Abstract
OBJECTIVE
To measure prospectively bone mineral density (BMD) of the Charcot and non-Charcot foot in 36 diabetic patients presenting with acute Charcot osteoarthropathy.
RESEARCH DESIGN AND METHODS
Calcaneal BMD was measured with quantitative ultrasound at presentation, at 3 months of casting, and at the time of the clinical resolution.
RESULTS
BMD of the Charcot foot was significantly reduced compared with BMD of the non-Charcot foot at presentation (P = 0.001), at 3 months of casting (P < 0.001), and at the time of clinical resolution (P < 0.001). Overall, from the time of presentation to the time of resolution there was a significant fall of BMD of the Charcot foot (P < 0.001) but not of the non-Charcot foot (P = 0.439).
CONCLUSIONS
Although the Charcot foot was treated with casting until clinical resolution, there was a significant fall of BMD only from presentation up until 3 months of casting.
Studies on bone mineral density (BMD) have shown a reduction of BMD of the Charcot foot compared with the contralateral non-Charcot foot (1–4). However, it is not known what happens to BMD in the natural history of the osteoarthropathy. The aim of this study was to measure prospectively the longitudinal changes of BMD of the Charcot and non-Charcot foot in patients presenting with acute Charcot osteoarthropathy.
RESEARCH DESIGN AND METHODS
We studied 36 consecutive patients (19 type 1 diabetic patients; 20 male subjects; mean age 54 years [95% CI 49.4–57.9]; mean duration of diabetes 22 years [16.9–26.3]) who presented to the Diabetic Foot Clinic between February 2002 and October 2008 with a red, hot, swollen foot and a skin temperature >2°C compared with that of the contralateral foot and who had no previous offloading treatment. Foot skin temperatures were measured by Dermatemp 1001 (Exergen, Watertown, MA). All patients were treated with offloading and cast immobilization until the temperature difference between the feet was <2°C at two consecutive monthly visits (5,6).
BMD of the calcaneum was measured by quantitative ultrasound (Sahara Clinical Bone Sonometer; Hologic, Waltham, MA) as described previously (1). BMD of the Charcot foot was compared with BMD of the non-Charcot foot at presentation, at 3 months, and at the time of the clinical resolution at the end of the casting treatment using a paired Student t test. One-way repeated-measures ANOVA was used to assess the longitudinal change of BMD and foot skin temperature difference between feet. Pairwise comparisons between the means for BMD at presentation, at 3 months, and at clinical resolution were carried out to assess the effect of time. Results are presented as means (95% CI). Differences were considered significant at P < 0.05. All subjects gave informed written consent to participate. The study was approved by King's College Hospital NHS Trust Research Ethics Committee and carried out in accordance with the Declaration of Helsinki.
RESULTS
BMD of the Charcot foot was significantly reduced compared with BMD of the non-Charcot foot at presentation (0.456 g/cm2 [95% CI 0.411–0.502] vs. 0.494 g/cm2 [0.456–0.533]; P = 0.001), at 3 months of casting (0.433 g/cm2 [0.389–0.476] vs. 0.482 g/cm2 [0.393–0.564]; P < 0.001), and at the time of clinical resolution (0.432 g/cm2 [0.388–0.477] vs. 0.479 g/cm2 [0.393–0.579]; P < 0.001).
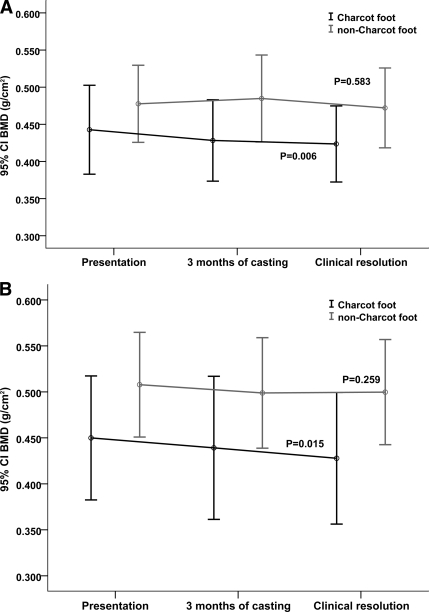
Time to clinical resolution was 8.2 months (95% CI 6.9–9.5). The multivariate analysis demonstrated a significant fall of BMD of the Charcot foot from the time of presentation to the time of clinical resolution (Wilks Λ = 0.525, P < 0.001). This was noted in both type 1 (Wilks Λ = 0.528, P = 0.006) (Fig. 1A) and type 2 diabetes (Wilks Λ = 0.497, P = 0.015) (Fig. 1B).
Figure 1.
Longitudinal changes of BMD of the Charcot and non-Charcot foot in patients with type 1 diabetes (A) and in patients with type 2 diabetes (B). Data are means (95% CI). P values indicate significance of the changes of BMD of the Charcot and non-Charcot foot from the time of presentation until the time of clinical resolution.
The pairwise comparisons between the different time points demonstrated a significant fall of BMD of the Charcot foot from presentation to clinical resolution (P = 0.015). The fall of BMD from presentation (0.456 g/cm2 [95% CI 0.411–0.502]) to 3 months of casting (0.433 g/cm2 [0.389–0.476]) was highly significant (P < 0.001), both in type 1 (P = 0.002) and type 2 diabetes (P = 0.004), but the subsequent fall of BMD from 3 months of casting to clinical resolution was not significant (0.433 g/cm2 [0.389–0.476] to 0.432 g/cm2 [0.388–0.477]; P = 0.949). This applied to both type 1 (P = 0.748) and type 2 diabetes (P = 0.832).
