Abstract
Hypothesis
Fractures and femoral reaming are associated with lung injury. The mechanisms linking fractures and inflammation are unclear; but tissue disruption might release mitochondria. Mitochondria are evolutionarily derived from bacteria and contain “Damage Associated Molecular Patterns” (DAMPs) like formylated peptides that can activate immunocytes. We therefore studied whether fracture reaming releases mitochondrial DAMPs (MTD) and how MTD act on immune cells.
Methods
Femur fracture reamings (FFx) from 10 patients were spun to remove bone particulates. Supernatants were assayed for mitochondrial DNA (mtDNA). Mitochondria were isolated from the residual reaming slurry, sonicated and spun at 12,000g. The resultant MTD were assayed for their ability to cause neutrophil (PMN) Ca2+ transient production, p44/42 MAPK phosphorylation, IL-8 release and matrix metalloproteinase-9 (MMP9) release with and without formyl peptide receptor-1 (FPR1) blockade. Rats were injected with MTD and whole lung assayed for p44/42 activation.
Results
mtDNA appears at many thousand fold normal plasma levels in FFx and at intermediate levels in patients’ plasma, suggesting release from fracture to plasma. FFx MTD caused brisk PMN Ca2+ flux, activated PMN p44/42 MAPK and caused PMN release of IL-8 and MMP9. Responses to MTD were inhibited by FPR1 blockade using Cyclosporin H and anti-FPR1. MTD injection caused P44/42 phosphorylation in rat lung.
Conclusions
FFx reaming releases mitochondria into the wound and circulation. MTD then activates PMN. Release of damage signals like MTD from FFx may underlie activation of the cytokine cascades known to be associated with facture fixation and lung injury.
Keywords: Innate immunity, formyl peptides, fractures, neutrophils
Acute lung injury and adult respiratory distress syndrome (ALI/ARDS) occur after fractures in a sporadic entity often termed “fat embolism syndrome” (FES). FES is hard to distinguish from ALI/ARDS occurring after sepsis, and may be associated with reamed nailing more than other methods of fixation. Current concepts emphasize that fracture hematomas are rich in inflammatory mediators 1–4 that can activate immune cells like neutrophils (PMN) that can injure the lung but it is unknown what the primary events are causing fractures to be rich in mediators. Understanding the events linking mechanical injury to immune organ dysfunction is essential if effective therapies are to be developed.
Bacteria can cause inflammation by releasing lipids (like LPS) or n-formyl peptides 5. Such “pathogen-associated molecular patterns” (PAMPs) 6 activate cells through “pattern-recognition receptors” like Toll-Like Receptor-4 (TLR4: responds to LPS) or the receptors FPR1 and FPRL-1 that respond to formyl peptides (FP) 7. Human genomic proteins are not formylated, but mitochondria resemble bacteria in many ways, having FP and circular DNA (mtDNA) with non-methylated repeats like bacterial DNA 5, 8, 9. These observations led to the conclusion that mitochondria were once free-living bacteria that became intracellular symbionts 10, 11. They also suggest that when mitochondrial molecular patterns are released from cells by injury they might activate immunity by mimicking bacterial motifs. Intrinsic molecular motifs like this are referred to as “damage-associated molecular patterns” (DAMPs) 12 or ‘alarmins’. We have shown that mitochondrial FP’s activate PMN 5 and that mitochondrial DNA is released in shock 8. We now hypothesized that mitochondrial FP and other mitochondrial DAMPs (MTD) are released when cells are disrupted during fracture injury or reaming. If so, they could activate immunocytes and contribute to fracture-related lung injury.
MATERIALS AND METHODS
Research compliance
Studies were performed under the supervision of the Institutional Review Board (IRB) of Beth Israel Deaconess Medical Center (BIDMC) and Harvard Medical School. Fracture reaming specimens were collected under waiver of consent for discarded materials. Consent was obtained for sampling and archiving of trauma plasma samples from the patients or their legally authorized representative whenever such consent was available. Animal experimentation was approved by the IACUC of BIDMC.
