricted access to infant blood samples, and lack of a detailed food intake assessment. In addition, our ELISA-based AGE tests, although highly validated (18), have been criticized by some authors (25).
In summary, we present evidence of a dynamic relationship of maternal and food-derived AGEs to circulating AGEs and to select inflammatory and metabolic markers in infants. Taken together, these data suggest that an elevated maternally dictated oxidant AGE burden in infants, if combined with AGE-rich infant foods, may negatively precondition infants to high oxidant stress and weaken innate resistance to or raise incidence of disease, such as diabetes in young individuals.
Acknowledgments
This work was supported by the National Institute on Aging (grants MERIT AG-23188 and AGE-09453 to H.V.).
No potential conflicts of interest relevant to this article were reported.
V.M. and C.P. designed the clinical study, obtained all the human samples, and reviewed/edited the manuscript. W.C., X.C., and L.Z. performed all of the animal studies, measured AGEs, and reviewed/edited the manuscript. G.E.S. provided significant advice and reviewed/edited the manuscript. H.V. and J.U. designed the overall study, analyzed the data, and wrote the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Dabelea D: The accelerating epidemic of childhood diabetes. Lancet 2009;373:1999–2000 [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Jr, Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF: Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 2008;31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riccardi G, Giacco R, Rivellese AA: Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 2004;23:447–456 [DOI] [PubMed] [Google Scholar]
- 5.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H: Advanced glycatione end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 2010;110:911–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlassara H, Uribarri J: Glycoxidation and diabetic complications: modern lessons and a warning? Rev Endocr Metab Disord 2004;5:181–188 [DOI] [PubMed] [Google Scholar]
- 7.Peppa M, He C, Hattori M, McEvoy R, Zheng F, Vlassara H: Fetal or neonatal low-glycotoxin environment prevents autoimmune diabetes in NOD mice. Diabetes 2003;52:1441–1448 [DOI] [PubMed] [Google Scholar]
- 8.Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, Fisher EA, Vlassara H: Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes 2002;51:2082–2089 [DOI] [PubMed] [Google Scholar]
- 9.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H: Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 2005;54:2314–2319 [DOI] [PubMed] [Google Scholar]
- 10.Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H: Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol 2008;173:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferrucci L, Tansman L, Striker GE, Uribarri J: Protection against loss of innate defenses in adulthood by low glycation end product (AGE) intake: role of a new anti-inflammatory AGE receptor-1. J Clin Endocrinol Metab 2009;94:4483–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ: Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 2002;99:15596–15601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H: Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 2007;62:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang JS, Lee TA, Lu MC: Prenatal programming of childhood overweight and obesity. Matern Child Health J 2007;11:461–473 [DOI] [PubMed] [Google Scholar]
- 15.Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C: Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res 2002;26:1584–1591 [DOI] [PubMed] [Google Scholar]
- 16.Zhao Z, Zhao C, Zhang XH, Zheng F, Cai W, Vlassara H, Ma ZA: Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology 2009;150:2569–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miele C, Riboulet A, Maitan MA, Oriente F, Romano C, Formisano P, Giudicelli J, Beguinot F, Van Obberghen E., Miele C, Riboulet A, Maitan MA, Oriente F, Romano CH, Formisano P, Giudicelli J, Beguinot F, Van Obberghen E: Human glycated albumin affects glucose metabolism in L6 skeletal muscle cells by impairing insulin-induced insulin receptor substrate (IRS) signaling through a protein kinase C α-mediated mechanism. J Biol Chem 2003;278:47376–47387 [DOI] [PubMed] [Google Scholar]
- 18.Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H: Oxidative stress-induced carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med 2002;8:337–346 [PMC free article] [PubMed] [Google Scholar]
- 19.Chekir C, Nakatsuka M, Noguchi S, Konishi H, Kamada Y, Sasaki A, Hao L, Hiramatsu Y: Accumulation of advanced glycation end products in women with preeclampsia: possible involvement of placental oxidative and nitrative stress. Placenta 2006;27:225–233 [DOI] [PubMed] [Google Scholar]
- 20.Tsukahara H, Ohta N, Sato S, Hiraoka M, Shukunami K, Uchiyama M, Kawakami H, Sekine K, Mayumi M: Concentrations of pentosidine, an advanced glycation end-product, in umbilical cord blood. Free Radic Res 2004;38:691–695 [DOI] [PubMed] [Google Scholar]
- 21.Dittrich R, Hoffmann I, Stahl P, Müller A, Beckmann MW, Pischetsrieder M: Concentrations of Nε-carboxymethyllysine in human breast milk, infant formulas, and urine of infants. J Agric Food Chem 2006;54:6924–6928 [DOI] [PubMed] [Google Scholar]
- 22.Sebeková K, Saavedra G, Zumpe C, Somoza V, Klenovicsová K, Birlouez-Aragon I: Plasma concentration and urinary excretion of Nε-(carboxymethyl)lysine in breast milk- and formula-fed infants. Ann NY Acad Sci 2008;1126:177–180 [DOI] [PubMed] [Google Scholar]
- 23.Birlouez-Aragon I, Pischetsrieder M, Leclere J, Morales FJ, Hasenkopf K, Kientssch-Engel R, Ducauze CJ, Rutledge D: Assessment of protein glycation markers in infant formulas. Food Chem 2004;87:253–259 [Google Scholar]
- 24.Bayol SA, Simbi H, Bertrand JA, Stickland NC: Offspring from mothers fed a “junk food” diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol 2008;13:3219–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed N, Mirshekar-Syahkal B, Kennish L, Karachalias N, Babaei-Jadidi R, Thornalley PJ: Assay of advanced glycation endproducts in selected beverages and food by liquid chromatography with tandem mass spectrometric detection. Mol Nutr Food Res 2005;49:691–699 [DOI] [PubMed] [Google Scholar]