Abstract
Background
Morbid obesity and diabetes cause diastolic dysfunction that can be detected by Doppler echocardiography. Metabolic syndrome subjects could demonstrate early diastolic dysfunction which may influence effort tolerance (VO2 max).
Methods and Results
32 subjects (17 males) who fulfilled 2 or more of the 5 metabolic syndrome criteria were studied. Average age was 37 ± 2 years. All were overweight-obese (mean BMI of 34.4 ± 0.7 kg/m2), 15 had BP > 130/85mmHg, 19 had elevated triglycerides (>150mg/dl) and 17 had low HDL cholesterol (males <40 mg/dl, females <50 mg/dl). Maximal exercise was performed using Bruce treadmill protocol with standard stress echocardiography and tissue Doppler. Maximal oxygen consumption (VO2 max) was measured using indirect calorimetry. LV filling pressure was indirectly derived from dividing pulse Doppler early mitral inflow velocity (E) by tissue Doppler early diastolic mitral annular motion (E′) or E/E′.
The group's average treadmill time was 8.06 ± 0.28 minutes, VO2 max was 28.6±1.1 ml/kg/min and 8.2 ±0.3 METs. None had evidence for myocardial ischemia, systolic or diastolic dysfunction with exercise. Mean resting E/E′ and post exercise E/E′ were 7.01 ± 0.04 and 7.41 ± 0.41 respectively. There was no significant correlation between resting E/E′ and VO2 max (r= -0.266, p=0.14). The post exercise E/E′ significantly correlated with VO2 max (r= -0.483, p=0.005) and METs (r= -0.487, p=0.005).
Conclusions
Diastolic function is preserved in early metabolic syndrome. Even in the normal diastolic function range, exercise E/E′ is inversely related to VO2 max. Further longitudinal studies are needed to determine if they develop diastolic dysfunction and related heart failure.
Introduction
Heart failure with normal ejection fraction (HFNEF) is more commonly seen in today's patient population.1 Current estimates in the US are that over 50% of all heart failure hospitalizations are due to HFNEF. 2 The long-term prognosis and morbidity and mortality outcomes are similar to those with systolic heart failure. 2-11 The etiology of HFNEF is uncertain, but most believe it is due to diastolic dysfunction of the left ventricle (LV) resulting in high LV filling pressures. Aging population and increasing morbidity due to obesity likely account for the higher incidence of HFNEF.
Morbid obesity and diabetes in the absence of hypertension can directly lead to left ventricular hypertrophy and diastolic dysfunction.12, 13 Whether metabolic syndrome subjects with milder forms of obesity, insulin resistance and hyperlipidemia also develop cardiac structural changes and early diastolic dysfunction is unclear.
Recent reports have shown the correlation of exercise capacity with Doppler echocardiography derived diastolic function. 14-17 Our study tested the hypothesis that a well characterized group of subjects with at least two of five metabolic syndrome criteria would have early findings of LV filling abnormalities. Further, we believe that these findings would correlate with their exercise capacity, as measured by maximal oxygen consumption (VO2 max).
Methods
60 subjects with no history of heart disease were screened. 24 did not have at least two criteria for the Metabolic Syndrome, 2 enrolled but did not complete the echocardiographic part of the study due to time conflicts and 2 decided not to participate after qualifying. Thirty two subjects (17 males, 15 females) completed the study. All subjects were informed of risks and benefits of participation and signed a written consent approved by our Institutional Review Board. Subjects were overweight to class II obese, (Body mass index [BMI] 25-39.9 kg/m2) and sedentary individuals, defined as not meeting the Surgeon General's recommendations which is for more than 150 min/wk of physical activity (Table 1). They fulfilled at least two of the five of the metabolic syndrome criteria as determined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III).18 According to NCEP ATP III, individuals must have at least three criteria to have the metabolic syndrome. Criteria include: elevated blood pressure (>130/85 mmHg), elevated plasma triglyceride concentrations (>150mg/dl), elevated fasting blood glucose (>100 mg/dl), elevated waist circumference (>88cm in females, >102cm in males), and reduced high density lipoprotein (HDL-C) concentrations (<50mg/dl in females, <40mg/dl in males). Inclusion in our study required presence of atleast two of these five metabolic criteria to limit confounding effects of diabetes and long standing hypertension. Subjects were excluded if they had a fasting blood glucose >125 mg/dl or known cardiovascular disease.
