Abstract
Background
Near-infrared spectroscopy (NIRS) monitoring of cerebral oxygen saturation (rSO2) has become routine in many centers, but no studies have reported the relationship of intraoperative NIRS to long-term neurodevelopmental outcomes after cardiac surgery.
Methods and Results
Of 104 infants undergoing biventricular repair without aortic arch reconstruction, 89 (86%) returned for neurodevelopmental testing at age 1 year. The primary NIRS variable was the integrated rSO2 (area under the curve) for rSO2 ≤ 45%; secondary variables were the average and minimum rSO2 by perfusion phase and at specific time points. Psychomotor (PDI) and Mental Development Indexes of the Bayley Scales, head circumference, neurologic examination, and abnormalities on brain MRI did not differ between subjects according to a threshold level for rSO2 of 45%. Lower PDI scores were modestly associated with lower average (r=0.23; P=0.03) and minimum rSO2 (r=0.22; P=0.04) during the 60 minute period following cardiopulmonary bypass (CPB), but not with other perfusion phases. Hemosiderin foci on brain MRI were associated with lower average rSO2 from post-induction to 60 minutes post-CPB (71±10 vs. 78±6%; P=0.01), and lower average rSO2 during the rewarming phase (72±12 vs. 83±9%; P=0.003) and during the 60 minute period following CPB (65±11 vs. 75±10%; P=0.009). In regression analyses adjusting for age ≤ 30 days, PDI score (P=0.02) and brain hemosiderin (P=0.04) remained significantly associated with rSO2 during the 60 minute period following CPB.
Conclusions
Perioperative periods of diminished cerebral oxygen delivery, as indicated by rSO2, are associated with one-year PDI and brain MRI abnormalities among infants undergoing reparative heart surgery.
Clinical Trial Registration Information
Keywords: heart defects, congenital; brain; oxygen; near-infrared spectroscopy; magnetic resonance imaging
Short Commentary.
This study explored whether intraoperative cerebral oxygen saturation (rSO2) measured by near-infrared spectroscopy (NIRS) was associated with neurodevelopmental outcomes at age one year among infants undergoing biventricular repair without aortic arch reconstruction. Lower Psychomotor Development Index (PDI) of the Bayley Scales was modestly associated with lower rSO2 during the 60 minute period following cardiopulmonary bypass (CPB) and at the time points 10 minutes of cooling, off-CPB, and 60 minutes post-CPB. Lower rSO2 from post-induction to 60 minutes post-CPB, and for the 60 minute period following CPB, was associated with hemosiderin foci on qualitative MRI analysis. No relationship could be demonstrated between rSO2 and Mental Development Index of the Bayley Scales, neurologic examination, or head circumference. The relationship of lower rSO2 with lower PDI score and greater risk of hemosiderin on brain MRI, even after adjustment for age ≤ 30 days or diagnosis group, suggests that periods of intraoperative and early postoperative decreased cerebral oxygen delivery are associated with adverse longer term neurodevelopmental outcomes.
Introduction
As operative mortality rates for virtually all forms of congenital heart disease (CHD) have declined, the focus has shifted to improving late functional outcome. Survivors of CHD have a significant prevalence of neurodevelopmental and behavioral disabilities. These have been linked, in part, to bypass-related variables, and include lower scores on the Bayley Scales of Infant Development and longer-term impairments in cognitive abilities, gross and fine motor function, visual-spatial skills, speech and language, executive function, and attention.1, 2 Identifying methods to reduce the frequency and severity of these adverse outcomes are therefore a high priority. Although neurodevelopmental outcomes of children with CHD are influenced by many factors, both innate and acquired, with cumulative effects,3 cerebral ischemia in the perioperative period is still an important cause of brain injury.4-9 Therefore, reliable methods for detecting cerebral ischemia may allow for management strategies to improve neurodevelopmental outcomes.
The ability of near-infrared spectroscopy (NIRS) to measure cerebral oxygenation was first demonstrated by Jobsis.10 Animal studies have demonstrated a relationship of NIRS during cardiopulmonary bypass (CPB) to early neurologic outcome.11, 12 In humans, Austin et al. reported that intraoperative interventions based on multimodality neurophysiologic monitoring, including NIRS, during pediatric cardiac surgery reduced the incidence of postoperative neurologic sequelae; however the relationship of NIRS findings to long-term neurologic outcome was not evaluated.13 Subsequent reports have shown that lower intraoperative cerebral oxygen saturation9 and prolonged low postoperative cerebral oxygen saturation4 are risk factors for white matter injury on brain magnetic resonance imaging (MRI). To monitor cerebral oxygen saturation, some investigators have advocated widespread adoption of NIRS during cardiac surgery.14 Indeed, NIRS monitoring has become routine in many centers. However, no studies have reported the relationship of intra-operative NIRS findings to long-term neurologic outcome after cardiac surgery.
Our group has shown that intraoperative cerebral oxygen saturation in infants undergoing biventricular repair varies according to diagnosis and accounts for very little of the variance in early postoperative outcome.15 Specifically, no significant associations were found between cerebral oxygen saturation and lactate at 60-min post-CPB, cardiac index at 6- and 18-h post-CPB, PRISM III scores, and length of intubation, intensive care, and hospital stay. Additionally, area under the curve analysis found no difference in early outcome between those subjects in whom the rSO2 remained above 45% versus those in whom the rSO2 decreased to ≤ 45%. The aim of this study is to evaluate the relationship of intraoperative cerebral oxygen saturation to neurologic outcome at age 1 year, assessed by neurodevelopmental evaluation and brain MRI. We hypothesized that lower levels of intraoperative cerebral oxygen saturation would be associated with worse neurodevelopmental outcome.
Methods
Patients and Study Design
With Institutional Review Board approval and parental informed consent, patients were enrolled between April 2001 and July 2004 at Children's Hospital Boston in a randomized trial of hemodilution to a hematocrit of 25% vs. 35% during hypothermic CPB.16 Eligibility criteria included reparative heart surgery at < 9 months of age in three diagnostic groups: (1) D-transposition of the great arteries (D-TGA); (2) tetralogy of Fallot (TOF) with or without pulmonary atresia or truncus arteriosus; and (3) ventricular septal defect (VSD) or complete common atrioventricular canal defect. Exclusion criteria included birth weight < 2.3 kg, recognizable phenotypic syndrome of congenital anomalies, extracardiac anomalies of greater than minor severity, previous cardiac surgery, or associated cardiovascular anomalies requiring aortic arch reconstruction or additional open surgical procedures before the planned developmental follow-up.
Anesthesia and Perfusion Methods
Methods for anesthesia and perfusion have been previously described.15, 17 Briefly, high dose opioid-relaxant anesthesia (fentanyl 100 mcg/kg) was supplemented with midazolam and/or isoflurane as tolerated. Bypass prime comprised whole blood and Plasma-lyte A pH 7.4 (Multiple Electrolytes Injection, Type 1, USP) to attain a hematocrit of 25% or 35% at the time of onset of low-flow CPB. A pH-stat strategy was used during core cooling, low-flow hypothermic perfusion, and rewarming up to 30°C. Full-flow CPB was approximately 2.5 L·min −1·m −2; some patients had periods of DHCA, and most had at least one period of low-flow CPB of approximately 0.75 L·min−1·m−2 when at deep hypothermia (rectal temperature <18°C). Methylprednisolone (30 mg/kg), phentolamine (0.2 mg/kg), and furosemide (0.25 mg/kg) were given at the initiation of CPB, and mannitol (0.5 g/kg) and phentolamine (0.2 mg/kg) at the onset of rewarming. Patients were rewarmed for at least 30 minutes and to a rectal temperature of 34°C.
