Summary
The insulin-like signaling (ILS) pathway regulates metabolism and is known to modulate adult lifespan in C. elegans. Altered stress responses and resistance to a wide range of stressors are also associated with changes in ILS and contribute to enhanced longevity. The transcription factors DAF-16 and HSF-1 are key effectors of the longevity phenotype. We demonstrate that increased intrinsic thermotolerance, due to lower ILS, is not dependent on stress induced transcriptional responses but instead requires active protein translation. Translation profiling experiments reveal genes that are post-transcriptionally regulated in response to altered ILS during heat shock in a DAF-16-dependent manner. Furthermore, several novel proteins are specifically required for ILS effects on thermotolerance. We propose that lowered-ILS results in metabolic and physiological changes. These DAF-16-induced changes precondition a translational response under acute stress to modulate survival.
Introduction
An insulin-like signaling (ILS) pathway has profound effects on C. elegans development, fertility, stress resistance, metabolism and lifespan. Hypomorphic mutations in the gene encoding the insulin receptor-like protein, DAF-2, increase worm lifespan by 100% and affect various developmental and reproductive traits (Kenyon et al., 1993; Kimura et al., 1997; Jenkins et al., 2004). Downstream signaling components also exhibit large effects on lifespan including AGE-1, a PI3-kinase (Johnson, 1990; Kimura et al., 1997; Wolkow et al., 2000), which normally limits lifespan and DAF-16, a FOXO-like transcription factor, which promotes lifespan extension (Kenyon et al., 1993;Ogg et al., 1997).
Long-lived mutant worms are also better able to survive multiple types of extrinsic stresses including, but not limited to, thermal (Lithgow et al., 1995; Gems et al., 1998) and oxidative stress (Larsen, 1993; Vanfleteren, 1993). Resistance to heat shock may be measured as either intrinsic (having no pre-stress) or acquired thermotolerance gained from a non-lethal pre-stress. Induction of heat shock proteins (HSP) following such pre-treatments is an established paradigm to increase survival; many HSPs exhibit molecular chaperone activity and maintain protein homeostasis during and following stress. However, to survive fluctuating stresses likely requires multiple mechanisms. The underlying molecular and metabolic mechanisms of intrinsic and acquired thermotolerance may comprise unique and shared processes. Moreover, how ILS modulates resistance is not understood. Microarray analysis of mRNA abundance has identified stress response genes as a class that distinguishes long-lived mutant worms from wild type in early adult life in the absence of stress (Murphy et al., 2003; McElwee et al., 2003; Fisher and Lithgow, 2006). Some of these genes are known to be direct targets of the transcription factor DAF-16 (Oh et al., 2006). Long-lived mutants show moderate constitutive increases in small HSP mRNA (Murphy et al., 2003; McElwee et al., 2003; Hsu et al., 2003; Halaschek-Wiener et al., 2005) and accumulate elevated small HSP levels following stress (Walker et al., 2001). However, to date no constitutively elevated HSP proteins have been observed in long-lived ILS mutants. How then do daf-2 mutants survive acute heat stress when wild types perish? We focused on mechanisms by which the ILS impacts stress responses and determines survival.
Results
ILS modulates thermotolerance
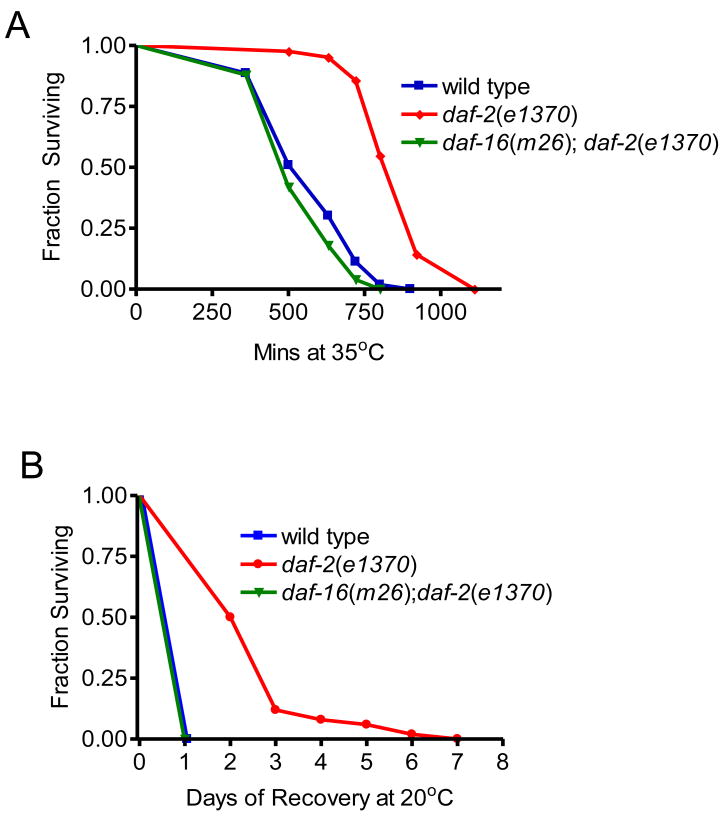
ILS determines intrinsic thermotolerance (referred to as thermotolerance), such that lowered ILS in daf-2 mutants significantly increases survival (Figure 1A). This increase is suppressed by mutation of daf-16, consistent with previous reports using daf-16(RNAi) knockdown (Hsu et al., 2003). We tested the physiological relevance of thermotolerance by returning populations to the optimal reproductive temperature following an acute stress (Figure 1B). daf-2(e1370) mutants not only survive for longer during heat shock, but continue to survive for many days following an acute heat shock (7h at 35°C), unlike wild type or daf-16(m26);daf-2(e1370). Furthermore, only daf-2(e1370) was observed to produce viable progeny (data not shown). This data is consistent with prior observation of age-1 mutant fertility following thermal stress (Lithgow et al., 1994). ILS enhances survival to both chronic and acute heat stress.
Figure 1. ILS increases Resistance and Survival to Heat Shock.
(A) daf-2(e1370) mutants have significantly increased thermotolerance (median survival 920 mins, n=42, p<0.0001) compared to wild type (630 min, n=53). Mutation of daf-16 suppresses thermotolerance increase (p<0.0001), daf-16(m26);daf-2(e1370) median survival 500 min, n=50. (B) daf-2(e1370) mutants have significantly increased survival (p<0.0001) following a 7h 35°C heat shock, compared to wild type or daf-16(m26);daf-2(e1370).
ILS modulates induced HSP mRNA
We postulated that daf-2 mutants would exhibit up-regulated transcription of HSP genes in response to an acute heat stress. We utilized quantitative real-time PCR (RT-PCR) to assay constitutive and heat shock-induced (2h at 35°C) HSP mRNA in individual worms. Initially, we determined that mRNAs corresponding to the house keeping genes, gpd-1 and gpd-2, were invariant in wild type, daf-2(e1370) and daf-16(m26);daf-2(e1370) under both control (CTL) and heat shock (HS) conditions (Figure S1A-B). These genes were then used to derive Calibrated Normalized Relative Quantity (Vandesompele et al., 2002; Hellemans et al., 2007) values of mRNA levels for candidate hsp genes. As expected mRNA for hsp-12.6, hsp-16.2 and hsp-70 (C12C8.1) were all markedly heat-induced (Figure 2). Mutation of daf-2 led to elevated stress-induced levels of all three genes in a daf-16-dependent manner. Similar data was observed for hsp-16.1, whilst minor effects on mRNA levels were seen for hsp-1, hsp-6 and none for the hsp-90 ortholog, T05E3.11 (Figure S1C-F). These results are consistent with previous qualitative data (Hsu et al., 2003) and indicate that daf-2 mutants induce higher levels of select HSP-encoding mRNAs. The mechanism of mRNA up-regulation and its contribution to thermotolerance were examined further.
