Abstract
To ensure correct meiotic chromosome segregation, sister chromatid cohesion (SCC) needs to be maintained from its establishment in prophase I oocytes before birth until continuation of meiosis into metaphase II upon oocyte maturation in the adult. Aging human oocytes suffer a steep increase in chromosome missegregation and aneuploidy, which may be caused by loss of SCC through slow deterioration of cohesin [1-3]. This hypothesis assumes cohesin expression in embryonic oocytes is sufficient to provide adequate long-term SCC. With increasing age, mouse oocytes deficient in the meiosis-specific cohesin SMC1β massively lose SCC and chiasmata [3, 4]. To test the deterioration hypothesis, we specifically and highly efficiently inactivated the mouse Smc1β gene at the primordial follicle stage shortly after birth when oocytes had just entered meiosis I dictyate arrest. In the adult, however, irrespective of oocyte age, chiasma positions and SCC are normal. Frequency and size of litters prove full fertility even in aged females. Thus, SMC1β cohesin needs only be expressed during prophase I prior to the primordial follicle stage to ensure SCC up to advanced age of mice.
Results and Discussion
Aneuploidy constitutes a major health problem as it leads to embryonic death or severe syndromes such as Down syndrome. Aneuploidy predominantly arises through errors in female meiosis, and loss of sister chromatid cohesion is thought to play a major role as it may lead to chromosome missegregation. In humans, the frequency of aneuploidy greatly increases with advancing age of the mother. The incidence of trisomy in clinically recognized pregnancies increases from less than 4 % at the age of 25 to more than 30 % at 40 years [1, 2].
To avoid chromosome missegregation during cell division two copies of each chromosome, sister chromatids, need to be properly positioned at the division plane and be attached to the spindle microtubules. To achieve correctly oriented attachment the sister chromatids have to stay connected to each other by SCC. In meiotic prophase I, recombination between non-sister chromatids creates obligate physical links between homologous chromosomes. These sites of exchange can be visualized cytologically as chiasmata [5].
In somatic cells, SCC depends on the essential cohesin complex consisting of four proteins, which in mammals are named SMC1α, SMC3, RAD21, and SA1 (or SA2). RAD21 closes the cohesin ring, pre-formed by the V-shaped SMC dimer. The precise function of SA1/SA2 remains to be elucidated (for recent reviews see [6, 7]. Meiotic cells express homologs of the SMC1α, RAD21, and SA proteins, called SMC1β, REC8 and STAG3, which associate with SMC3 to form additional cohesin complexes. The meiotic kleisin REC8 replaces RAD21, STAG3 replaces SA1/SA2, and SMC1β replaces SMC1α. Early in meiosis, however, the canonical cohesin featuring SMC1α is still present [8]. The meiosis-specific cohesins including the SMC1β-based cohesin complex are present in mouse spermatocytes and oocytes throughout meiosis [9-11]. At the metaphase-anaphase transition of the first meiotic division dissolution of SSC in chromosome arms allows separation of homologs, but sister chromatids remain linked together by centromeric cohesion. SCC at centromeres persists until anaphase II.
In mammals, oocytes start undergoing meiosis in the fetal ovary and then enter meiosis I arrest, a stage of quiescence called the dictyate, when they form the storage pool awaiting the hormonal signal for continuation of meiosis (reviewed in [12]). In the mouse, the first cohorts of oocytes leave dictyate arrest within a few weeks after birth and resume meiosis I by entering into metaphase I. Oocytes become again arrested at metaphase of the second meiotic division prior to ovulation. Anaphase II, and thus dissolution of the remaining SCC, happens upon fertilization. Thus, the time interval from establishment to final dissolution of SCC in female meiosis is remarkably long and in human may last for more than 40 years. Degradation of cohesin over time, accompanied by a failure to reload new cohesin complexes, may cause premature separation of sister chromatids leading to age-dependent increase in aneuploidy.