Near-Infrared Spectroscopy
Regional cerebral oxygen saturation (rSO2) was measured with the INVOS 5100B (Somanetics, Troy, MI) as previously described.15, 17 The INVOS is a continuous wave near-infrared spectrometer that uses two wavelengths of light (730 and 810 nm) to measure the ratio of oxyhemoglobin to total hemoglobin to derive oxygen saturation, the scale unit of which is percent (%). Pediatric SomaSensors® (Somanetics, Troy, MI) were placed on the right and left forehead following induction of anesthesia and data collected continuously up to 60 minutes post-CPB. As cerebral oximetry was not a routine part of our practice at the time of this study, perioperative management decisions were not made on the basis of cerebral oxygen saturation.
The primary variable selected for evaluation of the relationship between rSO2 and neurodevelopmental outcome at age 1 year was the integrated rSO2 or area under the curve (AUC) for rSO2 ≤ 45% (minutes*desaturation points ≤ 45%). Subjects who had at least one episode in which the rSO2 fell to ≤ 45% for a period of 1-minute (threshold level) were identified as having a decline in cerebral oxygen saturation to the defined cutoff value. Comparisons were then done between three groups of subjects: those in whom the rSO2 remained > 45% (Group I, AUC = 0 min%), and those in whom the rSO2 fell to ≤ 45% and dichotomized by the median integrated rSO2 ≤ 45%, which was 40 min%. Group II subjects had an AUC > 0 but < 40 min%, and Group III subjects had an AUC ≥ 40 min%. Integrated rSO2 was chosen over total duration of rSO2 ≤ 45% to better indicate the extent of desaturation below the threshold value. Laboratory and clinical studies suggest that near-infrared spectroscopy thresholds for cerebral injury are oxygen saturations in the range of 33% to 55%.4, 11, 18, 19 Cerebral oxygen saturations at these levels are associated with functional impairment (increased brain lactate, electroencephalogram (EEG) changes, decreased brain tissue adenosine triphosphate concentration),18 abnormal neurobehavioral examinations and brain histology,11, 19 and new or worsened ischemia on postoperative brain MRI.4 Because a nonlinear relationship between rSO2 and neurodevelopmental outcome may exist, similar analyses were done using 50% and 55% as possible threshold values.
Secondary NIRS variables included the average rSO2 and minimum rSO2 for the time period post-induction to 60 minutes post-CPB, and by perfusion phase: post-induction to on-CPB, onset cooling to onset last rewarming, onset last rewarming to off-CPB, and off-CPB to 60 minutes post-CPB. The rSO2 measurements at specified time-points17 were also used as secondary variables.
Neurodevelopmental Outcomes
Neurodevelopmental evaluation at one-year follow-up comprised neurologic examination (also performed preoperatively), developmental evaluation, and brain MRI. Neurologic examination was defined as abnormal if abnormalities were noted in any of the following categories: head circumference, mental status, special senses, cranial nerve motor function, or peripheral motor function. Development was assessed with the Bayley Scales of Infant Development (BSID-II), which yields scores on two indexes: the Psychomotor Development Index (PDI) and the Mental Development Index (MDI).20 PDI scores were the primary outcome for the hematocrit trial16 from which these secondary analyses are derived. Brain MRIs were obtained in a subset of participants. MRI sequences have been described in detail elsewhere.21 Briefly, these sequences were a sagittal localizer and an axial multiplanar gradient recalled (MPGR) acquisition sequence for detection of old products of hemorrhage, three-dimensional SPoiled Gradient Recalled (3D SPGR) and dual fast spin echo (FSE) sequences in the coronal plane for volumetric analysis, an axial diffusion tensor imaging (DTI) sequence, and a single voxel proton magnetic resonance spectroscopy (MRS) sequence. The percent of white matter relative to whole brain volume was calculated by dividing the sum of the myelinated and unmyelinated white matter volume by the volume of the entire brain (i.e., without CSF included). All sequences were evaluated for evidence of acquired or developmental abnormalities by a single pediatric neuroradiologist (RLR). A detailed description of the MRI data acquisition parameters and data processing techniques used to analyze the volumetric, DTI, and MRS data has been reported previously 16, 21
Statistical Analysis
Comparisons of perioperative characteristics, NIRS values, and neurodevelopmental outcomes across categories of integrated rSO2 ≤ 45% were made using analysis of variance for continuous variables and Fisher's exact test for categorical variables. Analyses of PDI and MDI scores also adjust for social class,22 and analyses of quantitative MRI variables also adjust for age at MRI evaluation. Partial Spearman rank correlation coefficients and linear regression, adjusting for social class, were used to assess associations between NIRS measures and PDI and MDI scores. Partial Spearman rank correlation coefficients, adjusting for age at MRI evaluation, were used to assess associations between NIRS measures and quantitative MRI measures. We used t-tests and logistic regression to assess associations between NIRS measures and binary MRI abnormalities. We also considered linear and logistic regression models that adjusted for neonatal status or diagnosis group. In this secondary analysis of data arising from a clinical trial, a sample size of 89 subjects provides 80% power to detect correlations of 0.30 or larger, in a two-sided 0.05 level test of the null hypothesis of no correlation.
Results
Of 104 infants who had NIRS monitoring in the primary study, 89 (86%) returned for testing at age 1 year. Of the remaining 15 families, 12 refused to participate, 2 lived outside the country and were not invited for one-year follow-up, and 1 agreed to questionnaires only. Subjects who returned for one-year follow-up were similar to those who declined with respect to perioperative characteristics and NIRS measurements.
Demographic and perioperative characteristics according to the primary variable, integrated rSO2 ≤ 45%, are shown in Table 1. NIRS data according to categories of integrated rSO2 ≤ 45% are shown in Table 2. The integrated rSO2 ≤ 45% was 0 min% for Group I, ranged from 0.3-39 min% for Group II, and from 60-383 min% for Group III. No infant had clinical seizures or stroke perioperatively. Group III had the longest total support time, cross clamp time, and duration of circulatory arrest, with trends toward longer low flow bypass and diagnosis of D-TGA. The NIRS groups did not differ in hematocrit at any time point, nadir temperature (tympanic 20±5 °C), lowest pO2 (99±80 mmHg), lowest pCO2 (33±4 mmHg), or highest pCO2 (761±4 mmHg).
