Abstract
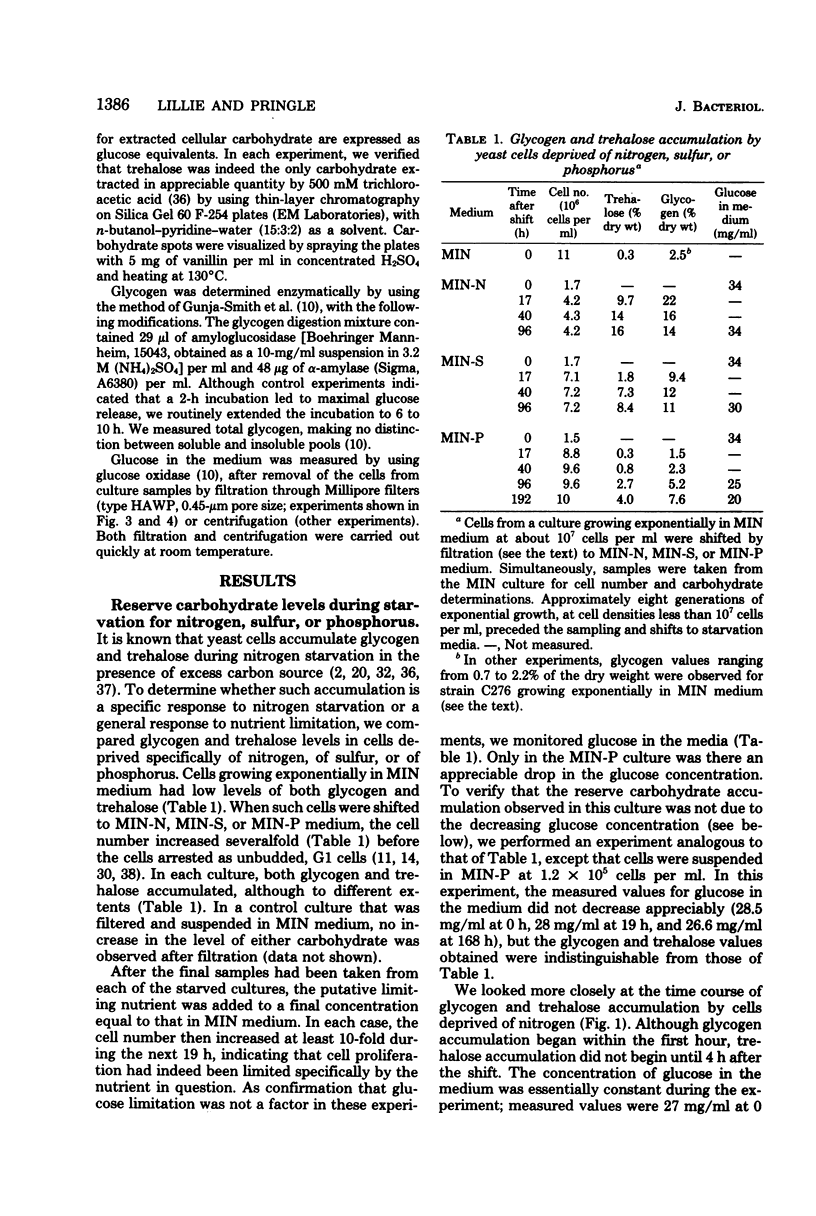
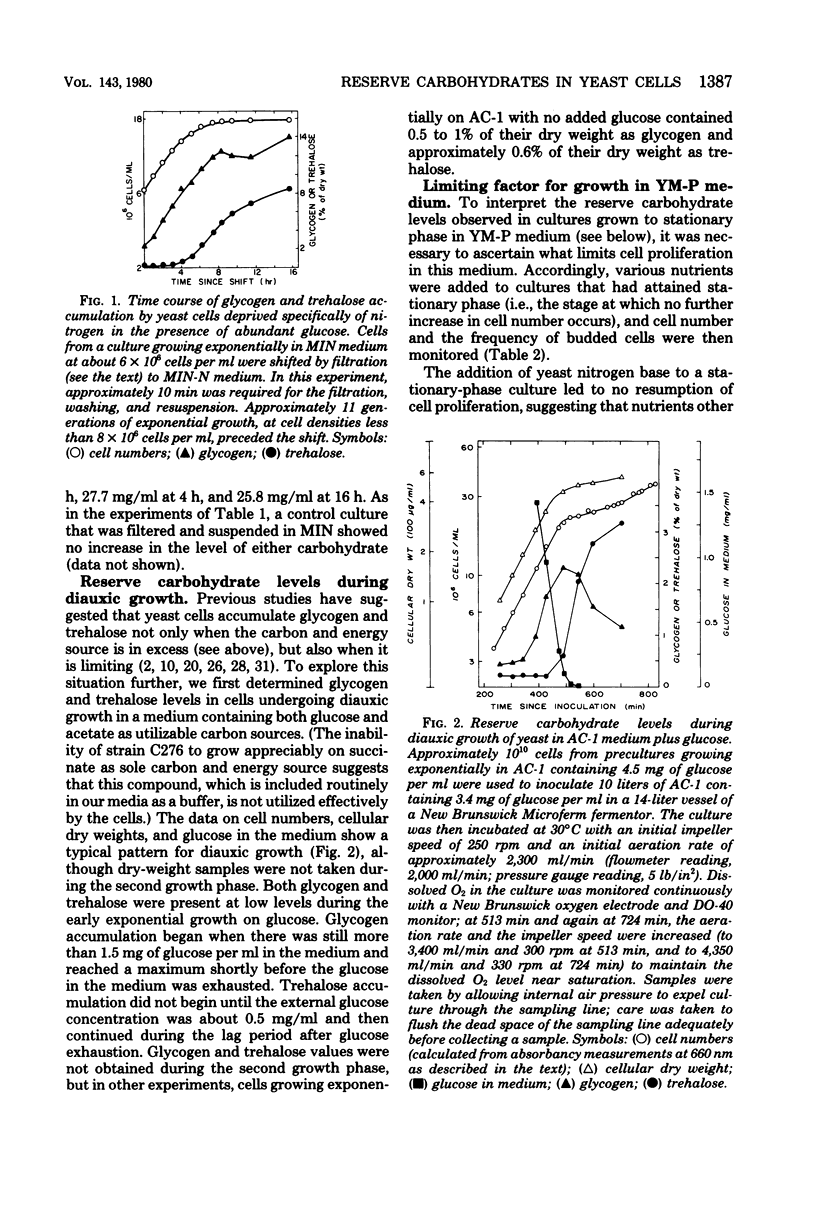
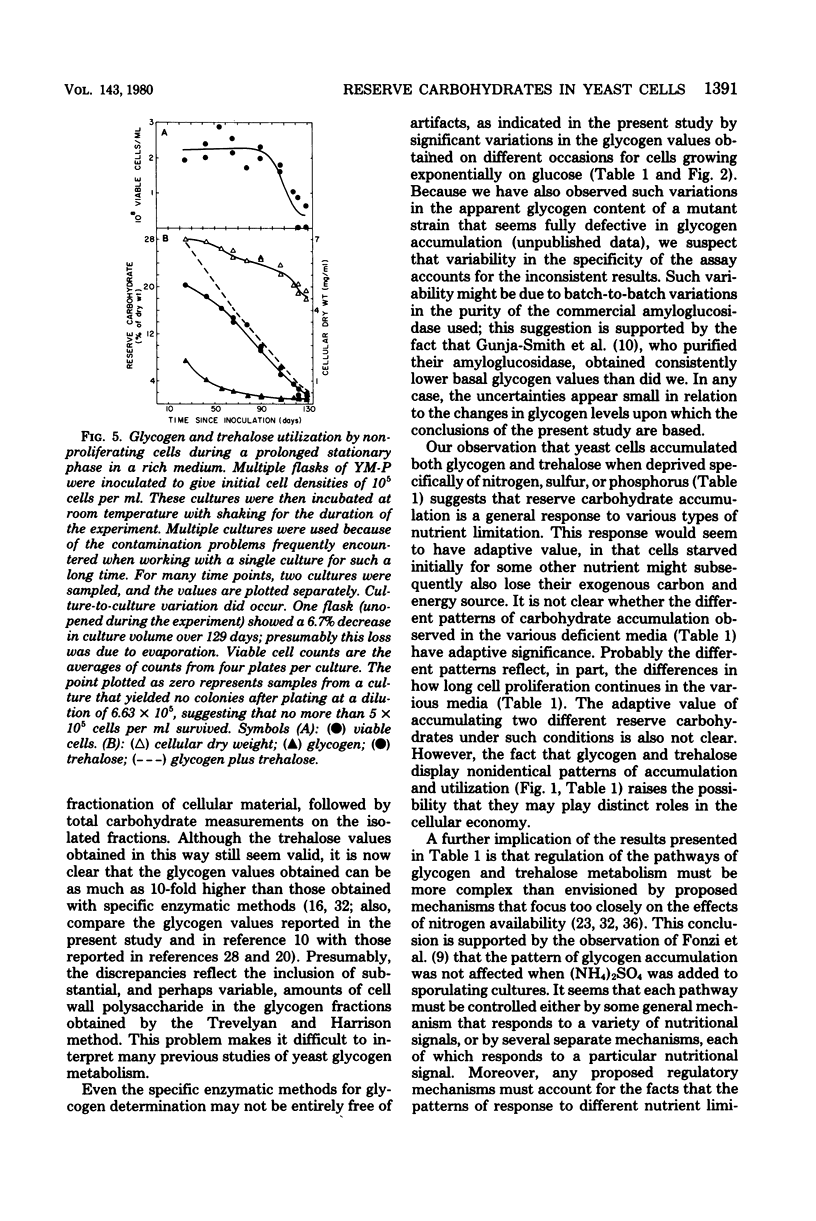
The amounts of glycogen and trehalose have been measured in cells of a prototrophic diploid yeast strain subjected to a variety of nutrient limitations. Both glycogen and trehalose were accumulated in cells deprived specifically of nirogen, sulfur, or phosphorus, suggesting that reserve carbohydrate accumulation is a general response to nutrient limitation. The patterns of accumulation and utilization of glycogen and trehalose were not identical under these conditions, suggesting that the two carbohydrates may play distinct physiological roles. Glycogen and trehalose were also accumulated by cells undergoing carbon and energy limitation, both during diauxic growth in a relatively poor medium and during the approach to stationary phase in a rich medium. Growth in the rich medium was shown to be carbon or energy limited or both, although the interaction between carbon source limitation and oxygen limitation was complex. In both media, the pattern of glycogen accumulation and utilization was compatible with its serving as a source of energy both during respiratory adaptation and during a subsequent starvation. In contrast, the pattern of trehalose accumulation and utilization seemed compatible only with the latter role. In cultures that were depleting their supplies of exogenous glucose, the accumulation of glycogen began at glucose concentrations well above those sufficient to suppress glycogen accumulation in cultures growing with a constant concentration of exogenous glucose. The mechanism of this effect is not clear, but may involve a response to the rapid rate of change in the glucose concentration.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck C., von Meyenburg H. K. Enzyme pattern and aerobic growth of Saccharomyces cerevisiae under various degrees of glucose limitation. J Bacteriol. 1968 Aug;96(2):479–486. doi: 10.1128/jb.96.2.479-486.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaich J. P., Senez J. C., Murgier M. Microcalorimetric study of glucose permeation in microbial cells. J Bacteriol. 