Abstract
Purpose
Recent developments in non-linear optical (NLO) imaging using femtosecond lasers provides a non-invasive method for detecting collagen fibers by imaging second harmonic generated (SHG) signals. However, this technique is limited by the small field of view (FoV) necessary to generate SHG signals. The purpose of this report is to review our efforts to greatly extend the FoV in order to assess the entire collagen structure using high resolution macroscopic (HRMac) imaging.
Methods
Intact human eyes were fixed under pressure and the whole cornea (13 mm diameter) excised and embedded in low melting point agar for vibratome sectioning (200–300 μm). Sections were then optically scanned using a Zeiss LSM 510 Meta and Chameleon femtosecond laser to generate SHG images. For each vibratome section, an overlapping series of 3-D data sets (466 × 466 × 150 μm) were taken covering the entire tissue (15 mm × 6 mm area) using a motorized, mechanical stage. The 3-D data sets were then concatenated to generate an NLO based tomograph.
Results
HRMac of the cornea yielded large macroscopic (80 Meg Pixels per plane), 3-dimensional tomographs with high resolution (0.81 μm later, 2.0 μm axial) in which individual collagen fibers (stromal lamellae) could be traced, segmented and extracted. 3-D reconstructions suggested that the anterior cornea is comprised of highly intertwined lamellae that insert into the anterior limiting lamina (Bowman’s Layer).
Conclusion
We conclude that HRMac using NLO based tomography provides a powerful new tool to assess collagen structural organization within the cornea.
Keywords: Cornea, optic nerve head, collagen, cornea, femtosecond laser, second harmonic signals
INTRODUCTION
The corneal stroma occupies approximately 90% of the corneal thickness in the human and is comprised predominantly of collagen. Corneal collagen uniquely forms small (32 nm), uniformly spaced fibrils that are organized into larger bundles or fibers termed ‘corneal lamellae’ that vary in size from 1–2 μm thick to 5–100s μm wide and are arranged in interweaving orthogonal layers throughout the stroma.1 The uniform size and spacing of the collagen fibrils is thought to provide for the transparent properties of the cornea,2 while the overall organizational pattern of the collagen fibers are thought to define the structural integrity and shape of the cornea that is required for refracting and focusing light back to the lens and retina. Since over 60% of the diopteric power of the eye is derived from the cornea, understanding how collagen fiber organization within the stroma defines shape and curvature is important to unlocking the biomechanical basis of refractive errors, the effects of surgery and the development of corneal ectasia.
For the most part, our understanding of collagen organization is derived from X-ray scattering studies that provide insight into the bulk orientation of collagen over the intact cornea. 3–5 These studies suggest that there is a superior-inferior and nasal temporal orientation with possible extension of “anchoring” lamellae from the limbus into the cornea along these meridians. 6, 7 While X-ray scattering has provided important clues into the organizational pattern, a more precise ‘blue print’ of collagen fiber structure is needed to develop more accurate biomechanical models.
Recently, ultrafast, femtosecond lasers that enable the focusing of very high intensity coherent light within small optical volumes using power levels well below the tissue destruction threshold has allowed for the generation of non-linear optical signals within biologic tissues including, two photon excited fluorescence (TPEF) and second harmonic generated signals (SHG).8 SHG has particular relevance to studying the structure of the cornea, since collagen fibrils generate strong SHG signals that can be imaged using optical microscopic techniques.
SHG imaging exploits a nonlinear optical property by which two longer-wavelength photons interact near simultaneously with non-centrosymmetric materials to generate a single photon that has exactly half the wavelength and twice the energy of the two initial photons. 9 This process is absorption-free, does not require fixation or sectioning of the tissue and does not use stains or dyes to visualize the structures. Hochheimer in 1982 was the first to show that SHG signals could be generated from the rabbit cornea,10 and more recent studies have shown that imaging of SHG signals can be used to establish collagen fiber orientation, as well as study the 3-dimensional collagen organization 11, 12.