To analyze a meiotic cohesin deficiency model we previously created a transgenic mouse with an allele of the Smc1β gene, Smc1β-, where exon 10 was replaced with the neo gene, causing loss of SMC1β [4]. Male and female Smc1β-/- mice are completely sterile. In prophase I, Smc1β-/- oocytes and spermatocytes show drastically shortened axial elements and synaptonemal complexes, incomplete synapsis, reduced numbers of MLH1 foci diagnostic for sites of recombination, and aberrant telomere structures [4, 13]. Spermatocytes die in pachytene whereas oocytes can survive until metaphase II but suffer a complete loss of sister chromatid cohesion at this stage [3, 4]. Thus, SMC1β is essential for continuous meiotic sister chromatid cohesion, and endogenous SMC1α cannot rescue a deficiency in SMC1β. Importantly, with increasing age of female Smc1β-/- mice, a dramatic loss of chiasmata holding the two pairs of sister chromatids together is observed. As Smc1β-/- oocytes get older loss of sister chromatid cohesion in metaphase I oocytes dramatically increases in parallel [3]. This strongly correlates with an increasingly distal localization of chiasmata on metaphase chromosomes, suggesting that without SMC1β mediated SCC chiasmata cannot be prevented from moving and slipping off the chromosomes. Indeed, the shorter mouse chromosomes show the highest frequency of loss of chiasmata, consistent with this hypothesis. Therefore, the SMC1β deficient mouse was considered a model that in certain respects reflects the age-dependent increase in aneuploidies seen in humans [14, 15]. However, why should human oocytes – proficient in SMC1β cohesin – suffer loss of cohesion?
One may speculate that in human oocytes, slow degradation of cohesin starts to cause significant increase in aneuploidy after several decades. Thus, a key question is whether cohesin that is loaded onto chromosomes at the initial stages of meiotic prophase I during embryogenesis is sufficient to maintain sister chromatid cohesion through years of the dictyate arrest.
In the Smc1β-/- mouse this question cannot be answered as the protein is absent from the onset of meiosis, and thus establishment of SMC1β-dependent SCC is initially impaired. We therefore addressed this question by generation and analysis of a mouse strain carrying a floxed Smc1β allele, Smc1βfl, which after Cre-mediated recombination becomes Smc1βex and lacks exon 10 similar to the loss-of-function allele Smc1β- [4] (Suppl. Fig. 1a). Cre recombinase is provided by a transgenic mouse strain expressing Cre under control of the GDF9 promoter [16]. In this strain, Cre is expressed and functional in oocytes at the primordial follicle stage, starting between day 1 and day 3 after birth [16]. Smc1β inactivation very soon after birth allows assessing the putative requirement for Smc1β expression during the dictyate arrest, which lasts for many months thereafter.
Smc1βfl/fl and Smc1βfl/- males and females were healthy and fertile. To obtain Smc1β-/fl or Smc1βfl/fl females expressing Cre in their oocytes they were crossed with Smc1β+/- GDF9-iCre males. Smc1βfl/- GDF9-iCre females are all fertile, which initially raised the question if inactivation of Smc1βfl by Cre-mediated excision was efficient and timely. Efficiency of Cre-mediated excision in the germline was evaluated by genotyping the progeny of Smc1βfl/- GDF9-iCre or Smc1βfl/fl GDF9-iCre females crossed with wt (Suppl. Fig. 1b, c). Among the 218 progeny of five Smc1βfl/- GDF9-iCre females mice genotyped by PCR, 110 inherited the loxP-containing allele and in 108 of them exon 10 was excised. Of the progeny of six Smc1βfl/fl GDF9-iCre females, whose oocytes contained two floxed alleles to be converted, all 183 genotyped mice inherited the excised allele. Overall, the conversion of Smc1βfl to Smc1βex by Cre-mediated recombination was 99% efficient.