Figure 2. ILS effects on Heat Shock Induced HSP mRNA levels.
Average expression levels of (A) hsp-12.6, (B) hsp-16.2 and (C) hsp-70. Relative expression levels were derived from Calibrated Normalized Relative Quantities using the geometric mean of 2 ‘house keeping’ genes, gpd-1 and gpd-4 and are plotted as arbitrary units (A.U) ±S.E.M. Data from n=15 individual worms for wild type and daf-16(m26);daf-2(e1370) groups, and n=14 for the daf-2(e1370) groups. For all genes and in all genotypes the Heat shock (HS) treatment (2h at 35°C) significantly increased relative mRNA levels (p<0.001) compared to genotype matched controls (CTL). Asterisks above CTL bars mark significance compared to wild type CTL, while those above HS bars are against wild type HS values (* p<0.05, ** p<0.01 and *** p<0.001).
Heat Shock Binding Activity of HSF-1 is unaltered by ILS
To investigate ILS modulation of the transcriptional response to heat shock we examined the activity of the heat shock factor (HSF-1). HSF-1 is a major transcriptional regulator of hsp genes and so we compared its specific DNA-binding activity between wild type and daf-2 mutants. Nuclear protein extracts were isolated from synchronous populations of adult hermaphrodites treated with or without a heat shock. Extracts were then incubated with radio-labeled ds-DNA containing three HSE (5′-nGAAn-3′) motifs found in alternating orientation to which HSF-1 will specifically bind (GuhaThakurta et al., 2002). The interaction between HSF-1 and the ds-DNA HSE probe was validated via an Electrophoretic Mobility Shift Assay (EMSA) in two ways. Firstly, we observe a marked increase in HSE binding activity from HS samples (Figure 3A). Secondly, we used two unlabelled ds-DNA with mutated HSE sequences in molar excess for competitive binding experiments. Scrambling the nucleotide sequence of all three HSEs or substituting a single base-pair in the middle HSE of our cold ds-DNA probe abolished their competitive binding (Figure 3A). These results demonstrate that our assay detects a heat shock-induced activity that binds specifically to the HSE sequences, and as such is consistent with C. elegans HSF-1. However, no alteration in HSE binding activity of HSF-1 in ILS mutants in control or HS treated samples was found (Figure 3B). These results are consistent with ILS-effects on HSP expression following acute stress acting via a pathway parallel to HSF-1.
Figure 3. ILS Does Not Alter HSF-1 DNA-Binding Activity and HSF-1 is Not Required for Thermotolerance.

(A) Validation of EMSA of HSF-1-HSE DNA binding following a HS. Lane 1 free ds-DNA probe; lane 2 control nuclear protein extract (NPE); lane 3 NPE from samples treated with a 4h 35°C HS. Lane 4, NPE from HS with molar excess of cold HSE, lane 5 NPE from HS with molar excess of cold HSE-mut1, lane 6 NPE from HS with molar excess of cold HSE-mut2 (see experimental procedures). Data shown is representative of replicate assays. (B) ILS does not alter HS induced DNA binding activity of HSF-1. Lane 1 free ds-DNA probe; lane 2 wild type NPE; lane 3 daf-2(e1370) NPE; lane 4 daf-16(m26) NPE. No heat shock controls (-), 1h of 35°C HS (+1) and 4h of 35°C HS (+4). Representative image of duplicate experiments. (C) Fluorometric analysis showing Relative Fluorescence Units (R.F.U) during recovery at 25°C of wild type and daf-2(e1370) mutants, which show unaltered GFP induction of a hsp-16.2:gfp transcriptional reporter and hence increased hsp-16.2 promoter activity following a HS compared to wild type. Treatments were controls (CTL), and HS of 33°C for 2h (HS). Error bars ± 2× S.E.M.. (D) Loss of HSF-1 activity abolishes mean GFP induction following a HS. Error bars ± 2× S.E.M. (E) Immunoblot detection of HSP-16.2 in wild type and hsf-1(sy441) mutants following an inducing HS. hsf-1(sy441) mutants express greatly reduced HSP-16.2. No HS control (-) and 2h 33°C HS with 6h recovery (+). Equivalent loading of total protein per lane demonstrated via detection of β-ACTIN. (F) Loss of HSF-1 does not affect intrinsic thermotolerance to 35°C heat stress, but does limit acquired thermotolerance. Kaplan-Meier survival curves show wild type and hsf-1(sy441) mutants have equivalent thermotolerance, median survival 420 min (wild type, n= 53) and 430 min (hsf-1(sy441), n=52) respectively (not significant). Pretreatment (30°C for 6h, followed by 12h recovery at 20°C, shown by dashed lines) significantly increases wild type thermotolerance, p<0.0001 (700 min, n=56). hsf-1(sy441) mutants also show a gain of thermotolerance following pretreatment, p<0.0001 (660 min, n=54), however, this gain is reduced compared to that observed in wild type, p<0.0001. (G) Loss of wild type HSF-1 activity does not affect daf-2(RNAi) gains in thermotolerance. Wild type and hsf-1(sy441) mutants have equivalent thermotolerance, median survival 540 min (wild type, n= 47) and 600 min (hsf-1(sy441), n=40) respectively (not significant). Pretreatment with daf-2(RNAi) significantly increases (p<0.0001) both wild type (1020 min, n=62) and hsf-1(sy441) thermotolerance (1020 min, n=45).
The hsp-16.2 5′-transcriptional promoter does not mediate ILS regulation
Since we failed to see differences in HSF binding activity, we then re-examined if the promoter of hsp-16.2 was sufficient to confer ILS regulation under heat shock. Previously we reported ILS effects on a hsp-16.48∷LacZ reporter, in which the hsp-16.48 promoter, exon 1 and intron were translationally fused to LacZ. Heat shock induced greater LacZ staining in lowered-ILS age-1(hx546) mutants compared to wild type (Walker et al., 2001). To investigate this further, we developed a non-invasive, real time assay of hsp-16.2 5′-transcriptional promoter (346 bp) activity in live adult worms (Figure S2A-C) using an established green fluorescent protein (GFP) reporter strain CL2070 (dvls70[pCL25(hsp-16.2:gfp) + pRF4]) (Link et al., 1999). The effect of mutations in ILS on the kinetics of GFP induction was examined by crossing the reporter construct into a series of mutants. The integrated hsp-16.2:gfp reporter itself did not alter the respective thermotolerance or longevity phenotypes of the ILS mutants (data not shown). Consistent with the EMSA results, we observed that daf-2(e1370) mutants showed no change to fluorescence after HS (Figure 3C and S2D). Similar results were observed using daf-2(e1368) and age-1(hx546) alleles (Figure S2E-F). Furthermore, loss-of-function daf-16(m26) and null daf-16(mu86) mutants also had unaltered HS induced fluorescence (Figure S2G-H). However, ILS regulation of expression was restored when we examined a translational fusion reporter of hsp-16.2∷GFP (which included promoter and ORF sequence) (Figure S2I-L). Following an inducing heat shock we observed robust fluorescence increases in daf-2(e1370) compared to a more modest induction in wild type.
These results are consistent with our observation that daf-2(e1370) mutants exhibit higher levels of multiple endogenous HSP mRNAs (Figure 2) and with earlier LacZ experiments (Walker et al., 2001). For hsp-16.2, it appears that ILS can act via a downstream transcriptional enhancer. We propose that ILS up-regulates stress responses via a mechanism independent of HSF-1 interactions with HSE promoter sequences.
We note that the elevated mRNA transcript levels of the endogenous hsp-16.2 gene, seen in the ILS-mutants, may also be due to altered mRNA stability. However, we determined that hsp-16.2 mRNA declines by similar rates following a heat stress in wild type, daf-2(e1370) and daf-16(mu86) mutant worms (Figure S2M-O). In addition, we note that ILS may also modulate specific mRNA levels via cis-acting effects from sequences outside the 5′-transcriptional promoter (Hsu et al., 2003).