Table I.
Various baseline clinical parameters among the metabolic syndrome cohort, n=32 reported as mean ±SE.
| Age (years) | 37± 2 |
| Males: Females | 17:15 |
| Weight (kg) | 104.1± 2.5 |
| BMI (kg/m2) | 34.4 ± 0.7 |
| Waist circumference (cm) | 114.7 ± 1.6 |
| Fasting triglycerides (mg/dl) | 180.4 ± 17.5 |
| Fasting blood glucose (mg/dl) | 98.3 ±1.6 |
| Systolic blood pressure (mmHg) | 128± 2 |
| Diastolic blood pressure (mmHg) | 84 ± 1 |
| HDL cholesterol (mg/dl) | 45.3 ± 2.1 |
| % body fat by DEXA | 34.8+ 1.4 |
| VO2 max (ml/kg/min) | 28.6±1.1 |
Subjects were weight stable (body weight change of less than ± 5%) and had not participated in a formal diet program within three months prior to the start of the study. Subjects also were non-smokers and were not taking any anti-hypertensive or weight altering medications, including over the counter supplements. These subjects were a cohort of volunteers from a larger long-term study on the effect of weight loss and weight regain on cardio-metabolic risk.
Treadmill Protocol
Using the standard Bruce treadmill protocol, stress testing was conducted on all of the subjects under close physician supervision. All subjects were exercised to volitional fatigue.19 Heart rate, blood pressure and ECG were monitored continuously. Reason for treadmill termination was documented and maximal oxygen consumption (VO2 max) was measured using the Parvo Medics TrueOne 2400 metabolic measurement system (Parvo Medics Inc., Sandy, UT) and a Hans Rudolph two-way non-rebreathing valve (Hans Rudolph Inc., Kansas City, MO). The highest oxygen consumption value obtained during the test was considered the subject's VO2 max. In order for this to be considered a valid measure, at least two of the following criteria were met: respiratory exchange ratio >1.15, a plateau in oxygen consumption (< 2 ml/kg/min increase in VO2 between progressive stages), and maximal heart rate within 10 beats of age predicted maximal heart rate (maximal heart rate = 220-age).
Stress Echocardiography
Using a Vivid I portable echocardiographic unit, 2D imaging was obtained using standard stress echocardiography protocol. An a priori decision was made to exclude patients with significant structural heart disease, manifested as cardiac chamber enlargement, dysfunction and valve disease and pulmonary hypertension. In our study group, all patients had normal structural heart function. Baseline wall motion analysis was performed in the apical four and two chamber views as well as the parasternal long and short axis views. Prior to exercise, pulse Doppler mitral inflow velocities as well as medial and lateral annular tissue Doppler (TDI) velocities were obtained from the apical 4-chamber view.
Repeat echocardiographic imaging with 2D imaging to evaluate global LV function and wall motion was performed within the first 60 seconds of termination of the maximal exercise. Pulse Doppler mitral inflow velocities and mitral annular tissue Doppler (TDI) velocities were then obtained from the apical 4-chamber view.