Recently, SHG imaging has been used to study the organization of collagen in the normal human and Keratoconus cornea 13, 14. These studies have shown that the human cornea contains a population of collagen lamellae that insert into the anterior limiting lamina (ALL, Bowmans’ Layer) and run transversely deeper into the corneal stroma at approximately 20° normal to the corneal surface (Figure 1, A–D). This transverse orientation is different to that of the conventional orthogonal-interwoven orientation of collagen lamellae, and bare similarity to the ‘sutural’ fibers present in the shark cornea;15 hence these structures have been termed ‘sutural’ collagen fibers. Interestingly, studies of keratoconus corneas show that regions of corneal thinning and ectasia apparently lack these ‘sutural’ collagen fibers (Figure 1, E), suggesting that they lend important structural support to the cornea that prevents ectasia.14 Based upon these findings, we have proposed that a better understanding of the organization, density and distribution of ‘sutural’ lamellae may provide important insights into their role in defining corneal biomechanics and corneal shape.
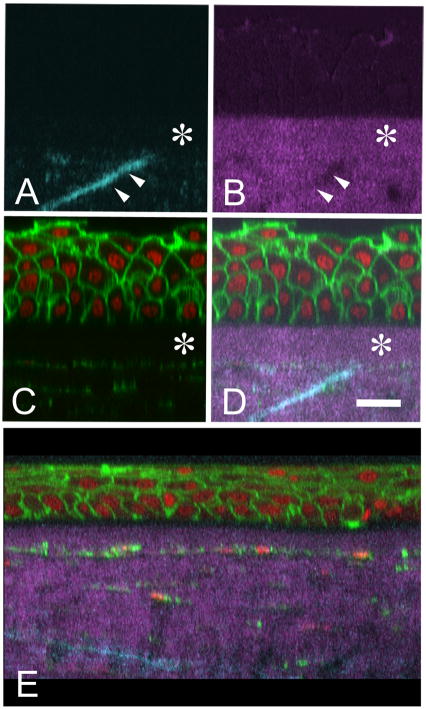
Figure 1.
Reconstructed cross-sectional images of normal human cornea showing forward (A. cyan), backward (B, magenta) SHG signal co-localized with Phalloidin to detect actin and Syto-59 to detect nuclei (C, Green and Red respectively). Co-localized image (D) shows that SHG imaging can detect the anterior limiting lamina (Bowman’s layer, asterisk) and the insertion of ‘sutural’ collagen fibers into the anterior limiting lamina (double arrows). Cross-sectional reconstruction of a keratoconus cornea (E) shows the absence of ‘sutural’ collagen fibers. Bar = 20 μm (Taken from Figure 4, J Cataract Ref Surg 32:1784, 2006.)
In order obtain data on the density and distribution of ‘sutural’ lamellae, a larger view of the collagen fiber organization is required. However, because the emission of SHG light is a function of the square root of the excitation power, second harmonic photons are only generated in the focal volume of the laser beam where the laser intensity is high enough to cause non-linear processes. As a result, sub-micron resolution can be achieved laterally, with an axial resolution on the order of approximately 1μm or 1 femtoliter volume. While this makes resolving the microstructure of collagenous tissue possible, the inverse relationship between magnification and field of view means that SHG images of any given tissue only cover a very small area and even the relatively small structures that comprise the human eye are much larger in comparison to the field of view.
To address this issue, we have evaluated a novel technique called High Resolution Macroscopy (HRMac) that combines SHG imaging with automated image acquisition and digital image reconstruction.16 HRMac is theoretically capable of generating large-scale, three-dimensional image mosaics several millimeters or even centimeters across while retaining the extremely high resolution obtained through NLO microscopy. Such an approach enables the study of both micro- and macrostructure simultaneously by allowing the traversal of different image scales to ‘zoom’ in and out of the sample and follow single collagen fibers. The resulting datasets can be further processed for quantitative analysis or for three-dimensional visualization and reconstruction of macroscopic portions of the tissue at microscopic resolutions.
MATERIALS AND METHODS
Human Corneas
Autopsy eyes were obtained from the San Diego Eye Bank following IRB approval at the University of California, Irvine and in accordance with the tenets of the Declaration of Helsinki. Globes were inspected to qualitatively assess corneal clarity to ensure the absence of any major visible defects or abnormalities. Eyes were then perfused and fixed under 20 mmHg pressure using 4% paraformaldehyde in phosphate buffered saline (PBS, pH: 7.2).
Corneas were then removed along with 1–2 mm of sclera and the Superior cornea marked by a cut at the anatomic 12 o’clock position to preserve orientation. The cornea was then bisected along the superior-inferior meridian, approximately 1mm off-center, and the tissue embedded vertically in low melting point agarose (Figure 2, A and B). Multiple consecutive slices, each approximately 300 μm thick, were then cut using a vibratome and stored in PBS.