HSF-1 is not required for Intrinsic Thermotolerance
Our results show that lowered-ILS mutants exhibit greater stress resistance and elevated induced HSP mRNAs, yet have unaltered HSF-1 DNA-binding activity, unaltered hsp-16.2 5′-transcriptional promoter activity and unaltered hsp-16.2 mRNA clearance following heat shock. To further define the role of HSF-1 function in determining thermotolerance during an acute stress in C. elegans we examined HSF-1 loss-of-function allele, hsf-1(sy441) (Hajdu-Cronin et al., 2004). Whilst this allele is not null, hsf-1(sy441) has greatly reduced stress induced HSP mRNA. When combined with the hsp-16.2:gfp reporter the hsf-1(sy441) mutants all but abolished GFP induction following an inducing HS (Figure 3D). Similarly, heat shock-induced HSP-16 expression was barely detectable in hsf-1(sy441) mutants compared to wild type (Figure 3E). However, despite being defective in HSP induction, the loss-of-function HSF-1 allele had no effect on thermotolerance when compared to wild type (Figure 3F).
Since this was a somewhat surprising observation we then further examined the role of HSF-1 in acquired thermotolerance. In C. elegans pretreating populations with a heat shock prior to a lethal stress is known to increase thermotolerance, via acquired thermotolerance (Lithgow et al., 1995; Cypser and Johnson, 2002). We examined different pretreatments and observed that 30°C for 6h followed by 12h recovery robustly increased wild type thermotolerance (Figure S2P). When given the same pretreatment, hsf-1(sy441) mutants still acquired an increase in thermotolerance although less than that seen in wild type (p<0.0001, Figure 4E), demonstrating that acquired thermotolerance is at least partially dependent on HSF-1.
Figure 4. Inhibition of Transcription Does Not Alter Thermotolerance.
(A) Effects of inhibition of transcription by α-amanitin pretreatment prior to inducing HS. Increasing concentrations of α-amanitin blocked HS induced GFP induction. Plotted is the Relative Fluorescence (R.F) versus time during recovery at 25°C. Error bars ±2× S.E.M. (B) Average expression levels of hsp-12.6, hsp-16.1 and hsp-16.2 following a heat shock (HS, 2h at 35°C), with and without a 1h pretreatment with α-amanitin (α-aman). Relative expression levels were derived from Calibrated Normalized Relative Quantities using the geometric mean of 2 ‘house keeping’ genes, gpd-1 and gpd-4 and are plotted as arbitrary units (A.U) ±S.E.M. Data from n=11 individuals. For all genes the heat shock (+, HS) treatment significantly increased relative mRNA levels (p<0.001) compared to controls (-). α-amanitin pretreatment significant reduced stress induced expression for all genes (p<0.001). (C) Immunoblot using anti-HSP-16.2 antibody of untreated (-) and heat shocked (+, HS) (33°C for 2h with a 12h recovery, HS) wild type populations with and without a pretreatment of 100ug/ml α-amanitin (as previously described). (D) Inhibition of transcription does not affect intrinsic thermotolerance in wild type, daf-2(e1370) or daf-16(m26) mutants. Kaplan-Meier survival curves show daf-2(e1370) mutants have significantly increased thermotolerance compared to wild type (p<0.0001) and daf-16(m26) mutants (p<0.0001), respectively. Pretreatment with 100 mg/ml α-amanitin (α-aman) did not alter thermotolerance of any genotype. Median survival were 480 min (wild type, n=50), 480 min (wild type + α-amanitin, n=50), 590 min (daf-2(e1370), n=51), 590 min (daf-2(e1370) + α-amanitin, n=52), 480 min (daf-16(m26), n=52), 590 min (daf-16(m26) + α-amanitin, n=50). (E) Inhibition of transcription significantly reduces resistance to the oxidative stressor juglone. Median survival of wild type was significantly reduced following pretreatment with 100 mg/ml α-amanitin (α-aman), p<0.0001. Median survival were 195 min (wild type, n=46) versus 135 min (wild type + α-amanitin, n=45). Median survival of daf-16(mu86) (185 min, n=47) was also significantly reduced compared to wild type, p<0.05.
We then reexamined hsf-1 mutant thermotolerance when ILS is reduced. When both wild type and hsf-1 mutants were pretreated with daf-2(RNAi) we observed an equivalent and significant increase in thermotolerance (Figure 3G). We conclude that the HSP-inducing activity of HSF-1 is not required for either wild type thermotolerance or the elevated thermotolerance from lowered ILS.
De novo Transcription is not required for ILS mediated Thermotolerance
Since the major transcriptional regulator of hsp gene expression, HSF-1, is not essential for thermotolerance we then assessed the requirement for de novo transcription in the determination of thermotolerance in wild type or altered ILS mutants. We utilized α-amanitin to inhibit de novo synthesis of mRNA during a lethal heat shock. Using a range of concentrations we determined that exposure to 100 μg/ml α-amanitin for 1h silenced the hsp-16.2:gfp transcriptional reporter following an inducing heat shock for at least 12h (Figure 4A). Pretreatment with α-amanitin at this dose dramatically reduced heat shock-induced mRNA for hsp-12.6, hsp-16.1 and hsp-16.2 in wild type (Figure 4B). Stress induced HSP-16 protein levels were also reduced by α-amanitin (Figure 4C). Exposure to α-amanitin transiently reduced adult motility, and we observed no effect on survival 12 or 24h after the inducing heat shock (data not shown).
Inhibiting transcription prior to a lethal heat shock had no effect on the thermotolerance of wild type or on ILS-mutants, daf-2(e1370) and daf-16(m26) (Figure 4D). These results demonstrate that intrinsic thermotolerance is not dependent on an induced transcriptional response. This observation is consistent with earlier observations of hsf-1 mutant thermotolerance with and without daf-2(RNAi) (Figure 3G).
We did note that chronic exposure to α-amanitin was detrimental. Wild type and daf-2(e1370) life spans were significantly reduced when cultured in 100 μg/ml α-amanitin (p<0.001, Figure S3). We also noted that while de novo transcription may be unimportant for C. elegans thermotolerance, this was not true for other stressors. We observed that transcription is required for wild type resistance to 500μM juglone, an oxidative stressor (Figure 4E). Loss of transcription significantly reduced wild type resistance compared to both untreated controls (p<0.001) and null daf-16(mu86) mutants (p<0.001).
Translation is required for ILS mediated Thermotolerance
Cycloheximide was then used to block protein synthesis prior to a lethal heat stress to examine the role of mRNA translation in thermotolerance. We initially determined a dose of cycloheximide that would silence stress-induced GFP translation from the hsp16.2:gfp reporter strain (Figure 5A). The highest dose used, 10mM cycloheximide, greatly reduced heat shock induced GFP expression. Although inhibition was not complete we used no higher concentration to avoid complication of generalized toxicity. Inhibition of translation prior to a lethal stress had no effect on wild type thermotolerance (Figure 5B and 5C). However, the increased thermotolerance of lowered-ILS mutants, daf-2(e1370) and age-1(hx546) were both completely abolished (p<0.0001). We concluded that translation is essential for lowered-ILS increases in thermotolerance.
Figure 5. Thermotolerance of ILS Mutants Determined by de novo Translation.
