Abstract
Obesity (Ob) and type 1 diabetes (T1DM) are associated with increased inflammation and oxidative stress, which are major pathogenetic pathways toward higher cardiovascular risks. While long-term exercise protects against systemic inflammation and oxidation, acute exercise actually exerts pro-inflammatory and oxidative effects, prompting the necessity for better defining these molecular processes in at-risk patients; in particular, very little is known regarding obese and T1DM children. We therefore examined key inflammatory and oxidative stress variables during exercise in 138 peripubertal children (47 Ob, 12.7±0.4 yr, 22F, BMI% 97.6±0.2; 49 T1DM, 13.9±0.2 yr, 20F, BMI% 63.0±3.6; 42 healthy, CL, 13.5±0.5 yr, 24F, BMI% 57.0±3.6), who performed 10 bouts of 2-min cycling ~80% VO2max, separated by 1-min rest intervals. Blood samples were drawn at baseline and peak-exercise. Ob displayed elevated baseline interleukin-6 (IL-6, 2.1±0.2 pg/mL, p<0.005) vs. CL (1.5±0.3), while T1DM displayed the greatest maximum exercise-induced change in IL-6 (1.2±0.3) than in both Ob (0.7±0.1, p< 0.001) and CL (0.6±0.1, p<0.0167). Myeloperoxidase (MPO) was elevated in T1DM (143±30 ng/mL, p<0.0167) vs. CL (89±10) and Ob (76±6), while increases in exercise only occurred in Ob and CL. Disparate baseline and exercise responses were also observed for 8-hydroxy-2′-deoxyguanosine, glutathione, and F2-isoprostane. This data show distinct patterns of dysregulation in baseline and adaptive immunologic and oxidative responses to exercise in Ob and T1DM. A full understanding of these alterations is required so that developing exercise regimens aimed at maximizing health benefits for specific dysmetabolic states can be achieved based on complete scientific characterization rather than empirical implementation.
Keywords: interleukin-6, myeloperoxidase, isoprostane, 8-hydroxy-deoxyguanosine, children
INTRODUCTION
Obesity (Ob) and type 1 diabetes mellitus (T1DM) are the two most prevalent pediatric dysmetabolic states in developed countries (1;2), associated with high incidence of long-term cardiovascular complications and mortality (3) Surprisingly, the precise biochemical steps linking obesity and T1DM to onset and progression of cardiovascular disease (CVD) remain relatively undetermined. Growing evidence, however, implicates the increased systemic inflammation and oxidative stress (4) caused by metabolic alterations such as high insulin, glucose, cortisol, lipoproteins. Altered leukocyte (WBC) function appears to play a central role, as activated WBC release pro-inflammatory cytokines (IL-1β, IL-6, TNFα, affecting platelet aggregation and the clotting cascade) (5;6) and oxidative enzymes (MPO, NADPH-oxidase, increasing free radicals harmful to the endothelium, DNA, lipids, and the sub-endothelial vascular tissues) (7;8). The molecular components of inflammatory and oxidative processes, which are numerous, complex and variably interconnected, likely display distinct alteration patterns in different dysmetabolic states. These patterns however remain unknown, especially in children and adolescents. In Ob and T1DM children, early disease onset means increased vulnerability to disease complications; age-specific characterization of the underlying mechanisms of CVD progression is therefore imperative in these subjects, whose physiologic, immunologic, and metabolic adaptation differ considerably from adults (9;10).
Physical activity can effectively prevent CVD in both Ob and T1DM (11). While the molecular mechanisms underlying this effect are sill unclear, modulation of immunologic, inflammatory, and oxidative molecules is likely involved. Weeks to months of exercise training can reduce plasma C-reactive protein, IL-6 (12), F2-isoprostane (F2-isoP, regarded as the most accurate marker of systemic lipid peroxidation) (13), improve lipid and glycemic profiles (14), and endogenous antioxidants (15). These long-term effects occur despite opposite, acute effects of exercise, during which pro-inflammatory (16;17) and oxidative markers (18) transiently increase, and antioxidants are depleted (18). This paradoxical dichotomy of acute and chronic exercise effects underscores the complexity of these processes, in which fine synchronization of diverse molecular interactions is responsible for cardio-protective effects. If such a delicate equilibrium is altered, as may occur in dysmetabolic states, the overall health benefits of exercise may be reduced. Also of importance is intensity and duration of exercise, as moderate intensity is less likely to illicit an inflammatory response compared to an intense exercise challenge (19). To date, in pediatric Ob and T1DM, adaptive immuno-modulatory and oxidative responses to exercise are largely unknown.
