Abstract
OBJECTIVE
Thiazolidinediones are used to treat type 2 diabetes. Their use has been associated with peripheral edema and congestive heart failure—outcomes that may have a genetic etiology.
RESEARCH DESIGN AND METHODS
We genotyped 4,197 participants of the multiethnic DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) trial with a 50k single nucleotide polymorphisms (SNP) array, which captures ∼2000 cardiovascular, inflammatory, and metabolic genes. We tested 32,088 SNPs for an association with edema among Europeans who received rosiglitazone (n = 965).
RESULTS
One SNP, rs6123045, in NFATC2 was significantly associated with edema (odds ratio 1.89 [95% CI 1.47–2.42]; P = 5.32 × 10−7, corrected P = 0.017). Homozygous individuals had the highest edema rate (hazard ratio 2.89, P = 4.22 × 10−4) when compared with individuals homozygous for the protective allele, with heterozygous individuals having an intermediate risk. The interaction between the SNP and rosiglitazone for edema was significant (P = 7.68 × 10−3). Six SNPs in NFATC2 were significant in both Europeans and Latin Americans (P < 0.05).
CONCLUSIONS
Genetic variation at the NFATC2 locus contributes to edema among individuals who receive rosiglitazone.
Although changes in lifestyle can prevent or delay diabetes (1), the majority of patients require multiple therapeutic strategies to prevent or treat the disease. Thiazolidinediones (TZDs) are a class of drugs used in the treatment of diabetes that derive their insulin sensitizing effects from the activation of the peroxisome proliferator–activated receptor γ (PPARγ) (2). TZDs can effectively control glycemia among diabetic patients (3). However, their use has been shown to cause an increase in peripheral edema and congestive heart failure (CHF) (4,5). Edema is the most commonly reported adverse drug reaction associated with TZDs, and this has been partly attributed to the 6–8% increase in plasma volume that occurs with their use (6). In addition, the observed increase in CHF associated with rosiglitazone may derive from a shared etiology.
An aim of the Diabetes REduction and Assessment with ramipril and rosiglitazone Medication (DREAM) trial was to determine whether rosiglitazone could prevent progression to diabetes among individuals with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) (7). Consistent with previous findings, a significant increase in edema and CHF among individuals receiving rosiglitazone was observed (7). The identification of genetic variants that predispose individuals to edema or CHF could lead to pretherapeutic screening procedures. To determine whether genetic variation contributes to the etiology of TZD-induced edema, we tested common single nucleotide polymorphisms (SNPs), capturing ∼2,000 cardiovascular/metabolic genes in DREAM trial participants receiving rosiglitazone.
RESEARCH DESIGN AND METHODS
The DREAM trial has been described in detail elsewhere (7). We tested 32,088 SNPs for an association with TZD-induced peripheral edema in 965 European individuals receiving rosiglitazone. Edema was defined as the presence of pitting edema at both ankles reported at any clinic visit, and individuals that withdrew from treatment due to edema were included. We used logistic regression for each SNP adjusted for age, sex, BMI, and the use of ACE inhibitors and calcium channel blockers (CCBs). Individuals taking diuretics were excluded from all analyses. The first 10 principal components of the shared alleles (identical by state) were included as covariates.
To test for interaction between the SNP and rosiglitazone, we performed a logistic regression analysis that included the main effects of the SNP, rosiglitazone, and their interaction term. Survival curves for each genotypic class and the corresponding hazard ratios (HRs) were calculated from a Cox proportional hazard analysis. A detailed description of all materials and methods is available in in an online appendix available at http://care.diabetesjournals.org/cgi/content/full/dc10-0452/DC1.
RESULTS
In our genetic substudy of DREAM, we observed an increase in edema among individuals who received rosiglitazone (n = 390 [22.3%]) versus placebo (n = 256 [14.5%]). Among the Europeans, 253 (26.2%) individuals receiving rosiglitazone experienced edema compared with 154 (16.1%) receiving placebo (P = 8.63 × 10−7) (supplementary Table 1). The clinical characteristics of the Europeans were not significantly different between the rosiglitazone and placebo arms (supplementary Table 2).
