Abstract
Background/Aims
The high risk and prevalence of dementia among patients with chronic kidney disease (CKD) and in those receiving hemodialysis (HD) may be preceded by mild cognitive impairment (MCI). We aimed to assess cognitive function in CKD and HD patients with no history of stroke or dementia, in order to identify and characterize early cognitive deficits.
Methods
24 CKD and 27 HD male outpatients without history of cerebrovascular or neurodegenerative disease underwent comprehensive neuropsychological testing in an observational cross-sectional study. Test results were used to categorize patients into MCI subtypes.
Results
All subjects scored ≥28 on the Mini-Mental State Examination. The prevalence of executive function was at least 25% in both groups and memory impairment occurred in 13% of the HD patients and 15% of those with CKD. MCI occurred in 76% of the group and HD patients showed a higher prevalence of MCI compared to CKD patients (89 vs. 63%) with a preponderance (>70%) of cases across both groups classified as non-amnestic MCI.
Conclusion
Predialysis CKD and HD patients have a high prevalence of MCI despite normal global cognitive function. MCI was more prevalent among the HD patients and deficits more frequently resulted in non-amnestic MCI.
Key Words: Cognition, Mild cognitive impairment, Cerebrovascular disease, Chronic kidney disease, Hemodialysis
Introduction
Advancing age and a heavy burden of cardiovascular risk factors among chronic kidney disease (CKD) and hemodialysis (HD) patients has focused attention on cerebrovascular morbidity in this population. Dementia prevalence in the HD population has been reported to be 30% and is frequently under recognized [1,2]. In patients with CKD prior to initiating dialysis, cognitive function has been correlated with the severity of renal disease and an increased risk of developing dementia [3,4]. Several factors may be responsible for the high prevalence of cognitive impairment in this population and may significantly impact cognition prior to the development of overt dementia.
Patients with CKD have a high prevalence of subcortical white matter lesions on neuroimaging and a high incidence rate for stroke [5,6]. White matter lesions result from subcortical small vessel disease and have been independently associated with severity of kidney disease [7]. Small vessel cerebrovascular disease leading to the development of subcortical white matter lesions is likely to be accelerated in CKD because these patients often have a clustering of traditional and emerging vascular risk factors, including hypertension, diabetes, hyperlipidemia, elevated oxidative stress, and an elevated inflammatory state. Small vessel cerebrovascular disease is the most common cause of vascular dementia with a pattern of cognitive deficits characterized by relatively preserved memory and impairment in domains related to attention, executive function and processing speed [8,9]. Current dementia screening tools, which rely heavily on memory deficits, may not identify patients with cognitive deficits characteristic of cerebral small vessel disease, particularly in the early stages.
Mild cognitive impairment (MCI) represents a transitional stage between cognitive changes of aging and dementia. Amnestic MCI with predominant memory deficits has gained a reputation as a predictor of incipient Alzheimer's disease [10]. Recent studies have introduced the non-amnestic MCI subtype that may serve as a potential prodrome of vascular dementia [11,12].
The purpose of our study was to perform detailed neuropsychological testing in outpatient CKD and HD patients with no history of dementia, stroke or neurodegenerative disease in order to identify and characterize cognitive deficits. We hypothesized that both groups would score significantly lower when compared to normative data, particularly in domains most affected by subcortical small vessel disease. We also predicted that HD patients would perform worse than predialysis CKD patients due to the increased cardiovascular risk as renal disease progresses and HD is initiated.
Materials and Methods
Study Design
This is an observational, cross-sectional study of stage III-IV CKD and HD patients attending the outpatient Renal and Hemodialysis Clinics at the James J. Peters Veterans Affairs Medical Center Bronx, N.Y. Patients were recruited from October 2006 to October 2008. Outpatients were referred for screening by their primary nephrologists. All participants provided signed informed consent in accordance with local institutional review board approval. Subjects included 27 of 80 patients on HD and 24 of approximately 200 patients with stage III or stage IV CKD.