We therefore designed this study to identify condition-specific alterations of inflammatory and oxidative mechanisms during exercise in 47 Ob and 49 T1DM children, as compared to 42 healthy, age-matched controls. Variables highly responsive to exercise were measured (IL-6, neutrophil counts, MPO) as well as nitrotyrosine (biomarker of nitric oxide oxidation); 8-hydroxy-2′-deoxyguanosine (8-OHdG) (by-product of DNA oxidation); and glutathione (GSH-420) (a major endogenous antioxidant).
METHODS
Subjects and Preliminary Visit
The study was approved by the UCI Institutional Review Board. Prior to enrollment, subjects and guardians were informed of all procedures and risks and signed consent and assent forms. In T1DM children, duration of diabetes was 5.2±0.6 years. Exclusion criteria included presence of acute or chronic illness (except diabetes in T1DM), known dysmetabolic or immunologic conditions (except obesity in Ob), recent physical injury or disability, medications (other than insulin in T1DM), and abnormal vital signs. To ensure balanced group composition, all participants completed a standard questionnaire assessing pubertal developmental (20).
Children with gender- and age-adjusted BMI (body mass index) ≥95th percentile, but otherwise healthy, were recruited as Ob (as per definition by the Centers for Disease Control and Prevention) (21); the control group (CL) included age-matched healthy children BMI 5th - 85th percentile. Skinfold thickness (using Slaughter et al equations for age<16 yr and Siri equation for ≥16 yr) (22) were performed to confirm all Ob children were presenting with excess % body fat rather than heavy skeletal muscles, bone, or visceral tissue.
All subjects, completed an incremental cycling test (Ergoline 800S, SensorMedics, Yorba Linda, CA): the workload was increased by 10-20 watts/min (~10% of predicted maximal work-rate/min based on age, gender, and body size) (23) until maximum exercise tolerance. Breath-by-breath gas exchange was collected via a standard metabolic cart (SensorMedics, Yorba Linda, CA) to calculate anaerobic threshold (AT) and maximal aerobic capacity (VO2max, the gold standard for cardiovascular fitness in both adults and children).
Main Exercise Study
At least 48 h after the preliminary visit, subjects arrived at the UCI ICTS ~7:30 am. Vital signs were recorded, and intravenous catheters were started on the median cubital veins of both forearms for blood draws and infusions of saline, insulin, and glucose (for T1DM). Euglycemia (4.4-6.1 mM) was verified for all subjects using a Beckman Glucose Analyzer II (Beckman Coulter, Fullerton, CA), and maintained for 90 min. In T1DM subjects not euglycemic at this time, euglycemia was first restored via i.v. insulin (9.0 nmol/hr insulin for every 5.6 mM glycemia above 8.3 mM), an then maintained for 90 min with an insulin infusion rate of ~6.0 nmol/hr or 110% of insulin pump basal rate was set to mimic physiologic conditions. I.V. dextrose was infused as needed to prevent plasma glucose concentration from falling below 4.4 mM.
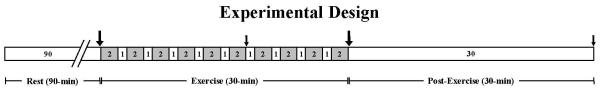
After 90 min of euglycemia, in all subjects baseline blood samples were drawn. Thereafter, subjects began pedaling on a stationary cycle ergometer at a work-rate between their AT and VO2max (~80% VO2max) for 2 min and then rested for 1 min; this was repeated 10 times (Fig. 1). This intermittent, intense cycling paradigm was chosen as it has previously been demonstrated to elevate inflammatory, immunologic, and metabolic responses to exercise in children (24;25), while simulating a real-life pattern of physical activity. Additional blood draws were taken during, peak-exericse, and 30 min after exercise (Fig. 1).
Fig. 1. Experimental design.
All groups rested for 90 min in euglycemic range (T1DM required insulin/glucose infusions to achieve euglycemia prior to the 90 min period and euglycemic clamp throughout the remainder of the study). Exercise consisted of 2-min cycling at ~80% VO2max followed by 1-min rest, completed 10 times. Blood samples were drawn before, during (at 18 min), peak-, and 30-min post-exercise. White bar, resting period; grey bar, cycling; large black arrow, blood draw for all analytes; small black arrow, additional small blood draws for quantification of IL-6.