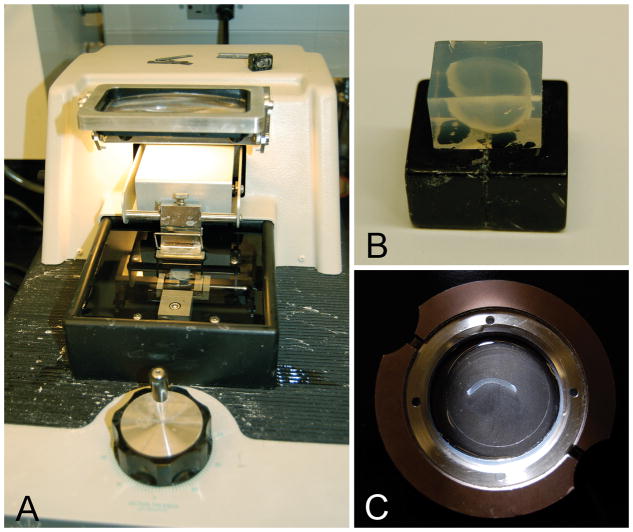
Figure 2.
Vibratome sectioning of the human cornea. (A) Vibratome with agarose block contain human cornea sample. (B) Agarose embedded human cornea. (C) Vibratome section of human cornea extending along the superior to inferior meridian.
Imaging
Vibratome sections were then mounted (Figure 2, C) and placed under a Zeiss 510 LSM and illuminated with 150 fs laser pulses generated by a Chameleon Titanium Sapphire laser (Coherent, Santa Clara, CA) tuned to 800 nm. The resulting 400 nm second harmonic light was collected in both forward and backward directions by the LSM’s detector array in two separate channels.
Image stacks of the cornea were acquired using a 20×/0.75 NA Zeiss Apochromat objective at 2 μm intervals to a depth of approximately 160 μm. Each image stack had a voxel resolution of 0.81 by 0.81 by 2 μm at 512 by 512 by 81 voxels (xyz). The entire slice covering the superior to the inferior limbus (13 mm by 6 mm) was imaged in multiple consecutive stacks using the Zeiss’ MultiTime macro function. The total number of stacks varied with corneal size and curvature and was typically around 200–300 individual stacks. Scanning a complete segment took approximately 24 hours.
To generate large-scale mosaics, image stacks were automatically concatenated into a single image stack after the scan and saved in Zeiss’ native .LSM format. The concatenation process takes adjacent image stacks and arranges them according to their position on the grid, using image registration algorithms to correct for stage and sample drift. (Figure 3).
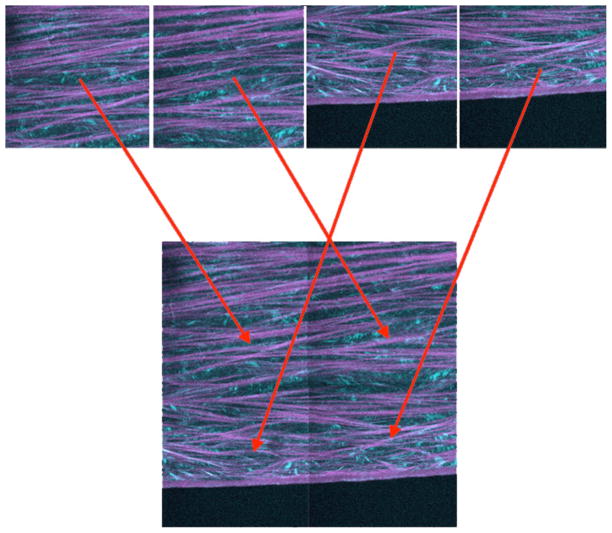
Figure 3.
Single-plane image concatenation of a 2×2 mosaic. Each picture represents a two-channel SHG image of human cornea (teal - forward scatter, purple - backscatter SHG). The image concatenation algorithm combines image stacks sequentially on a per-plane basis by using microscope stage coordinates to determine their position within the mosaic. An image registration algorithm attempts to correct for minor misalignments due to sample movement. Between 250 and 400 blocks were acquired to map the corneal cross-sections. Taken from Winkler et al, High resolution macroscopy (HRMac) of the eye using non-linear optical imaging. Proceeding of SPIE, Vol 7589: 758906.