Diastolic function measurements
In each patient E was the mean value of the pulse wave early diastolic mitral inflow velocity obtained over five cardiac cycles at the level of the mitral valve tip from the apical 4-chamber view. The early diastolic peak of the annular descent from both the medial and lateral walls was measured over 5 cycles. E′ represents the average of medial and lateral early diastolic mitral annular TDI descent. LV filling pressure was indirectly estimated from E/E′.20-23 E/E′ was measured both at rest and immediately following treadmill exercise in the supine position. Rest and stress E/E′ measurements below 10 are considered normal and E/E′ > 13 has been correlated with reduced exercise capacity (< 8 METs).15-16
Statistical Analysis
Pearson Product correlations were used to determine relationships between resting E/E′ and VO2 max and post exercise E/E′ and VO2 max. Pearson product correlations were also used to determine relationships between the five criteria for the metabolic syndrome, sex, BMI, and age with VO2 max. Linear regression analysis was used to determine the amount of variance in VO2 max attributed to post exercise E/E′ and other subject characteristic variables significantly correlated to VO2 max. Statistical analysis was done using SPSS (SPSS/11.0, SPSS, Chicago, IL, USA).
Results
The average age of the study group was 37 ± 2 years. Nineteen of the 32 had 3 or more characteristics of the metabolic syndrome. All the subjects were overweight- obese (mean body mass index, BMI, = 34.4 ± 0.7 kg/m2). Fifteen of the 32 subjects had elevated blood pressure greater than 130/85 mmHg. Nineteen subjects had elevated triglyceride concentrations over 150 mg/dl and 17 of the study group had reduced concentrations of HDL-C of less than 40 mg/dl in males and less than 50 mg/dl in females.
The average treadmill time was 8.06 ±0.28 minutes. The VO2 max achieved by the study was 28.6 ±1.1 ml/kg/min at a mean 8.2 ±0.3 METs. None of the 32 subjects demonstrated any clinical, ECG or echocardiographic evidence of cardiac ischemia. The reason for terminating treadmill stress was volitional exhaustion, shortness of breath, or nonspecific leg pains.
Table 1 shows the various baseline clinical parameters among the metabolic syndrome cohort, n=32 reported as mean ±SE. Table 2 shows the various echocardiographic Doppler parameters among the metabolic syndrome cohort, n=32. Mean resting E/E′ and post exercise E/E′ were 7.01 ± 0.37 and 7.41 ± 0.41 respectively. There was no significant correlation between resting E/E′ and VO2 max (r= -0.266, p=0.14) (figure 1). Figure 2 shows post exercise E/E′ significantly correlating with VO2 max (r= -0.483, p=0.005). Sex (r= -0.825, p=0.001) and diastolic blood pressure (r=0.357, p=0.045) also were correlated with VO2 max. Age, BMI, and other parameters of the metabolic syndrome were not associated with VO2 max (Table 3).
Table II.
Echocardiographic Doppler parameters among the metabolic syndrome cohort, n=32 reported as mean ±SE.
| Resting parameters | |
| Resting E (m/s) | 0.737 ± 0.025 |
| Resting A (m/s) | 0.64± 0.03 |
| Resting E′ medial annulus (m/s) | 0.102 ± 0.020 |
| Resting E′ lateral annulus (m/s) | 0.116 ± 0.005 |
| Resting E′ mean (m/s) | 0.109 ± 0.228 |
| Resting A′ (m/s) | 0.10± 0.003 |
| Resting E/E′ | 7.01 ± 0.37 |
| Post exercise parameters | |
| Post exercise E (m/s) | 1.01 ± 0.047 |
| Post exercise A (m/s) | 0.90± 0.03 |
| Post exercise E′ medial annulus (m/s) | 0.128 ± 0.006 |
| Post exercise E′ lateral annulus (m/s) | 0.1778 ±0.024 |
| Post exercise E′ mean (m/s) | 0.143 ± 0.006 |
| Post Exercise A′ (m/s) | 0.13 ± 0.004 |
| Post exercise E/E′ | 7.41 ± 0.41 |
Figure 1.