Laboratory Procedures
Neutrophil counts were quantified from peripheral whole blood using a Beckman Coulter LH750 System (Beckman Coulter, Fullerton, CA). Plasma samples were frozen at −80° C, and each tube was thawed only once on assay day. F2-IsoP concentrations were measured by gas chromatographic/negative ion chemical ionization mass spectrometry (GC/NICI-MS) with stable isotope dilution (26) at the Vanderbilt University Eicosanoid Core Laboratory. High sensitivity ELISA was used for IL-6 (R&D Systems, Minneapolis, MN), insulin (LINCO Research, St. Charles, MO), and 8-OHdG (preceded by standard ultrafiltration pretreatment) (Northwest Life Science Specialties, Vancouver, WA). MPO and nitrotyrosine were measured by ELISA, and GSH-420 by a kinetic, rate based high-sensitivity colorimetric technique (Northwest Life Science Specialties). A standard colorimetric method was used for free fatty acid (Zen-Bio, Research Triangle Park, NC) and glycerol (Sigma-Aldrich, St. Louis, MO). L-lactate concentrations were quantified using the YSI 2300 STAT PLUS Glucose and Lactate Analyzer (YSI, Yellow Springs, OH).
Statistical Analysis
Demographic information and results are presented as group mean±standard error (SE). For IL-6, logarithmic transformation was required to normalize the distribution of data prior to analyses; the mixed model, a statistical method for evaluating longitudinal data, was used across the 4 exercise time-points for each group and then between groups. For all other data, overall differences were first detected using ANOVA with the significance level at 0.05, followed by pairwise two-tailed Student’s t test with Bonferroni adjustment for multiple comparisons; paired, two-tailed Student’s t test was used to assess changes due to exercise within each group; differences in response to exercise between groups was performed with comparisons of absolute deltas. Statistical procedures were verified by UCI ICTS biostatisticians and completed using SAS 9 and JMP software (SAS Institute, Cary, NC).
RESULTS
Demographics and metabolic responses to exercise
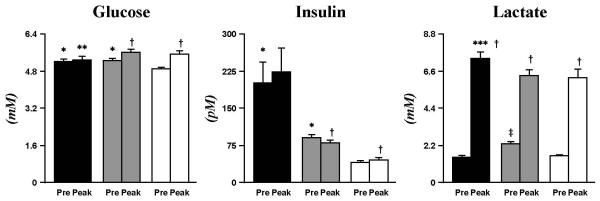
The characteristics of the 3 groups are listed in Table 1. Fasting plasma glucose (mM) in CL was 4.9±0.1, 5.2±0.1 in T1DM, and 5.3±0.1 in Ob; while all within normal glycemia, values in T1DM and Ob were significantly greater than CL (p<0.0167). Pre-exercise plasma insulin (pM) was significantly higher in T1DM (202±40, p<0.005) and Ob (90±6, p<0.005) than CL (40±3). In all groups, exercise induced quantitatively small changes in these variables which however reached statistical significance in the CL and Ob groups (Fig. 2).
TABLE 1.
Demographic features of the 3 experimental groups
| Age (Yr) | Gender (F/M) | Tanner | Height (cm) | Weight (kg) | BMI% | VO2max (L/min/kg) | |
|---|---|---|---|---|---|---|---|
| Ob | 12.7 ± 0.4 | 22/25 | 3.0 ± 0.2 | 157.0 ± 2.1 | *73.5 ± 3.0 | *97.6 ± 0.2 | *25.9 ± 0.8 |
| T1DM | 13.9 ± 0.2 | 20/29 | 3.3 ± 0.1 | 163.0 ± 1.7 | 55.8 ± 1.7 | 63.0 ± 3.6 | 39.6 ± 1.2 |
| CL | 13.5 ± 0.5 | 24/18 | 3.1 ± 0.2 | 156.9 ± 2.3 | 50.0 ± 2.2 | 57.0 ± 3.6 | 34.8 ± 1.0 |
T1DM, type 1 diabetic; Ob, obese; CL, healthy; F, female; M, male; BMI%, BMI percentile adjusted by age (in months) and gender
p <0.005 Ob vs. CL and T1DM. Data are means±SE.
Fig. 2. Plasma glucose, insulin, and lactate.
Baseline glucose and insulin were higher in T1DM (black bar) and Ob (grey bar) than CL (white bar). Exercise-induced changes in plasma glucose and insulin in Ob and CL, but not T1DM. All groups had an increase in lactate due to exercise. Data are mean±SE. * (p<0.0167), T1DM and Ob vs. CL at baseline; ‡ (p<0.005), Ob vs. CL and T1DM at baseline; † (p<0.05), exercise-induced changes within groups; ** (p<0.0167), exercise-induced patterns T1DM vs. CL; *** (p<0.005), exercise-induced increase T1DM vs. Ob.
Documenting that the relative intensity of exercise was similar across groups, the overall pattern of exercise-induced plasma lactate (mM) increase was similar across groups (T1DM, 1.5±0.1 to 7.4±0.4; Ob, 2.3±0.1 to 6.3±0.3; CL, 1.6±0.1 to 6.2±0.5; all p<0.005) (Fig. 2). The quantitatively small, albeit statistically greater increase observed in TIDM was probably related to greater reliance of this group on carbohydrate energy sources.
