Abstract
We previously showed that children (average age 9 y) with mildly elevated obstructive apnea-hypopnea indices (OAHI) retained CO2 at rest. Here, we report the results of a six-year follow up study on 14 children from that study.
Lung ventilation rate (VE) and the partial pressure of end-tidal CO2 (PETCO2) were measured during hypercapnic challenge.
OAHI fell from 7.5 ± 4.7 to 2.5 ± 1.8 from age 9 to age 15 (P < 0.001), despite an increase in BMI from 20 ± 4.6 to 26 ± 5.7 (P < 0.0001). Eupneic VE increased from 4.1 ± 0.31 to 5.9 ± 0.4 L/min/m2 (P< 0.01), while PET CO2 fell from 44.1 ± 0.8 to 33 ± 1.0 mmHg (P< 0.001). The VE - PETCO2 obtained during hypercapnia was left-shifted, such that VE at a PETCO2 of 50 mmHg increased from 24 L/min at age 9, to 36 L/min at age 15. Central respiratory drive did not change.
We hypothesize that somatic growth of the pharynx coupled with a regression of tonsillar tissue mass with age lead to enlargement of the upper airway lumen, a reduction in airway resistance and increased respiratory airflow at a given level of ventilatory drive.
INTRODUCTION
We previously showed that resting PETCO2 was higher in 6–12 year old children with relatively high obstructive apnea-hypoxia indices (OAHI) compared to age-matched controls with lower OAHI [1]. The initial studies were done in 1999 when the average age of our subjects was about 9 years. Here, we report the results of a six-year follow up study of 14 children from this original sample, now with an average age of 15 years. All of the children in our sample have intact tonsils and adenoids. The mechanism of the CO2 retention is unknown, but could be due to increased upper airway resistance, to blunted central chemoreceptor sensing and/or to altered regulation of central ventilatory motor output.
We were able to re-study 14 children from this original sample in 2006–2007, and our results are reported herein. Preliminary analyses from the entire TuCASA cohort indicate that the OAHI improves with age in children, even in the absence of adenoidectomy and tonsillectomy ([2]; and see Table 1). Here, we test the hypothesis that these improvements in the OAHI are associated with improvements in ventilatory control. We were particularly interested in changes in the resting pulmonary ventilation rate (VE), the resting PETCO2, and changes in the sensitivity to inspired CO2. Our unique longitudinal study shows that although the children increased their body-mass indices (BMIs) with age, they also had lower OAHIs, a marked diminution in resting PETCO2, and a substantial leftward shift in the VE- PETCO2 response curve.
Table 1.
Anthropometric and sleep data in 1999 and 2006.
| 1999 | 2006 | P value | |
|---|---|---|---|
| N | 14 | 14 | |
| Gender | 8M, 6F | 8M, 6F | |
| Age (y) | 8.7 ± 0.91 (7–10) | 15 ± 1.3 (12–17) | < 0.0001 |
| Weight (kg) | 38 ± 12 (24–67) | 71 ± 18 (49.6–101) | < 0.0001 |
| Height (cm) | 136 ± 8.3 (120–149) | 163 ± 7.1 (149–174) | < 0.0001 |
| BMI (Kg/m2) | 20 ± 4.6 (15–30) | 26.8 ± 5.7 (19–37) | < 0.0001 |
| BSA (m2) | 1.18 ± 0.21 (0.90–1.61) | 1.75 ± 0.22 (1.4–2.1) | <0.0001 |
| RDI (events/h) | 8.6 ± 5.3 (2.7–19) | 2.7 ± 1.8 (0.5–7.8) | 0.0018 |
| OAHI (events/h) | 7.5 ± 4.7 (1.8–17) | 2.5 ± 1.8 (0.3–7.6) | 0.001 |
| Total Sleep time (min) | 487 ± 56 (314–530) | 446 ± 51 (367–528) | NS |
| SaO2 nadir (%) | 89 ± 13 (87–91) | 91 ± 2.9 (86–94) | NS |
| SBP (mmHg) | 102 ± 1.3 (72–122) | 107 ± 9.9 (88–122) | NS |
| DBP (mmHg) | 65 ± 10 (50–88) | 61 ± 7.6 (48–74) | NS |
Values are mean ± SD, with range in parentheses. N, number of subjects; M, male, F, female; BMI, body mass index; BSA, body surface area; RDI, respiratory disturbance index; OAHI, obstructive apnea-hypopnea index; SaO2, oxygen saturation of arterial blood; SBP systolic blood pressure; DBP, diastolic blood pressure (see text for definitions).
MATERIALS & METHODS
Subjects
All methods used to recruit subjects and to collect the present data set were approved both by the University of Arizona Human Subjects Committee and the Tucson Unified School District Research Committee. In all cases, we obtained written informed consent from the parents, and assent from the children. Our initial sample (1999) was composed of subjects recruited through the Tucson Unified School District (TUSD), as described in detail previously [1]. For the present study we selected and attempted to contact all 50 subjects from our original cohort and asked them if they were interested in participating in a follow up study. Subjects that had adenoidectomy or tonsillectomy were excluded, and a total of 14 subjects that met our criteria agreed to participate (28% of the original cohort), with their anthropometric characteristics given in Table 1. The remaining children could not be located, had tonsillectomies or adenoidectomies (N= 19) and/or refused to participate.