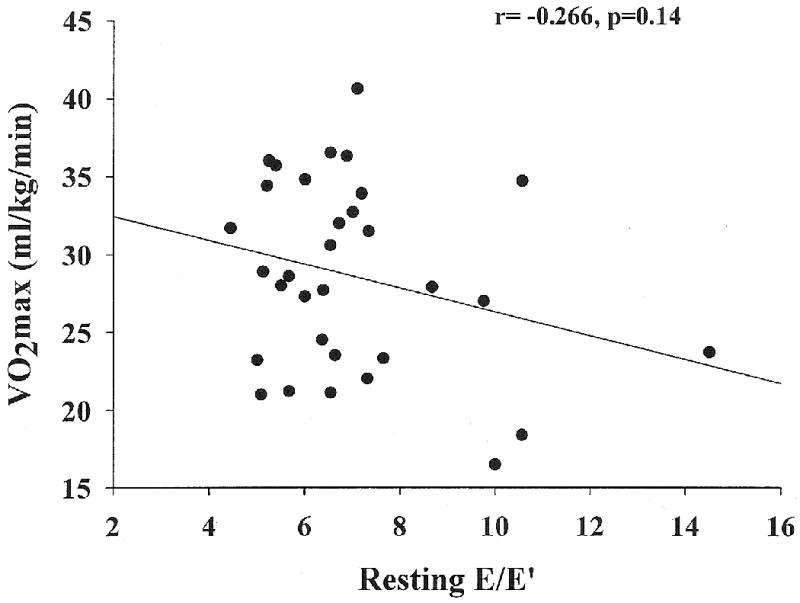
Relation between VO2 max and resting E/E′ obtained prior to exercise.
Figure 2.
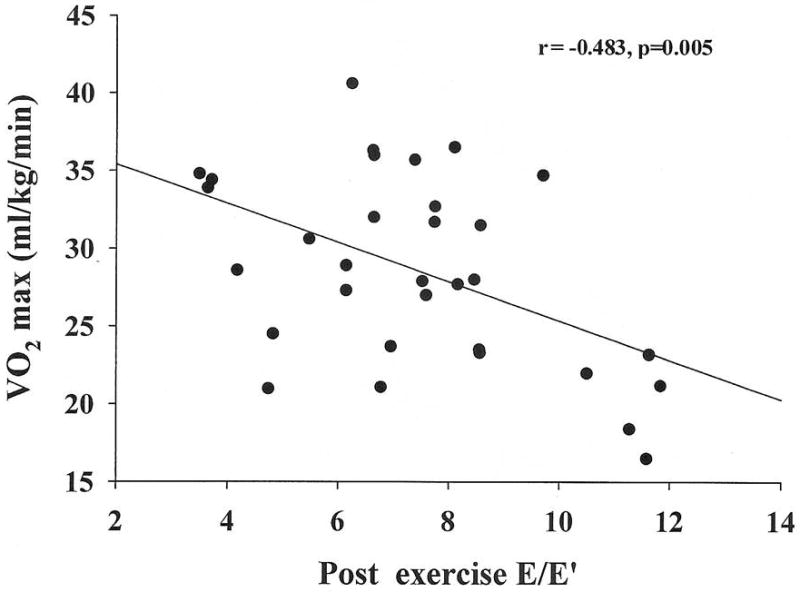
Relation between VO2 max and stress E/E′ obtained immediately following completion of maximal treadmill exercise.
Table III.
Simple correlations (Pearson r) between VO2 max (ml/kg/min) and variables.
| Characteristic | Pearson r | P |
|---|---|---|
| resting E/E′ | -0.266 | 0.141 |
| post exercise E/E′ | -0.483 | 0.005 |
| Sex | -0.825 | 0.001 |
| Age | -0.225 | 0.216 |
| BMI | -0.201 | 0.270 |
| Weight | 0.145 | 0.430 |
| Waist | -0.204 | 0.262 |
| SBP | 0.298 | 0.098 |
| DBP | 0.357 | 0.045 |
| Glucose | -0.163 | 0.372 |
| HDL-C | -0.002 | 0.991 |
| TG | 0.135 | 0.462 |
Separate linear regression models were created to examine how well post exercise E/E′ and other subject characteristics predicted VO2 max. Post exercise E/E′ alone accounted for approximately 20% of the variance in VO2 max (R2 adj=0.208, p=0.005) (Table 4). When sex was added to post exercise E/E′ in a second linear regression model, 70% of the variance in VO2 max was accounted for (R2 adj=0.703, p=0.001) (Table 4). Finally when a third model was created with post exercise E/E′, sex, and diastolic blood pressure, diastolic blood pressure contributed minimally to the model (R2 adj=0.714).