Image Processing
The SHG signals were separated into two individual channels and saved as individual stacks of .tiff files using the LSM toolbox plugin for ImageJ version 1.39 (National Institute of Health, http://rsb.info.nih.gov/ij/) running on a PC with dual Intel Xeon Quad Core CPUs clocked at 2.5 GHz per core with 16 GB of RAM running a 64 bit version of Windows XP Professional SP2. Stacks were then processed to reduce noise by applying a median filter with a 2px radius, and contrast and brightness were adjusted to achieve a maximum signal-to-noise ratio.
Three-dimensional reconstruction
To identify the lamellar structure in 3D, reconstructions were created using Amira 5.2 (Visage Imaging, Carlsbad, CA). The exported stack of .tiff images from the backscatter channel were imported into Amira and individual collagen fibers/lamellae were sectioned out by applying the “label field” module to the data using the paint brush tool to segment out and highlight specific fibers. To ensure continual highlighting of the same lamella, the segmentation process started in the middle of the stack and moved outwards. This method allowed for the identification of branching of collagen fibers in 3D.
RESULTS
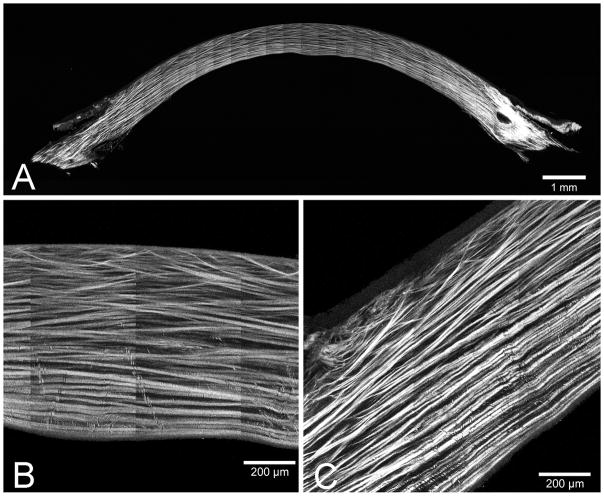
Figure 4A shows a down-sized image representing an 80 Meg Pixel image plane from an HRMac data set containing 81 separate planes covering 16500 × 6500 pixels (13.4 mm × 5.3 mm) and a file size of 64 Giga bytes. At this scale, the entire slice is visible, yet the details remain obscured. This is comparable to a standard low-power, stereo dissecting microscope image. The subsequent images show the slice at full resolution in the center of the cornea and at the limbus (Figure 4, B and C, respectively). The distinct collagen fiber/lamellar patterns are visible at this magnification. By scanning through different image planes, it is possible to follow any number of collagen fibers as the fibers traverse through the corneal stroma and to quantify various parameters.
Figure 4.
HRMac reconstruction of a human cornea cross-section. (A) Single 80 Meg pixel plane taken showing collagen fiber organization from superior to inferior limbus. (B) Zoomed view of the central cornea from the 80 Meg pixel plane showing insertion of anterior collagen fibers into the ALL (Bowman’s layer). (C) Zoomed view of the 80 Meg pixel plane showing the collagen fiber organization at the limbus.
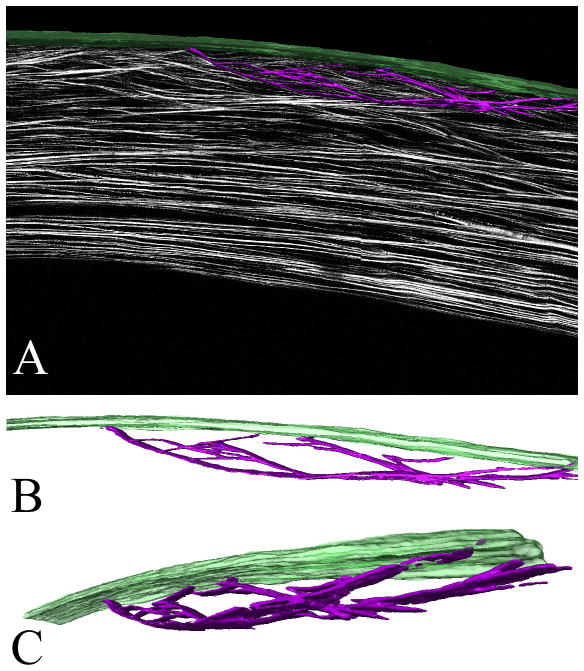
Depending on the size of the cross-section, HRMac images of the cornea are between 50 and 80 megapixels per slice. Current 3-D stacks consist of 25–80 slices each. Depending on the application these images can either be viewed in full or downsampled resolution to reduce file size. Due to the high resolution and the specificity of the SHG signal from collagen the lamellae can be semiautomatically separated from the background. Using Amira software, the lamellae can be reconstructed in 3-D and overlaid with the original images or viewed separately at different orientations (Figure 5, A). As seen in the rotation of the 3-D renderings (Figure 5, B and C), ‘sutural’ collagen fibers that insert into the ALL (Green), appear to branch many times along their length, and combine with other ‘sutural’ collagen fibers to return to the ALL, giving a ‘leaf spring’ or ‘bow’ appearance that may provide mechanical strength to the anterior cornea.