Demographic characteristics and chronic health conditions for both groups were obtained from the patient's electronic medical records. For CKD patients, the estimated glomerular filtration rate (eGFR), serum calcium (Ca), serum phosphate (PO4), and hemoglobin levels were recorded within 3 months of neuropsychological testing. eGFR was calculated using the Modification of Diet in Renal Disease study equation [13]. Serum intact parathyroid hormone (PTHi) levels were obtained within 6 months of neuropsychological testing. When more than one value was available, averages were calculated. Laboratory values from HD patients included hemoglobin, Ca level, PO4 level, PTHi and BUN for urea reduction ratio (URR) calculation. As part of routine clinical care, these labs are drawn monthly on dialysis days prior to the treatment. Values for Ca, PO4, PTHi and URR were calculated by averaging the monthly labs for 3 consecutive months prior to neuropsychological testing. All tests were performed at a single central laboratory using standard methods. Labs were drawn for thyroid-stimulating hormone (TSH) within 1 week of neuropsychological testing and values determined by radioimmunoassay for both groups. Measured blood pressure was determined by averaging the 3 clinical appointment blood pressure readings prior to neuropsychological testing.
Participants
Patients were considered for enrollment if they were fluent in English and able to complete baseline assessments. HD participants had to be receiving treatment for at least 3 months and have a 3-month average URR of ≥65% at the time of screening. Those receiving HD were dialyzed 3 days per week for 3–4 hours per session. Exclusion criteria were as follows: a history of stroke or dementia when questioned or if documented in the medical chart, a history of Parkinson's or neurodegenerative disease, liver function enzymes (AST and ALT) more than two times the upper limit of normal, or a hemoglobin level <10 g. Stage III CKD was defined as an eGFR 30–59 ml/min/1.73 m2 and stage IV CKD was defined as eGFR 15–29 ml/min/1.73 m2. All the subjects that completed testing were male. All attempts were made to perform neuropsychological testing on non-dialysis days in order to avoid the potential temporal relationship between cognitive function and time since last dialysis. However, 5 patients were tested on dialysis days due to scheduling difficulties.
Measurements
A 60-min battery of nine validated neuropsychological tests was administered to all study participants under supervision of a senior psychologist with specialized training in neuropsychology. The neurocognitive battery included: The Mini-Mental State Examination (MMSE) [14], The California Verbal Learning Test-II (CVLT-II) Standard Form[15], the Controlled Oral Word Association Test (COWAT-FAS) [16], animal category fluency, Digit Span [17], the Symbol Digit Modality Test (SDMT) [18], Trails A & B [19], Stroop Word & Color tests [20], and the Short Category Test (SCT) [21] (table 1).
Table 1.
Neurocognitive battery
| Test | Function assessed |
|---|---|
| Mini-Mental State Examination | Global cognitive status |
| Attention and processing speed | |
| Trails A | Visual attention |
| Digit Span | Auditory attention and memory |
| Symbol Digit Modalities Test | Visuomotor scanning and processing |
| Stroop Word | Speed of information processing |
| Stroop Color | Speed of information processing |
| Executive function | |
| Short Category Test | Concept formation and reasoning |
| Trails B | Set shifting |
| Stroop Color-Word | Cognitive flexibility |
| Language | |
| Controlled Oral Word Association Test (FAS) | Verbal fluency, phonemic naming |
| Animal naming | Verbal fluency, categorical naming |
| Memory | |
| California Verbal Learning Test-II | Verbal immediate, short-term, long-term and delayed recognition memory |
Neuropsychological Test Analysis and MCI Classification
In the absence of a matched control group, we compared group raw scores on each cognitive test to previously published normative data on age- and education-matched healthy controls. For between-group comparisons on each cognitive measure, raw test scores were converted to the published age- and education-standardized T scores. T scores that were ≥1.5 standard deviations (SD) below the mean were identified as impaired. To calculate the domain composite T scores, we first calculated a mean T score of all the completed cognitive tests within each particular domain (table 1) for each subject. Next, all subjects’ domain composite scores were averaged within each group to calculate the overall domain composite score for either CKD or HD groups. Results of neurocognitive tests were used to classify subjects with an MCI subtype by using diagnostic criteria utilized from the Cardiovascular Health Cognition Study and the Mayo Clinic Study of Aging as approximate guidelines [12,22,23]. Subjects with a composite score for long and short delayed memory tests ≥1.5 SD below age- and education-matched norms were classified as MCI amnestic type. Study participants who did not meet criteria for MCI amnestic type, but scored ≥1.5 SD below the norm on two or more tests in a non-memory domain (Attention/Processing Speed, Executive Function, or Language) were classified as MCI non-amnestic type. The MCI amnestic subtype was further categorized into single domain involvement if the memory domain was only impaired and multiple domain if the subject scored ≥1.5 SD below the norm on two or more tests within a non-memory domain. Subjects with non-amnestic MCI were further characterized into single domain if only one non-memory domain was impaired or multiple domain if the subject scored ≥1.5 SD below the norm on one or more tests in an additional non-memory domain.