To create the Smc1β- allele exon 10 was replaced with the neo gene, and consequently SMC1β protein disappeared [4]. As a result of splicing the neo sequence is removed from the transcript and exons 9 and 11 become joined as demonstrated by RT-PCR and sequencing of the PCR product (Suppl. Fig. 2a). Exon 10 codes for a part of the SMC1 hinge domain indispensable for SCC [6]. Thus, the deletion of exon 10 renders any residual Smc1β- transcript non-functional. We found that expression of the Smc1βex allele also leads to appearance of an mRNA containing the exon 9-exon 11 junction (Suppl. Fig. 2b), which provides an opportunity for testing for the presence of Smc1βex by RT-PCR.
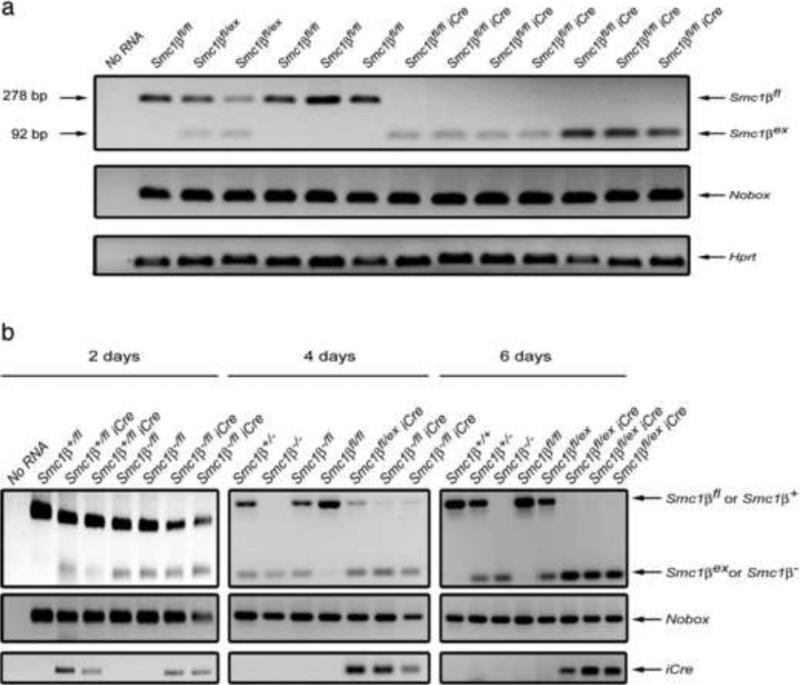
To prove that Cre recombinase mediated conversion of the Smc1βfl allele to the Smc1βex allele occurs in oocytes, we performed RT-PCR analysis of RNA isolated from single germinal vesicle stage oocytes collected from Smc1β-/fl or Smc1βfl/fl GDF9-iCre mice or their Cre-negative littermates (Fig. 1a). The mRNA transcribed from the Smc1βfl allele was present in Cre-negative oocytes, but was not detected in any of the Cre-expressing cells tested. We conclude that in Cre-expressing oocytes the Smc1β exon 10 deletion and degradation of the wild-type Smc1β transcript occurs before germinal vesicle breakdown.
Figure 1.
Absence of full-length Smc1β transcript in oocytes from the Smc1βfl/fl GDF-9-iCre mice. a. Total RNA was isolated from single oocytes and RT-PCR products generated with primers specific to Smc1β exon 9 and 11 were analyzed on an agarose gel (top panel). Mouse genotypes are shown above the lanes. In oocyte samples from the Smc1βfl/fl mice PCR generated the fragment of wild-type size (278 bp). In oocyte samples from the Smc1βfl/fl GDF-9-iCre mice only the truncated fragment (92 bp), which originated from the Smc1βex transcript, was amplified. Primers specific for an oocyte-specific gene Nobox (middle panel) and a housekeeping gene Hprt (bottom panel) were used as sample quality controls. b. Full length Smc1β transcript disappears from oocytes between days 4 and 6 postpartum due to Cre-mediated excision of exon 10 from the Smc1βfl allele. Total RNA was isolated from ovaries of different ages and analyzed by RT-PCR with primers specific to Smc1β exon 9 and 11 (top panels), an oocyte-specific gene Nobox as positive control (middle panels), and iCre (bottom panel). Mouse genotypes are shown above the lanes. In Cre-positive ovaries faint signals from the Smc1βfl allele still persist at day 4 post partum but completely vanish at day 6, whereas the intensity of the Smc1βex fragments increases.
