Abstract
Background:
Atherosclerosis (AS) is caused mainly due to the increase in the serum lipid, thrombosis, and injuries of the endothelial cells. During aviation, the incremental load of positive acceleration that leads to dramatic stress reactions and hemodynamic changes may predispose pilots to functional disorders and even pathological changes of organs. However, much less is known on the correlation between aviation and AS pathogenesis.
Methods and Results:
A total of 32 rabbits were randomly divided into 4 groups with 8 rabbits in each group. The control group was given a high cholesterol diet but no acceleration exposure, whereas the other 3 experimental groups were treated with a high cholesterol diet and acceleration exposure for 4, 8, and 12 weeks, respectively. In each group, samples of celiac vein blood and the aorta were collected after the last exposure for the measurement of endogenous CO and HO-1 activities, as well as the levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). As compared with the control group, the endocardial CO content and the HO-1 activity in aortic endothelial cells were significantly elevated at the 4th, 8th, and 12th weekend, respectively (P < 0.05 or <0.01). And these measures tended upward as the exposure time was prolonged. Levels of TC and LDL-C in the experimental groups were significantly higher than those in the control group, presenting an upward tendency. Levels of TG were found significantly increased in the 8-week-exposure group, but significantly declined in the 12-week-exposure group (still higher than those in the control group). Levels of the HDL-C were increased in the 4-week-exposure group, declined in the 8-week-exposure group, and once more increased in the 12-week-exposure group, without significant differences with the control group.
Conclusions:
Positive acceleration exposure may lead to a significant increase of endogenous CO content and HO-1 activity and a metabolic disorder of serum lipid in high-cholesterol diet–fed rabbits, which implicates that the acceleration exposure might accelerate the progression of AS.
Keywords: Endogenous carbon monoxide, Heme oxygenase-1, lipoprotein, positive acceleration exposure, total cholesterol
INTRODUCTION
Atherosclerosis (AS), the most important pathologic process leading to cardio-and cerebrovascular diseases, is suggested to be mediated by the increase in the serum lipid, thrombosis, and injuries of the endothelial cells.[1,2] During aviation, a kind of special occupation, pilots may experience an incremental load of acceleration that leads to dramatic stress reactions and hemodynamic changes. The positive acceleration exposure, especially repeated exposure, may cause accumulative adverse stress reactions in the body.[3] The situation may predispose pilots to functional disorders and even organic changes in various corporeal systems. For example, a pilot experienced a bout of idiopathic ventricular tachycardia originated from the outflow tract of the right ventricle during an aviation[4]; another pilot developed an onset of paroxysmal atrial fibrillation during a flight preparation[5]; and there were reports on myocardial infarction episodes during centrifuge simulation or aviation[6,7] As described by Zheng et al.,[8] among 39 pilots receiving coronary arteriography because of symptoms, such as choking or chest pain, 8 were confirmatively diagnosed as having coronary atherosclerotic heart disease. In accordance with related regulations, once a pilot is found developing coronary heart disease, regardless of the extent of the disease, the pilot must withdraw for permanent grounding. This sort of nonbattle withdrawal would significantly undercut the combat capability of the Air Forces. In this article, the authors treated AS model rabbits with positive acceleration exposures (+Gz) by using an animal centrifugal machine, and then observed the variation of endogenous carbon monoxide (CO) content, heme oxygenase-1 (HO-1) activity in the aortic endothelial cells, and serum lipid level. The study provided experimental evidences to delineate the correlation between aviation and AS pathogenesis.
MATERIALS AND METHODS
Establishment of AS model
A total of 32 healthy male adult purebred New Zealand rabbits (provided by the Animal Center of the Academy of Military Medical Sciences, Beijing, China), weighed between 1.0 and 1.5 kg, were fed with high fat diet with cholesterol powder 1.5 g daily for 3 months.[9] During the experiment, they were fed with high fat diet continuedly.
Grouping of experimental animals and sample collection
The rabbits were randomly divided into 4 groups with 8 rabbits in each group. The control group was given a high cholesterol diet but no acceleration exposure, whereas the other 3 experimental groups were treated with a high cholesterol diet and acceleration exposure for 4, 8, and 12 weeks, respectively. In each experimental group, routine disinfection, anesthesia, and laparotomy were performed for sample collection after the last acceleration exposure at the 4th, 8th, and 12th weekend, respectively. Approximately 100 mL of blood sample was drawn from the abdominal vein. The aorta was removed and frozen, and then was prepared with paraffin section, fixed with paraformaldehyde, desiccated at room temperature, and stored at −70°C for use.