Statistical Methods
Descriptive statistics are reported as means and SDs or percent as appropriate. Within each study group, single group t-tests were used to determine raw score differences between the CKD study participants and previously published normative (PN) data for age-matched, healthy controls. Multiple regression models were used to adjust for differences between the CKD and HD groups for diabetes mellitus (DM), hypertension (HTN), hyperlipidemia, coronary artery disease (CAD), congestive heart failure (CHF), peripheral arterial disease (PAD), PO4, Ca × PO4, PTHi and TSH. Independent sample t-tests were used to evaluate differences between the CKD and HD groups on individual cognitive test T scores and domain composite T scores. CKD and HD groups were compared on the demographic and clinical variables represented as a continuous number by using t-tests. For the dichotomous or categorical variables, χ2 tests were used to determine the significance of differences in prevalence between the groups. The statistical analyses were performed using StatView (SAS, 1998). Analysis of covariance (ANCOVA) was used to determine the differences between those with and without MCI for traditional and emerging cardiovascular risk factors.
In the CKD group, visual difficulties prevented 2 subjects from completing two or more of the following: Trails A, SDMT, Digit Span, Stroop Word, Stroop Color, Stroop Color-Word, and Trails B. Six patients refused to complete the Short Category Test out of frustration. In the HD group, 1 subject did not complete Stroop Color and Stroop Color-Word secondary to color vision deficiency. Scores from individual neurocognitive measures that were not completed were excluded from data analysis and not counted.
Results
Participants
Fifty-one male subjects participated in the study, 24 with CKD and 27 on HD. CKD subjects were significantly older than those receiving HD (72 ± 12 vs. 63 ± 11 years, p = 0.01). No other demographic variables were statistically different between the groups (table 2). Hypertension, diabetes, and hyperlipidemia were highly prevalent in both the CKD and HD groups, with no significant difference in proportion of these factors between groups. The HD group had significantly higher PO4, Ca × PO4 product, and PTHi. There were no differences between the groups for blood pressure (table 2). Overall, the HD group was adequately dialyzed, with an average 3-month URR of 71 ± 4% and no subject having less than 65%. Both groups had a prevalence of coronary artery disease (58% CKD, 37% HD), congestive heart failure (33% CKD, 22% HD) and peripheral arterial disease (21% CKD, 19% HD). There was no significant difference in prevalence between groups for CAD, CHF, and PAD. Hemoglobin levels were significantly different between CKD and HD groups (12.1 ± 1.6 vs. 11.4 ± 1.0, p = 0.04, respectively), and none were <10 g as per the exclusion criterion. Hemoglobin level was not found to significantly affect cognitive performance on the given tasks. The CKD group had a significantly higher TSH level than the HD group (2.3 ± 2.1 vs. 1.4 ± 0.8, p = 0.05).
Table 2.