Table 1. Preoperative and Operative Characteristics According to Categories of Integrated Cerebral Oxygen Saturation (rSO2) ≤ 45%.
| Variable | Integrated rSO2 ≤ 45% (min%) | |||
|---|---|---|---|---|
| 0 (N=66) |
0.3 – 39 (N=12) |
60 – 383 (N=11) |
P | |
| Preoperative Characteristics | ||||
| Birth weight (kg) | 3.5 ± 0.5 | 3.3 ± 0.6 | 3.3 ± 0.4 | 0.16 |
| Gestational age (wk) | 39.2 ± 1.3 | 39.2 ± 1.6 | 39.2 ± 1.0 | 1.0 |
| Sex (% male) | 64 | 42 | 55 | 0.35 |
| Race (% non-white) | 20 | 33 | 9 | 0.38 |
| Age at surgery (days), median (range) | 38 (2-200) | 64 (4-263) | 11 (7-198) | 0.35 |
| Neonatal status (% age ≤ 30 days) | 48 | 33 | 72 | 0.17 |
| Abnormal neurologic examination, n/total (%) | 27/47 (57) | 6/9 (67) | 4/10 (40) | 0.50 |
| Diagnosis, n (%) | 0.06 | |||
| TGA | 28 (42) | 2 (17) | 7 (64) | |
| TOF | 26 (39) | 5 (42) | 1 (9) | |
| VSD | 12 (18) | 5 (42) | 3 (27) | |
| Operative Characteristics | ||||
| Cross clamp time (min) | 65 ± 23 | 60 ± 29 | 94 ± 44 | 0.004 |
| Total support time (min) | 104 ± 30 | 101 ± 38 | 147 ± 69 | 0.002 |
| Total bypass time (min) | 95 ± 24 | 95 ± 36 | 122 ± 61 | 0.04 |
| Low-flow bypass time (min) | 48 ± 23 | 47 ± 31 | 67 ± 36 | 0.07 |
| Duration of circulatory arrest (min) | 8 ± 11 | 5 ± 13 | 25 ± 21 | <0.001 |
Values are expressed as mean ± standard deviation. P values are determined by analysis of variance for continuous variables and Fisher's exact test for categorical variables.
TGA = D-transposition of the great arteries
TOF = tetralogy of Fallot
VSD = ventricular septal defect
Table 2. Intraoperative Regional Cerebral Oxygen Saturation (rSO2) Variables According to Categories of Integrated rSO2 ≤ 45%.
| Variable | Integrated rSO2 ≤ 45% (min%) | |||
|---|---|---|---|---|
| 0 (N=66) |
0.3 – 39 (N=12) |
60 – 383 (N=11) |
P | |
| Integrated rSO2 ≤ 45% (min%) | 0 | 17 ± 14 | 148 ± 96 | <0.001 |
| Total duration ≤ 45% (min) | 0 | 6 ± 6 | 33 ± 19 | <0.001 |
| Longest duration ≤ 45% (min) | 0 | 6 ± 5 | 20 ± 7 | <0.001 |
| Post-induction – 60 min post-CPB | ||||
| Average rSO2 (%) | 79 ± 6 | 69 ± 7 | 67 ± 9 | <0.001 |
| Minimum rSO2 | 56 ± 7 | 40 ± 3 | 33 ± 7 | <0.001 |
| Post-induction – on-CPB | ||||
| Average rSO2 | 69 ± 10 | 58 ± 6 | 51 ± 7 | <0.001 |
| Minimum rSO2 | 60 ± 9 | 44 ± 8 | 41 ± 9 | <0.001 |
| Onset cooling – onset last rewarming | ||||
| Average rSO2 | 85 ± 7 | 79 ± 11 | 73 ± 11 | <0.001 |
| Minimum rSO2 | 67 ± 11 | 57 ± 13 | 43 ± 14 | <0.001 |
| Onset last rewarming – off-CPB | ||||
| Average rSO2 | 81 ± 10 | 74 ± 11 | 76 ± 13 | 0.06 |
| Minimum rSO2 | 71 ± 12 | 61 ± 10 | 65 ± 13 | 0.02 |
| Off-CPB – 60 min post-CPB | ||||
| Average rSO2 | 78 ± 9 | 63 ± 9 | 63 ± 13 | <0.001 |
| Minimum rSO2 | 68 ± 10 | 54 ± 11 | 49 ± 15 | <0.001 |
Values are expressed as mean ± standard deviation. P values are determined by analysis of variance.
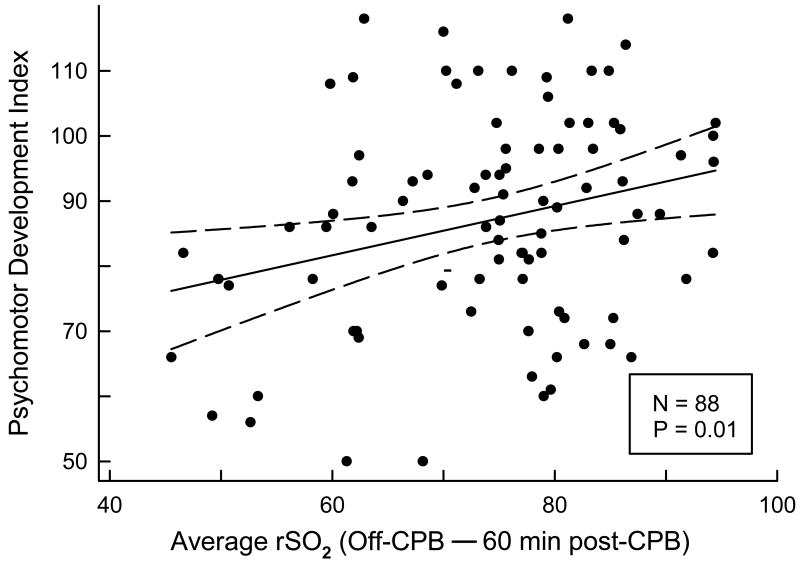
PDI and MDI scores for the entire cohort were 87±16 and 94±12, significantly lower than population norms with mean 100 (P<0.001 for each). PDI scores did not vary significantly among the three NIRS groups classified by integrated rSO2 ≤ 45% (Table 3). Furthermore, the partial Spearman correlation adjusting for social class found no relationship between integrated rSO2 ≤ 45% measured on a continuous scale and PDI score. When examined by perfusion phase, PDI was significantly correlated with average rSO2 (r=0.23; P=0.04) and minimum rSO2 (r=0.22; P=0.04) during the 60 minute period following cessation of CPB (“off-CPB - 60 minutes post-CPB”) (Figure 1), but not with any other perfusion phase. Evaluation of PDI with rSO2 at each time point found significant correlations at 10 minutes of cooling (r=0.24; P=0.03), at off-CPB (r=0.25; P=0.02), and at 60 minutes post-CPB (r=0.22; P=0.04), but no correlations at 6-h and 18-h post-CPB. PDI did not correlate with the difference in rSO2 between onset of cooling (64±14%) and at 10 minutes of cooling (86±9%). No correlation was found between any intraoperative NIRS variable and MDI score.