Patients and biologic samples
Femoral reamings were collected intra-operatively from 10 patients with diaphyseal femur fractures. Patients were 18–54 years old and had isolated closed injuries sustained in motor vehicle traumas. Specimens were kept at 4°C while processed. Specimens were spun to remove gross particulates. The residual cellular material was subjected to a standard mitochondrial isolation protocol using a kit (Pierce, Rockford, IL). Plasma specimens were obtained when consent for blood draw was available (n=5). Mitochondria were sonicated and spun at 12,000g. The supernatant was assessed for biologic activity of mitochondrial DAMPs (MTD). Samples of fracture fluid were also spun at 12,000g and the supernatants assayed by qPCR in triplicate to assay mtDNA.
Animals
Male Sprague-Dawley rats (250–350g, Charles River, Wilmington, MA) were acclimatized under barrier sustained conditions (25°C, 12 hour light/dark cycles, water and chow ad libitum). Animals were cannulated as per our published methods 13 and injected (n=3/group) with MTD from human fracture hematomas or media. Animals were sacrificed one hour later. Lungs were harvested and frozen at −80 C for later analysis.
Reagents and Chemicals
We purchase fMLP, EGTA, and DMSO from Sigma (St Louis, MO), Fura2-AM from Molecular Probes (Eugene, OR), anti-human formyl peptide receptor-1 (FPR1) from R&D (Minneapolis, MN), HBSS and PBS from GIBCO (Grand Island, NY), anti-human MMP-9 from Chemicon (Billerica, MA) and phospho-p44/42 MAP kinase (Thr202/Tyr204) and p44/42 MAP kinase antibodies from Cell Signaling (Danvers, MA).
Details of Mitochondrial Isolation
Mitochondria were isolated using a Mitochondria Isolation Kit for tissue (PIERCE, Rockford, IL) according to the manufacturer’s dounce-soft tissue protocol under sterile conditions at 4°C and then stored on ice for further processing.
Preparation of Mitochondrial DAMPs (MTD)
Mitochondria from 200mg of tissue were suspended in 1ml of buffer (HBSS for chemotaxis assays and HEPES for calcium assays). Protease inhibitor cocktail (1:100) was added. Suspensions were subjected to sonication on ice (VCX130-Vibra Cell, Sonics and Materials. Newtown, CT) at 100% amplitude, 3 times for 30 seconds each. The disrupted mitochondria were centrifuged at 12000 rpm for 10 min at 4°C. Supernatants were removed and stored at −20°C for experiments. Protein concentration was determined by BCA assay. We have previously noted that there are no interspecies differences in the responses to mitochondria between human and rat. Immune cells from rat and man respond equally to MTD from their own and the other species 5, 8, 9.
Neutrophil isolation
Detailed protocols are described elsewhere 13, 14. Briefly heparinized volunteer blood is centrifuged and platelet-rich plasma removed. The buffy coat and 2 cm of RBC are layered onto Polymorphoprep gradient (Robbins Scientific, Sunnyvale, CA) and spun (1500 rpm, 30 min). PMN are collected and osmolarity restored for 5 minutes with an equal volume of 0.45% NaCl. PMN are washed, centrifuged and a hypotonic lysis is done on ice to remove residual RBC. The preparation contains ≥98% neutrophils (cytospin) that are ≥99% viable (Trypan Blue). PMN pellets are resuspended in HBSS with 5% FBS for chemotaxis assays or in HEPES buffer with 0.1% BSA for calcium and oxidative burst experiments.
Calcium dye loading
PMN were incubated in 2μM fura-2AM (30 min, 37°C). Cells were divided into 200μl aliquots and kept on ice. Aliquots were re-warmed to 37°C prior to experiments, centrifuged, resuspended in 200 μl of HEPES and loaded into a cuvette containing 2.8 ml of the same buffer. Experiments were begun in “calcium-free” media (0.3mM EGTA added) and 1.8 mM CaCl2 was added to the media as indicated.
Spectrofluorometry
PMN [Ca2+]i was measured using fura-2AM in a spectrofluorometer (Fluoromax-2, Jobin-Spex, Edison, NJ) using our modifications of the methods of Grynkiewicz et al. (16). Fracture fluids were added to the media at up to 20%. Higher concentrations of admixed protein solutions cause unacceptable autofluorescence 14.