Polysomnography
Both in the 1999 study and the present one, children underwent unattended, nocturnal home polysomnography [3] using the Compumedics PS-2 system (Abbotsford, Victoria, Australia). The following signals were obtained: C3/A2 and C4/A1 electroencephalogram (EEG), right and left electrooculogram, a bipolar submental electromyogram, thoracic and abdominal displacement (inductive plethysmography bands), airflow (nasal/oral thermocouple), nasal pressure, electrocardiogram (single bipolar lead), snoring (microphone attached to a vest), body position (Hg gauge sensor), pulse oximetry (Nonin, Plymouth, MN) and ambient light (sensor attached to the vest to record on/off). Using Compumedics W-Series Replay, v 2.0, release 22, sleep stages were scored according to standard criteria [4]. The respiratory disturbance index (RDI) was defined as the number of respiratory events (apneas and hypopneas) per hour of the total sleep time irrespective of any associated oxygen desaturation or arousal. Polysomnograms with less than 4 hours of scorable oximetry were classified as failed studies and were repeated if the participant consented. Central apneas were scored if both airflow and thoracoabdominal effort were absent. However, central events that occurred after movement were not included. Obstructive apneas were identified if the airflow signal decreased to below 25% of the “baseline amplitude”. Hypopneas were scored if the magnitude of any ventilation signal decreased below approximately 70% of the “baseline” amplitude, as described previously [1]. Although more recent rules for scoring respiratory events have been published [5] we elected to score apneas and hypopneas using the same algorithm used in our 1999 study in order to make valid comparisons between the 2 time points, in each instance using the thermistor and/or the inductance plethysmography signal to score respiratory events.
The RDI that we routinely compute includes central apneas as well as obstructive apneas and hypopneas [3]. Based on the clinical and physiological uncertainty of central apneas in children [6, 7] we subtracted central events from the RDI to derive the OAHI. In our subjects this index represents primarily hypopneas. For example, in 1999 the number of frank obstructive events ranged from 0.1 – 0.8 per hour, except for one subject that averaged 7 obstructions per hour in 1999. In 2006 there were no frank obstructions except in one subject who had 2 obstructions per hour.
Ventilatory Control Protocol
For this portion of the study, participants were studied between 9:00 AM and 4:00 PM, and were instructed to refrain from caffeinated beverages and food for one hour prior to the time of their scheduled experiment. Subjects were studied while seated and listened to music through headphones throughout the entire protocol. Analog waveforms from transducers monitoring expiratory airflow, mask pressure and the fractional concentrations of O2 and CO2 were passed through an analog-to-digital converter (Spike II, Cambridge Electronic Design), sampled at 2500 Hz per channel, and stored on the hard drive of an IBM-compatible computer (details of the measurements and equipment are given below). Estimated oxygen saturation of arterial blood was monitored and recorded manually in all studies with a pulse oximeter (Ohmeda).
The investigators were blinded to the OAHI status of the subject when conducting experiments and analyzing data. For hyperoxic hypercapnia the subjects breathed from humidified Douglas bags filled with 3, 5 or 7 % CO2 in O2. Subjects started by breathing room air for 3–5 minutes, and then breathed, in succession, each of the three CO2-O2 mixtures for 3 minutes each, and recovered by breathing room air. In all conditions, airway occlusions were applied twice per minute, to obtain measurements of P0.1.
Measurement of pulmonary ventilation, inspired and expired gas concentrations and P0.1
Subjects breathed through a tight fitting mask that covered the nose and mouth, and that allowed free breathing through either the oral or nasal airway (Hans-Rudolph Pediatric rubber face mask). An additional rubber seal was created around the mask using Exaflex (GC America, Inc.) The mask was checked for leaks by instructing the subjects to hyperventilate while an investigator looked for leaks by sampling CO2 around the mask seal. When leaks were noted, they were sealed with additional Exaflex. The end-tidal CO2 on inspiration returned to zero under all conditions, indicating that the system dead space was sufficiently low to prevent rebreathing.
A low dead space non-rebreathing valve (Hans Rudolph 2600) was attached to the mask, and a short length of tubing and a pneumotachometer (Hans-Rudolph 4700) were placed on the expiratory side of the breathing valve for the measurement of expiratory airflow. The pressure drop across the pneumotachometer was measured with a differential pressure transducer with a ± 2.5 cmH20 diaphragm (Validyne MP 45). The pneumotachometer was calibrated with a precision Matheson Rotameter before each experiment. The respiratory period was measured from the flow signal, and used to compute breathing frequency (f). Expired flow was integrated by the computer off-line to derive expired tidal volume (VT), which was converted from ambient temperature, pressure and humidity conditions (ATPS) to body temperature, pressure and humidity conditions (BTPS), with the assumption that body temperature was 37 °C. Expired ventilation (VE) was computed offline as the product of VT and f. Breath-by-breath values for CO2 and O2 were measured with a rapidly responding analyzer (Raytech Instruments) that was connected to the non-rebreathing valve by small-bore tubing. The output of the analyzer was digitized and used to compute the end-tidal levels of CO2 and O2. Mask pressure was measured by connecting a short length of PE 200 tubing to the center of the non-rebreathing valve, and attaching the opposite end to a differential pressure transducer with a ± 56 cm H20 diaphragm (Validyne MP 45).