IL-6
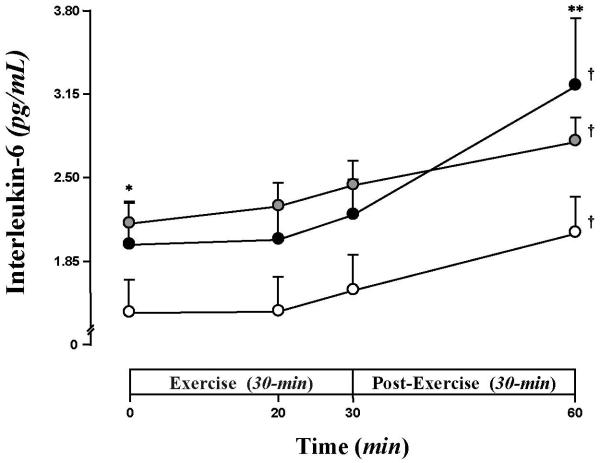
Baseline plasma IL-6 (pg/mL) was 1.5±0.3 in CL, 2.0±0.3 in T1DM, and 2.2±0.2 in Ob (p<0.005 vs CL) (Fig. 3). In all groups, IL-6 started to show slight increases at peak-exercise, and was significantly elevated at 30 min post exercise (T1DM 3.2±0.5, Ob 2.8±0.2, CL 2.1±0.3) (Fig. 3). The maximum exercise-induced change in IL-6 (30 min post vs baseline) was significantly greater in T1DM (1.2±0.3) than in both Ob (0.65±0.1, p< 0.001) and CL (0.62±0.13, p<0.0167).
Fig. 3. Baseline and exercise-induced increases in IL-6.
IL-6 was significantly elevated at baseline for Ob (grey circle) than CL (white circle), whereas T1DM (black circle) was intermediate. IL-6 increased significantly after exercise; IL-6 was higher in Ob than CL throughout the protocol, while T1DM displayed the greatest post exercise-induced change. Data are mean±SE. * (p<0.005), Ob vs. CL at baseline; † (p<0.005), exercise-induced increases within groups; ** (p<0.0167), exercise-induced patterns T1DM vs. CL and Ob.
MPO and neutrophil counts
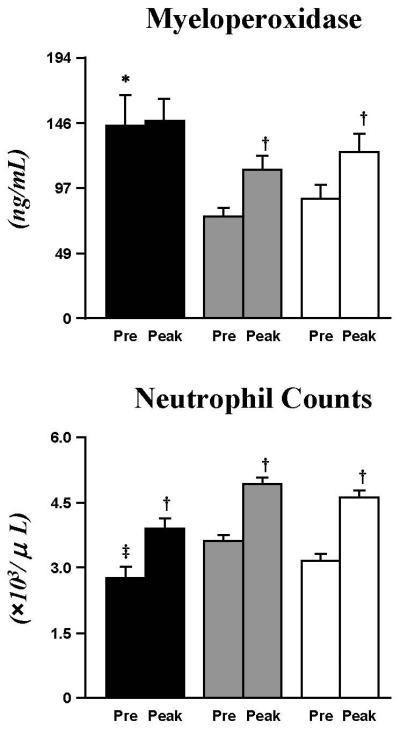
Baseline MPO (ng/mL) was markedly elevated in T1DM (143±23) as compared to CL (89±10, p<0.0167) and Ob (76±6, p<0.005) (Fig. 4). Following exercise, MPO increased significantly in Ob (111±10, p<0.005), and CL (123±14, p<0.005), but remained unchanged in T1DM (147±16) who, however, still displayed the highest peak-exercise absolute MPO concentration.
Fig. 4. Altered levels of MPO and neutrophils in T1DM.
Baseline MPO was significantly higher in T1DM (black bar) than CL (white bar) and Ob (grey bar), yet neutrophil counts were reduced compared to Ob. MPO increased following exercise in Ob and CL but not in T1DM, even when there was an increase in neutrophil counts for all 3 groups. Data are mean±SE. * (p<0.0167), T1DM vs. CL and Ob at baseline; ‡ (p<0.005), T1DM vs. Ob at baseline; † (p<0.005), exercise-induced increases within groups.
Baseline and exercise-induced changes in neutrophil counts (×103/μL) did not parallel MPO results. Baseline neutrophil counts in T1DM (2.8±0.2) were not different from CL (3.1±0.2), and lower than Ob (3.6±0.2, p<0.005) (Fig. 4), with comparable exercise-induced increases in all groups (p<0.005 vs baseline).