In contrast to the Charcot foot, the multivariate analysis in the non-Charcot foot indicated that there was a nonsignificant fall of BMD from the time of presentation (0.494 g/cm2 [95% CI 0.456–0.533]) to the time of resolution (0.479 g/cm2 [0.393–0.579], Wilks Λ = 0.947; P = 0.439), and this was present in both type 1 (Wilks Λ = 0.935, P = 0.583) (Fig. 1A) and type 2 diabetes (Wilks Λ = 0.799, P = 0.259) (Fig. 1B).
There was a significant fall of the foot skin temperature difference between the Charcot and non-Charcot foot from the time of presentation to the time of resolution (Wilks Λ = 0.423, P < 0.001). The foot skin temperature difference also fell significantly from 3.5°C (95% CI 3.1–4.1) at presentation to 2.2°C (1.8–2.2) at 3 months of casting (P < 0.001), and the latter further significantly reduced to 1.4°C (1.1–1.8) at clinical resolution (P = 0.001).
CONCLUSIONS
This study demonstrated that from the time of presentation to clinical resolution there was a significant fall of BMD of the Charcot foot but not of the non-Charcot foot. Although there was a significant fall of BMD of the Charcot foot at 3 months of casting compared with BMD at presentation, there was no further significant reduction of BMD from 3 months of casting up until clinical resolution, despite the ongoing casting of the Charcot foot.
Our study showed a fall of BMD at 3 months of casting in the Charcot foot both in type 1 and type 2 diabetes. The foot skin temperature of the Charcot foot was still 2°C greater compared with that of the non-Charcot foot, and this may have been related to inflammatory osteolysis that would have resulted in a fall of BMD (4). Increased levels of proinflammatory cytokines have been reported in patients with acute Charcot osteoarthropathy, and this may explain this observed reduction of BMD (7–9).
However, this fall of BMD may have been aggravated by the cast immobilization of the Charcot foot, and a recent case report has documented a fall of BMD in a total contact cast, highlighting the effect of immobilization (10). In our study, although there was a significant fall of BMD of the Charcot foot from presentation to 3 months of casting, this was not followed by a further significant fall of BMD up until clinical resolution despite the ongoing casting. Thus, it is more likely that the overall fall of BMD was related to inflammatory osteolysis rather than casting immobilization.
A limitation of this study is that we measured BMD of the calcaneum not BMD at the site of Charcot osteoarthropathy. Nevertheless, the calcaneum is a disease-responsive bone with a high metabolic turnover rate (11) and should have reflected overall changes of BMD in the foot.
In conclusion, although the Charcot foot was treated with casting until clinical resolution, there was a significant fall of BMD only from presentation until 3 months of casting. This may be related to the inflammatory osteolysis of Charcot osteoarthropathy.
Acknowledgments
N.L.P. was supported by Diabetes U.K. Grants BDA: RD 01/002284 and BDA: 05/0003025.
No potential conflicts of interest relevant to this article were reported.
N.L.P. and M.E.E. researched data, contributed to the discussion, wrote the manuscript, and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Petrova NL, Foster AV, Edmonds ME: Calcaneal bone mineral density in patients with Charcot neuropathic osteoarthropathy: differences between Type 1 and Type 2 diabetes. Diabet Med 2005;22:756–761 [DOI] [PubMed] [Google Scholar]
- 2.Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ: Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care 1995;18:34–38 [DOI] [PubMed] [Google Scholar]
- 3.Jirkovská A, Kasalický P, Boucek P, Hosová J, Skibová J, Kasalicky P, Boucek P, Hosova J, Skibova J: Calcaneal ultrasonometry in patients with Charcot osteoarthropathy and its relationship with densitometry in the lumbar spine and femoral neck and with markers of bone turnover. Diabet Med 2001;18:495–500 [DOI] [PubMed] [Google Scholar]
- 4.Sinacore DR, Hastings MK, Bohnert KL, Fielder FA, Villareal DT, Blair VP, 3rd, Johnson JE: Inflammatory osteolysis in diabetic neuropathic (charcot) arthropathies of the foot. Phys Ther 2008;88:1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong DG, Lavery LA, Liswood PJ, Todd WF, Tredwell JA: Infrared dermal thermometry for the high-risk diabetic foot. Phys Ther 1997;77:169–175 [DOI] [PubMed] [Google Scholar]
- 6.Petrova NL, Edmonds ME: Charcot neuro-osteoarthropathy: current standards. Diabete Metab Res Rev 2008;24(Suppl. 1):S58–S61 [DOI] [PubMed] [Google Scholar]
- 7.Jeffcoate WJ, Game F, Cavanagh PR: The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 2005;366:2058–2061 [DOI] [PubMed] [Google Scholar]
- 8.Jeffcoate WJ: Abnormalities of vasomotor regulation in the pathogenesis of the acute charcot foot of diabetes mellitus. Int J Low Extrem Wounds 2005;4:133–137 [DOI] [PubMed] [Google Scholar]
- 9.Petrova NL, Dew T, Musto R, Langworthy R, Sherwood R, Moniz C, Edmonds ME: The proinflammatory cytokines, TNF-alpha and IL-6, are linked with pathological bone turnover in the acute Charcot foot (Abstract). Diabet Med 2008;25:A22 [Google Scholar]
- 10.Hastings MK, Sinacore DR, Fielder FA, Johnson JE: Bone mineral density during total contact cast immobilization for a patient with neuropathic (Charcot) arthropathy. Phys Ther 2005;85:249–256 [PMC free article] [PubMed] [Google Scholar]
- 11.Langton CM, Palmer SB, Porter RW: The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med 1984;13:89–91 [DOI] [PubMed] [Google Scholar]