For the measurement of P0.1, a Hans-Rudolph automated balloon valve was attached to the inspiratory side of the non-rebreathing valve. The balloon was connected to a compressed air source, and was inflated or deflated with a solenoid valve and vacuum pump, respectively. The computer controlled the activation and deactivation of the solenoid valve and pump. The inspiratory port was occluded during expiration, and the occlusion was maintained for about 200 ms into the ensuing inspiration, allowing sufficient time to obtain measurements of P0.1 [8]. The computer measured the drop in mask pressure exactly 0.1 seconds after the onset of the occluded inspiratory effort, and denoted this value as P0.1.
Data processing and statistical analysis
Six-to-ten breaths obtained over the last 30–40 seconds under control conditions and at each level of hyperoxic hypercapnia, were used to calculate average values of VT, f, VE and the partial pressures of end-tidal O2 (PETO2) and CO2 (PETCO2). All P0.1 values obtained in each condition were averaged, so that each subject had a single P0.1 measurement for each of the experimental conditions. All P0.1 values were expressed in units of cmH2O.
The VE values for each subject were divided by the subject's body surface area (BSA, m2) to account for the large differences in size (and hence tidal volume) over the 6-year age range of our pediatric subject population. This analysis allowed us to compare the hypercapnic ventilatory responses of our subjects with previously published results in children of various ages.
To determine if the severity of SDB correlates with ventilatory drive, we first plotted both VE and P0.1 against PETCO2 for each subject, and computed the slope of the relation as an index of hypercapnic ventilatory drive using linear regression analysis. We then plotted each subject's hypercapnic response slope against their OAHI, and subjected the data to a linear regression analysis. To determine if resting CO2 retention was correlated with the OAHI, we plotted the resting level of PETCO2 for each subject against his or her OAHI. We used a simple linear regression model followed by analysis of variance (ANOVA, Sigma Stat 3.0) to determine if the relation was statistically significant, with significance defined as a P value < 0.05.
RESULTS
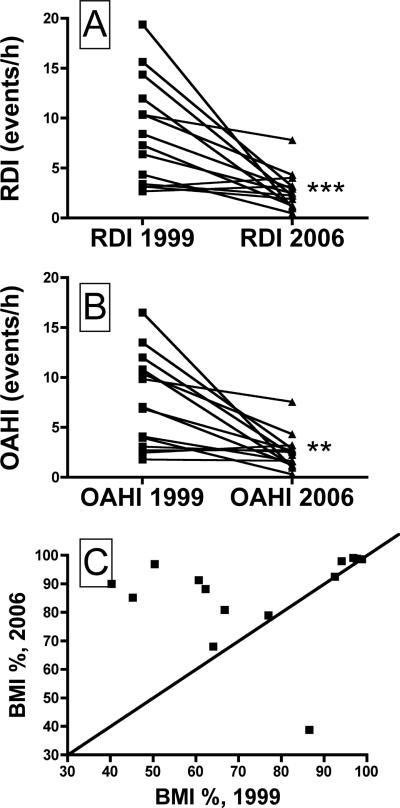
As shown in Table 1, the subject's average RDI and OAHI values were significantly lower in 2006 compared to 1999. Figures 1A and 1B show the RDI and OAHI values for each subject, and it is clear that these values fell with age in all but three subjects, in whom the values were very low to begin with. Weight, height, BMI and BSA all were significantly higher in 2006 compared to 1999 (Table 1). The BMI percentile adjusted for age, gender and ethnicity (calculated from the US Centers for Disease Control and Prevention growth charts, http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/index.htm) was higher in 2006 than in 1999 for 11 of the 14 subjects (Fig. 1C). Total sleep time and SaO2 nadir measured in the 1999 and 2006 nocturnal sleep studies did not differ significantly (Table 1).
Figure 1.
The respiratory disturbance index (RDI, panel A) and the obstructive apnea-hypopnea index (OAHI, panel B) recorded in 1999 and in 2006, for all 14 subjects. The OAHI declined in every subject. See Table 1 for average values, and text for description of how the RDI and OAHI values were calculated. **, Different than 1999 at P<0.01; ***, Different than 1999 at P<0.001. Panel C shows the BMI percentile values for each subject, in 1999 and 2006. The line of identity is shown, and it is clear that most subjects were in a much higher percentile in 2006 compared to 1999 (see text for details).