Characteristics of study participants
| CKD (min-max) | HD (min-max) | P | |
|---|---|---|---|
| Age, years | 72 ± 12 (39–87) | 63 ± 11 (45–82) | 0.01 |
| Education, years | 13 ± 4 (2–21) | 13 ± 1 (11–16) | 0.8 |
| Years on dialysis | N/A | 2.2 ± 2.3 (0.3–10) | |
| URR, % | N/A | 71 ± 4 (65–82) | |
| CKD status | |||
| Stage III | 16 (67) | N/A | |
| Stage IV | 8 (33) | N/A | |
| Stage V | N/A | 27 (100) | |
| Ethnicity | 0.2 | ||
| African-American | 10 (42) | 17 (63) | |
| Caucasian | 9 (38) | 5 (19) | |
| Other | 5 (21) | 5 (19) | |
| Traditional cardiovascular risk factors | |||
| Hypertension | 24 (100) | 24 (89) | 0.2 |
| Diabetes | 16 (67) | 14 (52) | 0.4 |
| Hyperlipidemia | 6 (25) | 5 (19) | 0.7 |
| Systolic BP mm Hg | 142 ± 16 | 145 ± 17 | 0.6 |
| Diastolic BP mm Hg | 74 ± 15 | 77 ± 13 | 0.4 |
| Emerging cardiovascular risk factors | |||
| PO4, mg/dl | 3.8 ± 0.8 | 5.4 ± 1.0 | <0.001 |
| Ca × PO4 | 33.4 ± 6.7 | 48.0 ± 11.0 | <0.001 |
| PTHi, ng/l | 128.1 ± 80.8 | 345.9 ± 269.7 | <0.001 |
HD = Hemodialysis; CKD = chronic kidney disease (stage III-IV); URR = urea reduction ratio; PO4 = serum phosphate; Ca × PO4 = calcium phosphate product; PTH¡ = parathyroid hormone (intact).
Raw Scores versus Published Norms
The raw scores compared with the published norms (PN) for the CKD and HD groups are presented (table 3). All subjects scored ≥28 on MMSE. In the CKD group, subjects scored significantly lower on 4 of 5 measures of attention and processing speed. In the domain of executive function, CKD subjects scored significantly lower than published norms on all measures. In the CKD group, only 1 of 4 measures of memory was significantly lower than the published norms. In the HD group, subjects scored significantly lower on all measures for each cognitive domain versus the published norms.
Table 3.
Raw scores on cognitive function tests versus published norms
| Test | CKD |
HD |
||||
|---|---|---|---|---|---|---|
| raw | PN | P | raw | PN | P | |
| MMSE | 28.9 ± 1.2 | 28.1 ± 1.8 | ||||
| Attention and processing speed | ||||||
| Trails A | 59.5 ± 24.2 | 47.2 ± 11.6 | 0.03 | 64.7 ± 24.8 | 39.5 ± 8.8 | <0.001 |
| Digit Span | 12.9 ± 2.4 | 15.2 ± 0.8 | <0.001 | 12.4 ± 3.3 | 15.9 ± 0.8 | <0.001 |
| SDMT | 29.8 ± 8.7 | 32.5 ± 9.5 | 0.3 | 29.1 ± 7.6 | 41.1 ± 10.1 | <0.001 |
| Stroop Word | 78.1 ± 13.5 | 97.6 ± 8.6 | <0.001 | 68.4 ± 11.7 | 99.1 ± 2.7 | <0.001 |
| Stroop Color | 45.8 ± 8.1 | 71.5 ± 4.8 | <0.001 | 49.9 ± 11.6 | 73.1 ± 2.2 | <0.001 |
| Executive function | ||||||
| Short Category Test | 43.4 ± 8.3 | 31.4 ± 2.9 | <0.001 | 38.3 ± 13.8 | 31.5 ± 2.7 | 0.02 |
| Trails B | 157.5 ± 56.8 | 115.6 ± 33.0 | 0.005 | 169.6 ± 93.6 | 92.8 ± 26.8 | <0.001 |
| Stroop Color-Word | 20.6 ± 4.4 | 32.9 ± 6.5 | <0.001 | 21.4 ± 7.7 | 35.1 ± 3.2 | <0.001 |
| Language | ||||||
| FAS | 30.3 ± 12.1 | 35.9 ± 6.1 | 0.05 | 25.4 ± 9.9 | 39.6 ± 4.2 | <0.001 |
| Animal naming | 15.6 ± 4.0 | 16.8 ± 2.2 | 0.2 | 13.2 ± 4.2 | 18.3 ± 2.2 | <0.001 |
| Memory | ||||||
| CVLT total recall | 34.5 ± 8.5 | 36.4 ± 6.0 | 0.4 | 33.7 ± 7.4 | 40.5 ± 5.8 | <0.001 |
| CVLT short delay recall | 6.1 ± 3.2 | 7.4 ± 1.4 | 0.08 | 5.9 ± 3.7 | 8.1 ± 1.1 | 0.006 |
| CVLT long delay recall | 6.7 ± 2.8 | 7.8 ± 1.3 | 0.09 | 6.6 ± 3.3 | 8.5 ± 1.0 | 0.008 |
| CVLT long delay recognition | 12.5 ± 2.5 | 14.0 ± 0.2 | 0.007 | 12.3 ± 2.3 | 14.0 ± 0.0 | <0.001 |
HD = Hemodialysis; CKD = chronic kidney disease; PN = published normative value.
