Abstract
Nonhuman adenovirus (Ad) vectors derived from bovine Ad serotype 3 (BAd3) or porcine Ad serotype 3 (PAd3) can circumvent pre-existing immunity against human Ad (HAd). We have previously reported differential transduction of human and nonhuman cells by these Ad vectors, and their distinct receptor usage and biodistribution. To compare the induction of innate immunity, vector toxicity and vector uptake by Kupffer cells (KCs) following intravenous administration of PAd3, BAd3, or HAd5 vectors in mice, we determined mRNA expression levels of proinflammatory chemokines and cytokines, and Toll-like receptors (TLRs) in the liver and spleen. Tissue toxicity of these vectors was assessed by comparing serum levels of liver-specific enzymes, histopathology and Kupffer cell (KC) depletion. Compared to the HAd5 vector, PAd3 and BAd3 vectors were more potent stimulators of innate immune responses as indicated by enhanced mRNA expression of TLRs and proinflammatory chemokines and cytokine genes. Histopathological changes in the liver were most pronounced in HAd5-inoculated mice while BAd3- or PAd3-inoculated mice revealed mild histologic changes that were confined to early time points. Inoculation with HAd5 or PAd3 vectors resulted in a significant (P <0.05) decline of the number of KCs in the liver. Together, these results extend our previous observations regarding distinct in vivo biology of nonhuman and human Ad vectors.
Keywords: bovine adenovirus, chemokines, gene therapy, innate immunity, Kupffer cells, nonhuman adenoviral vectors, porcine adenovirus, toll-like receptors, vector toxicity
1. Introduction
Vectors derived from adenoviruses (Ad) have demonstrated great promise as gene delivery systems for both gene therapy and recombinant vaccines (Edelstein et al., 2004; Jager and Ehrhardt, 2007). The ongoing clinical trials of Ad-based vectors in cancer therapy and vaccination for diseases such as pandemic influenza, Ebola virus infection, and human immunodeficiency virus-acquired autoimmune disease syndrome have highlighted the potential of Ad vectors for clinical applications (Pandey et al., 2010; Sharma et al., 2009b). Vectors based on human adenovirus (HAd) serotype 5 (HAd5) and HAd serotype 2 (HAd2) are widely used for gene therapy applications (Edelstein et al., 2004). However, because of the endemic nature of HAd5 and HAd2, pre-existing vector immunity may inhibit the levels and duration of transgene expression (Kass-Eisler et al., 1996). Additionally, the predominant hepatotropism of these vectors following systemic administration further limits their utility.
Depending on the dose and route of inoculation, Ad vectors are known to activate innate immunity leading to vector toxicity and subsequent elimination of transduced cell (Hartman et al., 2008; Muruve, 2004). The innate immune response is activated by pathogen-associated molecular patterns (PAMPs) of invading pathogens through pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs) (Hartman et al., 2008; Lee and Kim, 2007). This ensues a series of signaling events leading to the induction of proinflammatory chemokines and cytokines, which result in the elimination of invading pathogens and further activation of adaptive immune responses (Lee and Kim, 2007). Furthermore, following systemic administration, Ad vectors are rapidly removed by phagocytic cells such as Kupffer cells (KCs) in the liver, which additionally impairs the efficiency of gene delivery (Alemany et al., 2000; Tao et al., 2001; Wolff et al., 1997). Sequestration of Ad vectors by KCs results in the destruction of KCs and the limited distribution of Ad vectors to other tissues. Induction of proinflammatory molecules and the destruction of KCs following Ad inoculation result in tissue toxicity, especially hepatotoxicity as often indicated by elevated levels of liver enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (Brunetti-Pierri et al., 2004).
To circumvent some of these limitations and to expand the repertoire of Ad vectors, vectors based on less prevalent HAd serotypes such as HAd3, HAd11, and HAd35 and nonhuman Ads such as bovine Ad (BAd), porcine Ad (PAd), ovine Ad, canine Ad, simian Ad, and fowl Ad, are being developed as alternatives or supplements to HAd5 vectors (Bangari and Mittal, 2006; Stone and Lieber, 2006). We have earlier demonstrated that vectors based on PAd3 or BAd3 can evade anti-HAd5 immunity and have distinct receptor usage and in vivo tropism compared to those of HAd5 (Bangari and Mittal, 2005; Bangari et al., 2005b; Li et al., 2009; Moffatt et al., 2000; Sharma et al., 2009a).
In the present study, we assessed the induction of innate immune responses and tissue toxicity in mice following intravenous inoculation with a replication-defective PAd3 or BAd3 vector. Expression levels of genes coding for proinflammatory cytokines, chemokines and TLRs in the liver and spleen, and tissue toxicity were compared among PAd3, BAd3 and HAd5 vector-inoculated groups. We observed significant differences in the induction of innate immune response and tissue toxicity between nonhuman Ad and HAd5 vectors.
2. Materials and methods
2.1. Adenoviral vectors
Replication-defective HAd-GFP (Bangari and Mittal, 2004), PAd-GFP (Bangari and Mittal, 2004), and BAd-GFP (Bangari et al., 2005a) (Bangari et al., 2005b) vectors with deletions in early 1 (E1) region and carrying the green fluorescent protein (GFP) gene under the control of the human cytomegalovirus (CMV) promoter were propagated in 293 (human embryonic kidney cells expressing HAd5 E1) (Graham et al., 1977), FPRT HE1-5 (fetal porcine retina cells expressing HAd5 E1) (Bangari and Mittal, 2004), or FBRT HE1 (fetal bovine retina cells expressing HAd5 E1) (van Olphen et al., 2002), respectively. Virus purification was done by cesium chloride-density gradient centrifugation (Bangari and Mittal, 2004). The physical particle counts of purified stocks of HAd-GFP, PAd-GFP and BAd-GFP were estimated by spectrophotometry and expressed as vector particles (VP) per ml using a previously described method (Maizel, Jr. et al., 1968). Since plaque assays for these vectors were carried out in different cell lines, the efficiency of plaques formation varied widely with the virus and cell type combination. Furthermore, as the capsid proteins of Ads are mostly implicated in the induction of innate immunity, a dosage of equal particles of each virus was critical for comparative studies. Therefore, VP was selected instead of plaque-forming units for vector quantification to maintain consistency in vector dosage.