Table 3. Neurodevelopmental and Magnetic Resonance Imaging Evaluations at Age One-Year According to Categories of Integrated Cerebral Oxygen Saturation (rSO2) ≤ 45%.
| Variable | Integrated rSO2 ≤ 45% (min%) | |||
|---|---|---|---|---|
| 0 | 0.3 – 39 | 60 – 383 | P | |
| Bayley Scales of Infant Development | (N=66) | (N=12) | (N=11) | |
| Psychomotor Development Index | 88 ± 16 | 85 ± 20 | 86 ± 17 | 0.81 |
| Mental Development Index | 94 ± 11 | 91 ± 17 | 96 ±10 | 0.41 |
| Neurologic Examination | (N=62) | (N=12) | (N=10) | |
| Abnormal neurologic examination, n (%) | 37 (60) | 8 (67) | 5 (50) | 0.72 |
| Head circumference, Z-score | –0.02 ± 1.04 | 0.04 ± 0.86 | –0.16 ± 1.10 | 0.89 |
| Magnetic Resonance Imaging, Qualitative | (N=30) | (N=6) | (N=4) | |
| Brain hemosiderin, n (%) | 9 (30) | 3 (50) | 2 (50) | 0.46 |
| Focal stroke | 1 (3) | 0 | 0 | 1.0 |
| Periventricular leukomalacia | 2 (7) | 0 | 0 | 1.0 |
| Magnetic Resonance Imaging, Quantitative (volume in mL) | (N=30) | (N=6) | (N=4) | |
| Gray matter | 622 ± 62 | 606 ± 25 | 579 ± 55 | 0.49 |
| Subcortical gray matter | 32 ± 11 | 35 ± 8 | 43 ± 9 | 0.02 |
| Myelinated white matter | 81 ± 36 | 80 ± 31 | 75 ± 26 | 0.69 |
| Unmyelinated white matter | 177 ± 41 | 184 ± 57 | 148 ± 27 | 0.38 |
| Cerebrospinal fluid | 99 ± 36 | 101 ± 29 | 79 ± 10 | 0.57 |
| Brain | 912 ± 94 | 906 ± 70 | 844 ± 99 | 0.50 |
| Cerebrum | 811 ± 86 | 808 ± 66 | 748 ± 86 | 0.47 |
| Cerebellum | 101 ± 11 | 98 ± 9 | 96 ± 13 | 0.87 |
Values are expressed as mean ± standard deviation. P values are determined by analysis of variance for continuous variables and Fisher's exact test for categorical variables. P values for Bayley Scales also adjust for social class, and P values for quantitative magnetic resonance imaging variables also adjust for age at magnetic resonance imaging evaluation.
Figure 1.
Psychomotor Development Index (PDI) as a function of average rSO2 from off-CPB to 60 minutes post-CPB by scatter plot. The solid line was derived by unadjusted linear regression of the data, and the dashed lines delimit the 95% confidence interval. The linear regression P value shown is for the effect of average rSO2 from off-CPB to 60 minutes post-CPB on PDI, adjusting for social class.
Mean head circumference (Z-score) for the cohort at one-year follow-up was −0.03±1.01 and did not differ between the three integrated rSO2 ≤ 45% groups (Table 3). No correlation was found between any intraoperative NIRS variable and head circumference at age 1 year. Neurologic examination was abnormal in 50 subjects (60%) at age 1 year, but no difference in intraoperative NIRS variables was found between those subjects with a normal versus an abnormal neurologic examination.
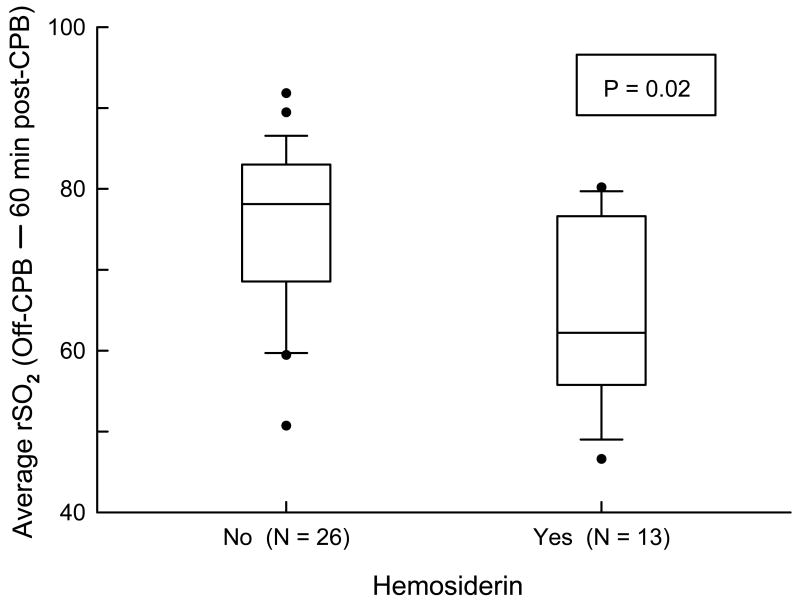
Brain MRI scans were obtained in 40 of the 89 subjects that had measurement of intraoperative cerebral oxygen saturation. Missing MRI scans were due to parental refusal usually related to the need for general anesthesia (n=47) and cancellations by the anesthesiologist relating to medical concerns (n=2). Subjects who had an MRI scan were similar, with respect to scores on the BSID-II and neurologic examination, to those subjects whose parents declined the MRI scan.21 There was no evidence of severe brain injury or major brain malformations. The majority of acquired abnormalities were tiny foci of signal abnormality on the MPGR sequence consistent with small foci of hemosiderin (Table 3). These foci were found in 14 of this subset of 40 subjects (35%), and were present throughout the entire brain, without predilection for any particular region or specific pattern of distribution. Analysis of the relationship according to integrated rSO2 ≤ 45% categories and qualitative MRI measures found no significant association with the presence of foci of hemosiderin or other developmental or acquired abnormalities. Similarly, we found no statistically significant relationship between integrated rSO2 ≤ 45% measured on a continuous scale and hemosiderin (P=0.10 via t-test). The number of subjects with focal stroke (n = 1) or periventricular leukomalacia (n = 2) was too small to determine whether any NIRS variables were associated with these specific brain lesions. With respect to the secondary NIRS variables, the average rSO2 from post-induction to 60 minutes post-CPB was lower in those subjects with hemosiderin (71±10 vs. 78±6%; P=0.01 via t-test). By perfusion phase, average rSO2 was lower during the rewarming phase (72±12 vs. 83±9%; P=0.003) and off-CPB to 60 minutes post-CPB (65±11 vs. 75±10%, P=0.009) (Figure 2) in those subjects with brain hemosiderin. Similarly, minimum rSO2 was lower during the rewarming phase (61±11 vs. 71±11%; P=0.006) and off-CPB to 60 minutes post-CPB (56±11 vs. 66±12%; P=0.02) in those subjects with brain hemosiderin. There were no statistically significant differences between those subjects with and without hemosiderin in average and minimum rSO2 during the prebypass and onset cooling to onset first rewarming phases, or with rSO2 at 10 minutes of cooling.
Figure 2.
Box plots of average rSO2 from off-CPB to 60 minutes post-CPB by hemosiderin status. A solid bar within the box represents the median value, the upper boundary of the box represents the 75th percentile, and the lower boundary of the box represents the 25th percentile. The vertical lines extend to the 10th and 90th percentiles, with more extreme observations plotted as filled circles. The logistic regression P value shown is for the effect of average rSO2 from off-CPB to 60 minutes post-CPB on hemosiderin.
Next, we analyzed the relationship between NIRS data and quantitative MRI measures to determine if specific NIRS variables predicted changes in brain volumes or white matter microstructure, adjusting for age at MRI evaluation (Table 3). NIRS variables were not strongly associated with regional brain volumes or tissue types, except for subcortical gray matter. There was an apparent paradoxical finding of increased subcortical gray matter volume in association with greater integrated rSO2 ≤ 45% when adjusted for age at MRI (r=0.33, P=0.04). Similarly, Spearman correlations found no relationships between integrated rSO2 ≤ 45% measured on a continuous scale with regional brain volumes or tissue types, except for subcortical gray matter (r=0.42, P=0.007). NIRS variables were not significantly correlated with measures of white matter microstructure (by DTI) or metabolite concentrations (by MRS).