(A) Effects of inhibition of translation by cycloheximide pretreatment prior to inducing HS. Increasing concentrations of cycloheximide reduced HS induced GFP induction. Plotted is the Relative Fluorescence (R.F) versus time during recovery at 25°C. Error bars ±2× S.E.M. (B) Inhibition of translation suppresses daf-2(e1370) gains of thermotolerance. Kaplan-Meier survival curve shows daf-2(e1370) mutants compared to wild type have significantly increased thermotolerance, median survival 360 min (wild type, n=53) versus 480 min (daf-2(e1370), n=57), p<0.0001. However, treatment with 10 mM cycloheximide (CHX), significantly reduces (daf-2(e1370) survival (360 min, n=55), p<0.0001. No effect was observed in wild type (360 min, n=54), not significant. (C) Inhibition of translation suppresses age-1(hx546) gains of thermotolerance. Kaplan-Meier survival curve shows age-1(hx546) mutants compared to wild type have significantly increased thermotolerance, median survival 360 min (wild type, n=53) versus 480 min (age-1(hx546), n=54), p<0.0001. Treatment with 10 mM cycloheximide (CHX), significantly reduces (age-1(hx546) survival (360 min, n=57), p<0.0001.
ILS mediated changes to Ribosomal loading on mRNAs under Heat Shock
We then considered that increased thermotolerance of lowered-ILS mutants was likely dependent on de novo translational regulation of, as yet, unknown gene products. To identify these critical genes and to better understand the physiological processes they determine, we used Translation State Array (TSA) Analysis (Dinkova et al., 2005). A schematic overview of this experiment is shown in Figure 6A. In summary, gene products actively translated under heat shock in an ILS-mutant background were identified on the basis of ribosome loading. mRNAs with the highest loading of ribosomes are consistent with mRNAs being translated efficiently. We isolated total RNA from Control (CTL) and Heat Shocked (HS) populations of wild type, daf-2(e1370) and daf-16(m26); daf-2(e1370) nematodes at four days of age. Since ILS-increased thermotolerance is dependent on DAF-16 (Figure 1) we included the daf-16(m26); daf-2(e1370) double mutant in this analysis to identify genes that behaved in a DAF-16 dependent manner. The duration of stress was limited to 2h at 35°C, so as to avoid lethality in any population (data not shown). Extracted RNA was fractioned based on ribosomal loading and pooled into ribosomal and free RNA (F1), light polysomes (F2) and heavy polysomes (F3). Each fraction was arrayed against a common total RNA (mixed stage) reference. A representative trace of A260 indicates RNA content across the fractions from wild type CTL and HS samples is shown in Figure 6A. As expected the heat shock treatment had a severe and generalized effect on ribosome loading, and hence global translation, by greatly reducing the proportion of mRNAs bound to polysomes. Within wild type, the abundance of transcripts in each fraction was positively correlated when compared pair-wise in CTL and HS treatments respectively (Figure S4A-B). Similar results were observed for the other genotypes assayed (data not shown), suggesting that mRNA abundance in any fraction scales, in general, with overall abundance. Furthermore, following the heat stress this relationship was preserved.
Figure 6. Translation State Array Analysis of Heat Shock Responses in C. elegans.
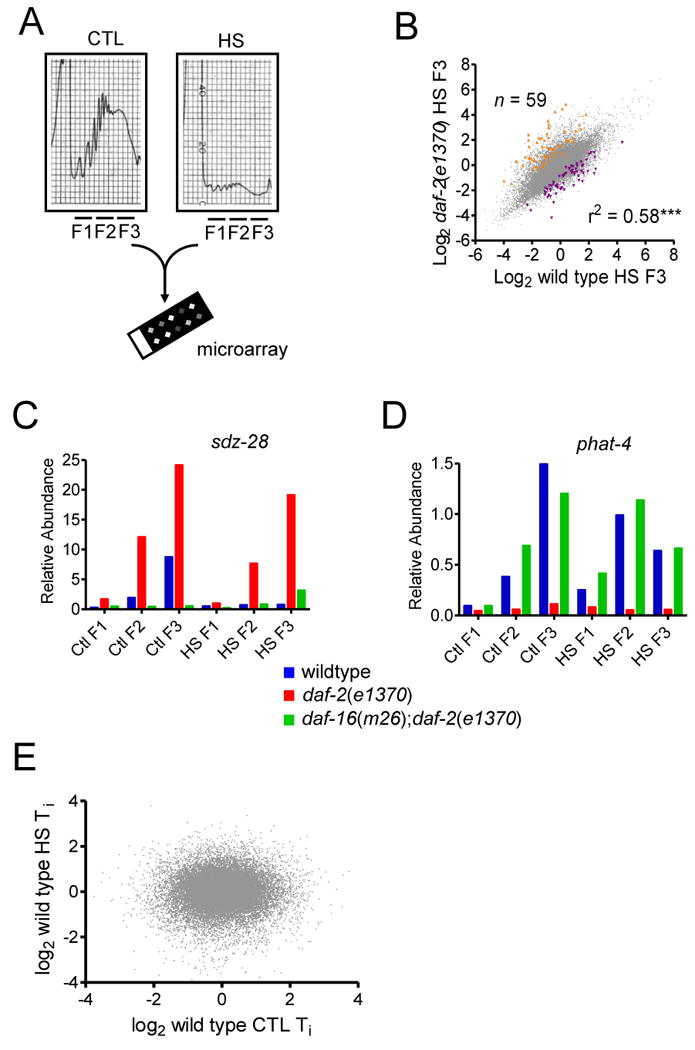
(A) Schematic of Translation State Array experimental design. (B) Scatter plot of log2 wild type HS F3 versus daf-2(e1370) log2 HS F3 versus. Transcripts with significantly altered abundance were identified via t-tests and a FDR ≤ 0.05. Transcripts significantly increased in daf-2(e1370) shown in orange (n = number of data points) and those decreased shown in purple, r2 denotes the correlation coefficient and ** p<0.01. (C) Plot of Relative Abundance of the sdz-28 transcript in each fraction (F1-F3) under control (CTL) and HS treatments (HS). Each bar represent mean of 4 biological replicates. (D) Plot of Relative Abundance of the phat-4 transcript in each fraction (F1-F3) under control (CTL) and HS treatments (HS). Each bar represent mean of 4 biological replicates. (E) Scatter plot of log2 wild type CTL Ti (i.e. F3/F2) versus log2 wild type HS Ti.
Comparisons of CTL versus HS data sets identified major changes to ribosomal loading and hence translational outputs. We identified genes with altered transcript abundance in F3 under HS compared to CTL in wild type. Using t-tests and a False Discovery Rate (FDR) (Benjamini and Hochberg, 1995) of ≤ 0.05, we identified 722 transcripts elevated in HS F3 compared to CTL F3 (Table S2 i). Many of the genes identified showed elevated levels in three HS fractions (F1-3) compared to CTL fractions. This finding is consistent with a transcriptional response to HS, and as expected this class of genes included HSP genes. For example, in wild type hsp-16.2 mRNA was increased >26 fold in all fractions following HS (Table S2 and Figure S4C). Despite the increased abundance of hsp-16.2 mRNA (and other HSP transcripts) during HS the induction does not impact on wild type or ILS thermotolerance during acute stress. Note that we also failed to detect a significant shift in ribosome loading for hsp-16.2 mRNA. This may be due the fact that a short mRNA cannot support high ribosome loading and consequently falls below our detection limit. In addition to mRNAs that are elevated in fraction 3, 776 transcripts with reduced-abundance in F3 under HS were observed (Table S2 ii).
Transcripts with elevated HS F3 abundance in daf-2(e1370)
A scatter plot of HS F3 wild type versus daf-2(e1370) abundance showed a significant positive correlation (r2=0.58, p<0.001) (Figure 6B). This indicates that under heat stress, the highly translated F3 fraction in wild type and daf-2(e1370) share many transcripts in common. However, using two-sample t-tests and a FDR significance cut-off of ≤0.05 we identified 59 transcripts with significantly increased abundance in daf-2(e1370) and 68 with decreased abundance (Table S3). Of the 59 transcripts showing elevated abundance in HS F3 vs CTL F3 10 contained either F-box, BTB/POZ and/or MATH-domains. This enrichment of proteins with domains involved in protein-protein interaction, and in particular with proteasomal process, may indicate a mechanism to increase targeted protein degradation.