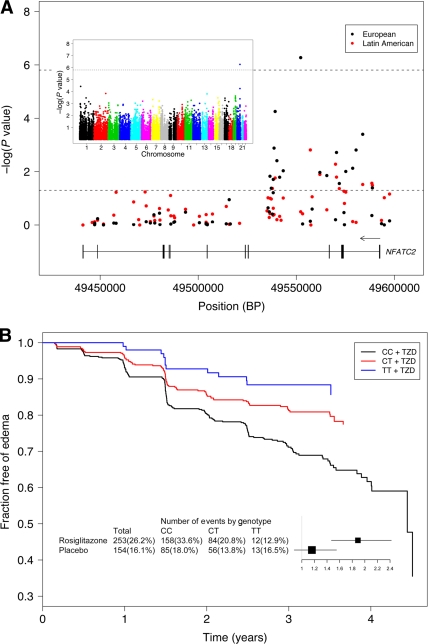
We tested 32,088 SNPs against edema in the Europeans receiving rosiglitazone. One SNP, rs6123045, in the nuclear factor of activated T-cells cytoplasmic calcineurin-dependent 2 (NFATC2) gene was significantly associated with edema (odds ratio [OR] 1.89 [95% CI 1.47–2.42]; P = 5.32 × 10−7, corrected P = 0.017) (Fig. 1A). The distribution of the observed versus the expected P values is shown in supplementary Fig. 1. We detected a significant interaction between rs6123045 and rosiglitazone treatment for edema in Europeans (P = 7.68 × 10−3). The effect of rs6123045, although in the same direction, was not significantly associated with edema in the placebo group (OR 1.16, P = 0.29) (Fig. 1B).
Figure 1.
A: Results of the association analysis between SNPs and TZD-induced peripheral edema at the NFATC2 locus. The −log of the P values are plotted against SNP location. P values were calculated from a logistic regression analysis adjusted for age, sex, BMI, and use of ramipril and CCBs, as well as the first 10 principal components of the alleles shared identity by state among the European and Latin American individuals. Individuals taking diuretics were excluded from the analysis. The dashed lines indicate Bonferroni corrected and nominal significance. Inset: Results of the initial association scan of 32,088 SNPs and TZD-induced peripheral edema in Europeans (n = 965) receiving rosiglitazone. The −log of the P values are plotted against SNP location for each chromosome. B: Survival curves estimated from the Cox proportional hazards model of time to the first occurrence of edema according to the rs6123045 genotype. European individuals homozygous for the risk allele (CC) have an increase in the rate to the first report of edema in comparison with the individuals heterozygous (CT) or homozygous (TT) for the protective allele (adjusted HR 1.76, P = 3.43 × 10−5 and adjusted HR 2.89, P = 4.22 × 10−4, respectively). Inset: The effect of the rs6123045 SNP on peripheral edema among European individuals receiving rosiglitazone or placebo. 33.6% (158 of 470) of individuals homozygous for the risk allele, 20.8% (84 of 403) of heterozygous individuals, and 12.9% (12 of 93) of individuals homozygous for the protective allele developed edema while receiving rosiglitazone compared with 18.0% (85 of 473), 13.8% (56 of 404), and 16.5% (13 of 79), respectively, while receiving placebo. The per-allele OR and 95% CI of the logistic regression analysis are shown.
A Cox proportional hazards analysis revealed that individuals homozygous for the risk allele had a decrease in the time to the first report of edema in comparison with individuals heterozygous or homozygous for the protective allele (HR 1.76, P = 3.43 × 10−5 and HR 2.89, P = 4.22 × 10−4, respectively) (Fig. 1B). The effect appears to be additive because heterozygous individuals had an increased rate of edema compared with homozygous individuals (HR 1.64, P = 0.11).