Western Blots
Rat lung homogenates, human PMN supernatants and lysates were boiled for 5 min in SDS sample buffer. Proteins were separated by SDS-PAGE using 4–20% Tris-glycine polyacrylamide gradient gels (Novex, San Diego, CA). Separated proteins were transferred to nitrocellulose (0.45-μm pore size; Immobilon-P, Millipore, Bedford, MA). MMP-9, Phospho-p44/42 and p44/42 MAPK were immunoblotted with specific antibodies and detected using enhanced chemiluminescence (ECL, Amersham, GE, Buckinghamshire, UK). Membranes were stripped and re-probed with β-actin (Santa Cruz, CA) to assess loading. Densitometry was performed with the Scion Image program.
Quantitative PCR
DNA was isolated from 200μl of volunteer plasma as controls. DNA was isolated from the same volume of reaming fluid of patients with femur fractures or their plasma when available. DNA isolation was performed using QIAamp DNA Mini Kit (Qiagen). DNA concentration and quality was measured with a Nanodrop spectrophotometer. 7.5μl of DNA was used for each PCR reaction. SYBR green PCR was performed on volunteer plasma, patient plasma, and fracture fluid using primers targeting cytochrome B (CytB) that were specific (on “BLAST” analysis) for mitochondrial DNA. Primers (1R: CGAAGTTTCATCATGCGGAG and 1F: ATGACCCCAATACGCAAAAT) were synthesized by Invitrogen.
Statistical Analyses
All [Ca2+]i transient results reported are measured as the mean change from basal [Ca2+]i in nanomoles per liter (nM). Quantitative PCR was assessed as cycle count number (Ct): increasing cycle number to detection reflects decreasing abundance of the target. Data was assessed for significance using Student’s (unpaired) t-test or Analysis of Variance (ANOVA) where appropriate. Post hoc analyses for ANOVA were selected by the SigmaStat program (Systat Software, Richmond, CA). Data are reported as the mean ± SEM and statistical significance is accepted at a p-value <0.05. All examples of molecular studies shown are representative of 3 or more replications.
RESULTS
MTD in fracture patients
mtDNA is a biomarker for the presence of MTD. We found that mtDNA was close to undetectable in volunteer plasma (Figure 1). Fracture fluids contained ~217 fold (>100,000 fold) more mtDNA than volunteer plasma. Circulating plasma from patients with fractures had 500–1000 fold more mtDNA than volunteer plasma.
Figure 1.
Quantitative PCR (qPCR) was performed for Cytochrome B in patients with femur fractures or healthy volunteer controls. Cytochrome B is a specific biomarker for the mitochondrial genome. In this analysis, lower PCR cycle values represent more rapid appearance of a detectable signal and thus higher concentration of DNA template in the specimen. Every cycle doubles the amount of DNA. So a difference of 10 cycles represents a 210 fold (i.e. 1024 fold) increase in starting material and so forth.
MTD activate PMN [Ca2+]i flux
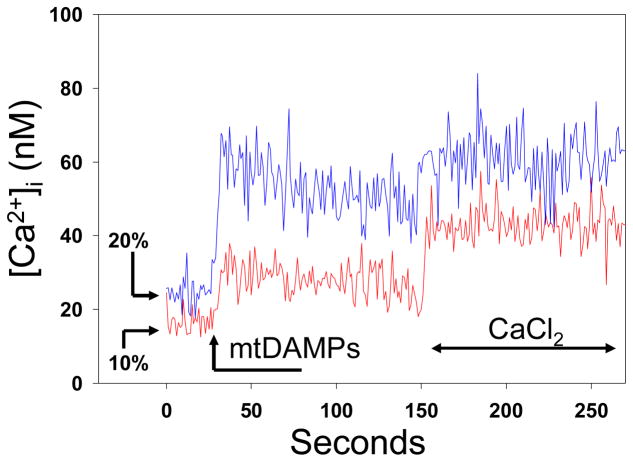
PMN were stimulated with fracture supernatants at 10% and 20% concentration. Experiments were performed in low-Ca2+ environment followed by re-calcification of the medium thus visualizing first intra-cellular Ca2+ flux and then Ca2+ entry into the cell (Figure 2). Fracture supernatants caused immediate (t=30s) Ca2+ release by endosomal stores followed by enhanced entry of Ca2+ into the cell (t=150s).
Figure 2.
Human neutrophils (PMN) were prepared for calcium studies by fura-loading in a cuvette with media low in calcium ([Ca2+]o ~50nM). We then added either 10% or 20% FFx supernatant. An immediate intracellular ‘spike’ in [Ca2+]i is seen. Further ‘store-operated’ Ca2+ entry is seen on re-calcification of the medium at t=150s. Responses are dose-related. Representative traces are shown, n=3/condition.