Experimental devices and reagents
The animal centrifuge was provided by the Air Forces Aeromedicine Institute (Beijing, China), with an arm length of 2.0 m, acceleration range 0.5–15 G, and G onset rate 0.1–6 G/s. The measurement reagent kits were provided by the Beijing Yili Fine Chemicals Ltd (Beijing, China).
Acceleration exposure of animals
The animals were exposed under +4 Gz for 3 consecutive rotations with each rotation lasting for 20 s. The G onset rate was set at 1 G/s and the interval between 2 rotations was 5 min. The centrifuge was performed 3 times a week. A weekly increment of +0.5 Gz was given until the acceleration was increased up to +6 Gz at the 5th week with each rotation lasting for 40 s. Samples of aorta and abdominal venous blood were collected after the last acceleration exposure at the 4th, 8th, and 12th weekend, respectively.
Determination of HO-1 activity
The aorta samples from the control group and the 3 experimental groups were prepared into a homogenate, and then mixed with buffer solution for deep freeze at −70°C and repeated freeze–thaw for 3 cycles. After centrifugation, the supernatant was mixed with the test reagent in lightproof container at 37°C for 1 h. The unit of measurement for HO-1 activity was pmol/(mg/h). Quantitative assay of protein was performed by using the Coomassie brilliant blue staining.
Measurement of CO content
Vascular rings of 3–5 mm in length were obtained from the aorta sample and were mixed with 2 mL of phosphate buffer (pH 7.4; 0.01 mol/L) for homogenate preparation; 0.2 mL of homogenate was taken in a cuvette and 0.2 mL of redistilled water was taken in another for control. In each cuvette, 2 mL of hemoglobin solution was added and mixed well, and 0.1 mL of sodium dithionite solution was added, mixed, and maintained still for 10 min. As compared with blank control, the optical density at 541 nm (OD, 541) and 555 nm (OD, 555) were measured by using the model 721A spectrophotometer.
Measurement of serum lipid
By using the Hitachi model 7600 automatic analyzer (produced in Ibaraki, Japan), total cholesterol (TC) and triglyceride (TG) were measured with double-reagent enzymatic method, and high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured using the double-reagent clearance method.
Statistical analysis
Statistical analysis was carried out using SPSS software (ver. 10.0, produced by IBM Company, Chicago, USA). Experimental data were treated by analysis of variance for determining differences among the groups. All data were given as mean ± standard deviation
(![]() ±SD).A P-value less than 0.05 was set as significance.
±SD).A P-value less than 0.05 was set as significance.
Ethical statement
The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996), with the ethical approval from the Hospital’s Expert Review Committee.
RESULTS
Effects of positive acceleration exposure on endogenous CO content in heart tissue and HO-1 activity in aortic endothelial cells
In the control group, after a high cholesterol diet for 12 weeks, no significant differences were observed in the endocardial CO content and HO-1 activity in the aortic endothelial cells. In the experimental groups, endocardial CO content and HO-1 activity in the aortic endothelial cells were significantly higher than those in the control at 4th, 8th, and 12th weekend, respectively. And these measures tended upward as the exposure time was prolonged, with significant differences among the experimental groups (P < 0.05) [Figures 1, 2].
Figure 1.
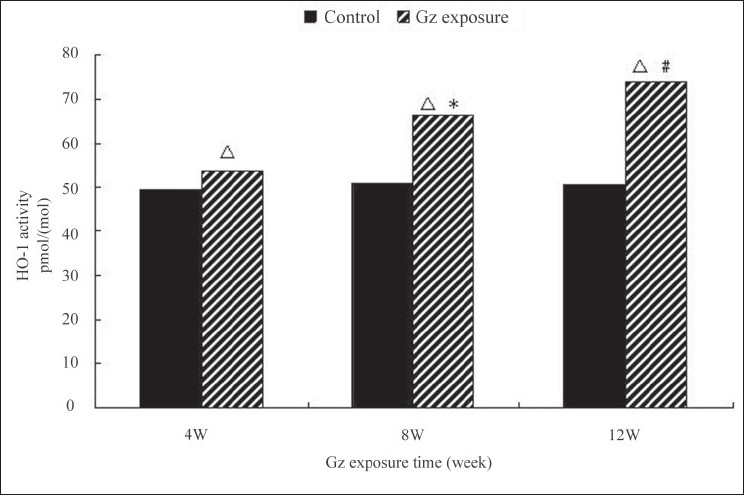
Changes of HO-1 activity after +Gz exposure in high cholesterol diet-fed rabbits. P < 0.01, between each +Gz exposure group and nonexposure group; *P < 0.05, between 4-week +Gz exposure group and 8-week +Gz exposure group; and #P < 0.05, between 8-week +Gz exposure group and 12-week +Gz exposure group
Figure 2.