Age and diagnosis group were highly collinear. Of 44 neonates (age ≤ 30 days), 37 (84%) had TGA, 5 (11%) had TOF, and 2 (5%) had VSD, whereas in 45 older infants 27 (60%) had TOF and 18 (40%) had VSD. Neonates and older infants were similar with respect to outcomes at age one year, including PDI score (90±15 vs. 85±17; P=0.13), MDI score (95±11 vs. 93±13; P=0.35), percentage with abnormal neurologic examination (22% vs. 28%; P=0.37) and head circumference (Z-score -0.02±0.98 vs. -0.03±1.06; P=0.97). Of the 40 subjects who underwent brain MRIs at one year of age, 2/17 neonates (11.8%) compared to 12/23 older infants (52.2%) had findings of brain hemosiderin (P=0.02).
In regression analysis adjusting for age ≤30 days (Table 4), PDI remained significantly associated with average rSO2 for the phase off- CPB to 60 minutes post-CPB (P=0.02), as well as at 60 minutes post-CPB (P=0.03), whereas there were trends (.05 < P <.1) in the associations between PDI and minimum rSO2 for the phase off-CPB to 60 minutes post-CPB (P=0.053), rSO2 at 10 minutes of cooling (P=0.07), rSO2 at off-CPB (P=0.06), and average rSO2 from post-induction to 60 minutes post-CPB (P=0.09). In models adjusting for age ≤30 days, diagnosis group, and social class, statistical significance or trends were retained for the associations between PDI and average rSO2 for the phase off-CPB to 60 minutes post-CPB (P=0.04), rSO2 at 60-minutes post-CPB (P=0.05), minimum rSO2 for the phase off-CPB to 60 minutes post-CPB (P=0.09), and rSO2 at off-CPB (P=0.09). Additional adjustment for ICU and hospital length of stay quartiles did not alter the associations between rSO2 and PDI.
Table 4. Effects of Cerebral Oxygen Saturation (rSO2) Variables on Psychomotor Development Index of the Bayley Scales of Infant Development.
| Psychomotor Development Index (N = 89*) Linear Regression Beta Coefficient (P value) |
|||
|---|---|---|---|
| rSO2 Predictor Variable | Adjusting for Social Class | Adjusting for Age ≤ 30 Days and Social Class | Adjusting for Age ≤ 30 Days, Diagnosis Group, and Social Class |
| Integrated rSO2 ≤ 45% (min%) | |||
| 0 | 0 | 0 | 0 |
| 0.3-39 | −2.9 (0.58) | −2.0 (0.70) | −0.7 (0.90) |
| 60-383 | −2.3 (0.67) | −3.5 (0.53) | −2.1 (0.70) |
| Integrated rSO2 ≤ 45% (per min%) | −0.043 (0.15) | −0.052 (0.08) | −0.047 (0.12) |
| Postinduction − 60-min post-CPB | |||
| Average rSO2 (per %) | 0.44 (0.04) | 0.38 (0.09) | 0.23 (0.22) |
| Minimum rSO2 | 0.24 (0.13) | 0.25 (0.11) | 0.21 (0.20) |
| Onset last rewarming – off-CPB | |||
| Average rSO2 | 0.24 (0.13) | 0.12 (0.60) | 0.03 (0.91) |
| Minimum rSO2 | 0.17 (0.24) | 0.03 (0.86) | −0.01 (0.98) |
| Off-CPB – 60 min post-CPB | |||
| Average rSO2 | 0.38 (0.01) | 0.35 (0.02) | 0.32 (0.04) |
| Minimum rSO2 | 0.31 (0.02) | 0.27 (0.053) | 0.24 (0.09) |
| rSO2 10 min after cooling | 0.43 (0.03) | 0.37 (0.07) | 0.31 (0.15) |
| rSO2 Off-CPB | 0.34 (0.02) | 0.33 (0.06) | 0.29 (0.09) |
| rSO2 60 min post-CPB | 0.32 (0.02) | 0.30 (0.03) | 0.28 (0.045) |
N = 89, except N = 88 for postinduction − 60 min post-CPB, off CPB − 60 min post-CPB, and 60 min post-CPB regressions.
In regression analysis adjusting for age ≤30 days, brain hemosiderin was significantly associated with average rSO2 for the phase off-CPB to 60-minutes post-CPB (P=0.04), and rSO2 at off CPB (P = 0.01) (Table 5). In addition, there was a trend toward significance for average rSO2 from post-induction to 60 minutes post-CPB (P = 0.054), as well as minimum rSO2 from off-CPB to 60-minutes post-CPB (P=0.06). Findings were generally similar for associations between rSO2 and brain hemosiderin after adjustment for diagnosis (Table 5). Finally, because age was so highly associated with diagnosis in this small group of children, we were unable to adjust for both diagnosis and age (the logistic regression models didn't converge).
Table 5. Effects of Cerebral Oxygen Saturation (rSO2) Variables on Hemosiderin.
| Hemosiderin (N = 40*) Logistic Regression Odds Ratio (P value) |
|||
|---|---|---|---|
| rSO2 Predictor Variable | Unadjusted | Adjusting for Age ≤ 30 Days | Adjusting for Diagnosis Group |
| Integrated rSO2 ≤ 45% (min%) | |||
| 0 | 1 | 1 | 1 |
| 0.3-39 | 2.3 (0.35) | 2.5 (0.38) | 2.9 (0.31) |
| 60-383 | 2.3 (0.43) | 10.1 (0.12) | 10.2 (0.11) |
| Integrated rSO2 ≤ 45% (per min%) | 1.01 (0.15) | 1.02 (0.07) | 1.02 (0.07) |
| Postinduction – 60 min post-CPB | |||
| Average rSO2 (per %) | 0.89 (0.02) | 0.90 (0.054) | 0.89 (0.03) |
| Minimum rSO2 | 0.94 (0.08) | 0.91 (0.04) | 0.91 (0.04) |
| Onset last rewarming – off-CPB | |||
| Average rSO2 | 0.91 (0.009) | 0.93 (0.11) | 0.92 (0.047) |
| Minimum rSO2 | 0.92 (0.01) | 0.94 (0.16) | 0.93 (0.07) |
| Off-CPB − 60 min post-CPB | |||
| Average rSO2 | 0.92 (0.02) | 0.93 (0.04) | 0.92 (0.03) |
| Minimum rSO2 | 0.93 (0.03) | 0.94 (0.06) | 0.93 (0.04) |
| rSO2 10 min after cooling | 0.96 (0.25) | 0.97 (0.47) | 0.97 (0.40) |
| rSO2 Off-CPB | 0.87 (0.003) | 0.88 (0.01) | 0.88 (0.006) |
| rSO2 60 min post-CPB | 0.94 (0.08) | 0.95 (0.16) | 0.94 (0.10) |
Logistic regression models for hemosiderin did not converge when adjusting for both age ≤ 30 days and diagnosis group (TGA, TOF, VSD) due to collinearity of age and diagnosis and small cell counts.
