Abstract
Background
Angioedema is a rare adverse effect of angiotensin converting enzyme (ACE) inhibitors that occurs more commonly in women and black Americans. Angioedema is thought to result from decreased degradation of vasoactive peptides. During ACE inhibition, bradykinin is primarily inactivated by aminopeptidase P (APP). Previous studies have provided conflicting data regarding serum APP activity in patients with a history of ACE inhibitor-associated angioedema. A single nucleotide polymorphism, −2399C>A (rs3788853, C-2399A), in XPNPEP2, the X-linked gene that encodes membranous APP, has been reported to associate with APP activity.
Objective
To test the hypothesis that the relationship between XPNPEP2 C-2399A genotype and APP activity or ACE inhibitor-associated angioedema is gender- and/or race-dependent.
Methods
We compared C-2399A genotype frequencies in 169 cases with a history of ACE inhibitor-associated angioedema and 397 ACE inhibitor-exposed controls. Controls were pre-specified to be 50% white, 50% black and 50% female. Cases and controls were group matched for age and smoking.
Results
XPNPEP2 C-2399A genotype associated with serum APP activity in both men and women. Serum APP activity was lower in men than in women, independent of genotype. XPNPEP2 −2399 A/ genotype was associated with an increased risk of angioedema in men [odds ratio 2.17 (1.09-4.32), P=0.03] in multivariate analysis. The A/ genotype was associated with angioedema in black men (P=0.03) but not in white men.
Conclusion
APP activity is lower in men and the XPNPEP2 C-2399A polymorphism associates with ACE inhibitor-associated angioedema in men but not women.
Keywords: Aminopeptidase P, Angioedema, Angiotensin-Converting Enzyme, XPNPEP2
INTRODUCTION
Forty million people take angiotensin-converting enzyme (ACE) inhibitors worldwide. A small proportion of patients who take ACE inhibitors, 0.1 to 0.7 percent, will develop angioedema, a potentially life-threatening side effect.[1, 2] Angioedema is characterized by swelling of the face, lips, tongue and airway and, if severe, can cause suffocation and death. Clinical risk factors for ACE inhibitor-associated angioedema include black American race, female gender, smoking, seasonal allergies, and immunosuppressant therapy.[3-5] Diabetes is associated with a decreased risk of ACE inhibitor-associated angioedema.[4, 5]
ACE inhibitor-associated angioedema is thought to result from defective degradation of the vasoactive peptides bradykinin (BK) and substance P.[6, 7] During ACE inhibition, BK and substance P are inactivated primarily by aminopeptidase P (APP) and dipeptidyl peptidase IV (DPPIV), respectively.[8, 9] APP-inactivated bradykinin metabolites are also further degraded by DPPIV.[8] Adam et al. have previously reported decreased APP activity in the sera of 39 white patients with a history of ACE inhibitor-associated angioedema, as compared to 39 ACE inhibitor-exposed controls.[10] Byrd et al. reported decreased DPPIV antigen and activity levels in the sera of 50 patients with a history of ACE inhibitor-associated angioedema as compared to 176 ACE inhibitor-exposed controls, but did not observe a relationship between case-control status and APP activity.[4]
Identifying genes associated with ACE inhibitor-associated angioedema can help to elucidate mechanism. A previous study investigating the role of genetic factors in the regulation of APP activity identified a single nucleotide polymorphism (SNP), C-2399A (rs3788853), in XPNPEP2, an X-linked gene that encodes for membranous APP.[11] This variant segregated with reduced serum APP activity in families in which the proband had ACE inhibitor-associated angioedema or anaphylactoid reaction during dialysis.[11] The frequency of the −2399A allele was also increased in 20 mixed-gender cases of ACE inhibitor-associated angioedema as compared to 60 ACE inhibitor-exposed controls.[11] We have now collected DNA from a large number of patients with ACE inhibitor-associated angioedema and ACE inhibitor-exposed controls. Because studies have provided conflicting data regarding serum APP concentrations in patients with a history of ACE inhibitor-associated angioedema and because the gene encoding for serum APP is X-linked, we tested the hypothesis that the relationship between XPNPEP2 C-2399A genotype and APP activity or ACE inhibitor-associated angioedema is gender- and/or race-dependent.