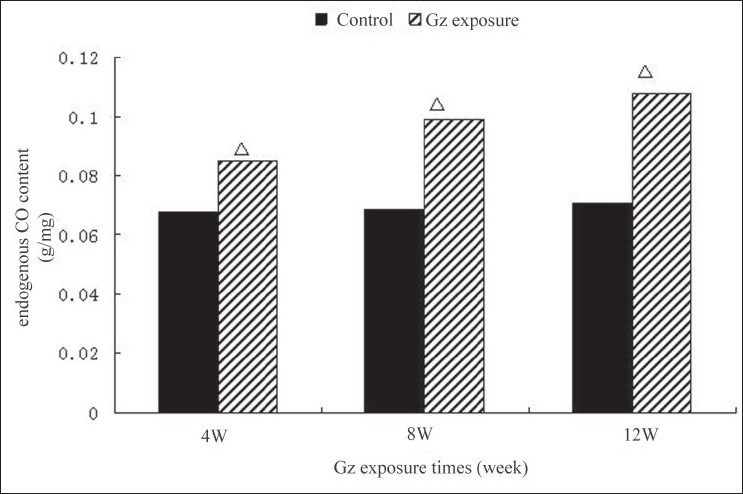
Changes of endogenous CO content after +Gz exposure in high cholesterol diet-fed rabbits. P < 0.01, between each +Gz exposure group and nonexposure group; *P < 0.05, between 4-week +Gz exposure group and 8-week +Gz exposure group; and #P < 0.05, between 8-week +Gz exposure group and 12-week +Gz exposure group
Effects of positive acceleration exposure on serum lipid metabolism
Total cholesterol
Following a high cholesterol diet, the levels of serum TC were elevated. Serum TC levels in +Gz exposure groups were higher than in the control group at 4th, 8th, and 12th weekend, respectively (P < 0.05). There were striking differences between the 4-week +Gz exposure group and the 8-week +Gz exposure group (P < 0.05), and between the 8-week +Gz exposure group and the 12-week +Gz exposure group (P < 0.05) in serum TC levels [Table 1].
Table 1.
Effects of +Gz exposure on TC and TG in high cholesterol diet–fed rabbits
| Group | TC |
TG |
||
|---|---|---|---|---|
| Control | +Gz exposure | Control | +Gz exposure | |
| n=8 | n=8 | n=8 | n=8 | |
| High cholesterol diet for 4 weeks | 1.68 ± 0.39 | 4.80 ± 1.50** | 1.06 ± 0.44 | 0.97 ± 0.34 |
| High cholesterol diet for 8 weeks | 3.92 ± 1.14 | 17.04 ± 1.74**## | 1.04 ± 0.47 | 1.78 ± 0.67*# |
| High cholesterol diet for 12 weeks | 4.26 ± 1.34 | 18.60 ± 2.18**# | 1.10 ± 0.54 | 1.26 ± 0.68* |
Notes: Between the control group and +Gz exposure group:
P < 0.05,
P < 0.01.
Between the 4-week +Gz exposure group and the 8-week +Gz exposure group, or between the 8-week +Gz exposure group and the 12-week +Gz exposure group:
P < 0.05,
P < 0.01.
Triglyceride
Following a high cholesterol diet, the levels of the serum TG were elevated. As compared with the control group, serum TG levels were decreased in the +Gz exposure group at the 4th weekend, whereas the levels significantly increased at the 8th weekend (P < 0.05). At the 12th weekend, the serum TG levels were still higher than in the control group but no significant differences were observed. There were significant differences (P < 0.05) between the 4-week +Gz exposure group and 8-week +Gz exposure group in the serum TG levels, but no remarkable difference (P > 0.05) between the 8-week +Gz exposure group and 12-week +Gz exposure group in serum TG levels [Table 1].