1968 May;95(5):1750–1757. doi: 10.1128/jb.95.5.1750-1757.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHESTER V. E. The dissimilation of the carbohydrate reserves of a strain of Saccharomyces cerevisiae. Biochem J. 1963 Jan;86:153–160. doi: 10.1042/bj0860153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna W. J., Magee P. T. Glycogenolytic enzymes in sporulating yeast. J Bacteriol. 1978 Jun;134(3):844–853. doi: 10.1128/jb.134.3.844-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. The teleonomic significance of biosynthetic control mechanisms. Cold Spring Harb Symp Quant Biol. 1961;26:1–10. doi: 10.1101/sqb.1961.026.01.005. [DOI] [PubMed] [Google Scholar]
- EATON N. R. Endogenous respiration of yeast. I. The endogenous substrate. Arch Biochem Biophys. 1960 May;88:17–25. doi: 10.1016/0003-9861(60)90192-2. [DOI] [PubMed] [Google Scholar]
- FALES F. W. The assimilation and degradation of carbohydrates by yeast cells. J Biol Chem. 1951 Nov;193(1):113–124. [PubMed] [Google Scholar]
- Fonzi W. A., Shanley M., Opheim D. J. Relationship of glycolytic intermediates, glycolytic enzymes, and ammonia to glycogen metabolism during sporulation in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):285–294. doi: 10.1128/jb.137.1.285-294.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja-Smith Z., Patil N. B., Smith E. E. Two pools of glycogen in Saccharomyces. J Bacteriol. 1977 May;130(2):818–825. doi: 10.1128/jb.130.2.818-825.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia C. F., Sols A., DelaFuente G. Specificity of the constitutive hexose transport in yeast. Eur J Biochem. 1968 Aug;5(3):321–329. doi: 10.1111/j.1432-1033.1968.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Cabib E. Yeast glycogen synthetase in the glucose 6-phosphate-dependent form. I. Purification and properties. J Biol Chem. 1974 Jun 25;249(12):3851–3857. [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar von Meyenburg H. Energetics of the budding cycle of Saccharomyces cerevisiae during glucose limited aerobic growth. Arch Mikrobiol. 1969;66(4):289–303. doi: 10.1007/BF00414585. [DOI] [PubMed] [Google Scholar]