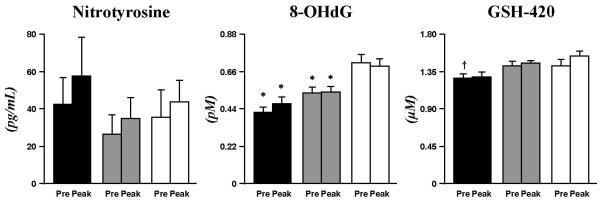
Other indices of oxidation
No significant difference in baseline or exercise-induced concentrations of nitrotyrosine was detected across experimental groups (Fig. 5). Plasma 8-OHdG (pM), however, was significantly lower both at baseline and following exercise in T1DM (0.42±0.03; 0.47±0.03, p<0.005) and Ob (0.54±0.03; 0.54±0.03, p<0.005) than CL (0.71±0.05; 0.70±0.04) (Fig. 5). The level of the endogenous antioxidant, GSH-420 (μM), was lower in T1DM (1.27±0.05, p<0.005) than CL (1.54±0.08) (Fig. 5); mean GSH-420 of Ob was intermediate (1.45±0.05), with no difference compared to the other 2 groups; no exercise effect was observed for any of the groups for GSH-420 (Fig. 5).
Fig. 5. Nitrotyrosine, 8-OHdG, and GSH-420.
Baseline and peak exercise 8-OHdG was significantly lower in T1DM (black bar) and Ob (grey bar) than CL (white bar). GSH-420 was reduced in T1DM vs. CL and Ob. No exercise-induced changes occurred for these oxidative molecules in all groups. Data are mean±SE. * (p<0.005), T1DM and Ob vs. CL. † (p<0.005), T1DM vs. Ob and CL.
Systemic lipid concentrations and peroxidation (Ob vs Control)
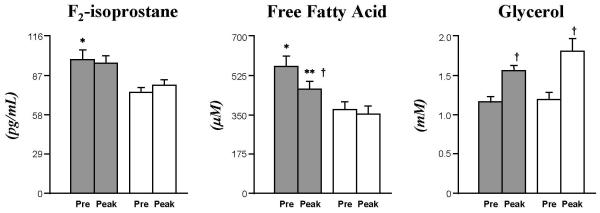
FFA concentrations (μM) were higher in Ob both at baseline (564±46 vs. 372±34, p<0.005) and at peak-exercise (464±35 vs. 352±34), despite a greater exercise-induced reduction in the Ob group (−101±21 vs. −21±23, p<0.05). Baseline glycerol (mM) was similar between Ob and CL (1.2±0.1 and 1.2±0.1, respectively), and a similarly significant exercise-induced increase (p<0.005) was observed for both groups.
The higher plasma FFA in the Ob group was paralleled by proportionally greater levels of systemic lipid peroxidation vs CL: F2-IsoP (pg/mL) at baseline were 99±7 vs. 75±4, p<0.005, and at peak-exercise 96±7 vs 80±4; in neither group did exercise induce significant changes in this variable.
DISCUSSION
In this study of 138 children, pediatric obesity and T1DM were clearly associated with altered inflammatory and oxidative responses to exercise. In both conditions, IL-6 was higher before, during, and after exercise; in addition, we observed widespread alterations of multiple indices of oxidative stress and metabolic control. To our knowledge, this is the first report simultaneously characterizing, with identical experimental procedures, adaptive responses to exercise in pediatric obesity and T1DM, together account for the large majority of dysmetabolic cases in pediatric populations.
IL-6 is an immuno-modulatory cytokine with documented pro- and anti-inflammatory (27;28), as well as metabolic effects (5). Chronic subclinical elevations of IL-6 and other pro-inflammatory markers are firmly associated with future cardiovascular morbidity and mortality (29;30). IL-6 is among the cytokines displaying the most robust and consistent increases after exercise, largely through mobilization from the skeletal muscle; IL-6 signaling is believed to stimulate hepatic glucose production and modulate exercise–induced leukocytosis (31;32), priming immune defenses against pathogens, while simultaneously shunting leukocytes and platelets away from atherosclerotic lesions (12). In contrast to acute exercise, long-term exercise training reduces resting levels of pro-inflammatory markers (C-reactive protein, IL-6, and TNF-α) (33). This dichotomy of seemingly contrasting effects underscores the complexity of inflammatory adaptation, requiring a delicate balance between opposing stimuli to produce physiological health effects. IL-6 fully embodies this equilibrium, apparently exerting both pro- and anti-inflammatory actions; elevated IL-6 levels, whatever its predominant effect in a given context, remain however clearly associated with the presence of inflammation, and this role as an inflammatory marker, rather than necessarily as an inflammatory promoter, render it very useful in identifying, and quantifying perturbations in inflammatory homeostasis.