METHODS
Case and Control Subjects
The study protocol was approved by the Vanderbilt Institutional Review Board, and all subjects provided written informed consent. Case and control subjects were identified as previously described.[4] Briefly, blood samples were obtained from 169 subjects with a history of ACE inhibitor-associated angioedema, defined as swelling of the face, lips, or pharynx while taking an ACE inhibitor but no history of angioedema when not taking an ACE inhibitor. Because of the difficulty in diagnosis, we excluded subjects with angioedema of the bowel. Samples were also collected from 397 control subjects who had been treated with an ACE inhibitor for at least 6 months without experiencing angioedema. Controls were pre-specified to be 50% black American, 50% white American and 50% female, 50% male. Cases and controls were group-matched by age and smoking status. Medical history, including the history of angioedema, was confirmed by a research nurse using a detailed case report form.
PCR
DNA extraction was performed using a standard automated protocol (Qiagen, Valencia, CA). Genotyping of the C-2399A SNP in XPNPEP2 was accomplished using allele-specific PCR described elsewhere.[12] Two standard PCRs were performed using one common (AACCCTCCCCACGTTGAATCA) and either of two allele-specific oligonucleotides (GCACTGCTGAAATAGCAGTTGTTAG and GCACTGCTGAAATAGCAGTTGTTAT), which differed only at the nucleotide at the 3′-end.[11] PCR products were visualized by electrophoresis on a 1.5% agarose gel.
APP activity
Sera was stored at −80°C until the time of assay. Serum APP activity was measured using a modified version of the assay previously described by Lefebvre et al.[13] Two or 4μl serum samples, plated in a 96-well format, were incubated at 37°C for 3h with 5μM L-lysyl(ε-2-aminobenzoyl)-L-prolyl-L-prolyl-4-nitroanilide [H-Lys(ε-Abz)-Pro-Pro-pNA] (Bachem, Torrance, CA), an internally quenched fluorescent substrate.[14] Fluorescence was measured at 5 to 7 time points in a Flexstation II 384 spectrofluorometer (Molecular Devices, Sunnyvale, CA). After the 3h reading, 1nmol of internal standard, Abz-Gly (Bachem, Torrance, CA), was routinely added to each well to normalize for serum and substrate quenching effects.
DPPIV activity
Serum DPPIV activity was measured as previously described.[13] Briefly DPPIV activity was assayed by incubating sera with a colorimetric substrate, L-glycyl-L-prolyl p-nitroanilide (Sigma-Aldrich, St. Louis, MO), at 37°C.
Statistical Analysis
Data are presented as mean ± standard deviation (SD), unless otherwise noted. Between- or among- group comparisons were made using χ2 testing for categorical values and ANOVA for continuous variables. Multivariate analysis was performed using binary logistic regression. A 2-sided P<0.05 was considered significant. Statistical analysis was performed using SPSS 17.0 (SPSS Inc, Chicago, IL).
RESULTS
Subject characteristics appear in Table 1. As specified in the methods, cases were pre-specified to be 50% black American and 50% female and matched for age and smoking status. Fifty-six percent of cases were women. The median ACE inhibitor exposure time for controls was 42 months. Only 16 controls took an ACE inhibitor for less than one year. The median exposure time for cases was 5 months (P<0.001). The prevalence of seasonal allergies was significantly higher in cases compared with control subjects. Month of ACE inhibitor initiation was similar in the two groups (P=0.26). Overall, there was no difference in serum APP activity between cases and controls (220.6 ± 162.2 vs 216.9 ± 161.1 pmoles/ml/min, P=0.9). Serum DPPIV activity was lower in case subjects as compared to controls, as previously reported for a subset of these subjects.[4]
Table 1.