We also investigated expression of Smc1β at various time points after birth to determine the kinetics of excision of the floxed allele (Fig. 1b). RT-PCR was performed on total RNA isolated from ovary, in which Smc1β is expressed only in oocytes. Provided the presence of the GDF9-iCre transgene, mRNA transcribed specifically from the Smc1βex allele can already be detected at day 2 after birth. A weak signal from the wild-type transcript was still observed at day 4 but was not found at day 6 and thereafter. At day 6 more than 90% of oocytes are at the primordial follicle stage and only a tiny fraction developed to primary and secondary follicles [17]. Thus, as expected Cre-mediated deletion of the Smc1β exon 10 and degradation of wild-type Smc1β mRNA happens early after birth at the primordial follicle stage.
In Smc1β-/- oocytes, all cohesion is lost in metaphase II and the mice are sterile at all ages [4]. Thus there is no other cohesin-like activity that could even partially complement SMC1β-deficiency. If re-loading of SMC1β would be necessary for maintenance of SCC in oocytes throughout adulthood and for progression through meiosis I and II, the Smc1βfl/- GDF9-iCre or Smc1βfl/fl GDF9-iCre female mice would be compromised in their fertility or be sterile. However, we observed full fertility of these mice (Table 1).
Table 1.
Loss of Smc1β expression in oocytes during the dictyate stage does not affect fertility. Females with the Smc1β gene inactivated in the oocytes by Cre (floxed + iCre) or females with intact Smc1β (control) at the specified age were mated with wt or Smc1β+/- males and the number of pups born during the 3-month mating trial was counted. The litter sizes and the litter frequencies as well as the age-dependence of litter size and litter frequencies were not significantly different between the two groups as analyzed by ANOVA (p values are between 0.3 and 0.9; SD = standard deviation).
| Genotype | Age, months | # of females | # of pups | # of litters | Litter size, mean (SD) | Offspring number, mean (SD) |
|---|---|---|---|---|---|---|
| Floxed +iCre | 2-3 | 4 | 91 | 9 | 10.1 (2.9) | 22.8 (7.3) |
| >3 <5 | 2 | 50 | 6 | 8.3 (2.3) | 25.0 (2.8) | |
| >5 <7.5 | 4 | 75 | 11 | 6.8 (2.7) | 18.8 (6.7) | |
| Control | 2-3 | 6 | 126 | 17 | 7.4 (2.9) | 21.0 (9.9) |
| >3 <5 | 6 | 121 | 17 | 7.1 (3.1) | 20.2 (6.9) | |
| >5 <7.5 | 4 | 72 | 9 | 8.0 (2.7) | 18.0 (4.5) |
Smc1βinactivation in oocytes of Smc1βfl/- GDF9-iCre or Smc1βfl/fl GDF9-iCre mice did not affect their fertility with respect to litter size or number of offspring at any age tested. Two-way ANOVA test showed that effects of age or genotype were statistically insignificant. Some Smc1βfl/- GDF9-iCre or Smc1βfl/fl GDF9-iCre were kept breeding for up to 15 months, and no difference to wild-type mice was observed. Thus, the conversion of the Smc1βfl allele to Smc1βex allele in dictyate arrested oocytes right after birth does not impair oocyte maturation.