- Kelly P. J., Catley B. J. A purification of trehalase from Saccharomyces cerevisiae. Anal Biochem. 1976 May 7;72:353–358. doi: 10.1016/0003-2697(76)90541-8. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Kleinzeller A. Affinity of the yeast membrane carrier for glucose and its role in the Pasteur effect. Biochim Biophys Acta. 1967 Feb 1;135(1):106–111. doi: 10.1016/0005-2736(67)90012-0. [DOI] [PubMed] [Google Scholar]
- Küenzi M. T., Fiechter A. Changes in carbohydrate composition and trehalase-activity during the budding cycle of Saccharomyces cerevisiae. Arch Mikrobiol. 1969;64(4):396–407. doi: 10.1007/BF00417021. [DOI] [PubMed] [Google Scholar]
- Küenzi M. T., Fiechter A. Regulation of carbohydrate composition of Saccharomyces cerevisiae under growth limitation. Arch Mikrobiol. 1972;84(3):254–265. doi: 10.1007/BF00425203. [DOI] [PubMed] [Google Scholar]
- Labbe-Bois R., Volland C., Forestier J. P., Labbe P. Protohaem synthesis by the yeast Saccharomyces cerevisiae during respiratory adaptation. Relationships with glycogen metabolism. Enzyme. 1973;16(1):9–20. [PubMed] [Google Scholar]
- Lerch K., Fischer E. H. Amino acid sequence of two functional sites in yeast glycogen phosphorylase. Biochemistry. 1975 May 6;14(9):2009–2014. doi: 10.1021/bi00680a031. [DOI] [PubMed] [Google Scholar]
- PANEK A. Synthesis of trehalose by baker's yeast (Saccharomyces cerevisiae). Arch Biochem Biophys. 1962 Sep;98:349–355. doi: 10.1016/0003-9861(62)90197-2. [DOI] [PubMed] [Google Scholar]
- Panek A. D. Adenosine triphosphate inhibition of yeast trehalase. J Bacteriol. 1969 Sep;99(3):904–905. doi: 10.1128/jb.99.3.904-905.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek A. D., Mattoon J. R. Regulation of energy metabolism in Saccharomyces cerevisiae. Relationships between catabolite repression, trehalose synthesis, and mitochondrial development. Arch Biochem Biophys. 1977 Sep;183(1):306–316. doi: 10.1016/0003-9861(77)90444-1. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Ingledew W. M. The relationship of acid-soluble glycogen to yeast flocculation. Can J Microbiol. 1975 Oct;21(10):1608–1613. doi: 10.1139/m75-235. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. Induction, selection, and experimental uses of temperature-sensitive and other conditional mutants of yeast. Methods Cell Biol. 1975;12:233–272. doi: 10.1016/s0091-679x(08)60959-0. [DOI] [PubMed] [Google Scholar]