Characteristics of Cases and Controls
| Characteristic | ACEi exposed controls (397) |
ACEi angioedema cases (169) |
|---|---|---|
| Blacks : Whites | 179 : 218 | 87 : 82 |
| Male : Female | 200 : 197 | 73 : 96 |
| Age (years) | 57.4 ± 11.4 | 57.3 ± 14.1 |
| Median ACE inhibitor exposure months (n) |
42 (299) | 5 (92)* |
| Diabetic : Non-diabetic | 147 : 247 | 54 : 112 |
| Smoker : Non-smoker | 102 : 293 | 49 : 116 |
| Seasonal allergies (yes:no) | 184:213 | 97:51* |
| APP activity (pmoles/ml/min) | 216.88 ± 161.07 (n=387) | 220.60 ± 162.19 (n=149) |
| DPPIV activity (nmoles/ml/min) | 30.45 ± 8.74 (n=373) | 28.24 ± 10.88 (n=144)† |
Data presented as means ± standard deviations.
P<0.001
P<0.05 versus control
XPNPEP2 C-2399A genotype frequencies were similar in white and black women and in white and black men (Table 2). Genotypes were in Hardy-Weinberg equilibrium among women. Age was similar among genotype groups (58.2 ± 13.3, 59.2 ± 11.7, 58.1 ± 12.2 years in C/C, C/A and A/A) women and (56.5 ± 11.7, 54.8 ± 10.9 years in C/ and A/) men. There was no association between XPNPEP2 C-2399A genotype and the prevalence of diabetes or smoking (both P>0.2).
Table 2.
XPNPEP2 C-2399A Variant Genotype and Allele Frequencies by Gender and ACE Inhibitor-Associated Angioedema Case-Control Status
| XPNPEP2 allele frequency | Patient Type |
|
|---|---|---|
| Control | Case | |
| Female | ||
| All | n=181 | n=93 |
| C/C, n (%) | 116 (64.1) | 56 (60.2) |
| C/A, n (%) | 53 (29.3) | 32 (34.4) |
| A/A, n (%) | 12 (6.6) | 5 (5.4) |
| OR (95% CI) | 1.18 (0.7-2.0) | |
| Blacks | n=84 | n=51 |
| C/C, n (%) | 55 (65.5) | 29 (56.9) |
| C/A, n (%) | 24 (28.6) | 19 (37.3) |
| A/A, n (%) | 5 (6.0) | 3 (5.9) |
| OR (95% CI) | 1.44 (0.7-2.9) | |
| Whites | n=97 | n=42 |
| C/C, n (%) | 61 (62.9) | 27 (64.3) |
| C/A, n (%) | 29 (29.9) | 13 (31.0) |
| A/A, n (%) | 7 (7.2) | 2 (4.8) |
| OR (95% CI) | 0.94 (0.4-2.0) | |
| Male | ||
| All | n=185 | n=71 |
| C/, n (%) | 153 (82.7) | 52 (73.2) |
| A/, n (%) | 32 (17.3) | 19 (26.8) |
| OR (95% CI) | 1.75 (0.9-3.3) | |
| Blacks | n=83 | n=32 |
| C/, n (%) | 72 (86.7) | 22 (68.8) |
| A/, n (%) | 11 (13.3) | 10 (31.3) |
| OR (95% CI) | 2.98 (1.1-7.9) | |
| Whites | n=102 | n=39 |
| C/, n (%) | 81 (79.4) | 30 (76.9) |
| A/, n (%) | 21 (20.6) | 9 (23.1) |
| OR (95% CI) | 1.16 (0.5-2.8) | |
Odds ratios (OR) and 95% confidence intervals (CIs) were calculated for C-2399A A/A + C/A vs C/C (reference) or A/ vs C/ (reference).