Observations regarding IL-6 are less well defined in children, whose physiologic and immunologic regulatory pathways may differ drastically from adults (10;34). Timmons et al., for instance, recently showed that exercise IL-6, TNF-α, and leukocytes response differ between men and boys, and between late- versus early-pubertal children; in general, increasing age is associated with higher levels of inflammatory markers (9;35). Elevated IL-6 in obese and T1DM children, as compared to controls, have been previously reported separately (36;37); however, this is the first time these populations are directly compared under identical experimental conditions, allowing both a qualitative and quantitative comparison. This is also the first time both inflammatory and oxidative responses are measured in parallel in these populations. While peak IL-6 values during our procedures shifted across groups (greatest in obese at baseline and during exercise, in T1DM 30 min post-exercise), control subjects consistently displayed lowest levels. Interestingly, all 3 groups exhibited similar increases immediately after exercise, indicating that obesity or T1DM per se does not induce greater IL-6 responses. Differences, however, existed. As baseline values were significantly higher in obese children, it could be argued that in this group the exercise-induced increase in IL-6, while quantitatively similar to the other groups, was proportionally smaller. Further, in T1DM a greater, late increase was noted 30 min post-exercise, consistent with prior observations (36). At this late time point, however, IL-6 displayed greater inter-individual variability in T1DM than in the other groups, consistent with the notion that in T1DM recurrent inflammatory exacerbations are controlled by the pattern of hyperglycemic fluctuations (37), which can be very variable across subjects or within the same subjects over time. Further, in T1DM the effect of exogenous insulin administration must be considered (good glycemic control in fact often results in quite high plasma insulin, as was the case in our study, see figure 2). As insulin has anti-inflammatory properties (38), if glycemic correction was not implemented, our T1DM subjects may have displayed even greater IL-6 elevations. Finally, as IL-6 is now also known to regulate substrate metabolism, (representing a potential signaling link between skeletal muscle, the central nervous system, and energy storage organs like the liver and adipose tissue) (39), a possible intriguing possibility is that IL-6 may be inappropriately secreted in T1DM during acute perturbations of carbohydrate metabolism, such as states of relative insulinopenia and/or hyperglycemia.
MPO is a heme catalytic protein emerged in recent years as a major biomarker of cardiovascular risk. It catalyzes production from hydrogen peroxide (H2O2) of the potent oxidant hypochlorous acid (HOCl), a bactericidal agent whose dysregulated secretion causes adjacent cellular damage (7). Chronic elevation of circulating MPO are associated with future development of cardiovascular diseases and mortality (8), and higher MPO during myocardial infarction or early stages of coronary artery disease (CAD) increase the risk of death. Through still undefined mechanisms, severe endothelial impairment in diabetic animals displayed leukocytes infiltration, exaggerated MPO activity and overproduction of HOCl (40). The MPO alterations reported in this study indicate that already at a very early stage in the progress of these conditions, underlying biochemical mechanisms leading to endothelial dysfunction and ultimately atherosclerosis may be at work.
As MPO is mostly neutrophil-derived, elevated systemic MPO should be paralleled by proportional increases in neutrophil counts. However, in this study, T1DM had the highest resting MPO concentrations, despite higher neutrophil counts in Ob children. This may reflect hyper-activation of T1DM neutrophils with exaggerated basal MPO secretion, possibly triggered by recurrent hyperglycemia. Indeed, increased systemic MPO levels have been reported in adult T1DM patients; and in culture of isolated neutrophils in hyperglycemic medium has been shown to induce a drop in intracellular MPO at glucose concentrations > 20 mMol/L (8;41). Consistent with this hypothesis, in our study, exercise induced a ~50% increase in both neutrophil counts and plasma MPO only in the CL and Ob groups. Conversely, MPO was unchanged in T1DM after exercise, suggesting that MPO secretion may have already been maximized to the point of being unresponsive to a strenuous exercise challenge (still, both pre- and peak-exercise MPO levels were higher in T1DM than peak-exercise levels of both Ob and CL).
As altered MPO signaling is related to broader systemic pro-oxidant effects, we performed an exploratory investigation of additional key biomarkers of oxidative stress. Levels of nitrotyrosine, both at rest and in response to exercise, reflecting the interaction of reactive oxygen species with nitric oxide, were not significantly different across groups, although lower levels were measured in the Ob group, consistent with previous reports (42;43). Similarly, GSH-420 displayed a fundamentally comparable pattern across groups, with only a moderate (albeit significant) reduction in basal levels in the T1DM group, possibly reflecting overconsumption of antioxidants, as previously reported in similar conditions (44). Interestingly, circulating 8-OHdG was found to be significantly lower in both T1DM and Ob children, as compared to controls; which appears counterintuitive in the presence of an overall increase in inflammatory and oxidative status. It should be noted, however, that children may differ significantly from adult obese or diabetic subjects, in which prior studies were conducted (45). As 8-OHdG reflects DNA oxidation, protection against these processes may become an absolute priority in a growing organism, eliciting tissue-specific increases in anti-oxidant efficacy. Confirmation of this speculation will obviously require additional work.