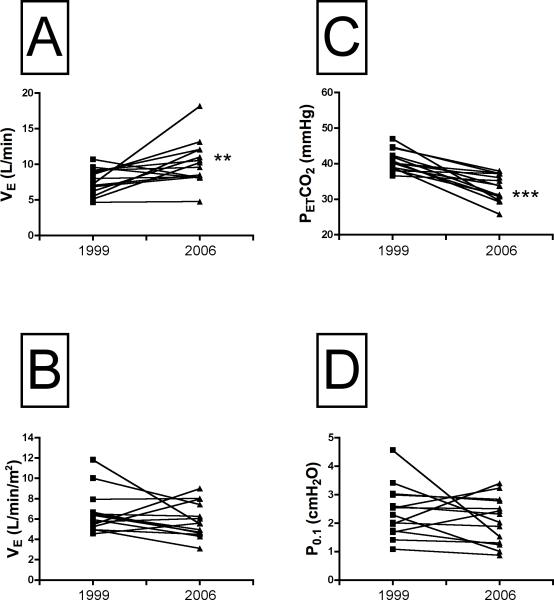
The absolute pulmonary ventilation rate measured under resting conditions increased with age (Fig. 2A), but was the same when it was expressed as a function of body surface area (P=0.26, Fig. 2B). The rise in resting ventilatory output was associated with a much lower PETCO2, from 44.1 ± 0.8 mmHg in 1999 to 33 ± 1.0 in 2006 (P<0.001; Fig. 2C). In contrast to the increase in lung ventilation with age, the resting P0.1 showed marked variability at both ages, and the mean values were not significantly different (Fig. 2D). It is important to point out here that our baseline P0.1 values are within the range reported for children of similar age by Marcus and colleagues [9].
Figure 2.
Resting values for absolute expired minute ventilation (VE, panel A), VE corrected for body surface area (BSA, panel B), the partial pressure of end tidal CO2 (PETCO2, panel C) and the mouth pressure measured 100 ms after the onset of inspiratory effort (P0.1, panel D). VE was higher in every subject in 2006 compared to 1999, but VE corrected for BSA (panel B) was the same in 1999 and 2006. PETCO2, declined in every subject (panel C), while P0.1 did not change (panel D). **, Different than 1999 at P<0.01; ***, different than 1999 at P<0.001.
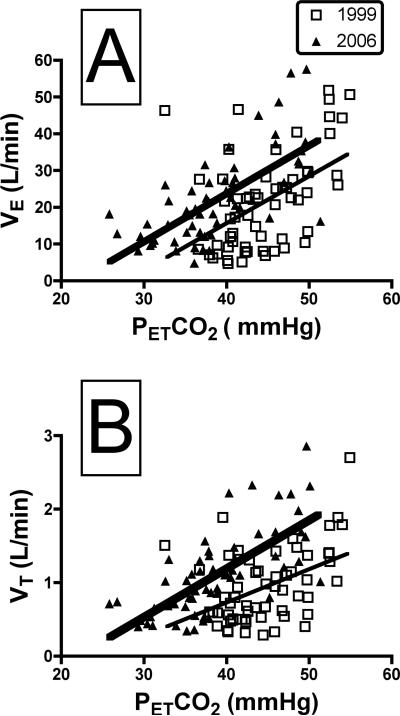
We estimated central CO2 sensitivity by conducting steady-state CO2 response tests at 3, 5 and 7% inspired CO2. The individual data points that were used to define the slope of the VE-PETCO2 response for all subjects are shown in Fig. 3A. It is clear from this graph that although the slopes in 1999 and 2006 are not different (1999, y = 1.28 (x) − 35.6; 2006, y = 1.31 (x) − 28.6, t= 0.08, P = 0.94), the curve is markedly left-shifted, such that VE was much higher at any given PETCO2 in 2006 compared to 1999 (Fig. 3A). For example, at a PETCO2 of 50 mmHg the VE estimated from the data shown in Fig. 3A would be 28.4 L/min in 1999 and 36.9 in 2006. Similarly, the average x-intercept calculated from the slopes, the so-called “apnea point” [10], was significantly different (1999, 33.1 ± 1.1; 2006, 26.4 ± 2.7, t = 2.41, P = 0.03). Figure 3B shows that the left-shift in the VE-PETCO2 response curves is due to a corresponding left-shift in the VT-PETCO2 response (1999, y = 0.05 (x) − 1.08; 2006, y = 0.07 (x) − 0.34, t = 1.43, P = 0.16), coupled with a frequency response that was identical in 1999 and 2006 (data not shown). To correct for changes in VE secondary to growth, we also examined the VE-PETCO2 response curves with VE expressed as a function of the body surface area. Again, there were no significant differences in the slope of the relationship (1999, y = 0.65 (x) − 19.6; 2006, y = 0.72 (x) − 15.3, t=0.36, P=0.72).
Figure 3.