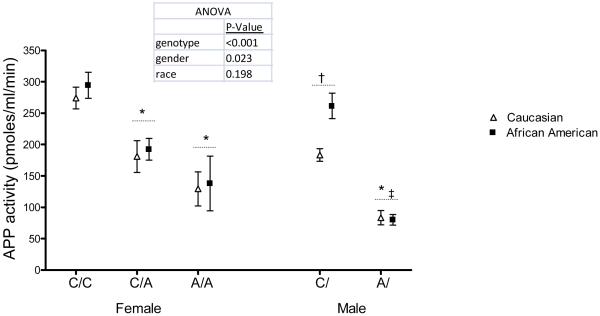
There was a significant association between XPNPEP2 C-2399A genotype and serum APP activity in both men and women (Figure 1). XPNPEP2 C-2399A genotype accounted for 9% of the variability of serum APP activity in women and 13% in men. Serum APP activity was lower in men compared to women, regardless of genotype. Serum APP activity was similar in older and younger women (P=0.19). There was no relationship between APP activity and age (P=0.27). Serum APP activity trended higher in black men compared to white men (P=0.058).
Figure 1.
C-2399A Variant Associates with APP Activity. For post-hoc analysis, *P<0.001 vs C/C or C/; †P<0.001 vs C/C women; ‡P<0.01 vs A/A women.
There was no association between XPNPEP2 genotype and case-control status, in all women (P=0.7), in white women (P=0.9) or in black women (P=0.6) when heterozygote was treated as intermediate (Table 2). There was also no association between XPNPEP2 genotype and angioedema in women when the −2399A allele was treated as dominant (i.e. A/A + C/A vs C/C). In contrast, among men, the frequency of the −2399 A/ genotype tended to be increased in cases compared to ACE inhibitor-exposed controls (P=0.07 for univariate analysis) for black and white males combined (Table 2). This was attributable to a significantly increased frequency of the XPNPEP2 −2399A allele in black males with a history of angioedema compared to ACE inhibitor-exposed black male controls (31.3% vs. 13.3%, P=0.03). The frequency of the −2399A allele was 23.1% in white males with angioedema versus 20.6% with ACE inhibitor-exposed controls, but this difference was not significant.
In multivariate analysis, the XPNPEP2 −2399 A/ genotype was associated with a 2.2-fold increased risk of angioedema in all males (P=0.03) after controlling for diabetes mellitus, seasonal allergies and DPPIV activity (Table 3). A history of seasonal allergies and DPPIV activity were associated with an increased risk of angioedema in both males and females in the multivariate analysis. When multivariate analysis was further stratified by race, the XPNPEP2 −2399 A/ genotype was associated with increased risk of ACE inhibitor-associated angioedema in black males (P=0.01) but not white males (P=0.53).
Table 3.
Multivariate Logistic Regression Model for ACE Inhibitor-Associated Angioedema
| Variable | Females | Males | ||||
|---|---|---|---|---|---|---|
| OR* | 95% CI | P value | OR | 95% CI | P value | |
| XPNPEP2 C-2399A genotype | 1.24 | 0.70-2.18 | 0.47 | 2.18 | 1.09-4.32 | 0.03 |
| Diabetes mellitus | 1.13 | 0.65-1.98 | 0.67 | 0.53 | 0.26-1.08 | 0.08 |
| Seasonal allergies | 2.57 | 1.44-4.59 | 0.001 | 2.11 | 1.14-3.90 | 0.02 |
| DPPIV activity | 0.97 | 0.96-0.98 | <0.001 | 0.97 | 0.95-0.98 | <0.001 |
Odds ratio (OR) and 95% confidence interval (CI) were calculated for A/A + C/A vs C/C genotypes for women. OR and 95% CI were calculated as A/ versus C/ genotype for men.
DISCUSSION
Angioedema is a rare, serious, adverse drug reaction that affects 40,000 to 280,000 patients who take ACE inhibitors a year.[1, 2] Associated risk factors, including black American race, female gender and smoking, suggest that both genetic and environmental factors influence the development of ACE inhibitor-associated angioedema.[3, 5, 6, 15-17] Previous studies suggest that altered degradation of the vasoactive peptides, bradykinin and substance P, contributes to the pathogenesis of angioedema.[7, 18] During ACE inhibition, aminopeptidase P inactivates bradykinin.[8] We examined the relationship between a previously-described functional polymorphism in XPNPEP2 in a large case-control study of ACE inhibitor-associated angioedema. We report that APP activity associates with XPNPEP2 C-2399A genotype in men and women, but that APP activity is lower in men compared to women regardless of genotype. Moreover, we observe an association between XPNPEP2 C-2399A genotype and ACE inhibitor-associated angioedema in men but not in women.