Shown in Figure 6C is an example of a gene with increased HS F3 abundance in daf-2(e1370) compared to wild type sdz-28 (SKN-1 Dependent Zygotic transcript-1) encodes an uncharacterized protein, containing BTB/POZ and MATH domains. In daf-2(e1370) sdz-28 mRNA levels are elevated in the translated fractions (F2 and F3), under both CTL and HS conditions. These effects appear dependent on DAF-16; daf-2 seems to regulate sdz-28 both at the level of transcription in a daf-16-dependent manner and during HS high F3 abundance is maintained, suggesting cellular processes maintain translation of this mRNA. RNAi knockdown of sdz-28 prior to a HS resulted in a partial suppression of daf-2(e1370) thermotolerance but with no effect in wild type (Figure S4D), suggesting sdz-28 encodes a component of the ILS effects on thermotolerance.
A similar result is observed for fbxb-22 (F-Box B protein-22), which encodes a protein containing an F-box motif predicted to mediate protein-protein interactions either with homologs of yeast Skp-1p or with other proteins (Figure S4E). Many of the genes identified in Table S3 appear to have mRNAs that are constitutively elevated in CTL F3 of daf-2(e1370) and this high abundance is maintained under heat shock, consistent with a direct involvement in modulating thermotolerance (data not shown).
For transcripts with reduced HS F3 abundance compared to CTL F3 in daf-2(e1370) we observed an overall decrease in abundance across all fractions (data not shown). This is consistent with transcriptional silencing, rather than shifts in ribosomal loading per se. As an example, phat-4 (PHAryngeal gland Toxin-related-4) abundance is significantly lowered in all daf-2(e1370) fractions, regardless of whether samples were heat shocked or not (Figure 6D). phat-4 appears to be ILS-regulated via daf-16-dependent transcriptional suppression.
mRNA Transcripts with elevated Translation Index under Heat Shock
We then focused on mRNA transcripts unique to daf-2(e1370) mutants that exhibited increased translation under heat stress. The ratio of F3 / F2 transcript abundance can be used to determine the translation index (Ti), an indice of translation efficiency. Higher Ti indicates greater ribosomal loading, which is consistent with increased translation. Comparisons of Ti across treatments and genotypes can then help identify key mRNAs likely to be under translational regulation. A scatter plot of wild type CTL versus HS log2 [Ti] showed no correlation (Figure 6E), indicating that during HS there are no systematic changes in Ti, consistent with major changes in how transcripts are being loaded with ribosomes and hence translated. Similarly, no correlation was observed for log2 CTL [Ti] versus log2 HS [Ti] in daf-2(e1370) or in daf-16(m26);daf-2(e1370) mutants (Figure S4F-G).
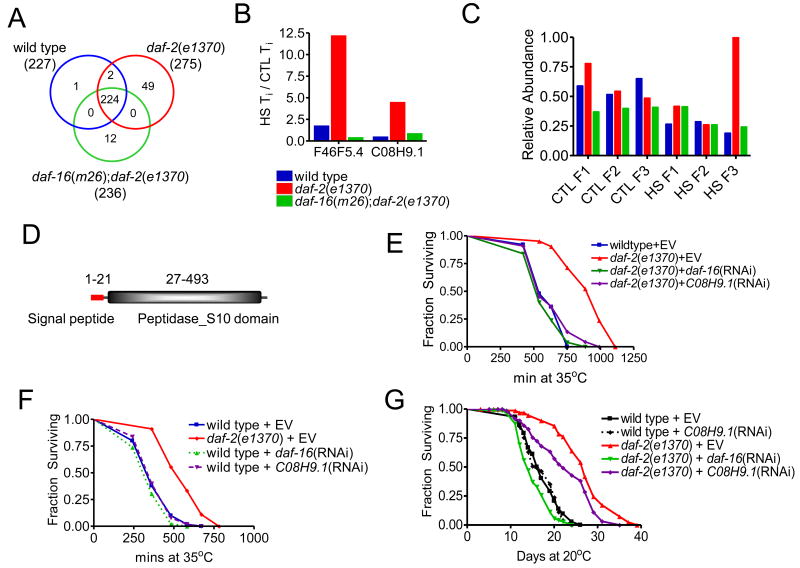
We then examined transcripts for which Ti specifically increased under stress compared to CTL in daf-2(e1370) mutants alone. We used the ratio of [HS Ti] / [CTL Ti] in all genotypes to identify transcripts that showed a re-distribution of mRNA from F2 to F3 specifically during heat shock, rather than an overall increase in transcript level (Table S4). Using two-sample t-tests and a FDR cut-off (Benjamini and Hochberg, 1995) ≤ 0.05 we found 227 transcripts in wild type, 275 in daf-2(e1370) and 236 in daf-16(m26); daf-2(e1370) that met this criterion. A Venn diagram illustrates the overlap of transcripts identified in each genotype (Figure 7A). The high number of transcripts common to all three genotypes (224) suggests we have observed a conserved response to the heat-induced stress. We observed 49 genes unique to daf-2(e1370) and then arbitrarily limited further analysis to genes for which the [HS Ti] to [CTL Ti] ratio in daf-2(e1370) was at least twice that in wild type or daf-16(m26); daf-2(e1370). Closer examination of the remaining five mRNA transcripts revealed two genes (F46F5.4 and C08H9.1) for which heat shock resulted in a DAF-16-dependent Ti increase. RNAi knockdown of F46F5.4 did not appear to affect thermotolerance of wild type or daf-2 mutants (data not shown) and was not examined further.
Figure 7. C08H9.1 is post-transcriptionally regulated by ILS and required for increased ILS-mediated thermotolerance.
(A) Venn diagram of transcripts identified as having significantly elevated Ti under HS compared to CTL. Shown is number of transcripts shared and unique to each genotype respectively. (B) Graph of the HS Ti / CTL Ti ratio for transcripts F46F5.4 and C08H9.1 in each genotype (as shown). (C) Relative abundance of C08H9.1 transcripts in each fraction, where F1, F2 and F3 represent unbound mRNA, light polysomes and heavy polysomes during control (CTL) and HS conditions (HS). Each bar represents the mean relative abundance measured in four biological replicates. (D) Schematic of the predicted domain organization of the C08H9.1 protein. (E) C08H9.1 is essential for increased daf-2(e1370) thermotolerance at 35°C. Kaplan-Meier survival curves showing daf-2(e1370) mutants have significantly increased thermotolerance compared to wild type, p<0.0001. Median survival: 540 min (wild type + empty vector (EV), n=50) versus 990 min (daf-2(e1370) + EV, n=42). (C08H9.1) RNAi suppression of elevated daf-2(e1370) thermotolerance (p<0.0001) is comparable to daf-16(RNAi). Median survival 540 min (daf-2(e1390) + daf-16(RNAi), n=50) and 540 min (daf-2(e1390) + C08H9.1(RNAi), n=44). (F) C08H9.1 does not modulate wildtype thermotolerance at 35°C. Kaplan-Meier survival curves showing daf-2(e1370) mutants have significantly increased thermotolerance compared to wild type, p<0.0001. Median survival: 360 min (wild type + empty vector (EV), n=50) versus 580 min (daf-2(e1370) + EV, n=45). Neither daf-16(RNAi) nor (C08H9.1) RNAi altered wild type survival. Median survival 360 min (wild type + daf-16(RNAi), n = 46) and 360 min (wild type + C08H9.1(RNAi), n=50). (G) C08H9.1 is required for daf-2(e1370) but not wild type longevity at 25°C. Kaplan-Meier survival curves showing daf-2(e1370) mutants are long lived compared to wild type, p<0.0001. Median lifespan: 17 (days) (wild type + empty vector (EV), n=72) versus 27 (daf-2(e1370) + EV, n=42). daf-16(RNAi) reduced daf-2(e1370) lifespan, p<0.0001, median life span: 14 (daf-2(e1390) + daf-16(RNAi), n=75). (C08H9.1)RNAi moderately reduced daf-2(e1370) lifespan, p<0.0026, median life span: 22 (daf-2(e1390) + C08H9.1(RNAi), n=65). In contrast, C08H9.1(RNAi) had no effect on wild type lifespan, median lifespan: 16 (wild type + C08H9.1(RNAi), n=72).