Panel A shows the VE- PETCO2 relation in 1999 (open squares) and 2006 (filled triangles). Each point represents data points obtained at rest, and in the steady state of breathing gas mixtures with 3, 5 and 7% inspired CO2 in all 14 subjects, as described in Methods. The slope of the relation was the same in 1999 and 2006 (1999, y=1.28 (x) − 35.6; 2006, y=1.31 (x) − 28.6, t= 0.08, P = 0.94), but the regression line obtained in 2006 was markedly left-shifted, as discussed in text. Panel B shows the relationship between tidal volume (VT) and PETCO2 in all subjects. As with VE, the slope of the relation was the same in 1999 and 2006 (1999, y=0.045 (x) − 1.1; 2006, y= 0.066 (x) − 1.45, t= 1.43, P = 0.16), but the curve in 2006 was markedly left-shifted. These data indicate that the left-shift in the ventilatory response to CO2 (panel A) was due to differences in the VT response, as the frequency response was unaltered (data not shown).
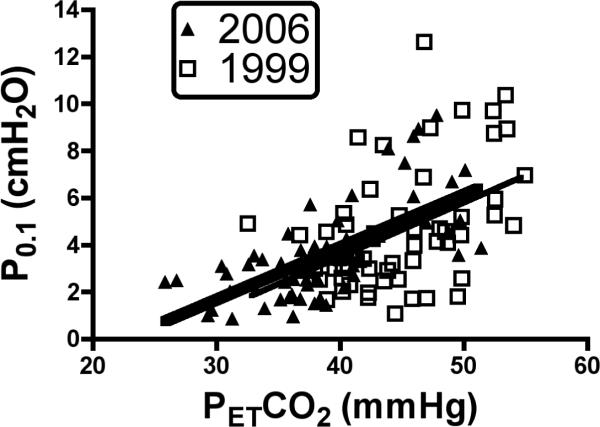
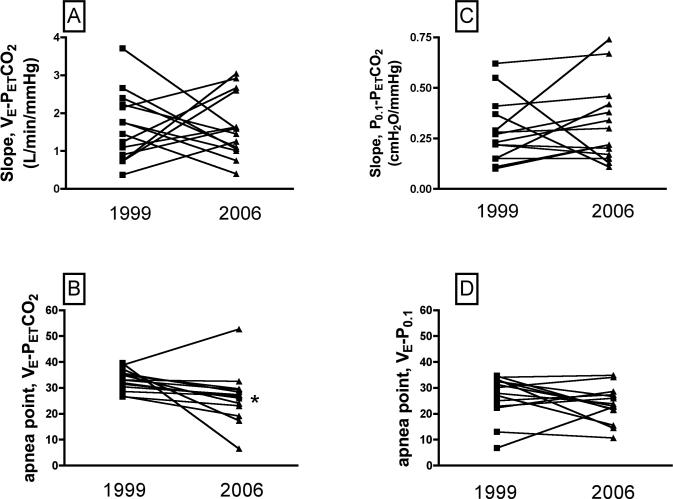
Interestingly, the P0.1-PETCO2 slopes in 1999 and 2006 (Fig. 4) were statistically identical (1999, y = 0.23 (x) − 5.7; 2006, y = 0.22 (x) − 5, t=0.16, P=0.87), and were only slightly left-shifted, such that the apnea point was the same in 1999 and 2006 (1999, 26.7 ± 2.2; 2006, 23.7 ± 1.8, t = 1.24, P = 0.24). Individual VE-PETCO2 and P0.1-PETCO2 slopes for each subject are shown in Fig. 5, A and C. With a few exceptions, the slopes did not change with age, and the group average slopes were the same in 1999 and 2006 (for VE-PETCO2, 1999 = 1.7 ± 0.24; 2006 = 1.65 ± 0.22, t = 0.14, P=0.89; for P0.1-PETCO2, 1999 = 0.28 ± 0.04; 2006 = 0.32 ± 0.05, t=0.6969, P=0.49). The individual apnea points for each subject are shown in Fig. 5 B and D. Statistical analysis of the individual apnea points revealed significant differences for the VE-PETCO2 curves (1999, 35.5 ± 1.3; 2006, 24.6 ± 3.2, t = 3.16, P = 0.0082), but not for the P0.1-PETCO2 curves (1999, 29.9 ± 2.5; 2006, 23.7 ± 2.0, t = 1.4, P = 0.19).
Figure 4.
Relationship between mouth occlusion pressures measured 100 ms after onset of inspiratory effort (P0.1) and PETCO2 in all subjects. The slopes calculated in 1999 and 2006 were the same (1999, y=0.23 (x) − 5.7; 2006, y=0.22 (x) − 5.0, t=0.16, P=0.87), suggesting that the central ventilatory response to CO2 did not change (see Discussion).
Figure 5.