2.2. Animal inoculation
Eight-to-ten-week-old female FVB/n mice were obtained from Harlan Laboratories (Indianapolis, IN). FVB/n mice were selected for the current study because of the availability of an immunocompetent tumor model with this mouse strain (Noblitt et al., 2005). The use of this mouse strain would allow us to extend our study for investigation of Ad vectors for cancer gene therapy. All animal inoculations were conducted in accordance with the guidelines and approval from Institutional Biosafety Committee and Institutional Animal Care and Use Committee. Mice were inoculated intravenously via tail vein with HAd-GFP, PAd-GFP, or BAd-GFP at a dose of 1010 VP per mouse in a volume of 100 µl PBS++ (Phosphate buffered saline supplemented with 0.01 % MgCl2 and 0.01 % CaCl2). Mice inoculated with PBS++ served as negative controls. Mice (3 animals per group) were euthanized at various time points (0.25, 0.5, 1, 2, 4, 8, and 16 days) post-inoculation, and serum samples were collected and evaluated for AST or ALT enzyme levels. The liver, spleen, lungs, heart and kidneys were either collected in 10% neutral buffered formalin or snap frozen and stored at −80°C.
2.3. Quantification of cytokines, chemokines and TLRs specific mRNA transcripts
Frozen tissue samples were used for RNA extraction. Total cellular RNA was isolated from 50 mg of the liver and spleen samples using RNA miniprep kit (Stratagene, Cedar Creek, TX). RNA samples were treated with DNase I to remove the residual DNA, if any. TaqMan® Gene Expression Assays (Applied Biosystems) mixture consisting of forward primer, reverse primer, and Taqman® minor groove-binding probe (labeled with 6-Carboxyfluorescein dye) specific to mouse CCL2, CCL3, CCL4, CCL5, CXCL2, IP-10, IFNγ, IL-6, TNFα, TLRs (TLR1 through TLR9), myeloid differentiation primary response gene 88 (MyD88) or TIR-domain-containing adapter-inducing interferon-β (TRIF) were used for the quantification of chemokines, cytokines and TLRs. Two hundred ng of total cellular RNA was processed for real-time RT-PCR using specific primers and probe and 1-step Brilliant qRT-PCR Master Mix Kit (Stratagene). For normalization of the target gene expression, similar real-time RT-PCR reactions targeting the endogenous 18S rRNA were simultaneously carried out in separate tubes. Reaction mixture consisted of 2 × qRT-PCR master mix, 250 nM each of respective forward and reverse primers, and 100 nM of Taqman probe along with other standard kit components. Each reaction was carried out in duplicate. The real-time RT-PCR was performed using the Mx3000 Thermocycler (Stratagene). The reaction conditions included cDNA synthesis step at 50°C for 30 min, followed by polymerase activation (95°C for10 min), and 45 cycles of denaturation (95°C for 15 sec) and annealing/extension (60°C for 1 min). The Ct values for individual reactions were determined and data were analyzed with MxPro software (Stratagene) to obtain the relative expression levels of various chemokines, cytokines, or TLRs. Quantification of expression levels of mRNA transcripts in the liver and spleen tissue samples was done by ΔΔCt method (Winer et al., 1999) and expressed in relation to the mean expression levels of respective genes observed in tissues from mock-inoculated mice at respective time points (referred to as calibrators). The difference in expression levels in relation to the time-matched calibrator was calculated as 2−ΔΔCt (where ΔΔCt = [Cttarget gene (unknown) – Ct18S rRNA (unknown)] - [Cttarget gene (calibrator) - Ct18S rRNA (calibrator)], Ct is the cycle number at which fluorescence signal crosses the threshold).
2.4. Histopathology and immunohistochemistry
Formalin-fixed tissues (liver, spleen, kidneys, lungs and heart) were paraffin-embedded and used for routine histopathology and immunohistochemistry. For histopathology, formalin-fixed, paraffin-embedded tissue blocks were sectioned at 5 µm, stained with hematoxylin and eosin and examined microscopically by two board-certified veterinary pathologists (HH and DSB).
For immunohistochemistry, formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated in xylene and alcohol according to standard procedures. For antigen retrieval, sections were immersed in hot (95°C) target retrieval solution pH 6.0 (DakoCytomation, Carpinteria, CA) for 30 minutes. Endogenous peroxidase activity was quenched by incubating sections in 3% hydrogen peroxide solution for 10 min. Endogenous biotin and avidin were blocked by sequential incubation in avidin and biotin solutions (Vector Laboratories, Burlingame, CA) for 15 min each. Subsequently, sections were incubated overnight at 4°C in a blocking buffer (PerkinElmer, Waltham, MA) supplemented with 5% goat serum.
The following day sections were incubated with a monoclonal rat anti-mouse F4/80 (pan-macrophage marker) primary antibody (Abcam, Cambridge, MA) at 1:10 dilution for 2 h followed by incubation with a goat anti-rat biotinylated secondary antibody (1:2500) for 1 h at room temperature in a humidified chamber. This step was followed by incubation with streptavidine-horseradish peroxidase conjugate (DakoCytomation) (1:100) for 30 min at room temperature. The signal was amplified by tyramide signal amplification kit (PerkinElmer). Color development was performed by aminoethyl carbazole (Red) substrate kit (Zymed Laboratories Inc., San Francisco, CA). The specimens were counter-stained with hematoxylin. Slides were mounted using Clearmount solution (Zymed Laboratories), dried and coverslipped with permount (Fisher Scientific, Pittsburgh, PA). For quantification of F4/80-positive cells (KCs), seven randomly selected overlapping fields were counted at 600 × magnification and the averages were calculated.
2.5. Evaluation of serum liver enzymes
Serum levels of liver-specific enzymes (AST and ALT) were determined using the VITROS 5.1 FS Chemistry System (Johnson & Johnson Gateway) at the Clinical Pathology Laboratory, Veterinary Teaching Hospital, School of Veterinary Medicine, Purdue University.
2.6. Statistical analyses
A two way ANOVA model was used to test the statistical significance between or within the groups. All statistical analyses were applied in PROC GLM with CONTRAST option in SAS 9.1. For all tests, P < 0.05 was considered significant.
3. Results
3.1. Induction of proinflammatory chemokines and cytokines expression in mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP
Intravenous inoculation of a host with Ad vectors results in activation of innate immune response which is often associated with toxicity and rapid vector clearance. Previous studies have reported the induction of a variety of proinflammatory cytokines and chemokines following the systemic administration of Ad vectors (Muruve et al., 1999; Muruve, 2004; Zaiss et al., 2002). Various serotypes of human Ad vectors differentially activate innate immune responses (Appledorn et al., 2008a; Iacobelli-Martinez and Nemerow, 2007). We have developed nonhuman Ad vectors based on PAd3 and BAd3 to circumvent some of the limitations associated with HAd vectors (Bangari and Mittal, 2004; Mittal et al., 1995).