The role of C08H9.1 in lowered-ILS Thermotolerance
We then focused our investigation on C08H9.1, which encodes a putative lysosomal serine carboxypeptidase. In daf-2(e1370) mutants C08H9.1 mRNA is significantly enriched in F3, the highly translated fraction, during stress (Figure 7B). This is consistent with a shift towards increased translation during heat shock in daf-2(e1370) mutants. This effect on C08H9.1 transcript abundance is DAF-16 dependent, as loss-of-function daf-16 mutation returns mRNA distribution abundance back to that of wild type. Under CTL conditions C08H9.1 transcripts are not elevated in F3 of daf-2(e1370), suggesting C08H9.1 ribosomal loading in daf-2(e1370) during heat shock is adaptive (Figure 7C). Analysis of the predicted C08H9.1 protein using Pfam (Finn et al., 2008) predicts a domain organization containing a short N-terminal signaling sequence and a S10 peptidase domain, with an overall homology with lysosomal cathepsin A (Figure 7D).
To validate the potential role of C08H9.1 in the determination of thermotolerance we utilized RNAi to knock down its expression for 24h prior to a lethal heat shock. In a resistant daf-2(e1370) background C08H9.1(RNAi)completely abolished the increase in thermotolerance (p<0.0001) (Figure 7E). This reduction in daf-2(e1370) thermotolerance was as effective as that from daf-16(RNAi), suggesting C08H9.1 is major determinant of ILS-effects on thermotolerance and is as important as DAF-16 in modulating thermotolerance. However, C08H9.1(RNAi) had no effect on wild type thermotolerance (Figure 7F), consistent with our observation that in wild type this transcript is not induced under heat stress and the ribosomal loading of C08H9.1 mRNA does not appear to be changed (Figure 7B-C). C08H9.1(RNAi) also had no effect on daf-16(m26);daf-2(e1370) thermotolerance (Figure S5A), consistent with C08H9.1 effects being dependent on DAF-16.
Chronic knockdown of C08H9.1 via RNAi over the entire lifetime had no measurable effect on wild type longevity. However, a modest but significant suppression of longevity (∼19% reduction in median lifespan, p<0.0001) was observed in daf-2(e1370) (Figure 7G), suggesting that in lowered ILS C08H9.1 function is specifically recruited to increase not only thermotolerance but aspects of longevity.
Discussion
Several findings support an association between longevity and cellular stress response, including HSP expression, in C. elegans. The Heat Shock Response (HSR) is a conserved and regulated genetic response to diverse environmental and physiological stressors that results in the rapid induction of genes encoding molecular chaperones (including HSPs) and additional proteins that protect and assist in recovery from the cellular damage associated with expression and accumulation of misfolded protein. The HSR and HSP induction have long dominated the stress literature as having critical roles in cellular protection against heat shock and other cytotoxic stressors (Morimoto, 2008).
In the nematode model, over-expression of HSF-1 extends life span (Hsu et al., 2003) and both lowered-ILS and dietary restriction effect on longevity are dependent on HSF-1 (Morley and Morimoto, 2004; Steinkraus et al., 2008). These observations provide support for the hypothesis that the rate of aging may be modulated by the ability of cells/tissues/organisms to withstand the effects of stress by maintaining protein homeostasis and hence normal function.
Here we have presented results consistent with a novel conceptual framework to further understand how whole organisms tolerate extreme heat stress and how ILS modulates this resistance. These results challenge the notion that transcriptional regulation of HSP genes is a preeminent mechanism of survival during acute heat shock. Although HSPs contribute to acquired thermotolerance, their stress induction appears not to be essential for wild type or ILS-mediated intrinsic thermotolerance in C. elegans. ILS mutants do have altered stress-induced expression of HSP, but this may not be the primary reason underlying their increased resistance to acute heat stress. In wild type, survival to a lethal 35°C heat shock is not altered when, prior to the stress, either transcription or translation is inhibited. This suggests that following a shift to extreme temperature, wild type C. elegans are limited in responses capable of modulating survival. However, lowered-ILS mutants have greatly increased survival. This resistance is not dependent on wild type HSF-1 activity or normal transcription, but rather, is dependent on de novo synthesis of novel proteins. We noted that the transcriptional inhibitor used, α-amanitin, did not completely inhibit all RNA polymerase II transcription under stress but our data strongly suggest that transcription of HSP genes does not predict thermotolerance. We propose that lowered-ILS (such as daf-2 mutation) results in DAF-16-induced changes in cells during permissive conditions that regulate a translational response during acute stress. This process may directly involve regulators of protein synthesis, such as the target of rapamycin (mTOR) - ribosomal S6 protein kinase (S6K1) pathway (Kaeberlein and Kapahi, 2009). However, the molecular details of how translational is regulated for specific transcripts requires further investigation.
Our findings on the role of HSF-1 on thermotolerance, appear at odds with data derived from RNAi experiments used to knock-down hsf-1 transcripts (Walker et al., 2003). hsf-1(RNAi) limits wild type thermotolerance whilst loss-of-function hsf-1(sy441) mutants exhibit no reduction of thermotolerance. The hsf-1(sy441) allele utilized in this study carries a G-to-A mutation that is predicted to cause a missense truncation of the last 86 amino acids (Hajdu-Cronin et al., 2004). This mutation clearly prevents heat induced HSP induction (Figure 4C and D), however, any additional activities of HSF-1 may be unaffected. hsf-1(RNAi) experiments examined thermotolerance in the F1 generation (Walker et al., 2003), such that maternal and developmental effects of hsf-1 knock-down could factor in reduced adult resistance. Indeed hsf-1(sy441) animals appear to escape the developmental defects reported for hsf-1(RNAi) (Walker et al., 2003; Hajdu-Cronin et al., 2004). However, hsf-1(sy441) does have a reduced life span compared to wild type (Hajdu-Cronin et al., 2004) (Figure S5B).
In contrast, acquired thermotolerance does require wild type HSF-1 activity. In Figure S2P we describe a heat shock-pretreatment regime that results in robust gains in acquired thermotolerance. This increased resistance is partially dependent on HSF-1 (Figure 3F), illustrating that prior HSP induction is a component of acquired thermotolerance. Although transgenic over-expression of hsf-1 extends C. elegans lifespan (Hsu et al., 2003) any effects on thermotolerance remain uncharacterized. Interestingly, daf-16(mu86) null mutants also show a robust increase in acquired thermotolerance (Figure S5C), similar to previous reports (Cypser and Johnson, 2002). This demonstrates that despite having reduced thermotolerance, daf-16 mutants can still acquire resistance, presumably via HSP induction. This is consistent with the observation that daf-16 mutants retain a robust induced increase in HSP mRNA expression (Figure 2 and S1) and HSF-1 DNA-binding activity (Figure 4B). Hypoxia inducing factor-1 (HIF-1) is an additional transcription factor which has been shown to have a role in the more gradual process of heat acclimation (Treinin et al., 2003). We observed that hif-1 loss-of-function mutants also have an impaired response to the more rapid acquired thermotolerance gains characterized in this study (Figure S5D). This data suggests acquired thermotolerance is partially dependent on both HSF-1 and HIF-1, yet not dependent on DAF-16.