Table IV.
Linear regression analysis: models created to account for variance in VO2 max (n=32).
| β | SE B | t score | p value | |
|---|---|---|---|---|
| Model 1a R2adj=0.208 post exercise E/E′ |
-0.483 | 0.417 | -3.02 | 0.005 |
| Model 2b R2adj=0.703 post exercise E/E′ |
-0.218 | 0.273 | -2.08 | 0.046 |
| Sex | -0.747 | 1.259 | -7.14 | 0.001 |
Model accounted for 20.8% of the variance in VO2 max (F=9.122, df=1, 30, p=0.005)
Model accounted for 70.3% of the variance in VO2 max (F=37.62, df=2,29, p=0.001)
Discussion
The major new finding from our study was the significant inverse correlation between post exercise Doppler derived indices of diastolic dysfunction and exercise capacity. Patients with higher post stress E/E′ levels demonstrated lower peak VO2max.
Methodological and Patient Considerations
Various factors contribute to functional capacity. Functional capacity is commonly quantified using VO2max, which is a product of cardiac output (heart rate times stroke volume) multiplied by arterial venous oxygen difference. Thus, the complex interplay of cardiac, pulmonary, peripheral muscle and cellular function determine the overall ability to exercise
In our study we chose a group of patients that had 2 or more of 5 criteria for metabolic syndrome. These patients were all otherwise healthy and capable of performing a treadmill exercise stress test. Thus, none of our patients had abnormal baseline indices for diastolic function. Also, following exercise stress, the E/E′ value did not increase as would be expected in subjects with early/occult diastolic dysfunction. Even when within normal range, E/E′ may still play a role in determining VO2 max.
Our metabolic syndrome cohort had less than expected treadmill times and below normal measured VO2 max despite the absence of any significant cardio-pulmonary disease. The mean VO2 max for the present group was below the 20th percentile for both sexes (males age 30-39: 20th percentile=36.2ml/kg/min, females age 30-39: 20th percentile =29.9 ml/kg/min.)18 Systolic as well as conventionally measured diastolic parameters were within normal range. Post treadmill, diastolic function as assessed by E/E′ remained below 13, suggesting the absence of any occult relaxation abnormalities. We had expected to detect elevated E/E′ post exercise values in at least a few of the subjects due to presence of obesity, diabetes and hypertension, or combinations thereof. Likely, our cohort was healthier and metabolic syndrome related cardiac changes might not have developed.
Interestingly, despite being within the normal range, post exercise E/E′ correlated significantly (and inversely) with METs and VO2 max. Resting E/E′ was normal also in this cohort and did not appear to predict effort tolerance. Subjects with abnormally elevated resting or stress E/E′ have reduced functional capacity.14-16 Although there are no prior studies for comparison, our results suggest that cardiac diastolic function is an important determinant of VO2 max in apparently healthy cohorts.
Comparisons to available literature
Recent studies suggest that E/E′ may be a reliable marker for LV filling pressure both at rest and post exercise. 14-16 This offers a non-invasive and widely available tool to investigate diastolic cardiac function at rest and post exercise along with estimating systolic function during stress echocardiography. Ischemia, hypertensive hypertrophy, fibrosis related to metabolic stresses and early infiltrative changes may all be expected to affect ‘stress diastolic cardiac function’ before standard echocardiographic abnormalities are evident. Doppler estimation of E/E′ may be valuable in quantifying diastolic function among subjects presenting with unexplained or excessive dyspnea than would be accounted for by underlying cardio-pulmonary conditions. Recent reports suggest that E/E′ elevation from a baseline normal value occurs among some dyspneic subjects with impaired relaxation.15-17 Both resting and post exercise E/E′ values > 15 predicted reduced VO2 max. Our data suggests that diastolic function, as estimated by stress E/E′, is normal among subjects with 2 or more of the characteristics for the metabolic syndrome. Post stress E/E′ in these ‘normal hearts’ appears to be inversely related to VO2 max.