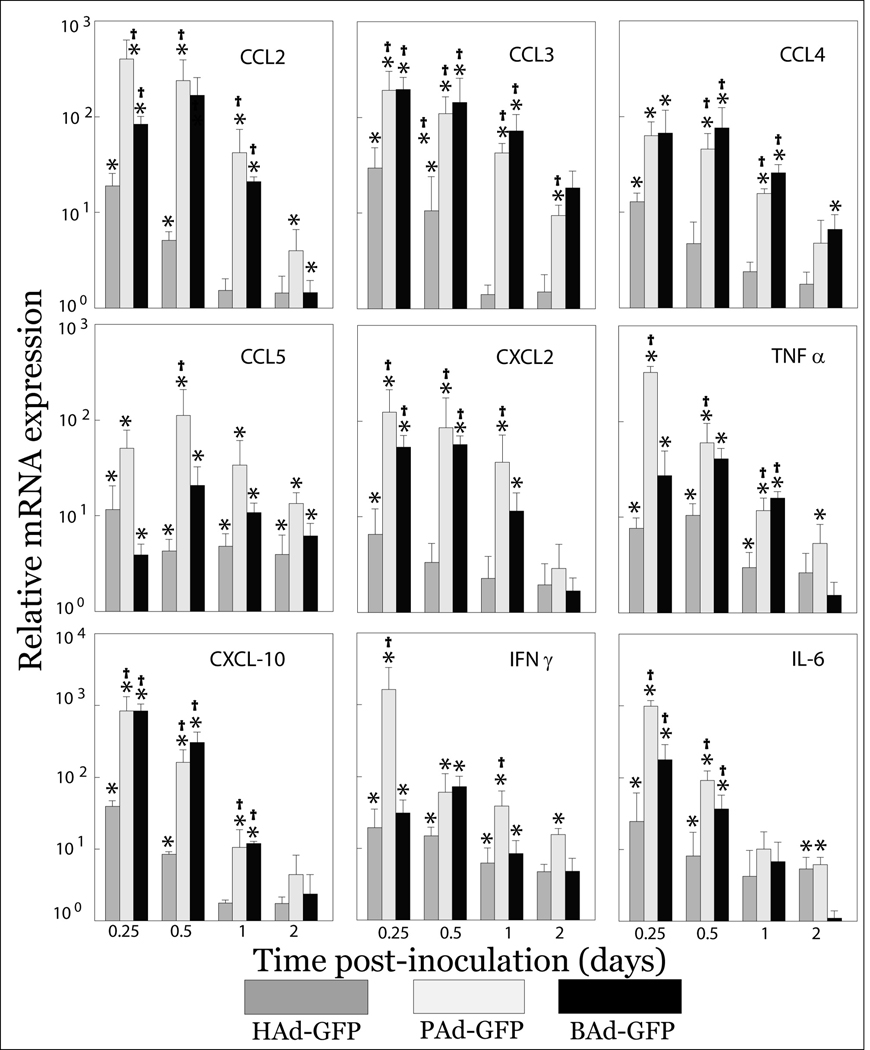
To investigate the differences in the induction of innate immune responses following systemic administration of these vectors, FVB/n mice were inoculated intravenously with 1010 vector particles (VP) of replication-deficient HAd-GFP, PAd-GFP or BAd-GFP, each of which carries the GFP transgene as a reporter. At 6, 12, 24 and 48 h post-inoculation, animals were euthanized and multiple tissues including the liver and spleen were collected. We have previously reported the in vivo biodistribution of HAd5-, BAd3- and PAd3- based vectors and their efficiency to express transgene (Sharma et al., 2009a). Since the majority of intravenously inoculated human or nonhuman Ads localize to the liver and spleen (Sharma et al., 2009a), these tissues were selected for assessment of cytokine and chemokine genes implicated in the induction of innate immune responses. Total cellular RNA extracted from the liver and spleen samples was analyzed for expression of various cytokines and chemokines mRNA by real-time reverse transcriptase polymerase chain reaction (RT-PCR). Significant (P < 0.05) increases in mRNA expression levels of various chemokines (CCL2, CCL3, CCL4, CCL5, CXCL2, CXCL10 and IP-10) and cytokines, (TNFα, IFNγ and IL-6) were observed in both the liver (Fig. 1) and spleen (data not shown) of vector-inoculated groups compared to the PBS-inoculated control group. In Ad vector inoculated animals, relative mRNA expression levels of proinflammatory cytokines and chemokines peaked at 6 h or 12 h post-inoculation and declined gradually thereafter. Relative mRNA expression levels of almost all the investigated cytokines and chemokines at 6 h post-inoculation were significantly (P < 0.05) higher in the nonhuman Ad-inoculated mice than in those HAd-GFP-inoculated mice. Between the two nonhuman Ad vectors, relative mRNA expression levels of these cytokines and chemokines were either comparable or marginally higher in mice inoculated with PAd-GFP.
Figure 1. Expression levels of proinflammatory cytokines and chemokines mRNA at different time points post-inoculation in the liver of mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP.
Real-time RT-PCR assays for various cytokines and chemokines together with 18S rRNA (as an endogenous control for normalization) were performed using 200 ng of total RNA and comparative quantification of cytokines and chemokines expression levels was achieved. Values are reported relative to mean expression levels observed with mock-inoculated mice at each time point post-inoculation. Values are shown as the mean ± standard deviation for three mice at each time point. *P < 0.05 versus expression level in PBS-inoculated mice. †P < 0.05 for PAd-GFP or BAd-GFP versus HAd-GFP at each time point.