We found that APP activity is decreased in males compared to females, even after controlling for XPNPEP2 C-2399A genotype. Regulation of APP by sex hormones could contribute to gender differences in enzyme activity. The effect of estrogen on APP expression or activity has not been reported. Progesterone increases APP activity.[19] Androgens increase APP activity in patients with hereditary angioedema, suggesting that increased testosterone concentrations in men cannot account for gender differences.[20] In addition, a prior study suggests XPNPEP2 escapes X-inactivation.[21]
XPNPEP2 C-2399A genotype was associated with ACE inhibitor-associated angioedema in all men and in black men, but not in white men. The risk of angioedema is increased in blacks compared to whites. Prior studies suggest that black Americans exhibit increased sensitivity to the effects of bradykinin-induced vascular permeability compared to white Americans.[22] Thus, black American men may be more susceptible to angioedema when the inactivation of bradykinin by APP is decreased. In addition, given the small sample size, we cannot exclude the effect of XPNPEP2 genotype in white men.
Despite the association between XPNPEP2 C-2399A genotype and ACE inhibitor-associated angioedema in men, we did not detect a difference in circulating APP activity overall between cases and controls. This is likely because, as reported previously [6], the majority of patients with ACE inhibitor-associated angioedema are women. Thus, while genetic variation in XPNPEP2 associates with angioedema, it is unlikely that variation in this X-linked gene accounts for the majority of cases of ACE inhibitor-associated angioedema.
Mechanistically, defects in multiple pathways may contribute to the pathogenesis of ACE inhibitor-associated angioedema. Bradykinin increases vascular permeability by stimulating its B2 receptor.[23] Bradykinin also stimulates the release of substance P from nerve terminals, and substance P increases vascular permeability via NK1 receptor stimulation.[23] In animal models, blocking either B2 or NK1 receptors prevents ACE inhibitor-associated angioedema.[7] Thus, variation in enzymes responsible for the inactivation of bradykinin (ACE, APP, neutral endopeptidase P (NEP)) or substance P (ACE, DPPIV, NEP), or for the receptor targets of bradykinin (B1 and B2) and substance P (NK1) could contribute to angioedema. We have previously reported that DPPIV activity is decreased in some patients with ACE inhibitor-associated angioedema.[4] Pharmacologic inhibition of both ACE and NEP increases the risk of angioedema when compared to ACE inhibition alone.[24] Genetic variation in the bradykinin B2 receptor influences bradykinin-mediated vasodilation during ACE inhibition.[25]
In summary, the XPNPEP2 C-2399A genotype associates with APP activity and the frequency of the loss-of-function XPNPEP2 −2399 A/ genotype is increased in men with ACE inhibitor-associated angioedema. Nevertheless, variation in this X-linked gene does not account for the pathogenesis of ACE inhibitor-associated angioedema in women, who comprise the majority of cases. The combination of multiple genetic and environmental factors that affect the degradation and action of vasoactive peptides during ACE inhibition likely contribute to ACE inhibitor-associated angioedema pathogenesis.
ACKNOWLEDGEMENTS
We thank Anik Décarie and Anthony Dematteo for technical support with the DPPIV and APP activity assays.