Scores are reported as mean ± SD.
Trails A, SDMT, Digit Span, STROOP Word n = 23 CKD, n = 27 HD; SCT n = 18 CKD, n = 27 HD.
STROOP Color, STROOP Color-Word n = 22 CKD, n = 26 HD; Trails B n = 22 CKD, n = 27 HD.
T Scores for Individual Tests: CKD versus HD
In order to compare groups, T scores for each neuropsychological test were calculated and reported (table 4). HD subjects scored significantly lower than CKD subjects on 3 of 5 measures of attention and processing speed and all measures of language. CKD subjects scored significantly lower than HD subjects on one test of executive function. There was no significant difference between the groups for measures of memory. When the T scores were adjusted for hypertension, diabetes, hyperlipidemia, CAD, CHF, PAD, PO4, Ca × PO4, PTHi and TSH, significant differences between the groups remained (data not shown). For the HD group, time since the last dialysis was not significantly associated with performance on the cognitive tests.
Table 4.
Cognitive function scores and prevalence of impairment by group
| Test | T score |
Impaired |
||||
|---|---|---|---|---|---|---|
| CKD | HD | P | CKD | HD | P | |
| Attention and processing speed | 40.0 ± 7.1 | 34.0 ± 5.2 | 0.001 | 6 (25) | 16 (59) | 0.02 |
| Trails Aa | 41.8 ± 16.8 | 32.1 ± 11.7 | 0.02 | |||
| Digit Spana | 45.0 ± 6.4 | 42.5 ± 7.4 | 0.2 | |||
| SDMTb | 46.4 ± 11.6 | 36.0 ± 10.2 | 0.001 | |||
| Stroop Wordb | 37.0 ± 9.8 | 28.3 ± 7.6 | 0.001 | |||
| Stroop Colorb | 29.1 ± 7.7 | 31.0 ± 8.9 | 0.4 | |||
| Executive function | 40.2 ± 7.7 | 38.9 ± 7.3 | 0.5 | 6 (25) | 8 (30) | 0.8 |
| Short Category Testa | 39.9 ± 7.0 | 46.5 ± 12.6 | 0.05 | |||
| Trails Ba | 40.2 ± 15.7 | 33.0 ± 13.7 | 0.09 | |||
| Stroop Color-Wordb | 38.5 ± 6.7 | 36.8 ± 7.3 | 0.4 | |||
| Languageb | 46.6 ± 8.8 | 38.9 ± 6.7 | <0.001 | 2 (8) | 8 (30) | 0.08 |
| FAS | 45.1 ± 11.0 | 38.9 ± 8.2 | 0.03 | |||
| Animal naming | 48.3 ± 11.0 | 38.9 ± 10.9 | 0.004 | |||
| Memorya | 45.7 ± 9.1 | 42.7 ± 8.2 | 0.2 | 3 (13) | 4 (15) | 0.9 |
| CVLT total recall | 48.3 ± 10.6 | 43.2 ± 8.2 | 0.06 | |||
| CVLT short delay recall | 46.7 ± 11.5 | 42.8 ± 11.3 | 0.2 | |||
| CVLT long delay recall | 46.9 ± 10.4 | 44.7 ± 9.6 | 0.4 | |||
| CVLT long delay recognition | 40.8 ± 13.2 | 39.8 ± 12.4 | 0.8 | |||
HD = Hemodialysis; CKD = chronic kidney disease (stage III–IV).