3.2. Induction of TLR expression in mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP
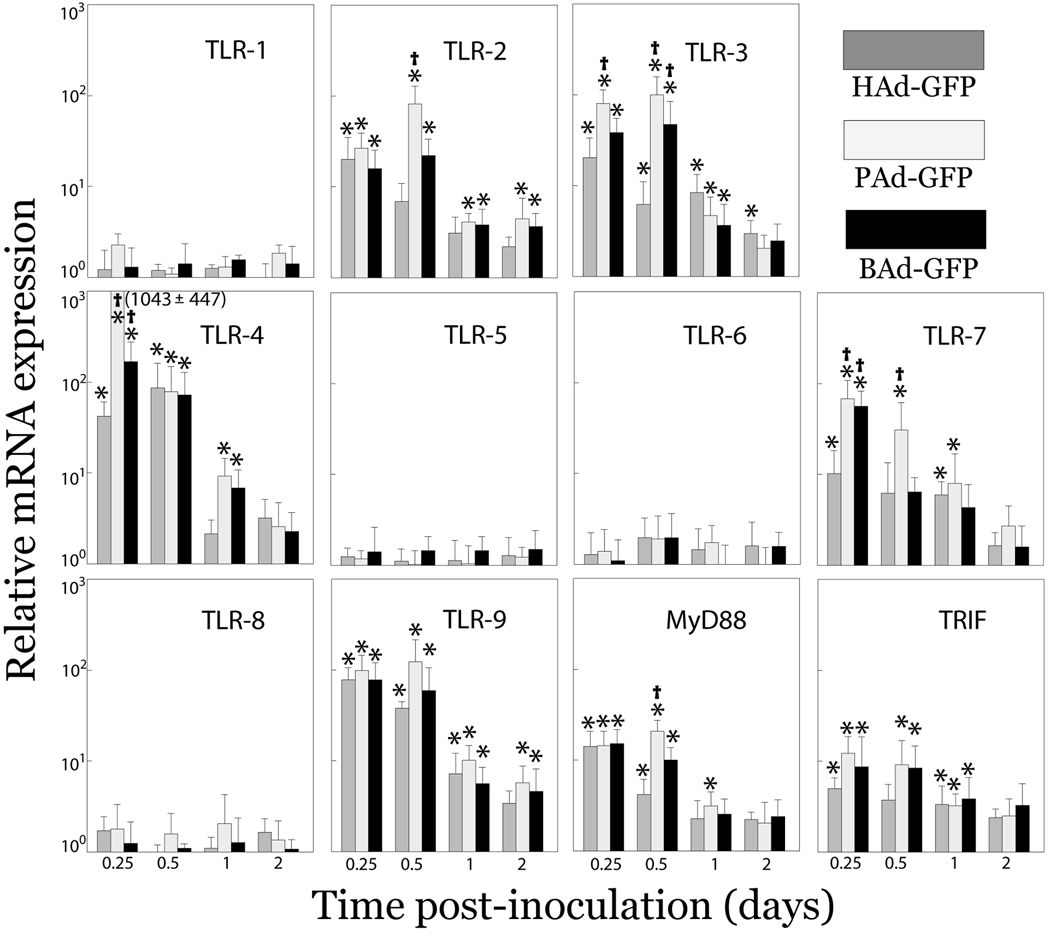
TLRs interact with various viral components to stimulate the host innate immune responses (Janssens and Beyaert, 2003). Following activation, TLRs initiate a signaling cascade through adaptor molecules such as MyD88 and/or TRIF that subsequently results in activation of innate as well as adaptive immune responses. In the present study, we examined relative mRNA expression levels of TLRs (TLR 1 through TLR 9), MyD88 and TRIF in the liver and spleen of mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP. A significant (P < 0.05) increase in relative mRNA expression levels of TLRs 2, 3, 4, 7 and 9, together with MyD88 and TRIF was observed in both the liver (Fig. 2) and spleen (data not shown) of Ad vector-inoculated mice compared to mock-inoculated controls. No significant changes were observed in mRNA expression levels of TLRs 1, 5, 6 and 8 compared to mock-inoculated mice. Relative mRNA expression of several of the TLRs peaked at 6 h or 12 h post-inoculation and gradually declined thereafter. Significantly higher mRNA expression levels of TLR 4 and 7 were observed in mice inoculated with nonhuman Ad vectors compared to those inoculated with HAd-GFP. Similar to mRNA expression levels of proinflammatory chemokines and cytokines, relative mRNA expression levels of TLRs in PAd-GFP-inoculated mice were either comparable or higher than those in BAd-GFP-inoculated mice.
Figure 2. mRNA expression levels of various TLRs and adaptor molecules (Myd88 and TRIF) at different time points post-inoculation in the liver of mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP.
Real-time RT-PCR assays for various TLRs, Myd88, or TRIF together with 18S rRNA (as an endogenous control for normalization) were performed using 200 ng of total RNA and comparative quantification of TLRs, Myd88 and TRIF expression levels was achieved. Values are reported relative to mean expression levels observed with mock-inoculated mice at each time point post-inoculation. Values are shown as the mean ± standard deviation for three mice at each time point. *P < 0.05 versus expression level in PBS-inoculated mice. †P < 0.05 for PAd-GFP or BAd-GFP versus HAd-GFP at each time point.
3.3. Evaluation of vector toxicity in mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP
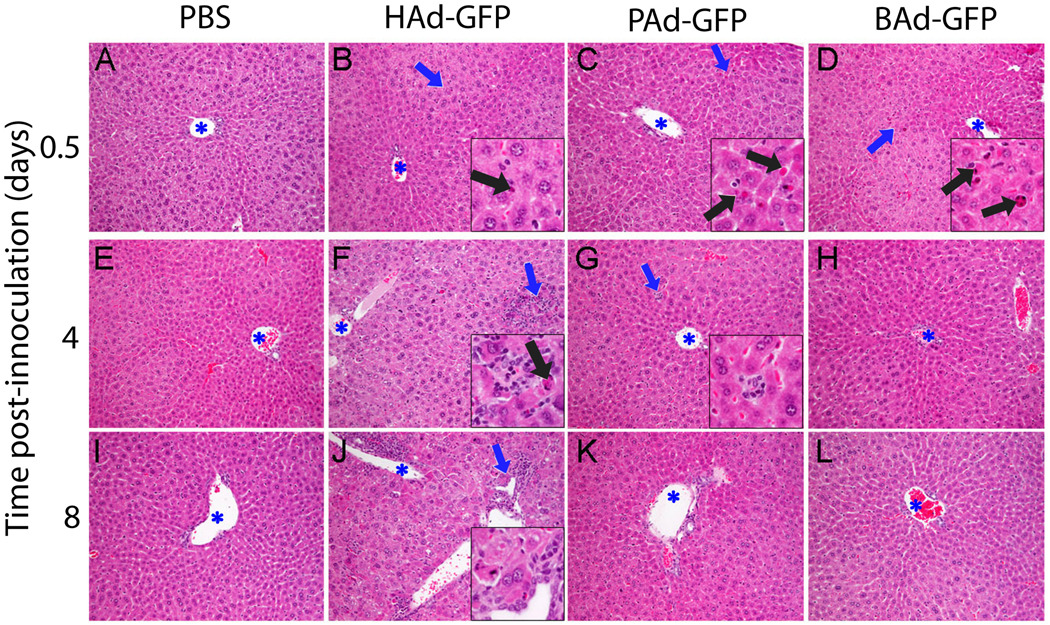
To examine vector toxicity in mice inoculated with HAd-GFP, PAd-GFP, or BAd-GFP, the liver, spleen, lungs, kidneys and heart were collected at 0.25, 0.5, 1, 2, 4, 8, and 16 days post-inoculation. The tissue samples were analyzed for histological changes by histopathology and for the number of KCs in the liver sections by immunohistochemistry. At earlier time points (6 to 24 h post-inoculation), histologic changes in the liver were minimal to mild in all Ad vector-inoculated mice (Fig. 3). These changes included scattered apoptotic hepatocytes with occasional infiltration by macrophages and fewer granulocytes. Apoptotic hepatocytes were observed at increased frequency in BAd-GFP-inoculated mice followed by PAd-GFP or HAd-GFP-inoculated mice (Fig. 3 B–D). The most striking liver histopathologic changes were observed in HAd-GFP-inoculated mice at later time points (4 or 8 d post-inoculation). At these time points, the livers of HAd-GFP-inoculated mice revealed multifocal hepatocellular degeneration, scattered apoptotic hepatocytes, portal lymphohistiocytic inflammation as well as scattered foci of inflammation (Fig. 3 F and J). In mice inoculated with PAd-GFP, BAd-GFP or PBS, the liver histology was unremarkable at these time points (Fig. 3). Rare clusters of macrophages (Fig. 3G) were observed within the hepatic parenchyma of some mice from all groups. Histologic changes in the spleen were comparable among all Ad vector-inoculated mice. Prominent germinal centers were present in the splenic white pulp of all Ad-inoculated mice at 8 and 16 days post-inoculation (data not shown). As expected, no significant histologic changes were observed in the control group at any time point.