Regardless, the loss of HSF-1 induced-transcriptional responses during heat shock or inhibition of all transcription prior to the stress does not change wild type or lowered-ILS mutant thermotolerance. These results suggest that a 35°C stress is so severe that wild type has no response capable of affecting survival. However, in lowered-ILS mutants with their increased thermotolerance, the translation of key mRNAs appears to be critical factor. Using TSA analysis we have identified several genes encoding novel proteins likely to be highly translated in response to HS in ILS-lowered mutants. Overall, the heat shock had a profound effect on the polysome profile of all genotypes, such that abundance of polysomes of all orders was markedly reduced with a concurrent increase in the unbound mRNA fraction (data not shown, see Figure 6A). Fraction 3 (F3) was a pool of the heaviest polysomes and represents mRNA most actively translated. We focused much of our analysis on F3 and observed that within each genotype there was a significant correlation in mRNA abundance between CTL F3 and HS F3. This suggests that despite global reduction in the proportion of mRNA bound to ribosomes due to heat shock, some transcripts remain in the fraction consistent with high translation.
Using TSA, 59 transcripts were identified with elevated abundance in daf-2(e1370) HS F3 compared to either HS F3 in wild type or daf-16(m26);daf-2(e1370) (Figure 6C and Table S3). Of these, 10 encoded proteins had either F-box or BTB/POZ and MATH domains, which function as adapters to target substrate proteins for ubiquitination. Polyubiquitination signals peptides for proteosomal degradation, whilst monoubiquitination acts to target substrates to lysosomes (Urbe, 2005). The high proportion of proteins with these domains in daf-2(e1370) HS F3 is consistent with an up-regulation of mechanisms to target specific proteins for degradation. Some of the increased thermotolerance of daf-2 mutants may be derived from targeting specific proteins for proteosomal or lysosomal degradation.
TSA analysis identified several genes specifically regulated by ILS for increased translation under HS and identified a novel gene not previously known to be critical for stress tolerance. Ribosomal loading on C09H8.1 transcripts is elevated in daf-2(e1370) under heat stress, consistent with an increase in translation of this peptide. C08H9.1 encodes a putative serine carboxypeptidase and belongs to the S10 family, a class of enzyme that catalyzes the hydrolysis of the C-terminal peptide bond in proteins or peptides at acidic pH values. C08H9.1 may function in the acidified lysosomal compartments of C. elegans, however, this needs to be experimentally verified. A role for lysosomes in determining thermotolerance is largely unexplored. However, serpin-6 (srp-6), a regulator of lysosmal peptidases, modulates survival to a range of stressors, including heat (Luke et al., 2007). Further investigation of lysosomal function and integrity may provide additional insight into survival and ILS-effects under heat stress.
DAF-2 effects on lifespan are pleiotropic, showing not only profound changes in aging rate but also correlated resistance to a wide variety of environmental stresses. This, in part, suggests that aging and stress tolerance may share common underlying molecular and metabolic processes. The efficient detection, capture and resolution of misfolded and aggregated-prone proteins has been proposed as such a mechanism (Morley and Morimoto, 2004). Expression of HSPs have also been proposed as having critical roles in these processes (Walker and Lithgow, 2003; Hsu et al., 2003). Furthermore, ILS mutants modulate the age-dependent aggregation of polyglutamine proteins (Morley et al., 2002) and the amyloidogenesis and cytoxicity of human-Aβ3-42 (Cohen et al., 2006; Florez-McClure et al., 2007; McColl et al., 2009). The exact roles of HSP in these disease models need further examination. However, this study demonstrates that thermotolerance may be determined by HSP dependent and independent mechanisms and that processes other than HSP expression are critical for ILS gains in intrinsic thermotolerance. We anticipate that the genes identified in this study that impact on thermotolerance may have wide roles in stress, biological aging and age-related disease pathology.
Experimental Procedures
Strains
All C. elegans strains and dihybrid crosses generated for this study are described in Table S1 and were cultured as described previously (Brenner, 1974). The hsp-16.2∷GFP translational fusion (pCL79) was constructed by recovering the hsp-16.2 promoter and coding sequence by genomic PCR and cloning this fragment into the Sph I/Bam HI sites of the promoterless GFP expression vector pPD95.77. After sequence confirmation PCL79 was coinjection with marker pRF4 [rol-6(su006)] into wild type worms. Strain CL1262 was recovered with an extrachromosomal transgenic array dvEx262 [pCL79(hsp-16.2∷GFP) + pRF4]. Following UV-integration of dvEx262 [pCL79(hsp-16.2∷GFP) + pRF4] strain GL400: rfls400 [pCL79(hsp-16.2∷GFP) + pRF4] was generated and backcrossed five times to wild type prior to further use. All experiments were performed on hermaphrodites cultured at 20°C.
Automated analysis of GFP expression
All strains were cultured as large populations, approximately 2×104 individuals, on 90mm NGM plates with concentrated E. coli (OP50; approximately 1×1011 cells/ml) in excess. All assays were performed on 4 day old egg-laying hermaphrodites cultured at 20°C. Worms were washed from the NGM plates with 10 ml S-basal medium and eggs and larvae removed via filtration through a 20μm filter (BD scientific) and transferred into a 15 ml tubes in 10ml S-basal. After gentle centrifugation the supernatant was aspirated. Approximately 5000 individuals were transferred to 60mm NGA plates with 500ml of concentrated OP50 and left to dry for 20 min. These plates were either left as controls at 25°C or heat shocked 33°C for 2h. Populations were washed from these plates as described above, with one additional wash and finally resuspended in 10ml of Sheath fluid (Union Biometrica Inc). Samples were then loaded into the sample cup of a COPAS BIOSORT (Union Biometrica Inc) at approximately 1 worm/μl. Five worms were dispensed per well, into black, 384-well, flat bottom microplates (Corning Inc.) containing 25 μl S-medium with 1×108 cells OP50/ml. Plates were sealed using VIEWseal film (E&K Scientific) to prevent evaporation. For each treatment group three columns were dispensed giving a total of 48 wells/replicates per treatment
Kinetic data for relative GFP expression during recovery from the HS was then collected using a fluorometer (Fluoroskan Ascent, Thermo Labsystems). Fluorescence was measured at 25°C every 1h over a 12h period, with a default integration time of 0.02 sec. The excitation and emission wavelengths were set to 485 and 538 nm respectively. Fluorescence was averaged across the 48 well-replicates for each treatment group and base line corrected (Prism v4.03, Graphpad), against wells containing buffer alone, and plotted against time as relative fluorescence units (RFU)
Inhibition of Transcription and Translation
For the inhibition of transcription, approximately one thousand 4-day-old adults were transferred into 24-well micro-titer plates containing 50-100 μg/ml α-amanitin (Sigma) in 1 ml of S-basal with 1×108 cells/ml of OP50. Worms were incubated at 25°C for 1hr with 175 rpm orbital shaking, then washed once with 10ml of S-basal and spotted onto a 60mm NGM plate with OP50 for approximately 10min to allow evaporation of excess liquid. For each treatment two plates of 25-30 adults were then heat shocked at 35°C as described above.
A similar protocol was used for inhibition of translation, whereby 4 day-old adults were incubated in 24-well micro-titre plates containing with 1- 10 mM cycloheximide (Sigma) in 1 ml of S-basal with 1×108 cells/ml of OP50.