N = 40, except N = 39 for off CPB − 60 min post-CPB and 60 min post-CPB regressions.
As only 23 subjects (26%) had a decline in rSO2 to ≤ 45%, analyses were also performed at threshold levels for rSO2 ≤ 50% (38 subjects) and ≤ 55% (58 subjects). No difference in PDI, MDI, neurologic examination, head circumference, or MRI variables was found between groups according to integrated rSO2 using these thresholds, nor was there a correlation between integrated rSO2 below these cut points and neurodevelopmental outcomes.
Discussion
We found a relationship between intraoperative cerebral oxygen saturation measured by NIRS and neurodevelopmental testing at one year of age among infants undergoing biventricular repair without aortic arch reconstruction. Lower PDI scores were associated with lower rSO2 during the 60 minute period following cardiopulmonary bypass and at the time points 10 minutes of cooling, off-CPB and 60 minutes post-CPB. No relationship could be demonstrated between rSO2 and MDI score, neurologic examination, or head circumference. The finding of hemosiderin foci on qualitative MRI analysis was associated with lower average rSO2 from post-induction to 60 minutes post-CPB and lower average and minimum rSO2 during rewarming and during the 60 minute period following cardiopulmonary bypass. The relationship of lower rSO2 with worse PDI score and brain hemosiderin on MRI suggest that periods of intraoperative and early postoperative impaired cerebral oxygen delivery may contribute to these findings.
NIRS devices measure oxygen saturation in a mixture of arterioles, capillaries, and venules in the tissue beneath the optical probe.23 Ischemia outside the optical field is not detected, and therefore NIRS reflects regional cerebral oxygenation. Cerebral oxygenation saturation thresholds associated with central nervous system injury in pediatric cardiac surgery are under investigation but yet to be clearly defined. Piglet studies show functional neurologic impairment at a threshold saturation of 33-44%,18 with the viability-time threshold for such injury to be a saturation of 35% for 2-3 hours at normothermia.19 Hagino et al. found that an average tissue oxygenation index (saturation) of <55% during 2 hours of low flow bypass at varying degrees of hypothermia in piglets was associated with both structural and functional neurologic injury.11 In neonates undergoing the Norwood procedure, prolonged low postoperative rSO2 (<45% for >180 minutes) was associated with new or worsened ischemia on early postoperative MRI.4 It is unclear whether a longer period of mild hypoxia-ischemia is more likely to cause brain injury than transient periods of worse hypoxia-ischemia. Factors that hinder the definition of threshold levels in pediatric cardiac surgery are the variability in cerebral oxygen saturation in children with CHD,24, 25 less frequent use and shorter durations of DHCA,5, 26 use of regional low flow perfusion as an alternative to circulatory arrest,5, 27 and the reduced incidence of acute clinical neurologic complications after open-heart operations in children.28, 29 Furthermore, devices currently approved for clinical use vary in measurement accuracy30 and do not measure intracellular oxygen utilization. The leftward shift of the oxyhemoglobin dissociation curve with hypothermia and alkalosis can result in normal or increased cerebral oxygen saturation values but be associated with abnormal or deficient intracellular oxygenation.31-34 In our cohort, and as reported by others, relatively high values of rSO2 were achieved during pediatric hypothermic bypass.17, 35-38 Thus, the prolonged periods of low cerebral oxygen saturation achieved in laboratory studies may not be seen in the clinical setting. Consequently, it will be difficult to define an intraoperative threshold level, particularly during hypothermia. The threshold, and hence NIRS sensitivity and specificity, is likely to vary according to specific bypass conditions. In the present study, we found no differences in hematocrit between the three NIRS groups and no relationship between randomized treatment assignment (25% vs. 35%) and decline in rSO2 to ≤ 45%.
The lack of association between our primary NIRS variable (integrated rSO2 ≤ 45%) and outcome could be due to too few subjects with sufficiently prolonged desaturation below the threshold level of 45%. The finding of a relationship between lower rSO2 in the early post-bypass phase with lower PDI and brain hemosiderin, even with adjustment for age ≤ 30 days or diagnosis, is consistent with the findings of studies demonstrating an association between early low postoperative oxygenation and adverse neurodevelopmental outcome6 and with abnormalities on MRI.4, 9 However, the cerebral rSO2 values in this study are far above values identified by clinical or experimental data to be indicative of inadequate oxygen delivery. As discussed above, capabilities of current NIRS monitors constitute a potential limitation in the study of the relationship of cerebral oxygenation to later neurologic outcomes.39 The light source in the INVOS 5100B (Somanetics, Troy, MI) is two light emitting diodes with a relatively wide bandwidth; in the FORE-SIGHT (CAS Medical Systems, Branford, CT) it is four laser diodes with a narrower bandwidth, and in the NIRO series (Hamamatsu Photonics, Hamamatsu, Japan), which is not approved for clinical use in the USA, it is three laser diodes. The INVOS and FORE-SIGHT currently display only a saturation value, while the NIRO additionally provides information on the blood concentration as well as concentration changes in oxygenated hemoglobin, deoxygenated hemoglobin, and total hemoglobin. NIRS technology continues to evolve and future development of better instruments and algorithms may include devices capable of multisite measurement of cerebral oxygenation, hemodynamics, and cellular energy status (cytochrome C oxidase).40-42 Limitations of NIRS monitors notwithstanding, the modest association between lower rSO2 after 10 minutes of cooling and lower one-year PDI score would support the previous hypotheses that cerebral oxygen availability is limited during cooling to deep hypothermia.43 Similarly, the modest relationships of lower rSO2 during the 60 minute period following cardiopulmonary bypass with worse PDI scores and more brain MRI abnormalities are consistent with the hypothesis that the early post-bypass phase is a vulnerable period for cerebral hypoxia-ischemia. Further studies are needed to determine whether NIRS-guided management in the early postoperative period can improve neurodevelopmental outcomes.