Figure 3. Representative photomicrographs of the liver sections of mice inoculated with PBS, HAd-GFP, PAd-GFP, or BAd-GFP.
Blue asterisks indicate lumen of portal veins and insets represent a high magnification of area indicated by blue arrows. Note scattered apoptotic hepatocytes (black arrows) in Ad-vector-inoculated mice liver at 0.5 day post-inoculation. Multifocal, random lymphocytic infiltration and hepatocellular degeneration/apoptosis as well as portal inflammation were observed only in HAd-GFP-inoculated mice at 4 and 8 days post-inoculation (panels F and J). Rare clusters of macrophages (inset, panel G) were occasionally found in mice from all groups. Stain: hematoxylin and eosin. Original magnification: 200 × (insets 600 ×)
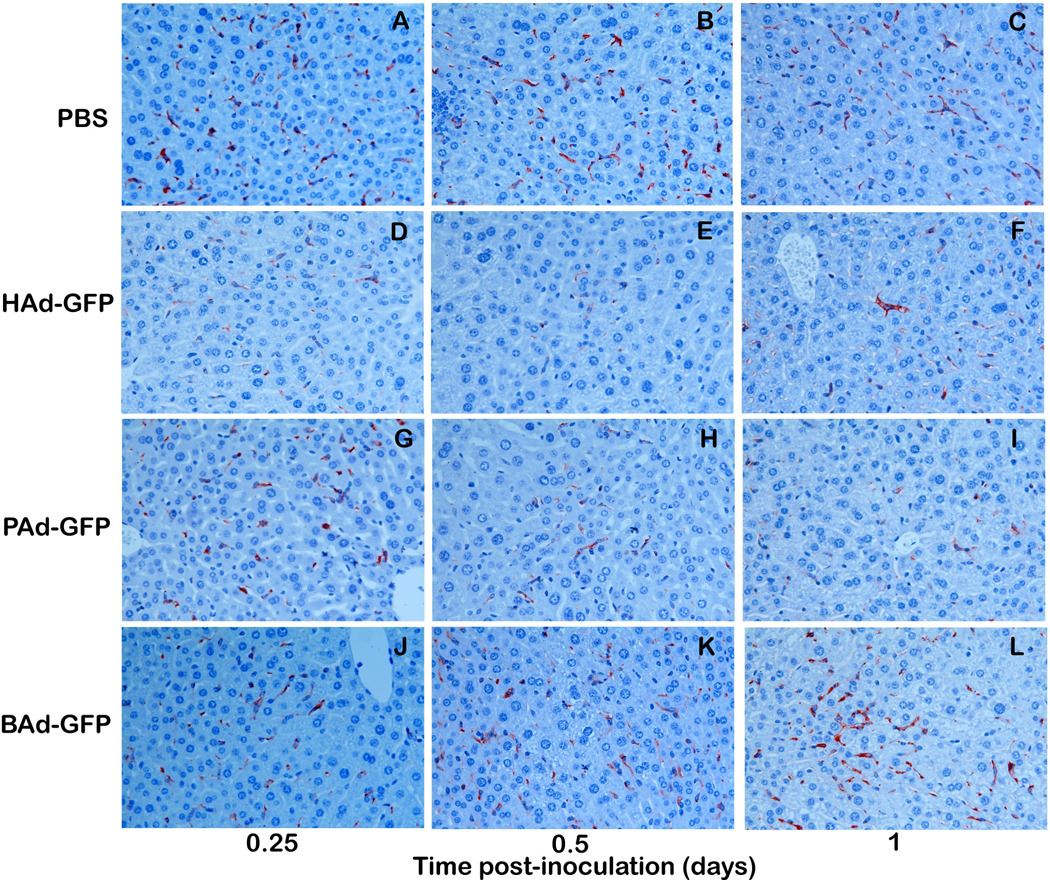
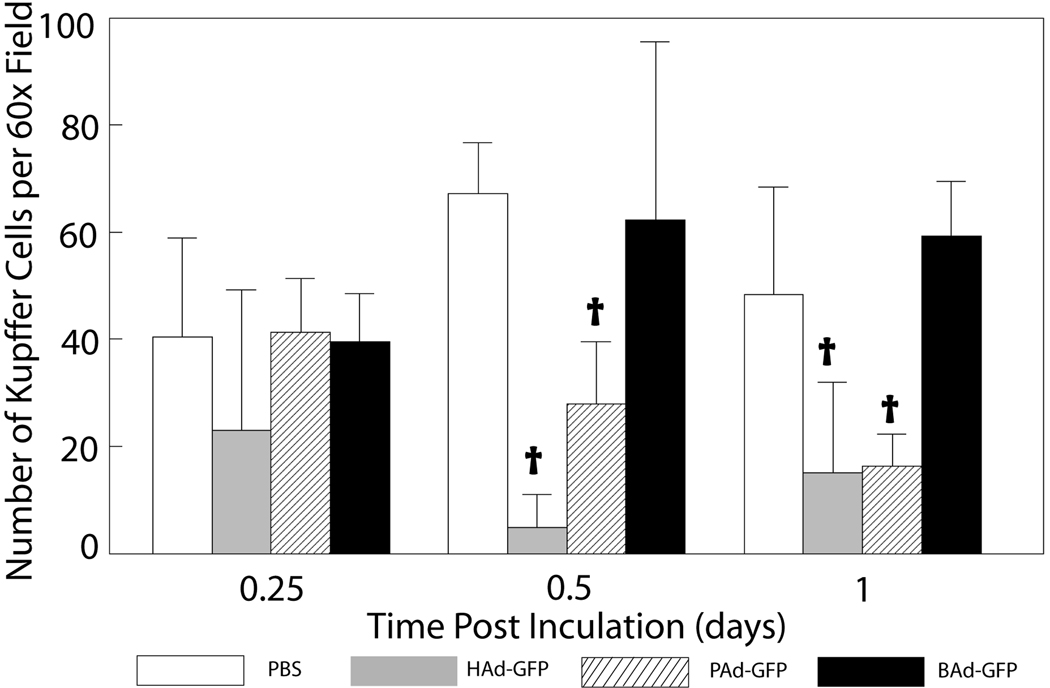
Immunohistochemical staining for KCs in the liver sections indicated significant decreases in the number of F4/80 immuno-positive KCs in HAd-GFP and PAd-GFP inoculated mice at 12 and 24 h post-inoculation compared to mock-inoculated controls (Figs. 4 a & b). In HAd-GFP-inoculated mice, KCs declined by approximately 50, 90 and 80% at 6, 12 and 24 h post-inoculation, respectively. At the same time points, the declines in KCs in PAd-GFP-inoculated mice were approximately 5, 60 and 70%, respectively. The decline in KCs in HAd-GFP-inoculated mice samples was significantly higher (P < 0.05) compared to that of mice inoculated with PAd-GFP at 12 h post-inoculation. No significant reduction (P > 0.05) in KCs was observed in BAd-GFP-inoculated mice at any time point (Fig. 4b).