Support: National Institutes of Health grants HL079184, UL1RR024975, GM007569
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- [2].Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- [3].Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60:8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- [4].Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, et al. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- [6].Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26:725–737. doi: 10.1016/j.iac.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [7].Emanueli C, Grady EF, Madeddu P, Figini M, Bunnett NW, Parisi D, et al. Acute ACE inhibition causes plasma extravasation in mice that is mediated by bradykinin and substance P. Hypertension. 1998;31:1299–1304. doi: 10.1161/01.hyp.31.6.1299. [DOI] [PubMed] [Google Scholar]
- [8].Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- [9].Russell JS, Chi H, Lantry LE, Stephens RE, Ward PE. Substance P and neurokinin A metabolism by cultured human skeletal muscle myocytes and fibroblasts. Peptides. 1996;17:1397–1403. doi: 10.1016/s0196-9781(96)00201-x. [DOI] [PubMed] [Google Scholar]
- [10].Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet. 2002;359:2088–2089. doi: 10.1016/S0140-6736(02)08914-6. [DOI] [PubMed] [Google Scholar]
- [11].Duan QL, Nikpoor B, Dube MP, Molinaro G, Meijer IA, Dion P, et al. A variant in XPNPEP2 is associated with angioedema induced by angiotensin I-converting enzyme inhibitors. Am J Hum Genet. 2005;77:617–626. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Germer S, Higuchi R. Single-tube genotyping without oligonucleotide probes. Genome Res. 1999;9:72–78. [PMC free article] [PubMed] [Google Scholar]
- [13].Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl Peptidase IV Activity in Patients With ACE-Inhibitor-Associated Angioedema. Hypertension. 2002;39:460–464. doi: 10.1161/hy0202.103054. [DOI] [PubMed] [Google Scholar]
- [14].Stockel-Maschek A, Stiebitz B, Koelsch R, Neubert K. A continuous fluorimetric assay for aminopeptidase P detailed analysis of product inhibition. Anal Biochem. 2003;322:60–67. doi: 10.1016/S0003-2697(03)00464-0. [DOI] [PubMed] [Google Scholar]
- [15].Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol. 1999;48:861–865. doi: 10.1046/j.1365-2125.1999.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- [17].Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract. 2004;10:499–509. doi: 10.1111/j.1365-2753.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- [18].Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621–622. doi: 10.1056/NEJM200208223470820. [DOI] [PubMed] [Google Scholar]
- [19].Cilia La Corte AL, Carter AM, Turner AJ, Grant PJ, Hooper NM. The bradykinin-degrading aminopeptidase P is increased in women taking the oral contraceptive pill. J Renin Angiotensin Aldosterone Syst. 2008;9:221–225. doi: 10.1177/1470320308096405. [DOI] [PubMed] [Google Scholar]
- [20].Drouet C, Desormeaux A, Robillard J, Ponard D, Bouillet L, Martin L, et al. Metallopeptidase activities in hereditary angioedema: Effect of androgen prophylaxis on plasma aminopeptidase P. J Allergy Clin Immunol. 2008;121:429–433. doi: 10.1016/j.jaci.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Prueitt RL, Ross JL, Zinn AR. Physical mapping of nine Xq translocation breakpoints and identification of XPNPEP2 as a premature ovarian failure candidate gene. Cytogenet Cell Genet. 2000;89:44–50. doi: 10.1159/000015560. [DOI] [PubMed] [Google Scholar]
- [22].Gainer JV, Nadeau JH, Ryder D, Brown NJ. Increased sensitivity to bradykinin among African Americans. J Allergy Clin Immunol. 1996;98:283–287. doi: 10.1016/s0091-6749(96)70151-3. [DOI] [PubMed] [Google Scholar]
- [23].Campos MM, Calixto JB. Neurokinin mediation of edema and inflammation. Neuropeptides. 2000;34:314–322. doi: 10.1054/npep.2000.0823. [DOI] [PubMed] [Google Scholar]
- [24].Coats AJS. Omapatrilat- the story of Overture and Octave. Int J Cardiology. 2002;86:1–4. doi: 10.1016/s0167-5273(02)00389-3. [DOI] [PubMed] [Google Scholar]
- [25].Van Guilder GP, Pretorius M, Luther JM, Byrd JB, Hill K, Gainer JV, et al. Bradykinin Type 2 Receptor BE1 Genotype Influences Bradykinin-Dependent Vasodilation During Angiotensin-Converting Enzyme Inhibition. Hypertension. 2008;51:454–459. doi: 10.1161/HYPERTENSIONAHA.107.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]