Translation State Array Analysis
Large scale populations of synchronous 5-day old young adults were used to generate polysomal profiles. Polysomes and ribosomal subunits were resolved using an adaptation of a previously described method (Dinkova et al., 2005). Briefly, 100 ml of gently pelleted worms were homogenized on ice in 300 ml of solublization buffer (300 mM NaCl, 50 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 1 mM EGTA, 200 mg heparin/ml, 400 U RNAsin/ml, 1.0 mM phenylmethylsulfonyl fluoride, 0.2 mg cycloheximide/ml, 1% Triton X-100, 0.1% Sodium Deoxycholate) via a Teflon homogenizer (60 strokes). An additional 700 ml of solublization buffer was added and the sample placed on ice for 10 min prior to centrifugation at 20,000g for 15 min at 4°C. A 0.9 ml aliquot of supernatant was applied to the top of a 10-50% sucrose gradient in high salt resolving buffer (140 mM NaCl, 25 mM Tris-HCl [pH 8.0], 10 mM MgCl2) and centrifuged in a Beckman SW41Ti rotor at 38,000 rpm for 90 min at 4°C. Polysomes were fractionated and collected using a Density Gradient Fractionation System (Teledyne Isco) with continuous monitoring of absorbance at 252 nm directly into Trizol LS reagent (Invitrogen Corp.) and RNA precipitated according to manufacturer's protocol.
RNA purified from each sample was amplified as previously described (McColl et al., 2008) via one round of linear amplification, and hybridized to full genome microarrays purchased from the University of Washington (http://genome.wustl.edu/projects/celegans/microarray/index.php), and washed and scanned as per previous studies (McColl et al., 2008; Golden et al., 2008). Resulting gene expression values were then analyzed for statistically significant differential expression as described below.
Statistics
Survival during heat stress was analyzed using a non-parametric (Mantel-Haenszel) Log rank test and presented as Kaplan-Meier survival curves (Prism™ software package). For the TSA analysis all full genome wide expression analyses involved comparisons of two groups and thus on a gene by gene basis, for each set, we performed two-sample t-tests on the log2 transformed expression measures to derive raw p-values. The Benjamini and Hochberg (Benjamini and Hochberg, 1995) was then used to report the inference adjusted for multiple comparisons (q-values), in our case controlling false discovery rate (FDR). Statistically significant differential expression was defined as FDR q-value ≤ 0.05, thereby controlling FDR at ≤ 0.05 for each set of comparisons.
Supplementary Material
Acknowledgments
We thank Krysta Felkey, Richard M. Neve, Gary K. Scott, and Nicole L. Jenkins for technical advice and critical comments. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center (University of Wisconsin), which is funded by the NIH National Center for Research Resources (NCRR). Knockout strains were provided by the C. elegans Reverse Genetics Core Facility (UBC, Canada) and the International C. elegans Gene Knockout Consortium. The COPAS BIOSORT system was a gift from The Glenn Foundation for Medical Research and The Herbert Simon Foundation. GM was supported by the Glenn Foundation for Medical Research /American Federation for Aging Research. GJL is supported by the NIH AG21069, AG22868, AG029631-01A1, ES016655, the Larry L Hillblom Foundation and UL1 RR024917. Array studies were supported by a Nathan Shock Center award to the Buck Institute, AG025798, and a Glenn award to SM from the Glenn foundation for Medical Research. GM planned and designed the project with consultation and support from PK and GJL. CDL and AIB provided material support. Gene expression studies were designed and carried out by GM and SM, and the TSA study was designed by GM, AR, PK and GJL and analyzed by GM, AEH and SM in the Genomics Core (Buck Institute). All other data was collected by GM, with assistance from SA. GM wrote the paper with contribution from all authors.
Abbreviations used
- ILS
Insulin-Like Signalling
- HS
Heat Shock
- HSR
Heat Shock Response
- HSP
Heat Shock Protein
- FDR
False Discovery Rate
- RFU
Relative Fluorescence Units
- Ti
Translation Index
Footnotes
Supplemental Information: Supplemental Experimental Procedures and data for this article including figures and tables can be found with this article online.
Accession Numbers: All microarray data are available in the Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo) via accession numbers (GSE numbers pending)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. (B).Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- Dinkova TD, Keiper BD, Korneeva NL, Aamodt EJ, Rhoads RE. Translation of a small subset of Caenorhabditis elegans mRNAs is dependent on a specific eukaryotic translation initiation factor 4E isoform. Mol Cell Biol. 2005;25:100–113. doi: 10.1128/MCB.25.1.100-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, Bateman A. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–D288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AL, Lithgow GJ. The nuclear hormone receptor DAF-12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long-lived worms. Aging Cell. 2006;5:127–138. doi: 10.1111/j.1474-9726.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Florez-McClure ML, Hohsfield LA, Fonte G, Bealor MT, Link CD. Decreased insulin-receptor signaling promotes the autophagic degradation of beta-amyloid peptide in C. elegans. Autophagy. 2007;3:569–580. doi: 10.4161/auto.4776. [DOI] [PubMed] [Google Scholar]
- Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden TR, Hubbard A, Dando C, Herren MA, Melov S. Age-related behaviors have distinct transcriptional profiles in Caenorhabditis elegans. Aging Cell. 2008;7:850–865. doi: 10.1111/j.1474-9726.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GuhaThakurta D, Palomar L, Stormo GD, Tedesco P, Johnson TE, Walker DW, Lithgow G, Kim S, Link CD. Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome Res. 2002;12:701–712. doi: 10.1101/gr.228902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu-Cronin YM, Chen WJ, Sternberg PW. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics. 2004;168:1937–1949. doi: 10.1534/genetics.104.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Khattra JS, McKay S, Pouzyrev A, Stott JM, Yang GS, Holt RA, Jones SJ, Marra MA, Brooks-Wilson AR, Riddle DL. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15:603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De PA, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Jenkins NL, McColl G, Lithgow GJ. Fitness cost of extended lifespan in Caenorhabditis elegans. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:2523–2526. doi: 10.1098/rspb.2004.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249:908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kapahi P. Cell signaling. Aging is RSKy business. Science. 2009;326:55–56. doi: 10.1126/science.1181034. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Larsen PL. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4:235–242. doi: 10.1379/1466-1268(1999)004<0235:doosri>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49:B270–B276. doi: 10.1093/geronj/49.6.b270. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke CJ, Pak SC, Askew YS, Naviglia TL, Askew DJ, Nobar SM, Vetica AC, Long OS, Watkins SC, Stolz DB, Barstead RJ, Moulder GL, Bromme D, Silverman GA. An intracellular serpin regulates necrosis by inhibiting the induction and sequelae of lysosomal injury. Cell. 2007;130:1108–1119. doi: 10.1016/j.cell.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ. Pharmacogenetic Analysis of Lithium-induced Delayed Aging in Caenorhabditis elegans. J Biol Chem. 2008;283:350–357. doi: 10.1074/jbc.M705028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G, Roberts BR, Gunn AP, Perez KA, Tew DJ, Masters CL, Barnham KJ, Cherny RA, Bush AI. The Caenorhabditis elegans Aβ1-42 model of Alzheimer disease predominantly expresses Aβ3-42. J Biol Chem. 2009;284:22697–22702. doi: 10.1074/jbc.C109.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinin M, Shliar J, Jiang H, Powell-Coffman JA, Bromberg Z, Horowitz M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol Genomics. 2003;14:17–24. doi: 10.1152/physiolgenomics.00179.2002. [DOI] [PubMed] [Google Scholar]
- Urbe S. Ubiquitin and endocytic protein sorting. Essays Biochem. 2005;41:81–98. doi: 10.1042/EB0410081. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J. 1993;292(Pt 2):605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E. Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. FASEB J. 2003;17:1960–1962. doi: 10.1096/fj.03-0164fje. [DOI] [PubMed] [Google Scholar]
- Walker GA, White TM, McColl G, Jenkins NL, Babich S, Candido EP, Johnson TE, Lithgow GJ. Heat shock protein accumulation is upregulated in a long-lived mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2001;56:B281–B287. doi: 10.1093/gerona/56.7.b281. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.