Figure 4.
a) Immunohistochemistry for Kupffer cells (KCs) in the liver sections of mice inoculated with PBS, HAd-GFP, PAd-GFP, or BAd-GFP. Formalin-fixed, paraffin-embedded liver tissue sections were processed for immunohistochemical analysis as described in material and methods. Anti-F4/80 labeled KCs stained in brown color could be observed (600 × magnification). b) Quantitative estimation of KCs in the liver sections at various time points. KCs in seven randomly selected non-overlapping fields were counted from each mouse liver section. Values are reported as the mean ± standard deviation for three mice at each time point. †P < 0.05 for PAd-GFP, BAd-GFP or HAd-GFP versus PBS-inoculated mice at each time point.
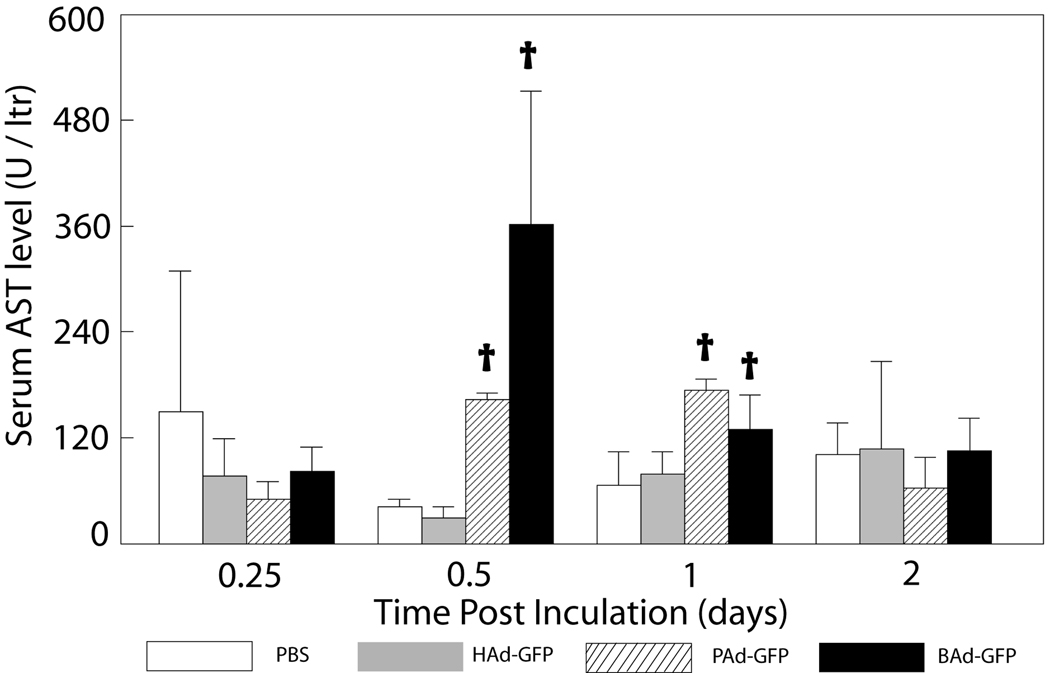
Hepatic toxicity due to vector administration was assessed by evaluating serum AST and ALT levels At 0.5 and 1 day post-inoculation, serum AST (Fig. 5) and ALT (data not shown) levels were mildly to moderately elevated in PAd-GFP and BAd-GFP inoculated mice. Although significantly higher (P<0.05) than those in mock-inoculated mice, these values were increased by less than 2-fold in PAd-GFP inoculated mice or less than 4-fold in BAd-GFP inoculated mice compared to the reported reference range (AST 95.6 ± 63.6 IU/L, ALT 59.5 ± 12 IU/L) for these enzymes in the FVB/n mouse strain (Mouse Phenome Database (2010)).
Figure 5. Serum levels of AST enzyme in mice inoculated with PBS, HAd-GFP, PAd-GFP, or BAd-GFP.
Values are reported as the mean ± standard deviation for three mice at each time point. †P < 0.05 for PAd-GFP, BAd-GFP or HAd-GFP versus PBS-inoculated mice at each time point.
4. Discussion
In the current study, we examined the induction of innate immune response and vector toxicity in mice inoculated intravenously with human or nonhuman Ad vectors. Induction of a variety of proinflammatory chemokines and cytokines including CCL2, CCL3, CCL4, CCL5, CXCL2, CXCL10, IP-10, IFNγ, IL-6, and TNFα following systemic administration of Ad vectors has previously been reported (Muruve, 2004; Muruve et al., 1999; Zaiss et al., 2002). Several studies have demonstrated the role of TLRs in innate immune response to Ad vectors (Appledorn et al., 2008b; Hartman et al., 2007a). In the present study, PAd-GFP was the most potent inducer of various cytokines and chemokines followed by BAd-GFP, while the HAd-GFP-induced mRNA expression levels of cytokines and chemokines were the lowest of the three vectors.
The observed differences in innate immune responses following systemic administration of PAd3, BAd3, or HAd5 vectors further highlight their distinct biological properties such as receptor usage, vector genome sequence, vector structural (capsid) proteins, in-vivo tropism etc. It is believed that the molecular events leading to the induction of immunoregulatory genes are triggered due to virus-cell interaction (Tibbles et al., 2002). Ad serotypes that utilize distinct cellular receptors differ in their intracellular trafficking and localization (Leopold and Crystal, 2007; Rogee et al., 2007). For instance, CD46-utilizing Ads tend to accumulate in lysosomes unlike subgroup C Ads that traffic rapidly to the nuclear envelope (Iacobelli-Martinez and Nemerow, 2007). Consequently, CD46-utilizing Ads preferentially activate TLR9 that is also located in lysosomes. Moreover, the CD46-utilizing Ads show altered expression and/or activity of transcription factors responsible for the activation of proinflammatory cytokine genes (Iacobelli-Martinez and Nemerow, 2007).