The major limitation is the inability to demonstrate diastolic dysfunction, namely elevation of E/E′ > 13 at rest or post exercise, in a subset of our subjects. This may be due to the fact that our subjects were relatively young and otherwise healthy obese individuals with only half meeting 3 of 5 currently accepted metabolic syndrome criteria.
Conclusions
Cardiac diastolic function is preserved in early metabolic syndrome. Even when within normal range, Doppler derived post stress E/E′ correlates with VO2 max, but the diminished exercise capacity is not primarily due to exercise-induced elevation in LA pressure. Diastolic indices during stress echocardiography may help evaluate dyspnea but further research is needed to define its role in the clinical management of cardio-metabolic syndrome subjects.
Acknowledgments
Funding Sources
Supported by NIH R01 DK067036 to TR Thomas and VISN 15 Veterans Administration Research award to A Chockalingam.
References
- 1.Nishimura RA, Jaber W. Understanding “diastolic heart failure”: the tip of the iceberg. J Am Coll Cardiol. 2007;49:695–697. doi: 10.1016/j.jacc.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 4.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 6.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ, Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 8.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–1518. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 9.Leite-Moreira AF. Current perspectives in diastolic dysfunction and diastolic heart failure. Heart. 2006;92:712–718. doi: 10.1136/hrt.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. doi: 10.1016/j.jacc.2005.09.032. HYPERLINK “ http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=16458127&ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum”. [DOI] [PubMed]
- 11.Rakowski H, Appleton C, Chan KL, Dumesnil JG, Honos G, Jue J, Koilpillai C, Lepage S, Martin RP, Mercier LA, O'Kelly B, Prieur T, Sanfilippo A, Sasson Z, Alvarez N, Pruitt R, Thompson C, Tomlinson C. Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography. The Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiog. 1996;9:736–760. doi: 10.1016/s0894-7317(96)90076-0. [DOI] [PubMed] [Google Scholar]
- 12.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- 13.Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320–323. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 14.Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation. 2004;109:972–977. doi: 10.1161/01.CIR.0000117405.74491.D2. [DOI] [PubMed] [Google Scholar]
- 15.Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: A novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise doppler echocardiography. J Am Soc Echocardiogr. 2005;18:63–68. doi: 10.1016/j.echo.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significant of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Talreja DR, Nishimura RA, Oh JK. Estimation of left ventricular filling pressure with exercise by Doppler echocardiography in patients with normal systolic function: a simultaneous echocardiographic-cardiac catheterization study. J Am Soc Echocardiogr. 2007;20:477–479. doi: 10.1016/j.echo.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (adult treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.ACSM's Guidelines for Exercise Testing and Prescription. 7th. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 79–100. [Google Scholar]
- 20.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 21.Lindstrom L, Wranne B. Pulsed tissue Doppler evaluation of mitral annulus motion: A new window to assessment of diastolic function. Clin Physiol. 1999;19:1–10. doi: 10.1046/j.1365-2281.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu Y, Uematsu M, Shimizu H, Nakamura K, Yamagishi M, Miyatake K. Peak negative myocardial velocity gradient in early diastole as a noninvasive indicator of left ventricular diastolic function: comparison with transmitral flow velocity indices. J Am Coll Cardiol. 1998;32:1418–1425. doi: 10.1016/s0735-1097(98)00394-5. [DOI] [PubMed] [Google Scholar]
- 23.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007:2539–2550. doi: 10.1093/eurheartj/ehm037. Epub 2007 Apr 11. [DOI] [PubMed] [Google Scholar]