When Smc1βex/+ mice were crossed with Smc1β+/-, their male and female Smc1βex/- descendants were sterile as assessed in extensive breeding trials. Similarly, breeding of Smc1βex/- mice, direct descendants of Smc1βfl/- GDF9-iCre or Smc1βfl/fl GDF9-iCre breeding with Smc1β+/-, with wild-type mice did not produce any litters in multiple crosses over > 5 months. Histological examination of the Smc1βex/- testes confirmed that spermatogenesis was arrested at the same stage as in the Smc1β-/- mutant (Suppl. Fig. 3). This demonstrates that the Smc1βex allele does not complement the Smc1β- allele and thus is non-functional, and again illustrates the efficiency of Cre-mediated Smc1β deletion. We conclude that expression of SMC1β cohesin is essential only in embryonic, pre-dictyate oocytes, and dispensable at later stages of oocyte development.
While the Smc1βfl/- GDF9-iCre and Smc1βfl/fl GDF9-iCre female mice retained full fertility despite loss of Smc1β expression very soon after birth, their oocytes may still be affected by a mild impairment of meiotic SCC, which may become apparent only with increasing age. Therefore, we analyzed the position of chiasmata in oocytes from Smc1βfl/- GDF9-iCre and Smc1βfl/fl GDF9-iCre mice with a focus on older animals (6 to 8 months) to assess a potential age-dependent effect. At that age, the Smc1β-/- females show massive oocyte loss, and about 80% of those oocytes that still exist show loss of sister chromatid cohesion and of chiasmata [3]. However, oocytes obtained from the Smc1βfl/- GDF9-iCre and Smc1βfl/fl GDF9-iCre females did not show any increased loss of chiasmata, i.e. appearance of univalents, and the position of chiasmata were not skewed towards the more distal locations as seen previously very prominently in the Smc1β-/- mice [3] (Table 2, Fig. 2). We conclude that expression of the Smc1β gene during and after dictyate arrest is not required for maintenance of sister chromatid cohesion and chiasma position in aging mice. Our results do not exclude that SMC1β could, in principle, be re-loaded during the long period of arrest of oocytes would it be expressed, but there is no evidence for such a mechanism. In Smc1β-/- oocytes, cohesin is partially intact during meiotic prophase I, but SCC is entirely lost upon oocyte maturation [4]. The early, SMC1β-independent cohesion, likely accomplished by the SMC1α complex still present early in meiosis, is not sufficient to maintain cohesion and cannot rescue loss of chiasmata and of SCC in Smc1β-/- or Smc1βex/- oocytes. Real-time PCR analysis of the Smc1a transcript level did not show any significant increase in single oocytes from Smc1βfl/fl GDF9-Cre mice compared to Cre-negative oocytes collected from 3-month or 6-month old mice. Neither genotype nor age affected Smc1a expression (data not shown; two-way ANOVA text, p-values >0.2).
Table 2.
Analysis of chiasma positions and univalent chromosomes in metaphase I spread oocytes. Chiasma position was determined in 940 bivalents (47 oocytes) from five Smc1βfl/- GDF9-iCre mice and 740 bivalents (37 oocytes) from five Smc1βfl/- mice. Univalents were counted in 63 oocytes from eight Smc1βfl/- GDF9-iCre mice and 56 oocytes from eight Smc1βfl/- mice. The animals were seven to eight months old. The results did not differ significantly between the two genotypes.
| Mouse genotype | Chiasma position | Univalents/oocyte | ||
|---|---|---|---|---|
| Proximal (%) | Interstitial (%) | Distal (%) | ||
| Smc1βfl/- GDF9-iCre | 15.1 | 56.2 | 28.6 | 0.3 |
| Smc1βfl/- | 11.8 | 61.1 | 27.1 | 0.4 |
| χ2 = 5.637, p = 0.060 | t-test p=0.37 | |||
Figure 2.