Similarly, CAR-fiber (Tamanini et al., 2006) or RGD-integrin (Liu et al., 2003) interactions have been shown to result in the activation of inflammatory responses. Tropism-modified Ads with altered CAR interaction or having additional RGD-binding domains resulted in differential gene expression following infection (Volk et al., 2005), further highlighting the influence of Ad entry pathway and the differences of viral capsids on activation of immune responses. Both BAd3 and PAd3 utilize cellular receptors distinct from HAd5 receptors (i.e., CAR and integrins) (Bangari and Mittal, 2005; Bangari et al., 2005a; Li et al., 2009). Moreover, vectors derived from these nonhuman Ads demonstrated distinct biodistribution following intravenous delivery, indicating their distinct receptor usage (Sharma et al., 2009). Details of intracellular trafficking and localization of BAd3 and PAd3 following receptor binding are not currently known.
Ad capsid proteins (e.g., hexon or fiber) have been implicated in expression of proinflammatory mediators (Molinier-Frenkel et al., 2002; Tamanini et al., 2006). The variations in capsid components of HAd5, PAd3 and BAd3 may also be responsible for the differential activation of proinflammatory gene expression. Ad DNA also appears to be involved in the activation of innate immunity (Muruve et al., 2008; Zhu et al., 2007). Cells infected with wild type HAd5, HAd5 with E1 and E3 deletions or helper-dependent HAd5 had a differential transcriptome dysregulation compared to uninfected cells (Martina et al., 2007). Furthermore, specific DNA sequences contained within the Ad vector may influence the induction of adaptive immune responses (Martina et al., 2007). Although the transgene expression cassette in all three vectors used in our study was identical, differences in the genomic nucleotide sequences of HAd5, PAd3 and BAd3 may have some contribution to their abilities to induce chemokine and cytokine mRNA expression following systemic delivery. Differential chemokine and cytokine response following the systemic delivery of Ad vectors may also result from different concentrations of the vector preparation. To eliminate such a possibility, we used equal number of viral particles for each of the three vectors.
As a part of the innate immune response following Ad infection, a number of TLRs and associated proteins such as MyD88 and TRIF are activated/upregulated (Appledorn et al., 2008a; Appledorn et al., 2008b; Hartman et al., 2007a; Hartman et al., 2007b; Iacobelli-Martinez and Nemerow, 2007; Zhu et al., 2007). Various TLRs are activated by unique PAMPs of viral components such as single-stranded or double-stranded RNA and CpG motifs in viral DNA. Activation of TLRs initiates a signaling cascade that results in activation of transcription factors, including NFκB, AP-1, and STAT1 (Hartman et al., 2007b). These transcription factors in turn upregulate expression of several cytokines and chemokine genes and stimulate innate as well as adaptive immune responses. As expected, upregulation of mRNA expression levels of several TLRs (TLR 2, 3, 4, 7 and 9) was observed that mirrored the induction of cytokines and chemokines genes, indicating that Ad vector inoculation activated the host TLR machinery. However, relative mRNA expression levels of TLRs 1, 5, 6 and 8 were minimally elevated in Ad vector-inoculated mice, suggesting the selective involvement of TLRs for immune activation following Ad vector inoculation.
Recent studies have also implicated the role of TLR-independent mechanisms such as RLR (RIG-I-like receptor), NALP3 (NATCHT- leucine-rich repeat- and pyrin-domain-containing protein 3 also known as NLRP3 or cryopyrin) and ASC (apoptosis-associated speck-like protein containing a CARD) components of inflammasomes in Ad-mediated induction of innate or adaptive immune responses (Cheng et al., 2007; Hartman et al., 2008; Muruve et al., 2008; Nociari et al., 2007; Zhu et al., 2007). The role of such TLR-independent mechanisms in Ad-mediated activation of innate immune response was not investigated in the current study.
Hepatotoxicity following systemic delivery of Ad vectors reflects a preferential vector biodistribution to the liver. In the present study, hepatotoxic changes were minimal to mild at earlier time points. Scattered apoptotic hepatocytes were observed in mice inoculated with Ad vectors but were slightly more frequent in BAd-GFP-inoculated mice. Serum levels of liver-specific enzymes were only assessed at 0.25, 0.5 and 1 days post-inoculation and were slightly elevated in PAd-GFP- and BAd-GFP-inoculated mice. Histologic changes indicative of hepatotoxicity were most prominent in HAd5-inoculated mice at 4 to 16 days post-inoculation. At these time points, liver histology in mice inoculated with PAd-GFP or BAd-GFP was unremarkable. Whether these observations reflect low hepatotoxicity of these nonhuman Ad vectors as compared to the HAd5 vector will require further investigations at higher vector doses.
We observed a significant reduction in the number of KCs in HAd-GFP- or PAd-GFP-inoculated mice, but there was no significant change in the KC number in BAd-GFP-inoculated mice. The decline in KCs in HAd-GFP-inoculated mice is in agreement with previous reports (Manickan et al., 2006; Schiedner et al., 2003; Xu et al., 2008). Interestingly, relatively prolonged persistence as well as efficient transgene expression by BAd-GFP was observed in various tissues in our previous study (Sharma et al., 2009). This could be due to the reduced vector uptake and clearance by the KCs. The clearance of HAd5-based vectors by KCs does not involve their interaction with HAd5 cell receptors (CAR or integrins), virus-binding to platelets, or vitamin K-dependent coagulation factors (Smith et al., 2008; Xu et al., 2008). However, the scavenger receptors, known for their ability to recognize negatively charged materials, have been implicated in Ad uptake by KCs (Xu et al., 2008). In general, Ad capsids bear an overall negative charge that varies with amino acid composition among various serotypes. Interestingly, hexon proteins of HAd5 and PAd3 are highly negatively charged (−21.3 and −21.4, respectively), while the BAd3 hexon carries a considerably lower negative charge (−5.6) at pH 7. The low negative charge on the BAd3 capsid might allow evasion of KCs, whereas, HAd5 and PAd3 are efficiently taken up by KCs via scavenger receptors. As we have previously reported, minimal or no sequestration of a BAd3 vector by KCs in the liver probably allows it to reach other tissues such as the lungs, kidneys and heart (Sharma et al., 2009). Opsonization of Ad particles by natural IgM antibodies and complement has also been implicated in their clearance by KCs (Xu et al., 2008). However, no detectable antibodies against HAd5, BAd3 or PAd3 were present in naïve mice in the present study (data not shown).
Our results suggest that PAd3 and BAd3-based vectors are stronger inducers of innate immune response than HAd5-based vectors. The activation of innate immunity and the release of cytokines and chemokines following Ad administration create an inflammatory milieu and provide a potent adjuvant effect for immune activation against Ad-delivered antigens. Potent induction of chemokine and cytokine genes by BAd3 and PAd3 vectors will be advantageous in their application as vaccine vectors. The prolonged persistence of BAd3 vectors together with their natural adjuvant-like properties and ability to evade sequestration by KCs will make them attractive candidates for gene delivery. Consistent with this hypothesis, our BAd3-vector based avian influenza vaccine study in BALB/c mice resulted in significantly higher levels of humoral and cell-mediated immune responses compared to a HAd5-based vaccine (Singh et al., 2008). Clearly, further investigation on the mechanisms underlying the observed differences in innate immunity and vector toxicity among these nonhuman and human Ad vectors will be critical for their development for clinical applications.