Among Europeans receiving rosiglitazone, rs6123045 was not significantly associated with diabetes or death or with cardiovascular end points, including CHF, myocardial infarction, stroke, angina, or a composite of these outcomes (data not shown).
rs6123045 was not significantly associated with TZD-induced edema in Latin Americans. However, six SNPs in NFATC2 were significant in both Europeans and Latin Americans (Fig. 1A). A haplotype defined by these SNPs is significant in both populations (OR 0.45 [95% CI 0.30–0.66]; P = 2.26 × 10−5 and OR 0.34 [95% CI 0.13–0.90]; P = 1.47 × 10−2 in Europeans and Latin Americans, respectively) (data not shown). All significantly associated SNPs were in Hardy-Weinberg equilibrium (P > 0.05).
CONCLUSIONS
We demonstratedthat variation within NFATC2 contributes to TZD-induced edema. rs6123045 was significantly associated with TZD-induced edema among Europeans when corrected for multiple testing. The significant interaction between rs6123045 and rosiglitazone treatment for edema (P = 7.68 × 10−3) among the European DREAM participants highlights its contribution to the etiology of TZD-induced edema. rs6123045 was not significantly associated with TZD-induced edema in Latin Americans. However, six SNPs in the same region are associated with TZD-induced edema and define a significantly associated haplotype in both populations.
Previous studies of either TZD-induced or dual PPAR agonist–induced edema (8–11) were smaller in size (n ≤ 730) and tested fewer genes (≤ 222). None of these studies analyzed the NFATC2 gene. Of the 38 SNPs previously associated with edema, 20 are captured by the ITMAT-Broad-CARE (IBC) array used in our study, and we were thus able to directly test them. However, we were unable to replicate any of these associations in Europeans (P > 0.05).
The NFATC2 gene encodes a cytoplasmic component of the NFAT transcription complex. Four NFAT cytoplasmic component proteins (NFATc1-c4) are known, and these are translocated to the nucleus after being dephosphorylated by the phosphatase calcineurin (12). Treatment of cardiomyocytes with rosiglitazone inhibited endothelin-1 induced calcineurin activity, suppressed the nuclear translocation of NFATc4, and enhanced the association of PPARγ with calcineurin/NFATc4 (13). The constitutive activation of either calcineurin or NFATc4 in mice leads to cardiac hypertrophy and heart failure (14). In addition, Nfatc2 null mice are protected from calcineurin-induced cardiac hypertrophy (15). In the context of these findings, our results are provocative and constitute a step toward elucidating the etiology of the CHF associated with TZD use (5).
Identifying the specific genetic variants interacting with TZDs and resulting in edema or cardiovascular events may have important clinical consequences and enable genetic variant directed use of TZD drugs among people with dysglycemia.
Supplementary Material
Acknowledgments
This work was funded by a grant from the Heart and Stroke Foundation of Ontario (NA 5754) and a Canadian Institutes of Health Research (CIHR) university/industry grant (ISO8000) with industry partners sanofi-aventis and GlaxoSmithKline (GSK). The main DREAM trial was funded by CIHR, GSK, sanofi-aventis, and King Pharma.
S.Y. has received grants, lecturing and consulting fees, and H.C.G. has received honoraria for scientific advice and for scientific presentations from GSK, the manufacturer of rosiglitazone.
S.A. holds the Heart and Stroke Foundation of Ontario Michael G. DeGroote endowed Chair in Population Health and the May Cohen Eli Lilly endowed Chair in Women's Health at McMaster University. H.C.G. holds the Population Health Research Institute Chair in Diabetes Research sponsored by Aventis. S.Y. is supported by an endowed chair from the Heart and Stroke Foundation of Ontario. B.K. holds a British Heart Foundation Personal Chair in Cardiology. S.D.B. holds a graduate scholarship from the McGill University Health Centre Research Institute and the Gerald Clavet fellowship. R.D. is a recipient of a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award from the CIHR. No other potential conflicts of interest relevant to this article were reported.