Individual VE-PETCO2 (panel A) and P0.1-PETCO2 (panel C) slopes in 1999 and 2006, for each of the 14 subjects. These data demonstrate the variability between subjects with respect to the VE-PETCO2, and the P0.1-PETCO2 relation, and that on average there was no age-dependent change in either of these relationships. Panels B and D show the apnea points computed from VE-PETCO2 and P0.1-PETCO2, curves, respectively. The apnea point derived from the VE-PETCO2 curve was significantly lower in 2006 compared to 1999 (panel B), consistent with the left shift in the VE-PETCO2 calculated from the composite data (Fig. 3A). The apnea points derived from the P0.1-PETCO2 curve were the same in 1999 and 2006, consistent with the lack of change in central ventilatory drive, as shown for the composite data in Fig. 4. *, Different than 1999 at P<0.05.
DISCUSSION
This is the first longitudinal study of developmental alterations in the ventilatory control of children with sleep-disordered breathing in early-childhood. Our subjects were, on average, 9 years of age on initial study, and 15 years when the current study was completed. Our main finding is that subjects now have much lower OAHIs and retain much less CO2 than they did in 1999, despite similar mass-specific resting ventilation rates and a substantial increase in BMI. We also showed that, on average, the slope of either the VE-PETCO2 curve or the P0.1- PETCO2 curve were not significantly different in 1999 and 2006, indicating that CO2 sensitivity during steady state hypercapnic challenge was unchanged. However, the VE-PETCO2 response was markedly left-shifted in 2006 compared to 1999, indicating greater VE at a given PETCO2, despite no change in CO2 sensitivity. This left-shift also resulted in much lower apnea points [10]. Interestingly, there was no left-shift in the P0.1- PETCO2 curve. As discussed below, our observations are most likely explained by changes in mechanical factors, e.g., a decrease in airflow resistance, rather than changes in the central control of breathing. That the left-shift in the VE-PETCO2 response curve was the result of changes in tidal volume rather than breathing frequency also supports the idea that the effects were mechanical and not central.
In theory, the increased lung ventilation rate at a given PETCO2 with no change in the sensitivity to CO2 could be explained by a reduction in airway resistance. This is due in large part to somatic growth of the airway as children grow taller [11]. Using the regression equations derived by Zapletal and Chalupova [11], we estimate that nasopharyngeal resistance in our subjects would have fallen by 23% on the basis of the change in height alone (136 cm tall in 1999 vs. 163 inches tall in 2006, Table 1). In addition, it is well known that tonsil size declines with age after reaching a peak size between 4 and 8 years of age [12]. There have been anecdotal suggestions that enlarged tonsils, and thus a narrow pharyngeal airway, predispose children to nasal breathing, which in turn leads to hypoventilation through the high-resistance nasal pathway. When our subjects were studied initially, in 1999, we found that their resting PETCO2 during wakefulness was significantly elevated, and that it was correlated with the OAHI [1]. Our previous MRI studies showed that a sub-sample of the group studied in 1999 had large adenoids, tonsils and soft palates [13]. We analyzed those data by computing the sum of the cross-sectional areas of these soft tissue structures and expressed the sum as a percentage of the nasopharyngeal cross sectional area. We found that the children with sleep-disordered breathing had a narrow nasopharynx as a result of increased soft tissue mass. The present data suggest that this ratio is now smaller. In other words, if their pharyngeal airway grew at a faster rate than the surrounding soft tissue structures, the lumen of the nasopharynx would be enlarged, leading to lower airway resistance. Although we do not have MRI data as part of this study, the increased ventilation and lack of CO2 retention in the absence of a change in sensitivity to PETCO2 is consistent with a larger upper airway lumen. The results of this study and our earlier one are consistent with other data showing hypoventilation and CO2 retention in young children with enlarged tonsils and sleep disordered breathing [14, 15].
We observed that both the RDI and OAHI decreased over the approximate 6-year interval between PSGs in these subjects. Preliminary examination of data from the entire TuCASA cohort confirms this finding [2]. In contrast to our findings, a previous study in Thai children showed that 5 of 7 children with obstructive sleep apnea had a higher OAHI over a 3-year interval [16]. However, PSG was performed in these children because they had symptoms of OSA, and thus there may have been some selection bias. As discussed above, we suspect that in our study the observed decrease in RDI and OAHI is related to somatic growth of the pharynx, coupled with regression of tonsillar tissue with age. At the initial TuCASA examination, children were studied between the ages of 6 and 11 years. This is the age range where some children will have large tonsils resulting in an elevated RDI and OAHI. With normal regression in tonsil size as they become adolescents, there should be a decrease in RDI and OAHI as we have found.
Although our observations are consistent with changes in airway resistance, the exact mechanism of the CO2 retention during quiet breathing at an average age of 9 years, but not approximately six years later remains unknown. One possibility is that the young children “chose” to hypoventilate rather than fight the increased flow resistance and thus higher work of breathing that would have been required to drop their PETCO2. This is consistent with the strategy employed by highly trained athletes during peak exercise, wherein they allow themselves to become hypoxic and relatively hypercapnic rather than consume the extra energy that would be required to elevate alveolar ventilation sufficiently to fully correct the blood gas and acid base derangements [17].