Acknowledgements
This work was supported by Public Health Service grant CA110176 from the National Cancer Institute. We are thankful to Jane Kovach for her excellent secretarial assistance and Ching-Yun Chang for help with statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Mouse Phenome Database. http://www.jax.org/phenome.
- Alemany R, Suzuki K, Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J.Gen.Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Appledorn DM, Kiang A, McBride A, Jiang H, Seregin S, Scott JM, Stringer R, Kousa Y, Hoban M, Frank MM, Amalfitano A. Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 2008a;15:885–901. doi: 10.1038/gt.2008.18. [DOI] [PubMed] [Google Scholar]
- Appledorn DM, Patial S, McBride A, Godbehere S, van RN, Parameswaran N, Amalfitano A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J.Immunol. 2008b;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Porcine adenovirus serotype 3 internalization is independent of CAR and alpha(v)beta(3) or alpha(v)beta(5) integrin. Virology. 2005;332:157–166. doi: 10.1016/j.virol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem.Biophys.Res.Commun. 2005a;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- Bangari DS, Sharma A, Mittal SK. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochemical and Biophysical Research Communications. 2005b;331:1478–1484. doi: 10.1016/j.bbrc.2005.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M, Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum.Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhong J, Chung J, Chisari FV. Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc.Natl.Acad.Sci.U.S.A. 2007;104:9035–9040. doi: 10.1073/pnas.0703285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989–2004-an overview. J.Gene Med. 2004;6:597–602. doi: 10.1002/jgm.619. [DOI] [PubMed] [Google Scholar]
- Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J.Leukoc.Biol. 2010 doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J.Gen.Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Black EP, Amalfitano A. Adenoviral infection induces multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology. 2007a;358:357–372. doi: 10.1016/j.virol.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, Amalfitano A. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses vivo. J.Virol. 2007b;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J.Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager L, Ehrhardt A. Emerging adenoviral vectors for stable correction of genetic disorders. Curr.Gene Ther. 2007;7:272–283. doi: 10.2174/156652307781369074. [DOI] [PubMed] [Google Scholar]
- Janssens S, Beyaert R. Role of Toll-like receptors in pathogen recognition. Clin.Microbiol.Rev. 2003;16:637–646. doi: 10.1128/CMR.16.4.637-646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu.Rev.Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Leopold PL, Crystal RG. Intracellular trafficking of adenovirus: many means to many ends. Adv.Drug Deliv.Rev. 2007;59:810–821. doi: 10.1016/j.addr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Li X, Bangari DS, Sharma A, Mittal SK. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology. 2009;392:162–168. doi: 10.1016/j.virol.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ, Muruve DA. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum.Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, Byrnes AP. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol.Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Martina Y, Avitabile D, Piersanti S, Cherubini G, Saggio I. Different modulation of cellular transcription by adenovirus 5, DeltaE1/E3 adenovirus and helper-dependent vectors. Virus Res. 2007;130:71–84. doi: 10.1016/j.virusres.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mittal SK, Prevec L, Graham FL, Babiuk LA. Development of a bovine adenovirus type 3-based expression vector. J.Gen.Virol. 1995;76:93–102. doi: 10.1099/0022-1317-76-1-93. [DOI] [PubMed] [Google Scholar]
- Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- Molinier-Frenkel V, Lengagne R, Gaden F, Hong SS, Choppin J, Gahery-Segard H, Boulanger P, Guillet JG. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J.Virol. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Hum.Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum.Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Noblitt LW, Bangari DS, Shukla S, Mohammed S, Mittal SK. Immunocompetent mouse model of breast cancer for preclinical testing of EphA2-targeted therapy. Cancer Gene Therapy. 2005;12:46–53. doi: 10.1038/sj.cgt.7700763. [DOI] [PubMed] [Google Scholar]
- Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J.Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, Singh N, Sambhara S, Mittal SK. Egg-independent vaccine strategies for highly pathogenic H5N1 influenza viruses. Hum.Vaccin. 2010;6:178–188. doi: 10.4161/hv.6.2.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogee S, Grellier E, Bernard C, Loyens A, Beauvillain JC, D'Halluin JC, Colin M. Intracellular trafficking of a fiber-modified adenovirus using lipid raft/caveolae endocytosis. Mol.Ther. 2007;15:1963–1972. doi: 10.1038/sj.mt.6300283. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Bloch W, Hertel S, Johnston M, Molojavyi A, Dries V, Varga G, van RN, Kochanek S. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum.Gene Ther. 2003;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- Sharma A, Bangari DS, Tandon M, Pandey A, HogenEsch H, Mittal SK. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 2009a;386:44–54. doi: 10.1016/j.virol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tandon M, Bangari DS, Mittal SK. Adenoviral vector-based strategies for cancer therapy. Curr.Drug ther. 2009b;4:117–138. doi: 10.2174/157488509788185123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against Human Adenovirus. Mol.Ther. 2008;16:965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Xu Z, Tian J, Stevenson SC, Byrnes AP. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum.Gene Ther. 2008;19:547–554. doi: 10.1089/hum.2008.004. [DOI] [PubMed] [Google Scholar]
- Stone D, Lieber A. New serotypes of adenoviral vectors. Curr.Opin.Mol.Ther. 2006;8:423–431. [PubMed] [Google Scholar]
- Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, Cabrini G. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J.Virol. 2006;80:11241–11254. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol.Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Spurrell JC, Bowen GP, Liu Q, Lam M, Zaiss AK, Robbins SM, Hollenberg MD, Wickham TJ, Muruve DA. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J.Virol. 2002;76:1559–1568. doi: 10.1128/JVI.76.4.1559-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olphen AL, Tikoo SK, Mittal SK. Characterization of bovine adenovirus type 3 E1 proteins and isolation of E1-expressing cell lines. Virology. 2002;295:108–118. doi: 10.1006/viro.2002.1389. [DOI] [PubMed] [Google Scholar]
- Volk AL, Rivera AA, Page GP, Salazar-Gonzalez JF, Nettelbeck DM, Matthews QL, Curiel DT. Employment of microarray analysis to characterize biologic differences associated with tropism-modified adenoviral vectors: utilization of non-native cellular entry pathways. Cancer Gene Ther. 2005;12:162–174. doi: 10.1038/sj.cgt.7700776. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal.Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Wolff G, Worgall S, van RN, Song WR, Harvey BG, Crystal RG. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J.Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Tian J, Smith JS, Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J.Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J.Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J.Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]