S.D.B., J.C.E., S.A., H.C.G., S.Y., V.M., A.M., and R. Di. researched the data. S.D.B., J.C.E., and S.A. wrote the manuscript. S.D.B., J.C.E., S.A., V.M., S.Y., H.C.G., B.K., R. Di., and R. Do reviewed and edited the manuscript. S.D.B., J.C.E., S.A., C.X., S.Y., H.C.G., B.K., and R. Do contributed to the discussion.
The authors thank Daniel Gaudet for helpful discussions and leadership in initiating the EpiDREAM genetics study; Brendan Keating, Lise Coderre, and Allan Sniderman for helpful discussions; and the research staff and all individuals who participated in the DREAM trial.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 2.Yki-Järvinen H: Thiazolidinediones. N Engl J Med 2004;351:1106–1118 [DOI] [PubMed] [Google Scholar]
- 3.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL: Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000;23:1605–1611 [DOI] [PubMed] [Google Scholar]
- 4.Berlie HD, Kalus JS, Jaber LA: Thiazolidinediones and the risk of edema: a meta-analysis. Diabetes Res Clin Pract 2007;76:279–289 [DOI] [PubMed] [Google Scholar]
- 5.Lago RM, Singh PP, Nesto RW: Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129–1136 [DOI] [PubMed] [Google Scholar]
- 6.Cho S, Atwood JE: Peripheral edema. Am J Med 2002;113:580–586 [DOI] [PubMed] [Google Scholar]
- 7.DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR: Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 8.Ruaño G, Bernene J, Windemuth A, Bower B, Wencker D, Seip RL, Kocherla M, Holford TR, Petit WA, Hanks S: Physiogenomic comparison of edema and BMI in patients receiving rosiglitazone or pioglitazone. Clin Chim Acta 2009;400:48–55 [DOI] [PubMed] [Google Scholar]
- 9.Geese WJ, Achanzar W, Rubin C, Hariharan N, Cheng P, Tomlinson L, Ordway N, Dracopoli NC, Delmonte T, Hui L, Krishnan B, Cosma G, Ranade K: Genetic and gene expression studies implicate renin and endothelin-1 in edema caused by peroxisome proliferator-activated receptor gamma agonists. Pharmacogenet Genomics 2008;18:903–910 [DOI] [PubMed] [Google Scholar]
- 10.Hansen L, Ekstrøm CT, Tabanera Y, Palacios R, Anant M, Wassermann K, Reinhardt RR: The Pro12Ala variant of the PPARG gene is a risk factor for peroxisome proliferator-activated receptor-gamma/alpha agonist-induced edema in type 2 diabetic patients. J Clin Endocrinol Metab 2006;91:3446–3450 [DOI] [PubMed] [Google Scholar]
- 11.Spraggs C, McCarthy A, McCarthy L, Hong G, Hughes A, Lin X, Sathe G, Smart D, Traini C, Van Horn S, Warren L, Mosteller M: Genetic variants in the epithelial sodium channel associate with oedema in type 2 diabetic patients receiving the peroxisome proliferator-activated receptor gamma agonist farglitazar. Pharmacogenet Genomics 2007;17:1065–1076 [DOI] [PubMed] [Google Scholar]
- 12.Crabtree GR, Olson EN: NFAT signaling: choreographing the social lives of cells. Cell 2002;109(Suppl.):S67–S79 [DOI] [PubMed] [Google Scholar]
- 13.Bao Y, Li R, Jiang J, Cai B, Gao J, Le K, Zhang F, Chen S, Liu P: Activation of peroxisome proliferator-activated receptor gamma inhibits endothelin-1-induced cardiac hypertrophy via the calcineurin/NFAT signaling pathway. Mol Cell Biochem 2008;317:189–196 [DOI] [PubMed] [Google Scholar]
- 14.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN: A calcineurin- dependent transcriptional pathway for cardiac hypertrophy. Cell 1998;93:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, Wehrens XH, De Windt LJ: NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem 2008;283:22295–22303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.