Although brain hemosiderin was associated with a lower average rSO2 from post-induction to 60 minutes post-CPB and lower average and minimum rSO2 during rewarming and during the 60 minute period following cardiopulmonary bypass, other NIRS measures were not strongly predictive of brain injury defined by MRI analysis. Periventricular leukomalacia is commonly seen in neonates after cardiac surgery but rarely detected in older infants,44 and higher flow rates associated with higher rSO2 may cause hyperperfusion injury to these deep brain structures.45 Important limitations pertain to our analyses of the relationship between NIRS levels of oxygenation and neurodevelopmental outcome and qualitative and quantitative findings on brain MRI. First, NIRS monitoring was only begun following induction of anesthesia and monitored continuously up to 1 hour post-CPB, with time-point measurements at 6 hours and 18 hours post-CPB, precluding a more comprehensive evaluation of pre- and postoperative cerebral oxygenation and outcome. Second, although 89 subjects with NIRS data returned for neurodevelopmental follow-up at one year of age, only 40 of those subjects underwent an MRI scan. This limited the power to detect significant associations between NIRS and MRI measures. Moreover, because age at surgery was closely correlated with diagnosis, it was not possible to determine whether diagnosis (and conduct of CPB) or age at surgery was the main factor associated with hemosiderin. Third, the power to detect associations may be limited since a decline in rSO2 to ≤ 45% for a duration of at least one minute occurred in only 26% of our subjects. Although paradoxically associated with a higher volume of subcortical gray matter, greater integrated rSO2 ≤ 45% was not associated with cortical gray matter, white matter, or brain volume, suggesting that the higher volume of subcortical gray matter may be an artifact of population differences in brain volume or the difficulty of accurately segmenting this small brain region. Moreover, the ability to detect tiny strokes and subtle periventricular white matter injury by MRI is diminished at one year of age. Thus, the low rates of stroke and white matter injury in our cohort may underestimate the true incidence of such lesions. Fourth, during the period of the study, not all patients with TOF were routinely tested for 22q11 microdeletions. Fifth, we did not correct for multiple comparisons as the primary NIRS variable was not significantly associated with the neurodevelopmental outcomes. Because we view analyses of the secondary NIRS variables as exploratory, we similarly did not correct for multiple comparisons. Finally, our results may not be generalizable to higher-risk populations, such as those undergoing the Norwood procedure, and to centers using other protocols for vital organ support, such as regional perfusion.9, 45
In conclusion, this study of infants undergoing biventricular repair without aortic arch reconstruction found an association between rSO2 at early cooling and during the 60 minute period following cardiopulmonary bypass with PDI score, as well as an association between lower intraoperative rSO2 with hemosiderin foci on qualitative MRI analysis. However, our study design and the strong association among potentially explanatory variables, including neonatal age and diagnosis group, do not permit us to assess which factors are causal. Future prospective multi-center studies are needed to define NIRS thresholds of injury in the clinical setting, determine the costs and benefits of an expensive technology, and evaluate NIRS monitoring and brain imaging in varying cardiac surgical populations.
Acknowledgments
We are indebted to the following individuals from Children's Hospital Boston, Boston, Massachusetts: Department of Neurology - Gene Walter for monitoring and data collection; Department of Cardiology - Ludmila Kyn for database and statistical programming; Donna M. Donati, Donna M. Duva, and Lisa-Jean Buckley for data management; and Kathleen M. Alexander, for project coordination.
Sources of Funding: Supported by grants HL 063411 and RR 02172 from the National Institutes of Health, the Farb Family Fund, and Children's Hospital Medical Center Anesthesia Foundation intramural funds.
Footnotes
Disclosures
Barry D. Kussman, MBBCh – Research support through (i) NIH (SBIR) grant to CAS Medical Systems, Inc. for validation of FORE-SIGHT® near-infrared spectroscopy cerebral oximeter in pediatric subjects >$10k; (ii) CAS Medical Systems, Inc. sponsored study for validation of FORE-SIGHT® near-infrared spectroscopy monitor for viscerosomatic applications in pediatric subjects >$10k.
Jane W. Newburger, MD, MPH – (i) Three NIH grants on long-term neurodevelopmental function in patients with transposition of the great arteries, tetralogy of Fallot, and Fontan, respectively- all grants >$10k; (ii) Honoraria for grand rounds at academic medical centers <$10k; (iii) Expert witness <$10k.
Peter C. Laussen, MBBS – Honoraria for Visiting Professor; <$10k.
David C. Bellinger, PhD – (i) NIH grant on children's chemical exposures >$10k; (ii) Honoraria for lectures, document reviews <$10k; (iii) Expert witness >$10k.
Richard Robertson, MD – NIH grant on magnetic resonance of early preterm experience >$10k.
Janet S. Soul, MD – (i) March of Dimes on cerebral visual impairment in children born prematurely >$10k; (ii) Harvard CTSC for phase 1 trial of bumetanide >$10k; (iii) Children's Hospital Boston for pilot trial of bumetanide to treat neonatal seizures >10k; (iv) Charles Hood Foundation for pilot trial of bumetanide to treat neonatal seizures >10k; Honoraria – American Academy of Neurology Annual Meetings <10k; expert witness <10k.
Richard A. Jonas, MD – NIH R01 grant >$10k
David Wypij, PhD – None
James A. DiNardo, MD – None
Frank A. Pigula, MD – None
References
- 1.Bellinger DC, Wypij D, duDuplessis AJ, Rappaport LA, Jonas RA, Wernovsky G, Newburger JW. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–1396. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Wernovsky G. Neurodevelopmental outcomes in hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:39–47. doi: 10.1053/j.pcsu.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Bellinger DC. Brain injury in congenital heart disease. Circulation. 2006;113:183–185. doi: 10.1161/CIRCULATIONAHA.105.594804. [DOI] [PubMed] [Google Scholar]
- 4.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, Pearl JM, Khoury PR, Kurth CD. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130:1523–1530. doi: 10.1016/j.jtcvs.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg CS, Bove EL, Devaney EJ, Mollen E, Schwartz E, Tindall S, Nowak C, Charpie J, Brown MB, Kulik TJ, Ohye RG. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg. 2007;133:880–887. doi: 10.1016/j.jtcvs.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman GM, Mussatto KA, Brosig CL, Ghanayem NS, Musa N, Fedderly RT, Jaquiss RD, Tweddell JS. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2005;130:1094–1100. doi: 10.1016/j.jtcvs.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 7.Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110:563–578. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 8.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–114. [PubMed] [Google Scholar]
- 9.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, Azakie A, Karl T, Miller SP. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 10.Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 11.Hagino I, Anttila V, Zurakowski D, Duebener LF, Lidov HG, Jonas RA. Tissue oxygenation index is a useful monitor of histologic and neurologic outcome after cardiopulmonary bypass in piglets. J Thorac Cardiovasc Surg. 2005;130:384–392. doi: 10.1016/j.jtcvs.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto T, Hatsuoka S, Stock UA, Duebener LF, Lidov HG, Holmes GL, Sperling JS, Munakata M, Laussen PC, Jonas RA. Prediction of safe duration of hypothermic circulatory arrest by near-infrared spectroscopy. J Thorac Cardiovasc Surg. 2001;122:339–350. doi: 10.1067/mtc.2001.115242. [DOI] [PubMed] [Google Scholar]