An important observation resulting form our study is that, while in general inflammatory and oxidative processes were increased in both pediatric obesity and T1DM, different components appeared to be altered in each condition. While a marked MPO elevation predominated in T1DM, we hypothesized that greater levels of systemic lipid peroxidation would be a dominant feature in Ob. Indeed, in the latter group, marked metabolic alteration (FFA, glucose, insulin) were paralleled by a significant increase in plasma F2-IsoP, currently considered the gold-standard biomarker of systemic oxidation of lipids (46) (unfortunately, due to sample volume constraints, this could not be measured in T1DM). Interestingly, within our Ob study group no clear association was observed between levels of inflammatory/oxidative markers and severity of central adiposity, as opposed to simple whole body fat mass. While apparently in contrast with prior reports indicating the importance of central vs peripheral fat mass distribution in the pathogenesis of obesity-related complications (47), this finding probably simply reflects the substantial homogeneity of our obese population, in which central adiposity was predominant.
A message that should transpire clearly from our work, is that physical exercise remains a critically useful tool exerting multiple beneficial health effects in healthy subjects and patients with a broad range of clinical conditions, including obese and diabetic children. The reported alterations in oxidative and inflammatory processes may somewhat reduce these effects, but a considerable preventive action against vascular complication is retained. Regular exercise should therefore be encouraged as an integral part of the management of these conditions; future studies, further clarifying the characteristics of altered response in specific groups of patients, can help identify appropriate format, type and duration of exercise paradigms.
In summary, this is the first study, to our knowledge, to comprehensively characterize the differences in the inflammatory and oxidative stress responses to exercise, between the two most common pediatric dysmetabolic conditions, Ob and T1DM. While both groups exhibited overall increases in inflammatory and oxidative status, alterations in several molecular components of these processes appeared to be condition-specific. Future clarification of these biochemical alterations is imperative so to develop exercise regimens able to maximize the cardio-protective effects of physical activity in specific populations of pediatric patients.
Fig. 6. Elevated baseline F2-IsoP and FFA, and altered FFA response to exercise in Ob.
Ob (grey bar) had elevated F2-IsoP and FFA compared to CL (white bar) at baseline. FFA decreased in Ob, while glycerol increased for both groups at end-exercise. Data are mean±SE. * (p<0.005), Ob vs. CL at baseline; † (p<0.005), exercise-induced changes within each group; ** (p<0.05), exercise-induced patterns Ob vs. CL.
ACKNOWLEDGEMENTS
The authors thank all UCI ICTS personnel for their support. This study was funded by NIH grants M01-RR00827-28 and K-23 RR018661-01 and Juvenile Diabetes Research Foundation grant #11-2003-332.
Reference List
- (1).Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004 Jun 16;291(23):2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- (2).DIAMOND Project Group Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006 Aug;23(8):857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- (3).Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001 Dec 13;414(6865):813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- (5).Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005 Dec;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- (6).Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002 Oct 15;106(16):2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- (7).Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007 Nov;152(6):838–54. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005 Jun;25(6):1102–11. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- (9).Timmons BW, Tarnopolsky MA, Bar-Or O. Immune responses to strenuous exercise and carbohydrate intake in boys and men. Pediatr Res. 2004 Aug;56(2):227–34. doi: 10.1203/01.PDR.0000132852.29770.C5. [DOI] [PubMed] [Google Scholar]
- (10).Williams DA, Xu H, Cancelas JA. Children are not little adults: just ask their hematopoietic stem cells. J Clin Invest. 2006 Oct;116(10):2593–6. doi: 10.1172/JCI30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Robertson K, Adolfsson P, Scheiner G, Hanas R, Riddell MC. Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2009 Sep;10(12):154–68. doi: 10.1111/j.1399-5448.2009.00567.x. [DOI] [PubMed] [Google Scholar]
- (12).Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005 May 17;45(10):1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- (13).Davison GW, George L, Jackson SK, et al. Exercise, free radicals, and lipid peroxidation in type 1 diabetes mellitus. Free Radic Biol Med. 2002 Dec;33(11):1543–51. doi: 10.1016/s0891-5849(02)01090-0. [DOI] [PubMed] [Google Scholar]
- (14).Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007 Dec;14(6):837–43. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- (15).Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267(2 Pt 2):R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- (16).Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999 Feb 15;515(Pt 1):287–91. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nemet D, Oh Y, Kim HS, Hill M, Cooper DM. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002 Oct;110(4):681–9. doi: 10.1542/peds.110.4.681. [DOI] [PubMed] [Google Scholar]