Scores are reported as mean ± SD. Impairment recorded as n (%).
Trails A, SDMT, Digit Span, STROOP Word n = 23 CKD, n = 27 HD; SCT n = 18 CKD, n = 27 HD.
STROOP Color, STROOP Color-Word n = 22 CKD, n = 26 HD; Trails B n = 22 CKD, n = 27 HD.
Age-adjusted.
Age- and education-adjusted (T scores).
Composite T Scores and Prevalence of Impairment by Domain: CKD versus HD
HD subjects had significantly lower composite T scores in the domains of attention and processing speed and language (34 ± 5 vs. 40 ± 7, p < 0.01, and 39 ± 7 vs. 47 ± 9, p < 0.01, respectively). The proportion of subjects with attention and processing speed impairment was significantly greater in the HD compared with the CKD group (59 vs. 25%, p = 0.01). The prevalence of executive function impairment was high in both groups (30 and 25%). Memory impairment was least prevalent among HD patients and present in 13% of those with CKD (table 4).
Prevalence and Subtype of MCI
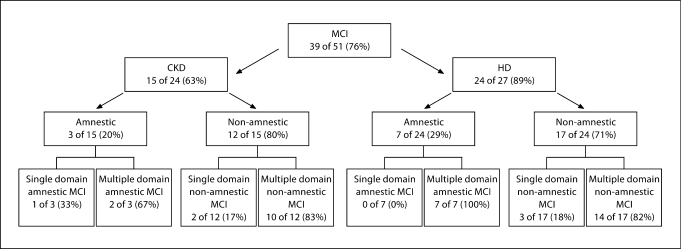
A flow chart of the prevalence and subtype of MCI is presented (fig. 1). Of those subjects with MCI, 26% (10 of 39) met the criteria for amnestic MCI and all but 1 of these had multiple domain involvement. The remaining 74% (29 of 39) were characterized as having non-amnestic MCI, of whom 83% (24 of 29) had multiple domain involvement.
Fig. 1.
Mild cognitive impairment by subtype. Diagnostic criteria: Amnestic MCI single domain was defined as ≥1.5 SD below the norm on delayed recall composite score. Amnestic MCI multiple domain was defined as ≥1.5 SD below the norm on delayed recall composite score and 2 tests ≥1.5 SD below the norm in any one domain other than memory. Non-amnestic MCI single domain was defined as 2 tests ≥1.5 SD below the norm in any one domain other than memory. Non-amnestic MCI single domain was defined as 2 tests ≥1.5 SD below the norm in any one domain other than memory and 1 test ≥1.5 SD below the norm in an additional domain other than memory.
Traditional and Emerging Cardiovascular Risk Factors: MCI versus Non-MCI
No significant differences were found between the groups with MCI and without MCI or between MCI subtypes for PO4 level, Ca × PO4 product, PTHi level, TSH level, measured blood pressure or history of hypertension, diabetes, and hyperlipidemia, CAD, CHF, and PAD (data not shown).
Discussion
In our CKD and HD outpatients with normal global cognitive function and no history of dementia, stroke or neurodegenerative disease, a high prevalence of neuropsychological deficits was detected compared to normative samples. Examination of clinically meaningful deficits with significant impairment meeting the criteria for MCI revealed that these deficits were most common in non-memory domains. These results are supported by an earlier study of HD patients without a clinical history of dementia or stroke where cognitive dysfunction was primarily subcortical in nature with a relative sparing of cortical domain function [24].
MCI is a transitional state between normal cognition and the earliest clinical features of dementia. Amnestic and non-amnestic subtypes have been identified based on specific domain involvement with each subtype influencing the subsequent development of Alzheimer's or vascular dementia [25]. For example, amnestic MCI has been established as a prodrome of Alzheimer's disease, whereas non-amnestic MCI more frequently predicts the development of vascular dementia.