MTD activate PMN P44/42 MAP-kinases
PMN activated by mitochondria from FFx (10μg protein/ml, 10min) were assessed for phosphorylation of p44/42 mitogen-associated protein kinase (MAPK). Total p44/42 and β-actin were used as internal standards. MTD cause brisk phosphorylation of p44/42-MAPK (Figure 3). Stimulation after treatment with cyclosporin H (CsH, 1μM, a specific inhibitor of FPR1 7) completely inhibited activation. Thus FFx activate PMN P44/42 MAPK via formyl peptide receptors.
Figure 3.
Neutrophils were exposed to MTD derived from FFx (MTD) and assayed by western blot for the phosphorylation of p44/42 Mitogen Associated Protein Kinase. DAMPs from FFx rapidly activated this key PMN kinase. Activation was strongly inhibited by cyclosporin H, an inhibitor of the FPR1 receptor. Total p44/42 and β-actin are shown as controls. *P<0.05 (ANOVA/Tukey’s test)
MTD cause PMN to release matrix metalloproteinase-9 (MMP-9)
Excessive MMP release in inflammation contributes to bystander organ injury via many pathways 15. We found PMN released MMP-9 in a dose-dependent fashion when stimulated by MTD from femoral reamings (Figure 4). As with p44/42-MAPK, the effect was completely reversed by CyH. MMP-9 release was completely blocked by anti-FPR1 antibodies. These findings show PMN degranulation of MMPs is activated by MTD in FFx through formyl peptide receptors, likely FPR1. (n=3 replications/condition, *p< 0.05 by ANOVA/Tukey’s test).
Figure 4.
PMN release MMP9 after exposure to FFx MTD. PMN were exposed to FFx MTD (10 min) at the concentrations noted. Supernatants were assayed for MMP-9 by western blot. MTD caused brisk degranulation of MMP-9 (*p< 0.05; ANOVA/Tukey’s test). Release was inhibited by CsH, an inhibitor of FPR1, or by monoclonal antibodies to FPR1 (αFPR1)
FFx MTD cause PMN IL-8 release
Activated PMN release IL-8 which activates PMN and recruits further PMN. Both IL-8 and responses to it are increased in ALI/ARDS 14, 16. Here, PMN were induced to produce IL-8 by exposure to MTD from femoral reamings (Figure 5). The dose response suggests this may occur at the concentrations found in circulating plasma during reaming.
Figure 5.
PMN produce IL-8 after exposure to MTD from FFx reamings. 10% and 20% MTD caused brisk release of IL-8 at 4 hours (ANOVA p<0.01; post hoc Holm/Sidak p<0.05).
Pulmonary inflammation in response to fracture MTD
MTD from human femoral reaming was injected into rats IV. Rats were sacrificed one hour later. Whole lung homogenates were prepared and assayed for phosphorylation of p44/42 (Figure 7). We found brisk phosphorylation of p44/42, suggesting FFx MTD cause ingress of activated inflammatory cells into the lung.
DISCUSSION
Activation of innate immune phagocytic function is required for the clearance of injured tissue that must precede wound repair. What we see here is that the same primary mediators initiating local innate immunity can also precipitate systemic activation of innate immunity, clinically manifest as SIRS. We believe SIRS is universal after major fracture/soft-tissue injury, but that its intensity varies widely. This may reflect factors related to the local wound or reflect variable host response. But in any case, fracture/soft-tissue injury and its management can contribute to clinical SIRS indistinguishable from sepsis without an identifiable infection. Moreover, these manifestations can include ALI or ARDS.
Here, we show femoral fracture wounds (and their repair by reamed nailing) can release mitochondrial debris into the wound and into the systemic circulation. Since mitochondria are evolutionarily derived from bacteria 10, 11, we hypothesized their release by cellular disruption would expose the host to immunologically active ‘danger signals’. Our findings demonstrate that femur fractures and reamed repairs do indeed release mitochondrial DAMPs and that these FFx-derived DAMPs are capable of activating innate immune cells and causing pulmonary inflammation in an animal model.