- (18).Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- (19).Murtagh E, Boreham C, Nevill AM, et al. Acute Responses of Inflammatory Markers of Cardiovascular Disease Risk to a Single Walking Session. J Physical Activity and Health. 2005 Jul;2(3):324–32. [Google Scholar]
- (20).Peterson AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988 Apr;17(2):117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- (21).Barlow SE, Expert Committe Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120(4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- (22).Janz KF, Nielsen DH, Cassady SL, et al. Cross-validation of the Slaughter skinfold equations for children and adolescents. Med Sci Sports Exerc. 1993 Sep;25(9):1070–6. [PubMed] [Google Scholar]
- (23).Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–34. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- (24).Eliakim A, Nemet D, Zaldivar F, et al. Reduced exercise-associated response of the GH-IGF-I axis and catecholamines in obese children and adolescents. J Appl Physiol. 2006 May;100(5):1630–7. doi: 10.1152/japplphysiol.01072.2005. [DOI] [PubMed] [Google Scholar]
- (25).Rosa JS, Schwindt CD, Oliver SR, et al. Exercise leukocyte profiles in healthy, type 1 diabetic, overweight, and asthmatic children. Pediatr Exerc Sci. 2009 Feb;21(1):19–33. doi: 10.1123/pes.21.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoco. 2007;2(1):221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- (27).Steensberg A, van Hall G, Osasa T, et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000 Nov;529(1):237–42. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005 Apr;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- (29).Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005 Apr 21;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- (30).Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003 Nov;108(19):2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- (31).Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007 Sep;103(3):1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- (32).Zaldivar F, Wang-Rodriguez J, Nemet D, et al. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J Appl Physiol. 2006 Apr;100(4):1124–33. doi: 10.1152/japplphysiol.00562.2005. [DOI] [PubMed] [Google Scholar]
- (33).Goldhammer E, Tanchilevitch A, Maor I, et al. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005 Apr;100(1):93–9. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- (34).Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc Immunol Rev. 2009;15:6–41. [PubMed] [Google Scholar]
- (35).Timmons BW, Tarnopolsky MA, Snider DP, Bar-Or O. Immunological changes in response to exercise: influence of age, puberty, and gender. Med Sci Sports Exerc. 2006 Feb;38(2):293–304. doi: 10.1249/01.mss.0000183479.90501.a0. [DOI] [PubMed] [Google Scholar]
- (36).Galassetti PR, Iwanaga K, Crisostomo M, Zaldivar FP, Larson J, Pescatello A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatr Diabetes. 2006 Feb;7(1):16–24. doi: 10.1111/j.1399-543X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- (37).Rosa JS, Flores RL, Oliver SR, et al. Resting and exercise-induced IL-6 levels in children with Type 1 diabetes reflect hyperglycemic profiles during the previous 3 days. J Appl Physiol. 2010 Feb;108(2):334–42. doi: 10.1152/japplphysiol.01083.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Aljada A, Ghanim H, Saadeh R, Dandona P. Insulin inhibits NFkappaB and MCP-1 expression in human aortic endothelial cells. J Clin Endocrinol Metab. 2001 Jan;86(1):450–3. doi: 10.1210/jcem.86.1.7278. [DOI] [PubMed] [Google Scholar]
- (39).Pedersen BK, Febbraio M. Muscle-derived interleukin-6--a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun. 2005 Sep;19(5):371–6. doi: 10.1016/j.bbi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- (40).Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Nature. 2002 Jun;296(5577):2391–4. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- (41).Galassetti PR, Littler P, Bachman G, Ibardolaza M, Oliver SR, Zaldivar FP., Jr. Exposure to High Glucose Concentrations Alters Human Neutrophil Function and Myeloperoxidase Content. Diabetes. 2010;59(suppl 1) Ref Type: Abstract
- (42).Li Z, Rodríguez-Iturbe B, Ni Z, et al. Effect of hereditary obesity on renal expressions of NO synthase, caveolin-1, AKt, guanylate cyclase, and calmodulin. Kidney Int. 2005 Dec;68(6):2766–72. doi: 10.1111/j.1523-1755.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- (43).Galassetti P, Zaldivar F, Cooper DM. Reduced Basal and post-Exercise Nitrotyrosine and Increased Inflammatory Cytokines in Obese Children. Diabetes. 2004;53(S2):A417. [Google Scholar]
- (44).Darmaun D, Smith SD, Sweeten S, et al. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes. 2005 Jan;54(1):190–6. doi: 10.2337/diabetes.54.1.190. [DOI] [PubMed] [Google Scholar]
- (45).Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996 Feb;347(8999):444–5. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- (46).Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem. 2008 Jun 6;283(23):15533–7. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand. 2005 Jan;183(1):13–30. doi: 10.1111/j.1365-201X.2004.01385.x. [DOI] [PubMed] [Google Scholar]