A high prevalence of MCI was identified in the overall group and among HD patients versus CKD patients with a preponderance of cases classified as non-amnestic MCI. It is noteworthy that even among the amnestic MCI cases, all but 1 subject also had non-memory deficits. In fact, most of our MCI cases had multiple non-amnestic domain involvement, which has been shown to increase the risk of developing vascular dementia and increase the risk of mortality versus amnestic MCI [25]. Although all patients had a normal MMSE, their profile of neuropsychological deficits are likely to affect planning, sequencing, organization, and mental flexibility all of which are required for independence in activities of daily living and compliance with complex medical regimens. Because subcortical vascular dementia secondary to cerebral small vessel disease has a relatively insidious onset with gradual cognitive decline often associated with non-memory domains, relying on routine neuropsychological instruments that require memory deficit for diagnosis could underdiagnose non-amnestic deficits, increasing the renal patient's risk for treatment non-compliance and failure. Early detection of non-amnestic deficits characteristic of cerebral small vessel disease may provide an opportunity for interventions aimed to slow disease progression, such as more vigorous control of blood pressure, dyslipidemia, and other metabolic perturbations as well as cognitive therapy. Future trials should then be designed to evaluate the efficacy of interventions to slow the progression of MCI to overt dementia.
When compared to CKD patients, our HD patients scored significantly lower on several measures of attention and mental processing speed and language with no significant change on any measure of memory between groups. Impairments in the domains of attention and processing speed, executive function, and language with a relative preservation of memory is reminiscent of the cognitive changes associated with the early manifestations of vascular dementia secondary to small vessel cerebrovascular disease [26]. This pattern of cognitive deficit has been associated with manifestations of cerebral small vessel disease such as white matter lesions [27,28,29,30,31]. A potential link between kidney disease and subcortical cognitive deficits comes from evidence that albuminuria has been associated with executive function deficits and volume of white matter hyperintensities [32]. Subcortical white matter lesions are highly prevalent in advanced CKD and associated with renal disease severity [7]. Significantly lower measures of subcortical cognitive function in the HD group versus the CKD group, despite comparable memory scores, suggests that worsening cognitive deficits related to small vessel disease may parallel the progression of renal disease.
In our patients, the limited number of traditional and emerging cardiovascular risk factors identified was not found to be associated with the lower cognitive scores in the HD group compared with the CKD group. These cardiovascular risk factors were equally prevalent between patients with and without MCI. However, mechanisms for cognitive deficits characteristic of cerebral small vessel disease among CKD and HD patients may involve the cumulative effect of multiple vascular risk factors. As renal function declines, levels of nitric oxide synthase inhibitor asymmetric dimethyl-L-arginine increase, suppressing the synthesis of nitric oxide [33]. Nitric oxide is an inhibitor of vascular smooth muscle cell proliferation, platelet aggregation, and a potent vasodilator. Endothelial dysfunction resulting from reduced nitric oxide production in cerebral small vessels may contribute to the development of chronic ischemic damage of subcortical structures. Endothelial dysfunction has been associated with white matter hyperintensities in older adults and with a decrease in vasodilatory capacity of the cerebral cortex [34]. Cerebral small vessel damage is likely to be exacerbated in patients with advancing CKD through increased levels of inflammation [35], advanced glycation end products [36,37], and oxidant stress [38] that occur as renal function deteriorates. In patients receiving HD, frequent and repetitive hypotensive episodes during treatment may cause further damage to ischemia-sensitive frontal-subcortical circuits because small vessel arteriosclerosis, calcification, and nitric oxide deficiency may likely interfere with normal mechanisms of autoregulation and preservation of blood flow to these important anterior cerebral structures. The significantly decreased carotid blood flow that appears to improve following a single dialysis treatment and the decreased cerebral oxygenation that has been reported in HD patients are not yet fully understood [39]. In patients with advancing kidney disease, cumulative small vessel damage with subsequent damage to subcortical white matter structures may lead to early subcortical cognitive deficits. Even though our patients had no clinical history of stroke, significant underlying cerebrovascular disease cannot be excluded since neuroimaging was not available. The observed non-amnestic deficits may be an indicator of underlying cerebrovascular damage since kidney disease has been shown to independently predict subclinical brain infarcts [40]. As renal function deteriorates and patients require HD, the cognitive deficits are likely to progress and ultimately result in overt vascular dementia.