Cessation of Smc1β expression in dictyate stage oocytes does not impair sister chromatid cohesion. Metaphase I chromosome preparations from adult females were stained with Hoechst 33342. In oocytes from Smc1βfl/fl iCre females after birth Smc1βfl alleles were converted to non-functional Smc1βex alleles. Oocytes from Smc1β-/ex iCre females lacked SMC1β throughout all stages of meiosis. Both in Smc1βfl/fl (left) and Smc1βfl/fl iCre (middle) oocytes from 8-month-old females no unpaired bivalents were found. In contrast, in Smc1β-/ex oocytes from a five-month-old female (right) most of the chromosomes were present as univalents or single chromatids, as observed previously in SMC1β-deficient females [3]
These data show that SMC1β cohesin – shown earlier to be essential for sister chromatid cohesion and chiasma maintenance – needs only be expressed at the initial stage of meiosis. Recent observations in age-accelerated mouse strains fit this model, for there is a decline in chromosome-associated SMC1β with increasing age in these mice, which suffer from enhanced rates of meiotic aneuploidies [10]. Together, the data presented here show that in oocytes the key requirements for SMC1β cohesin are met prior to the primordial follicle stage. Thus, the decline in faithful chromosome segregation observed with increasing age in humans is very likely not due to a failure to continuously express and reload SMC1β – unless mice and humans differ fundamentally in this respect. The most straightforward interpretation based on data presented here is that slow degradation of SMC1β-mediated cohesion, established early in embryonic meiosis, significantly contributes to the age-dependent increase in aneuploidy.
Experimental Procedures
Generation of mice for conditional inactivation of Smc1β
To generate an allele of the Smc1β gene suitable for conditional targeting we modified the targeting vector used by us previously [4] by inserting the Smc1β exon 10 flanked by loxP sites and the FRT-flanked tk-neo selection cassette between the long and short arms of homology (Suppl. Fig. 1a). The tk negative selection marker was replaced by the diphteria toxin A fragment (DTA) gene controlled by the PGK promoter and polyadenylation signal. The sequence of the targeting vector is available upon request. Mice carrying the Smc1β allele with the floxed exon 10 and the insertion of the FRT-flanked tk-neo (Smc1βfl::neo) were generated as described previously [4]. To remove the tk-neo selection marker the Smc1βfl::neo/+ mice were crossed with the ACTB::Flpe mice [18] (Suppl. Fig. 1a). In the progeny the FRT-flanked neo was deleted by the FLP recombinase and the resulting Smc1βfl allele was transmitted through the germline. Smc1β+/- mice [4] were crossed with GDF-9-iCre transgenic mice [16], and the resulting Smc1β+/-; GDF-9-iCre males were mated with Smc1bfl/+ females to obtain Smc1βfl/-; GDF-9-iCre females. Mice were genotyped by PCR analysis of tail DNA using Taq polymerase (Invitrogen, Carlbad, CA) and primers shown in Supplementary Figures 1b and 1c. Primers were designed by Primer3 software [19]. Sequences of the primers are listed in the Suppl. Table 1. iCre PCR primers and conditions were as described [16]. Smc1β PCR conditions were as follows: denaturation at 94°C 3 min, 12 cycles of denaturation at 94°C 30 sec, annealing at 60°C-0.5°C/cycle for 30 sec, elongation at 72°C 2 min, followed by 25 cycles of 94°C for 30 sec, 54°C for 30 sec, 72°C for 2 min, final elongation at 72°C for 2 min.