Our findings support the novel paradigm that fracture/soft-tissue injury contribute to the genesis of clinical SIRS by local release of intracellular, mitochondrial-derived DAMPs. Our current studies are limited to evaluation of PMN activation and biochemical studies performed in rats treated with MTD. Moreover, other intracellular motifs can act as ‘alarmins’ 17, 18 and such molecules are also likely to participate in local wound repair as well as systemic inflammation. Thus biologic response modifications based upon the effects of MTD alone would be premature and must be approached with caution.
We therefore hypothesize that release of MTD from injured tissues forms a link between tissue trauma and sterile SIRS that can predispose to inflammatory lung injury. More research is required to determine the extent to which release of mitochondrial products by mechanical disruption or other forms of tissue injury is responsible for systemic PMN activation and the evolution of SIRS after clinical injury.
Figure 6.
MTD from FFx reamings were injected into rats intravenously. One hour later rats were sacrificed and whole lung homogenates were assayed for activation of p44/42 by western blot. We found brisk phosphorylation of p44/42, suggesting the onset of pulmonary inflammation. *P=0.002 vs naïve (ANOVA/Holm-Sidak).
Acknowledgments
This work was supported by NIH/NIGMS Grant 2 R01GM059179 and by Department of Defense Hypothesis award DR080924/W81XWH-09-1-0472 (CJH)
Footnotes
Presented at the 25th annual meeting of the Orthopedic Trauma Association, October 7–8, 2009. San Diego, CA.
References
- 1.Hauser CJ, Zhou X, Joshi P, et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J Trauma. 1997;42(5):895–903. doi: 10.1097/00005373-199705000-00021. discussion 903–4. [DOI] [PubMed] [Google Scholar]
- 2.Hauser CJ, Joshi P, Zhou X, et al. Production of interleukin-10 in human fracture soft-tissue hematomas. Shock. 1996;6(1):3–6. doi: 10.1097/00024382-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Pape HC, Griensven MV, Hildebrand FF, et al. Systemic inflammatory response after extremity or truncal fracture operations. J Trauma. 2008;65(6):1379–84. doi: 10.1097/TA.0b013e31818c8e8c. [DOI] [PubMed] [Google Scholar]
- 4.Kobbe P, Vodovotz Y, Kaczorowski DJ, et al. The role of fracture-associated soft tissue injury in the induction of systemic inflammation and remote organ dysfunction after bilateral femur fracture. J Orthop Trauma. 2008;22(6):385–90. doi: 10.1097/BOT.0b013e318175dd88. [DOI] [PubMed] [Google Scholar]
- 5.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial Peptides Activate Neutrophils Via FPR-1. J Trauma. 2010:68. doi: 10.1097/TA.0b013e3181dcd28d. in press. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel-Seifert K, Seifert R. Cyclosporin H is a potent and selective formyl peptide receptor antagonist. Comparison with N-t-butoxycarbonyl-L-phenylalanyl-L-leucyl-L-phenylalanyl-L-leucyl-L-phenylalanine and cyclosporins A, B, C, D, and E. J Immunol. 1993;150(10):4591–9. [PubMed] [Google Scholar]
- 8.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 MAP-Kinase. Shock. 2010:33. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14(3):255–74. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 11.Lang BF, Gray MW, Burger G. Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet. 1999;33:351–97. doi: 10.1146/annurev.genet.33.1.351. [DOI] [PubMed] [Google Scholar]
- 12.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 13.Lee C, Xu DZ, Feketeova E, et al. Attenuation of shock-induced acute lung injury by sphingosine kinase inhibition. J Trauma. 2004;57(5):955–60. doi: 10.1097/01.ta.0000149495.44582.76. [DOI] [PubMed] [Google Scholar]
- 14.Hauser CJ, Fekete Z, Livingston DH, Deitch EA. Chemokine stimulation of human neutrophil [Ca2+]i signaling in biologic environments. Shock. 1998;10(5):324–8. doi: 10.1097/00024382-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Gaggar A, Li Y, Weathington N, et al. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L96–L104. doi: 10.1152/ajplung.00492.2006. [DOI] [PubMed] [Google Scholar]
- 16.Simms HH, D’Amico R. Polymorphonuclear leukocyte dysregulation during the systemic inflammatory response syndrome. Blood. 1994;83(5):1398–407. [PubMed] [Google Scholar]
- 17.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 18.Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7(8):774–8. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]