- Pringle J. R., Mor J. R. Methods for monitoring the growth of yeast cultures and for dealing with the clumping problem. Methods Cell Biol. 1975;11:131–168. doi: 10.1016/s0091-679x(08)60320-9. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Two forms of yeast glycogen synthetase and their role in glycogen accumulation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):967–974. doi: 10.1073/pnas.66.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman L. B., Cabib E. Regulation of glycogen synthesis in the intact yeast cell. Biochemistry. 1969 Aug;8(8):3332–3341. doi: 10.1021/bi00836a030. [DOI] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O., Bulla L. A., Jr, St Julian G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J Bacteriol. 1972 Mar;109(3):1232–1238. doi: 10.1128/jb.109.3.1232-1238.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. 5. The trehalose content of baker's yeast during anaerobic fermentation. Biochem J. 1956 Feb;62(2):177–183. doi: 10.1042/bj0620177b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. 7. Yeast carbohydrate fractions. Separation from nucleic acid, analysis, and behaviour during anaerobic fermentation. Biochem J. 1956 May;63(1):23–33. doi: 10.1042/bj0630023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger M. W., Hartwell L. H. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L. E., Pringle J. R. Transient G1 arrest of S. cerevisiae cells of mating type alpha by a factor produced by cells of mating type a. Exp Cell Res. 1974 Nov;89(1):175–187. doi: 10.1016/0014-4827(74)90200-6. [DOI] [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem Biophys Res Commun. 1975 Feb 3;62(3):553–560. doi: 10.1016/0006-291x(75)90434-9. [DOI] [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3',5'-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem Biophys Res Commun. 1974 Feb 4;56(3):580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]