High-density lipoprotein cholesterol
Following a high cholesterol diet, the levels of serum HDL-C were elevated. As compared with the control group, serum HDL-C levels were elevated in the +Gz exposure group at the 4th weekend, whereas the levels decreased at the 8th and 12th weekend. The serum HDL-C levels were decreased in the 8-week +Gz exposure group than in the 4-week +Gz exposure group, and were elevated in the 12-week +Gz exposure group than in the 8-week +Gz exposure group, but no significant differences were seen among these groups [Table 2].
Table 2.
Effects of +Gz exposure on HDL-C and LDL-C in high cholesterol diet–fed rabbits
| Group | HDL-C |
LDL-C |
||
|---|---|---|---|---|
| Control | +Gz exposure | Control | +Gz exposure | |
| n=8 | n=8 | n=8 | n=8 | |
| High cholesterol diet for 4 weeks | 0.69 ± 0.21 | 0.78 ± 0.19 | 0.72 ± 0.43 | 2.81 ± 1.23 |
| High cholesterol diet for 8 weeks | 0.87 ± 0.19 | 0.62 ± 0.21# | 2.58 ± 1.12 | 15.60 ± 1.61**## |
| High cholesterol diet for 12 weeks | 1.01 ± 0.31 | 0.77 ± 0.24 | 2.75 ± 1.25 | 17.25 ± 2.11* |
TC, total cholesterol; TG, triglyceride, Notes: Between the control group and +Gz exposure group:
P < 0.05,
P < 0.01.
Between the 4-week +Gz exposure group and the 8-week +Gz exposure group, or between the 8-week +Gz exposure group and the 12-week +Gz exposure group:
P < 0.05,
P < 0.01.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Low-density lipoprotein cholesterol
Following a high cholesterol diet, the levels of the serum LDL-C were elevated. The serum LDL-C levels in +Gz exposure groups were higher than those in the control group at the 4th, 8th, and 12th weekend, respectively, with significant difference between the 8-week group and the control (P = 0.003). There were significant differences between the 4-week +Gz exposure group and the 8-week +Gz exposure group (P = 0.001), and between the 8-week +Gz exposure group and the 12-week +Gz exposure group (P = 0.116) in the serum LDL-C levels [Table 2].
DISCUSSION
With the improvement of aircraft’s mobility and increasingly complicated airbattle environment, pilots who have a high-protein, high-fat dietary structure have presented a remarkably increasing morbidity of cardiovascular diseases in recent years. According to a survey made by Poland Air Forces, among 229 pilots most of them were found to have hyperlipidemia, and the severity of the disease was mild in 40.6% of cases, moderate in 30.4%, and severe in 7.4%—the average Atherogenic Index of Plasma being greater than 5.[10] Another investigation on the military or civilian pilots killed in flying accidents in the USA showed that 43.82% of the pilots had cardiovascular disorders, and 37.6% of them had coronary artery stenosis.[11] In China, the occurrence of AS was found to be 10 years earlier in military pilots than in the general population, mostly attributing to the potential threats of latent AS and functional changes in the coronary artery.[12]
The HO-1 is the initial enzyme and rate-limiting enzyme of hemoglobin catabolism. Under the influence of anoxia and other stimulating factors, the synthesis and release of endogenous CO, which is derived from HO metabolism, is a kind of adaptation reaction in cells. Endogenous CO acts not only as a vasodilatation factor but also as an inhibitor to the proliferation of vascular smooth muscle cells, playing a critical role in maintaining the tissue’s oxygen supply and reperfusion.[13] Moreover, endogenous CO is an important messenger molecule that regulates physiological and pathologic processes in various systems,[14] such as the activation of inflammatory cells, the production of cytokines, and the activation of their actions.[15] In this study, we found that the endocardial CO content and the HO-1 activity in the heart and aorta in AS model rabbits were gradually elevated as atherosclerotic process deteriorated and the +Gz exposure time was prolonged. The HO-1, 32 kDa heat shock protein, can be expressed in response to various stimuli, such as stress, hemoglobin, amino acids, and some cytokines (IL-1, IL-6, oxidatively modified low-density lipoprotein),[16] being a potent and effective inflammation inhibitor and immunoregulator that has prophylactic effect on cardiovascular system.