The left-shifted ventilatory response to PETCO2 is often considered to be due to an “extra” stimulus to breathe. If this were the case we would have expected a left-shift in the relation between P0.1 and PETCO2, which we did not observe (Fig. 4). Complicating the relation between P0.1 and PETCO2 is that the former can be influenced by both respiratory muscle strength and end-expiratory lung volume. Weak inspiratory muscles lead to lower P0.1 values during hypercapnic challenges, although the effects are small until the PETCO2 exceeds 60 mmHg [18]. This would have little or no impact on our data as the PETCO2 values were less than 60 mmHg in every case (Figs. 3 and 4). Inspiratory muscle strength increases by about 20% from age 9 to 15 in boys [19], suggesting that changes in strength alone as the subjects grew would result in slightly higher P0.1 values in 2006 compared to 1999 (Figure 4), but this was not seen.
The P0.1 can also be influenced by changes in end-expiratory lung volume, with lower volumes associated with a greater P0.1, due to improved muscle length-tension properties and thus improved mechanical advantage [20, 21]. However, the pertinent issue is the end-expiratory lung volume as a percentage of an individuals total lung capacity, as this dictates the length-tension relationship of the respiratory muscles for that particular system [22]. End-expiratory lung volume as a percentage of total lung capacity increases from approximately 46% in 9 year olds (the average age of our subjects in 1999), to 53% in 15 year olds (average age in 2006), corresponding to a volume increment of about 400 ml [23]. It has been shown that an increase in FRC of 500 ml reduces respiratory muscle pressure development by about 10% [22]. In our subjects this would translate into at most an 8% decrease in the P0.1 (400/500 × 10%), which would result in only a negligible shift in the P0.1-PETCO2 curve (Figure 4). Obesity can reduce end-expiratory lung volume independently of age and height, although the effects are small and variable [24, 25] except in severe obesity [26]. Most of our subjects were in a higher BMI percentile in 2006 than they were in 1999 (Fig. 1C), with some of them exhibiting severe obesity (i.e., BMI values at or above the 95th percentile). This could also contribute to a slight leftward shift in the P0.1-PETCO2 relation, but again, this was not observed. Finally, although it is possible that hypercapnia could increase airway resistance to a variable extent across the subject population, the P0.1 is uninfluenced by airway resistance and behavioral adjustments in ventilatory output [8, 22]. Taken together, our longitudinal data support the contention that the elevated resting PETCO2 and the left-shifted VE-PETCO2 curve in younger children is the result of reduced flow resistance, and likely not to the addition of an “extra” excitatory stimulus to breathe.
The functional consequences of the elevated eupneic PETCO2 when the children were younger are unknown. Given that the apnea point was significantly higher in 1999 than in 2006, one might surmise that the tendency for apnea was greater in the young children. However, the difference between the apnea point and the eupneic PETCO2 was the same in 1999 and 2006 (1999, 7.7 ± 1; 2006, 6.9 ± 3, P=NS). This difference has been called the CO2 reserve, and it has been suggested that a smaller reserve increases the propensity for apnea in adult human subjects [27]. Our subjects had higher RDI values in 1999 than in 2006 despite a similar CO2 reserve, suggesting that the CO2 reserve may not predict a predisposition to apnea in children.
In conclusion, we have examined changes in the control of breathing from childhood to adolescence in a group of subjects that had mild sleep-disordered breathing as young children. The main finding is that the rate of pulmonary ventilation at a given PETCO2 was much higher, and the eupneic PETCO2 much lower at an average age of 15 compared to an average age of 9. This occurred in the absence of changes in sensitivity to inspired CO2, suggesting that upper airway resistance dropped as the children grew, leading to improved alveolar ventilation in the absence of significant changes in central ventilatory drive.
ACKNOWLEDGMENTS
This study was supported by NIH grant HL 62373.