RT-PCR on single oocyte samples or ovary RNA
Total RNA was isolated from oocytes using RNeasy Micro Kit (Qiagen, Valencia, CA). Single oocyte from 7- month-old animals were frozen in 80 μl of lysis buffer RLT. For real-time PCR oocytes from 3-month- or 6-month-old animals were frozen in pools of ten in 80 μl of buffer RLT. Upon defrosting poly-A RNA carrier was added to 0.25 ng/μl. To lyse cells the mixture was pipetted up and down 10 times. RNA was eluted with 14 μl of elution buffer. Total RNA was isolated from ovaries or liver using RNeasy Mini kit (Qiagen, Valencia, CA). 2 ovaries were homogenized in 350 μl of lysis buffer RLT. RNA was eluted in 40 ul of water. Reverse transcription (RT) was performed in final volume of 20 μl using Superscript™ III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's instruction with either 4 -9 μl of single oocyte RNA and random hexamer primers, or 500 ng of total ovary RNA and oligo dT15 primer. For single oocyte cDNA PCR amplification was carried out using Ex Taq™ (Takara Bio USA, Madison, WI) with 5 μl of single oocyte cDNA in a 20 μl reaction according to the manufacturer's instruction. PCR conditions were as follows: cDNA was denatured at 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and elongation at 72°C for 30 s. Final elongation step was at 72°C for 1 min. For ovary cDNA samples PCR was performed using using DreamTaq Green DNA Polymerase (Fermentas, St. Leon-Rot, Germany) with 1 μl of a 1:10 cDNA dilution in a 25 μl reaction according to the manufacturer's instruction. The PCR program for Smc1β and iCre was: 95°C for 2 min, 35 cycles of 94°C for 1 min, 55°C for 30 sec, 72°C 45 sec, final elongation at 72°C for 5 min. For Nobox, 25 cycles of the same program were used. Sequences of the primers are listed in the Supplemental Table 1. Real-time PCR amplification was done using the LightCycler 480 II Instrument (Roche Applied Science, Indianapolis, IN) with PerfeCTa SYBR Green FastMix™ (Quanta Biosciences, Gaithersburg, MD). Amplification was carried out with 5 μl of oocyte cDNA in a 20-μl reaction according to the manufacturer's instruction. The cDNA was denatured at 95°C for 2 min. PCR conditions were as follows: denaturation at 95°C for 10 s, annealing at 56°C for 15 s and elongation at 72°C for 20 s. Fluorescence was acquired at the end of the elongation step. To confirm specificity we carried out melting curve analysis after 45 cycles and analyzed PCR products by agarose gel electrophoresis. Quantification was done using LightCycler Software Version LCS480 1.5.0.39. We generated standard curves using serial dilutions of total ovary cDNA. We used the Hprt transcript as a reference to allow comparisons of Smc1a levels between individual oocyte samples. Relative quantification results were expressed as the Smc1a: Hprt ratio. Real-time PCR was performed in triplicates for each sample. 14 samples from 3-month-old and 8 samples from 6-month-old Smc1βfl/fl; GDF-9-iCre or Smc1βfl/-; GDF-9-iCre mice and the same number of samples from Cre-negative Smc1βfl/fl or Smc1βfl/- animals were analyzed.
Oocyte isolation, culture and chromosome spreads
Oocytes from adult mice in dioestrus stage were isolated and matured in M2 medium at 37 °C for 8 hours. Oocytes with germinal vesicle breakdown were incubated 5 min in 0.75% sodium citrate before they were fixed in methanol : acetic acid (3 : 1) and dropped onto slides. After drying at room temperature chromosomes were stained with Hoechst 33342 (1μg/ml in PBS; Sigma) and mounted with Vectashield (Vector Laboratories, Burlingame, CA).
Mating trials
Depending on the age at the start of breeding breeding females were divided into three groups: from 2 to 3-month-old, older than 3 months but younger than 5 months, and older that 5 months but younger than 8 months. Each female was housed with one male for three months, and the number of pups in each litter was recorded. The matings that tested oocytes upon the Smc1β gene inactivation by Cre consisted of Smc1βfl/fl GDF9-iCre or Smc1βfl/- GDF9-iCre females mated with Smc1βfl/fl, Smc1β+/-, or Smc1β+/+ males. The control crosses were reciprocal. In addition, some Smc1βfl/fl GDF9-iCre or Smc1βfl/- GDF9-iCre females were left with such males, which were occasionally exchanged, for up to 15 months.