- 13.Austin EH, 3rd, Edmonds HL, Jr, Auden SM, Seremet V, Niznik G, Sehic A, Sowell MK, Cheppo CD, Corlett KM. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 1997;114:707–715. 717. doi: 10.1016/S0022-5223(97)70074-6. discussion 715-706. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman GM. Neurologic monitoring on cardiopulmonary bypass: what are we obligated to do? Ann Thorac Surg. 2006;81:S2373–2380. doi: 10.1016/j.athoracsur.2006.02.076. [DOI] [PubMed] [Google Scholar]
- 15.Kussman BD, Wypij D, DiNardo JA, Newburger JW, Mayer JE, Jr, del Nido PJ, Bacha EA, Pigula F, McGrath E, Laussen PC. Cerebral oximetry during infant cardiac surgery: evaluation and relationship to early postoperative outcome. Anesth Analg. 2009;108:1122–1131. doi: 10.1213/ane.0b013e318199dcd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newburger JW, Jonas RA, Soul J, Kussman BD, Bellinger DC, Laussen PC, Robertson R, Mayer JE, Jr, del Nido PJ, Bacha EA, Forbess JM, Pigula F, Roth SJ, Visconti KJ, du Plessis AJ, Farrell DM, McGrath E, Rappaport LA, Wypij D. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135:347–354. e341–344. doi: 10.1016/j.jtcvs.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 17.Kussman BD, Wypij D, DiNardo JA, Newburger J, Jonas RA, Bartlett J, McGrath E, Laussen PC. An evaluation of bilateral monitoring of cerebral oxygen saturation during pediatric cardiac surgery. Anesth Analg. 2005;101:1294–1300. doi: 10.1213/01.ANE.0000180205.85490.85. [DOI] [PubMed] [Google Scholar]
- 18.Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood Flow Metab. 2002;22:335–341. doi: 10.1097/00004647-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Kurth CD, McCann JC, Wu J, Miles L, Loepke AW. Cerebral oxygen saturation-time threshold for hypoxic-ischemic injury in piglets. Anesth Analg. 2009;108:1268–1277. doi: 10.1213/ane.0b013e318196ac8e. [DOI] [PubMed] [Google Scholar]
- 20.Bayley N. Bayley scales of infant development. 2nd. San Antonio, Texas: The Psychological Corporation; 1993. [Google Scholar]
- 21.Soul JS, Robertson RL, Wypij D, Bellinger DC, Visconti KJ, du Plessis AJ, Kussman BD, Scoppettuolo LA, Pigula F, Jonas RA, Newburger JW. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374–381. doi: 10.1016/j.jtcvs.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 23.Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 24.Fenton KN, Freeman K, Glogowski K, Fogg S, Duncan KF. The significance of baseline cerebral oxygen saturation in children undergoing congenital heart surgery. Am J Surg. 2005;190:260–263. doi: 10.1016/j.amjsurg.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Kurth CD, Steven JL, Montenegro LM, Watzman HM, Gaynor JW, Spray TL, Nicolson SC. Cerebral oxygen saturation before congenital heart surgery. Ann Thorac Surg. 2001;72:187–192. doi: 10.1016/s0003-4975(01)02632-7. [DOI] [PubMed] [Google Scholar]
- 26.Hanley FL. Religion, politics…deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2005;130:1236. doi: 10.1016/j.jtcvs.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 27.Ohye RG, Goldberg CS, Donohue J, Hirsch JC, Gaies M, Jacobs ML, Gurney JG. The quest to optimize neurodevelopmental outcomes in neonatal arch reconstruction: the perfusion techniques we use and why we believe in them. J Thorac Cardiovasc Surg. 2009;137:803–806. doi: 10.1016/j.jtcvs.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 28.Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002;73:1752–1758. doi: 10.1016/s0003-4975(02)03534-8. [DOI] [PubMed] [Google Scholar]
- 29.Trittenwein G, Nardi A, Pansi H, Golej J, Burda G, Hermon M, Boigner H, Wollenek G. Early postoperative prediction of cerebral damage after pediatric cardiac surgery. Ann Thorac Surg. 2003;76:576–580. doi: 10.1016/s0003-4975(03)00468-5. [DOI] [PubMed] [Google Scholar]
- 30.Thavasothy M, Broadhead M, Elwell C, Peters M, Smith M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia. 2002;57:999–1006. doi: 10.1046/j.1365-2044.2002.02826.x. [DOI] [PubMed] [Google Scholar]
- 31.Dexter F, Hindman BJ. Theoretical analysis of cerebral venous blood hemoglobin oxygen saturation as an index of cerebral oxygenation during hypothermic cardiopulmonary bypass. A counterproposal to the “luxury perfusion” hypothesis. Anesthesiology. 1995;83:405–412. doi: 10.1097/00000542-199508000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Dexter F, Kern FH, Hindman BJ, Greeley WJ. The brain uses mostly dissolved oxygen during profoundly hypothermic cardiopulmonary bypass. Ann Thorac Surg. 1997;63:1725–1729. doi: 10.1016/s0003-4975(97)00297-x. [DOI] [PubMed] [Google Scholar]
- 33.du Plessis AJ, Newburger J, Jonas RA, Hickey P, Naruse H, Tsuji M, Walsh A, Walter G, Wypij D, Volpe JJ. Cerebral oxygen supply and utilization during infant cardiac surgery. Ann Neurol. 1995;37:488–497. doi: 10.1002/ana.410370411. [DOI] [PubMed] [Google Scholar]
- 34.Greeley WJ, Kern FH, Meliones JN, Ungerleider RM. Effect of deep hypothermia and circulatory arrest on cerebral blood flow and metabolism. Ann Thorac Surg. 1993;56:1464–1466. doi: 10.1016/0003-4975(93)90731-v. [DOI] [PubMed] [Google Scholar]
- 35.Andropoulos DB, Stayer SA, McKenzie ED, Fraser CD., Jr Regional low-flow perfusion provides comparable blood flow and oxygenation to both cerebral hemispheres during neonatal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2003;126:1712–1717. doi: 10.1016/s0022-5223(03)01027-4. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman GM, Stuth EA, Jaquiss RD, Vanderwal PL, Staudt SR, Troshynski TJ, Ghanayem NS, Tweddell JS. Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg. 2004;127:223–233. doi: 10.1016/j.jtcvs.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Kurth CD, Steven JM, Nicolson SC. Cerebral oxygenation during pediatric cardiac surgery using deep hypothermic circulatory arrest. Anesthesiology. 1995;82:74–82. doi: 10.1097/00000542-199501000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Kussman BD, Gauvreau K, DiNardo JA, Newburger JW, Mackie AS, Booth KL, del Nido PJ, Roth SJ, Laussen PC. Cerebral perfusion and oxygenation after the Norwood procedure: comparison of right ventricle-pulmonary artery conduit with modified Blalock-Taussig shunt. J Thorac Cardiovasc Surg. 2007;133:648–655. doi: 10.1016/j.jtcvs.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 39.Smith M, Elwell C. Near-infrared spectroscopy: shedding light on the injured brain. Anesth Analg. 2009;108:1055–1057. doi: 10.1213/ane.0b013e31819a0301. [DOI] [PubMed] [Google Scholar]
- 40.Elwell CE, Cope M, Edwards AD, Wyatt JS, Delpy DT, Reynolds EO. Quantification of adult cerebral hemodynamics by near-infrared spectroscopy. J Appl Physiol. 1994;77:2753–2760. doi: 10.1152/jappl.1994.77.6.2753. [DOI] [PubMed] [Google Scholar]
- 41.Tachtsidis I, Tisdall MM, Leung TS, Pritchard C, Cooper CE, Smith M, Elwell CE. Relationship between brain tissue haemodynamics, oxygenation and metabolism in the healthy human adult brain during hyperoxia and hypercapnea. Adv Exp Med Biol. 2009;645:315–320. doi: 10.1007/978-0-387-85998-9_47. [DOI] [PubMed] [Google Scholar]
- 42.Tisdall MM, Tachtsidis I, Leung TS, Elwell CE, Smith M. Near-infrared spectroscopic quantification of changes in the concentration of oxidized cytochrome c oxidase in the healthy human brain during hypoxemia. J Biomed Opt. 2007;12:024002. doi: 10.1117/1.2718541. [DOI] [PubMed] [Google Scholar]
- 43.Jonas RA, Bellinger DC, Rappaport LA, Wernovsky G, Hickey PR, Farrell DM, Newburger JW. Relation of pH strategy and developmental outcome after hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1993;106:362–368. [PubMed] [Google Scholar]
- 44.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 45.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, Heinle JS, Graves DE, Fraser CD., Jr Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]