[17] Under pathologic conditions, HO-1 might help fight stress in the tissues and cells of the cardiovascular system and maintain the integrity and stability of the cardiovascular system.[14] Endogenous CO is derived from bilirubin under catalysis by HO.[18] In this study, the increase of endogenous CO content and HO-1 activity may be a result of a prophylactic reaction of the body, in which stress, myocardial ischemia, and hypoxia caused by +Gz exposure lead to adaptive increment of endogenous CO to improve the blood supply to the tissues. However, when HO-1 is overexpressed, vasoconstriction may be attenuated, the cell proliferation following vascular damage may be inhibited, and the progress of AS might be delayed.[19]
With the morbidity rate of hyperlipidemia in pilots being higher than the general population, attention should be paid to the effective prevention and treatment of cardiovascular diseases in this special cohort. As described by Zhang et al.,[20] serum lipid analysis of 400 military pilots in China showed abnormality in 63.8% of them. In another investigation of 250 pilots on risk factors of cardiovascular diseases made by Ma et al.,[21] a high risk of cardiovascular diseases was demonstrated in pilots and the condition was found to deteriorate with age. Among the risk factors, hyperglycemia and hyperlipidemia were observed in younger population than before. TC, TG, and LDL-C are the risk factors for cardiovascular diseases, and HDL-C is negatively correlated with the occurrence and severity of cardiovascular diseases. In this study, we had successfully simulated repeated and long-lasting +Gz exposure in animal models, and recorded a gradual elevation of TC and LDL-C in AS model rabbits. We found a tendency of decline prior to the elevation of TG, and although decreased at the 12th weekend of +Gz exposure, the TG levels were still higher than those in the control group. The levels of HDL-C presented a tendency of "increase-decrease-increase," and although no significant differences were recorded among the +Gz exposure groups and between the +Gz exposure group and the control group, HDL-C levels remained lower compared with those of the control group at the 12th weekend of +Gz exposure. This study demonstrated the correlation between +Gz exposure and lipid metabolism disorders. Both high concentration of TC, LDL-C, and TG and low level of HDL-C can directly or indirectly impair aortic endothelial cells and result in dysfunction of endothelial cells,[22] which is considered as the early period of pathologic process of AS.[23] It is now known that AS is actually a series of inflammatory reactions on the basis of impairment of vascular endothelial cells.[24,25]When vascular endothelium is impaired, vasoactive substances (such as endothelin and nitrogen monoxide) released from the endothelial cells are unbalanced, which stimulate the proliferation of smooth muscle cells and the expression of cell adhesion molecules.[26] In addition, continuous +Gz exposure may promote the activation of blood platelets,[27] facilitating the progress of thrombosis. Because all the above-mentioned factors play important roles in the pathologic process of AS, +Gz exposure may effect the progress of AS. During aviation, complicated and special environment may cause various stress reactions in pilots. Moreover, the advancement in age, tensions from flight training and tasks, and increasing psychological stress may lead to dysfunction of lipid metabolism. Therefore, the morbidity risk of thrombotic diseases is much higher in pilots than in the general population. The prevention of thrombotic diseases in pilots is of great significance to ensure flying safety and to prolong pilot’s service years.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Goldschmidt-Clermont PJ, Creager MA, Losordo DW, Lam GK, Wassef M, Dzau VJ. Atherosclerosis 2005: Recent discoveries and novel hypotheses. Circulation. 2005;112:3348–53. doi: 10.1161/CIRCULATIONAHA.105.577460. [DOI] [PubMed] [Google Scholar]
- 2.Praticò D. Antioxidants and endothelium protection. Atherosclerosis. 2005;181:215–24. doi: 10.1016/j.atherosclerosis.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Martin DS, D’Aunno DS, Wood ML, South DA. Repetitive high G exposure is associated with increased occurrence of cardiac valvular regurgitation. Aviat Space Environ Med. 1999;70:1197–200. [PubMed] [Google Scholar]