Figure 5.
3-D reconstructed sutural lamellae in the human cornea. (A) 1 mm central corneal region from a HRMac corneal cross-section after segmentation in Amira. Bowman’s layer is rendered in green, the surtural lamellae are purple. (B) and (C) show the extracted sutural lamellae rendering in two different rotations. These images can be panned, zoomed and rotated at will. Taken from Winkler et al, High resolution macroscopy (HRMac) of the eye using non-linear optical imaging. Proceeding of SPIE, Vol 7589: 758906.
DISCUSSION
We have demonstrated that HRMac represents a novel approach to imaging tissues in 3-D on a macroscopic scale with sub-micron resolution and that this imaging paradigm can be used to characterize the structural organization of collagen fibers throughout the cornea or other tissues. For the cornea, the insertion of collagen fibers into the ALL has been previously detected using transmission electron microscopy by Komai et al.1 and others.17, 18 However, these previous studies have used transmission electron microscopy, which have a very limited FoV and hence were not able to trace the extension of collagen fibers from the ALL deeper into the corneal stroma. Using HRMac, collagen fibers that inserted into the ALL showed extensive branching, or intertwining, with other collagen fibers suggesting the collagen fibrils contribute to multiple collagen fibers or lamellae as they traverse through the cornea. Another findings was that intertwining of collagen fibers leads to multiple attachments to the ALL as they branch and divide in the anterior stroma. As discussed by Bron, the anterior stromal collagen is known to have extensive anteroposterior interweaving, but it is thought to only occasionally insert into to the ALL and contribute to the ‘anterior corneal mosaic’.19 Our observations suggest that insertion into the ALL is much more frequent, and that with collagen intertwining, the anterior region of the cornea may have markedly different mechanical properties. In this respect, studies by Muller et al evaluating swollen human corneas has shown that the anterior 100–120 μm of stroma is resistant to swelling and maintains the corneal curvature.20 While it has been proposed that the interwoven nature of the anterior cornea is responsible for this mechanical property, this region also corresponds to the region containing ‘sutural’ collagen fibers that extends to a depth of approximately 130 μm. Therefore, it is likely that the presence of ‘sutural’ collagen fibers that insert into the ALL and exhibit extensive fiber intertwining may explain these observations.
With regard to HRMac, this imaging paradigm combines the high resolution of NLO imaging with automated image acquisition techniques to generate continuous macro-scale 3-D datasets with microscopic resolution. Additionally, detailed investigations of the whole tissue are possible at the micron scale in order to build-up a ‘blueprint’ of collagen organization and its relationship to the macroscopic corneal shape which can extend for many millimeters. Past studies have evaluated small, limited regions using electron microscopy, 17, 18 while others have scanned the entire cornea using x-ray diffraction.4, 5 Both approaches, however, require discontinuous sampling, resulting in loss of information between data. Furthermore, it has yet to be shown that this disconnection between micro- and macrostructure using high-resolution scans of small regions and discontinuous sampling can provide the information required in order understand how collagen organization in the cornea controls corneal shape. HRMac, which allows for continuous sampling of the corneal collagen at the micron level could perhaps bridge this gap, and may constitute the next step towards a better understanding of the lamellar organization and how it relates to corneal biomechanics.
A current weakness in the application of HRMac is the inability of laser light to penetrate deeply into certain tissues. For the cornea, the high transparency in the infrared region makes it possible to scan approximately 200 microns deep into the section. However, other tissues that are more opaque and absorb more light would have a more limited scan depth. There are two approaches to overcoming this problem, first is to mechanically separate the tissue into thinner slices that would allow for imaging far beyond the laser penetration depth. This can be achieved by using vibratome sections as for the cornea, or semi-thin plastic sections and array tomography as we have done for the optic nerve head.21 Another promising approach would be to use higher numerical aperture objectives with longer working distances. These objectives are becoming available, although with higher numerical aperture comes reduced field of view, which would extend the time required to scan the entire tissue.