Histology
For hematoxylin and eosin staining testes were fixed in Bouin's solution (Sigma-Aldrich Chemie, Munich, Germany) at 4°C overnight and washed several times in 70% EtOH. Tissues were then embedded in paraffin and cut in sections of 8 μm. Staining was performed according to a standard protocol.
Statistical analysis
Two-way ANOVA was done using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA).
Supplementary Material
Acknowledgments
We thank Dr. Hans Jörg Fehling for providing the FRT-neo-FRT cassette, Dr. Austin J. Cooney and Dr. A. Francis Stewart for providing the GDF9-iCre and ACTB::FLPe mouse strains, respectively.
We thank Dr. Attila Toth for discussions and critical reading of the manuscript. This work was supported by grants from the NIGMS (E.R., GM062517) and the DFG (R.J., SPP1384 grant JE150/10-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- SMC1β cohesin is required for mammalian oocyte sister chromatid cohesion
- prenatal SMC1β cohesin suffices for oocyte sister chromatid cohesion
- postnatal cohesin is not required to prevent loss of oocyte chiasmata and aneuploidy
- no age-dependent loss of cohesion or chiasmata upon postnatal depletion of SMC1β
References
- 1.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 2.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–1355. doi: 10.1038/ng1672. [DOI] [PubMed] [Google Scholar]
- 4.Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R. Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol. 2004;6:555–562. doi: 10.1038/ncb1135. [DOI] [PubMed] [Google Scholar]
- 5.Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- 6.Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- 7.Wood AJ, Severson AF, Meyer BJ. Condensin and cohesin complexity: the expanding repertoire of functions. Nat Rev Genet. 2010;11:391–404. doi: 10.1038/nrg2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eijpe M, Heyting C, Gross B, Jessberger R. Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci. 2000;113(Pt 4):673–682. doi: 10.1242/jcs.113.4.673. [DOI] [PubMed] [Google Scholar]
- 9.Revenkova E, Eijpe M, Heyting C, Gross B, Jessberger R. Novel meiosis-specific isoform of mammalian SMC1. Mol Cell Biol. 2001;21:6984–6998. doi: 10.1128/MCB.21.20.6984-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Keefe DL. Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod Biomed Online. 2008;16:103–112. doi: 10.1016/s1472-6483(10)60562-7. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Cruz R, Brieno MA, Roig I, Grossmann M, Velilla E, Pujol A, Cabero L, Pessarrodona A, Barbero JL, Garcia Caldes M. Dynamics of cohesin proteins REC8, STAG3, SMC1{beta} and SMC3 are consistent with a role in sister chromatid cohesion during meiosis in human oocytes. Hum Reprod. 2010 doi: 10.1093/humrep/deq180. Advance Access published online on July 15. [DOI] [PubMed] [Google Scholar]
- 12.Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update. 2008;14:143–158. doi: 10.1093/humupd/dmm043. [DOI] [PubMed] [Google Scholar]
- 13.Adelfalk C, Janschek J, Revenkova E, Blei C, Liebe B, Gob E, Alsheimer M, Benavente R, de Boer E, Novak I, et al. Cohesin SMC1beta protects telomeres in meiocytes. J Cell Biol. 2009;187:185–199. doi: 10.1083/jcb.200808016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliland WD, Hawley RS. Cohesin and the maternal age effect. Cell. 2005;123:371–373. doi: 10.1016/j.cell.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Bickel SE. Aging (not so) gracefully. Nat Genet. 2005;37:1303–1304. doi: 10.1038/ng1205-1303. [DOI] [PubMed] [Google Scholar]
- 16.Lan ZJ, Xu X, Cooney AJ. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol Reprod. 2004;71:1469–1474. doi: 10.1095/biolreprod.104.031757. [DOI] [PubMed] [Google Scholar]
- 17.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, Woodruff TK. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 19.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.