REFERENCES
- 1.Fregosi RF, Quan SF, Jackson AC, Kaemingk KL, Morgan WJ, Goodwin JL, Reeder JC, Cabrera RK, Antonio E. Ventilatory drive and the apnea-hypopnea index in six-to-twelve year old children. BMC Pulm Med. 2004;4(1):4. doi: 10.1186/1471-2466-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin J, Silva G, Kaemingk K, Sherrill D, Morgan W, Fregosi R, Quan S. Comparison of child vs. parent report of sleep related symptoms in adolescent children--The Tucson Childrens Assessment of Sleep Apnoea Study (TuCASA) Sleep Biol Rhythms. 2007;5(Suppl 1):A69. [Google Scholar]
- 3.Goodwin JL, Enright PL, Kaemingk KL, Rosen GM, Morgan WJ, Fregosi RF, Quan SF. Feasibility of using unattended polysomnography in children for research--report of the Tucson Children's Assessment of Sleep Apnea study (TuCASA) Sleep. 2001;24(8):937–944. doi: 10.1093/sleep/24.8.937. [DOI] [PubMed] [Google Scholar]
- 4.Rechtshaffen AKA. A manual of standardized terminology: Techniques and scoring systems for sleep stages of human subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles, CA: 1968. [Google Scholar]
- 5.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 6.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164(1):16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 7.Tang JP, Rosen CL, Larkin EK, DiFiore JM, Arnold JL, Surovec SA, Youngblut JM, Redline S. Identification of sleep-disordered breathing in children: variation with event definition. Sleep. 2002;25(1):72–79. doi: 10.1093/sleep/25.1.72. [DOI] [PubMed] [Google Scholar]
- 8.Whitelaw WA, Derenne JP, Milic-Emili J. Occlusion pressure as a measure of respiratory center output in conscious man. Respir Physiol. 1975;23(2):181–199. doi: 10.1016/0034-5687(75)90059-6. [DOI] [PubMed] [Google Scholar]
- 9.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87(2):626–633. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham DJ, Shaw DG, Lahiri S, Lloyd BB. The effect of maintained ammonium chloride acidosis on the relation between pulmonary ventilation and alveolar oxygen and carbon dioxide in man. Q J Exp Physiol Cogn Med Sci. 1961;46:323–334. doi: 10.1113/expphysiol.1961.sp001550. [DOI] [PubMed] [Google Scholar]
- 11.Zapletal A, Chalupova J. Nasal airflow and resistance measured by active anterior rhinomanometry in healthy children and adolescents. Pediatr Pulmonol. 2002;33(3):174–180. doi: 10.1002/ppul.10066. [DOI] [PubMed] [Google Scholar]
- 12.Akcay A, Kara CO, Dagdeviren E, Zencir M. Variation in tonsil size in 4- to 17-year-old schoolchildren. J Otolaryngol. 2006;35(4):270–274. doi: 10.2310/7070.2005.0118. [DOI] [PubMed] [Google Scholar]
- 13.Fregosi RF, Quan SF, Kaemingk KL, Morgan WJ, Goodwin JL, Cabrera R, Gmitro A. Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children. J Appl Physiol. 2003;95(5):2030–2038. doi: 10.1152/japplphysiol.00293.2003. [DOI] [PubMed] [Google Scholar]
- 14.Marcus CL, Gozal D, Arens R, Basinski DJ, Omlin KJ, Keens TG, Ward SL. Ventilatory responses during wakefulness in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1994;149(3 Pt 1):715–721. doi: 10.1164/ajrccm.149.3.8118641. [DOI] [PubMed] [Google Scholar]
- 15.Waters KA, McBrien F, Stewart P, Hinder M, Wharton S. Effects of OSA, inhalational anesthesia, and fentanyl on the airway and ventilation of children. J Appl Physiol. 2002;92(5):1987–1994. doi: 10.1152/japplphysiol.00619.2001. [DOI] [PubMed] [Google Scholar]
- 16.Anuntaseree W, Kuasirikul S, Suntornlohanakul S. Natural history of snoring and obstructive sleep apnea in Thai school-age children. Pediatr Pulmonol. 2005;39(5):415–420. doi: 10.1002/ppul.20207. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73(3):874–886. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 18.Lin KH, Wu HD, Chang CW, Wang TG, Wang YH. Ventilatory and mouth occlusion pressure responses to hypercapnia in chronic tetraplegia. Arch Phys Med Rehabil. 1998;79(7):795–799. doi: 10.1016/s0003-9993(98)90358-6. [DOI] [PubMed] [Google Scholar]
- 19.Stefanutti D, Fitting JW. Sniff nasal inspiratory pressure. Reference values in Caucasian children. Am J Respir Crit Care Med. 1999;159(1):107–111. doi: 10.1164/ajrccm.159.1.9804052. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald RS, Garfinkel F, Silbergeld E, Loscutoff SC. Factors in the interpretation of mouth occlusion pressure during measurements of chemosensitivity. Chest. 1976;70(1 Suppl):145–149. doi: 10.1378/chest.70.1_supplement.145. [DOI] [PubMed] [Google Scholar]
- 21.Garfinkel F, Fitzgerald RS. The effect of hyperoxia, hypoxia and hypercapnia on FRC and occlusion pressure in human subjects. Respir Physiol. 1978;33(2):241–250. doi: 10.1016/0034-5687(78)90073-7. [DOI] [PubMed] [Google Scholar]
- 22.Whitelaw WA, Derenne JP. Airway occlusion pressure. J Appl Physiol. 1993;74(4):1475–1483. doi: 10.1152/jappl.1993.74.4.1475. [DOI] [PubMed] [Google Scholar]
- 23.Mansell AL, Bryan AC, Levison H. Relationship of lung recoil to lung volume and maximum expiratory flow in normal children. J Appl Physiol. 1977;42(6):817–823. doi: 10.1152/jappl.1977.42.6.817. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21(3):176–183. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88(4):361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubern B, Tounian P, Medjadhi N, Maingot L, Girardet JP, Boule M. Pulmonary function and sleep-related breathing disorders in severely obese children. Clin Nutr. 2006;25(5):803–809. doi: 10.1016/j.clnu.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165(9):1251–1260. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]