Acknowledgments
Support: NEI Grants EY07348, EY016663, EY018665, Discovery Eye Foundation and Research to Prevent Blindness, Inc.
Footnotes
This work has been previously presented at the SPIE Phontics West meeting in San Franciscio, CA, USA, January 24-26, 2010, and the International Symposium in Yamaguchi, Japan, March 20-21, 2010.
References
- 1.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991 Jul;32(8):2244–2258. [PubMed] [Google Scholar]
- 2.Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meek KM, Boote C. The organization of collagen in the corneal stroma. Exp Eye Res. 2004 Mar;78(3):503–512. doi: 10.1016/j.exer.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Meek KM, Quantock AJ. The use of X-ray scattering techniques to determine corneal ultrastructure. Prog Retin Eye Res. 2001 Jan;20(1):95–137. doi: 10.1016/s1350-9462(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 5.Meek KM, Tuft SJ, Huang Y, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005 Jun;46(6):1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- 6.Aghamohammadzadeh H, Newton RH, Meek KM. X-ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure. 2004 Feb;12(2):249–256. doi: 10.1016/j.str.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Boote C, Dennis S, Huang Y, Quantock AJ, Meek KM. Lamellar orientation in human cornea in relation to mechanical properties. J Struct Biol. 2005 Jan;149(1):1–6. doi: 10.1016/j.jsb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11014–11019. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franken P, Hill A, Peters C. generation of optical harmonics. Physical Rev Lett. 1961;7:118–119. [Google Scholar]
- 10.Hochheimer BF. Second harmonic light generation in the rabbit cornea. Appl Opt. 1982:1516–1518. doi: 10.1364/AO.21.001516. [DOI] [PubMed] [Google Scholar]
- 11.Stoller P, Kim BM, Rubenchik AM, Reiser KM, Da Silva LB. Polarization-dependent optical second-harmonic imaging of a rat-tail tendon. J Biomed Opt. 2002 Apr;7(2):205–214. doi: 10.1117/1.1431967. [DOI] [PubMed] [Google Scholar]
- 12.Yeh AT, Nassif N, Zoumi A, Tromberg BJ. Selective corneal imaging using combined second-harmonic generation and two-photon excited fluorescence. Opt Lett. 2002 Dec 2;27(23):2082–2084. doi: 10.1364/ol.27.002082. [DOI] [PubMed] [Google Scholar]
- 13.Morishige N, Petroll WM, Nishida T, Kenney MC, Jester JV. Noninvasive corneal stromal collagen imaging using two-photon-generated second-harmonic signals. J Cataract Refract Surg. 2006 Nov;32(11):1784–1791. doi: 10.1016/j.jcrs.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morishige N, Wahlert AJ, Kenney MC, et al. Second-harmonic imaging microscopy of normal human and keratoconus cornea. Invest Ophthalmol Vis Sci. 2007 Mar;48(3):1087–1094. doi: 10.1167/iovs.06-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman JN, Benedek GB. The relationship between morphology and transparency in the nonswelling corneal stroma of the shark. Invest Ophthalmol Vis Sci. 1967;6:574–600. [PubMed] [Google Scholar]
- 16.Winkler M, Jester BE, Nien-Shy C, Chai D, Brown DJ, Jester JV. High resolution macroscopy (HRMac) of the eye using non-linear optical imaging. Proceeding of SPIE. 2010;7589:758906, 758901–758907. [Google Scholar]
- 17.Radner W, Mallinger R. Interlacing of collagen lamellae in the midstroma of the human cornea. Cornea. 2002 Aug;21(6):598–601. doi: 10.1097/00003226-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Radner W, Zehetmayer M, Aufreiter R, Mallinger R. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea. 1998 Sep;17(5):537–543. doi: 10.1097/00003226-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Bron AJ. The architecture of the corneal stroma. Br J Ophthalmol. 2001 Apr;85(4):379–381. doi: 10.1136/bjo.85.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001 Apr;85(4):437–443. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler M, Jester B, Nien-Shy C, et al. High resolution three-dimensional reconstruction of the collagenous matrix of the human optic nerve head. Brain Res Bull. Feb 15;81(2–3):339–348. doi: 10.1016/j.brainresbull.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]