- 4.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38:S20–7. [PubMed] [Google Scholar]
- 5.Miyamoto Y, Shimazu H, Nakamura A. Plasma catecholamine and cortisol concentrations during acceleration stress. Eur J Appl Physiol Occup Physiol. 1995;70:407–12. doi: 10.1007/BF00618491. [DOI] [PubMed] [Google Scholar]
- 6.Cayce WR, Zerull RG. Myocardial infarction occurring at the conclusion of centrifuge training in a 37-year-old aviator. Aviat Space Environ Med. 1992;63:1106–8. [PubMed] [Google Scholar]
- 7.Han JL, Ma ZR, Liu YL, Gao YC. Coronary disease onset during aviation: A case study. Hang Kong Jun Yi. 2003;31:6. [Google Scholar]
- 8.Zheng J, Xu SX, Liu CZ, Xiao XG, Tao YH, Chen TX. Clinical study on 39 pilot cases underwent coronary angiography. Chin J Aerospace Med. 2003;14:220–2. [Google Scholar]
- 9.Yu L, He ZY. The research on endogenous carbon monoxide and expression of carbon monoxide synthase during atherosclerosis. Chin J Arterioscler. 1999;7:120–4. [Google Scholar]
- 10.Taneja N, Wiegmann DA. Prevalence of cardiovascular abnormalities in pilots involved in fatal general aviation airplane accidents. Aviat Space Environ Med. 2002;73:1025–30. [PubMed] [Google Scholar]
- 11.Zawadzka-Bartczak E, Kopka L, Gancarz A. Antioxidatie enzyme profiles in fighter pilots. Aviat Space Environ Med. 2003;74:654–8. [PubMed] [Google Scholar]
- 12.Zhao K, Cui QW. Cardiovascular diseases. In: Li ZG, editor. Aerospace medicine. Beijing: People’s Military Medical Press; 1992. pp. 702–10. [Google Scholar]
- 13.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 14.Durante W, Schafer AI. Carbon monoxide and vascular cell function. Int J Mol Med. 1998;2:255–62. doi: 10.3892/ijmm.2.3.255. [DOI] [PubMed] [Google Scholar]
- 15.Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–82. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibahara S. Heme oxygenase-regulation of and physiological implication in heme catabolism. In: Fujita S, editor. Regulation of heme protein synthesis. Medina, Ohio: Alpha Med Press; 1994. pp. 103–16. [Google Scholar]
- 17.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, et al. Adenovirus-mediated heme oxygenase gene transfer inhibits the development of atherosclerosis in apolipoprotein E deficient mice. Circulation. 2001;104:1519–25. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 18.McCoubrey WK, Jr, Ewing JF, Maines MD. Human heme oxygenase-2: characterization and expression of a full-length cDNA and evidence suggesting that the two HO-2 transcripts may differ by choice of polyadenylation signal. Arch Biochem Biophys. 1992;295:13–20. doi: 10.1016/0003-9861(92)90481-b. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa K, Sugawara D, Wang Xp, Suzuki K, Itabe H, Maruyama Y, et al. Heme oxygenase-1 inhibits atherosclerotic lesion formation in LDL-receptor knockout mice. Circ Res. 2001;88:456–7. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JY, Gao YX. Analysis on the types of lipid abnormality in fighter pilots. J Prev Med Chin PLA. 2007;25:98–100. [Google Scholar]
- 21.Ma HY, Zhou JL, Wei LZ, Zhang JY, Ma WJ. Investigation on risk factors of cardiovascular diseases in 250 pilots. J Prev Med Chin PLA. 2004;22:40–1. [Google Scholar]
- 22.Liu YJ, Zhang T, Zhang Y, Ren P, Lin GH, Shu JM, et al. The evaluation of brachial artery endothelial function with color Doppler flow imaging. China J MIT. 2000;16:234–6. [Google Scholar]
- 23.Arbustini E, Grasso M, Diegoli M, Bellini O, Ghio S, De Servi S, et al. Morphologic changes induced by acetylcholine infusion in normal and atherosclerotic coronary arteries. Am J Cardiol. 1993;71:1382–90. doi: 10.1016/0002-9149(93)90597-6. [DOI] [PubMed] [Google Scholar]
- 24.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- 25.Lu JC, Feng DX, Yin XC. Advances in Chlamydia pneumoniae infection and atherosclerosis. China J Modern Med. 2004;14:48–54. [Google Scholar]
- 26.Luo Y, Xie XM. [Correlation between nuclear factor-χB, soluble inter-cellular adhesion molecule-1and coronary atherosclerosis] China J Modern Med. 2004;14:78–80. [Google Scholar]
- 27.Jin Z, Geng XC, Su X, Li Q, Wang H, Li BH. The effect of +Gz stress on platelet function. Chin J Aerospace Med. 2004;15:8–10. [Google Scholar]