The strength of our study is that a relatively healthy group of CKD and HD patients with no clinical history of dementia, stroke or neurodegenerative disease was evaluated, allowing us to identify a profile of cognitive deficits that may be helpful in determining the future progression to dementia. We also required that all patients had hemoglobin levels >10 g, normal liver function tests, and adequate dialysis in order to eliminate these confounders as causes of cognitive impairment. Nevertheless, our study had a relatively small number of subjects and other limitations. For example, while stroke was excluded by history and medical record review, imaging was not available. In addition, all of our subjects were male which limits generalization of our findings to female patients. Another possible limitation may have been in the diagnosis of MCI based on neurocognitive scores in the absence of a subjective clinical complaint of memory or cognitive decline.
In conclusion, we found evidence of significant cognitive impairment in CKD and HD outpatients with normal global cognitive function and no history of stroke or neurodegenerative diseases. These impairments were predominantly in non-memory domains, thus indicating a potential vascular etiology. In patients with advanced CKD, amnestic MCI may precede overt dementia and be underdiagnosed with routine screening measures such as the MMSE. Early recognition of MCI prior to the development of overt dementia may provide an opportunity to intervene and slow the progression of disease.
Acknowledgements
Funding for this project was provided by the VA VISN 3 Seed Grant Program, VARR&D CDA 2 #B5050W, VARR&D Center of Excellence #B4162C and NIH/NIA #P50AG005138.
References
- 1.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30:41–49. doi: 10.1016/s0272-6386(97)90563-1. [DOI] [PubMed] [Google Scholar]
- 2.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 3.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W. Kidney function and cognitive impairment in us adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Vea A, Salvado E, Bardaji A, Gutierrez C, Ramos A, Garcia C, Compte T, Peralta C, Broch M, Pastor R, Angelet P, Marcas L, Sauri A, Oliver JA. Silent cerebral white matter lesions and their relationship with vascular risk factors in middle-aged predialysis patients with CKD. Am J Kidney Dis. 2006;47:241–250. doi: 10.1053/j.ajkd.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134:83–88. doi: 10.1016/0022-510x(95)00226-7. [DOI] [PubMed] [Google Scholar]
- 7.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55–61. doi: 10.1161/STROKEAHA.107.493494. [DOI] [PubMed] [Google Scholar]
- 8.Looi JC, Sachdev PS. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology. 1999;53:670–678. doi: 10.1212/wnl.53.4.670. [DOI] [PubMed] [Google Scholar]
- 9.Roman GC, Royall DR. Executive control function: a rational basis for the diagnosis of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(suppl 3):S69–S80. doi: 10.1097/00002093-199912003-00012. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- 11.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 14.Folstein MFFS, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. ed 2 (CVLT-II) San Antonio: Psychological Corp; 2000. [Google Scholar]
- 16.Benton AL, Hamsher KD. Multilingual Aphasia Examination. ed 3. Iowa City: AJA Associates; 1994. [Google Scholar]
- 17.Wechsler D. Wechsler Memory Scale, ed 3. Administration and Scoring Manual. San Antonio: Psychological Corp; 1997. [Google Scholar]
- 18.Smith A. Symbol Digit Modality Test (SDMT) Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 19.Reitan RWD. The Halstead-Reitan Neuropsychological Test Battery. Tuscon: Neuropsychology Press; 1988. [Google Scholar]
- 20.Golden JC. Stroop Color and Word Test. Chicago: Stoeling Corp; 1978. [Google Scholar]
- 21.Wetzel L, Boll T. Short Category Test, Booklet Format. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- 22.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.Pereira AA, Weiner DE, Scott T, Chandra P, Bluestein R, Griffith J, Sarnak MJ. Subcortical cognitive impairment in dialysis patients. Hemodial Int. 2007;11:309–314. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 26.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 27.De Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- 29.Almkvist O, Wahlund LO, Andersson-Lundman G, Basun H, Backman L. White-matter hyperintensity and neuropsychological functions in dementia and healthy aging. Arch Neurol. 1992;49:626–632. doi: 10.1001/archneur.1992.00530300062011. [DOI] [PubMed] [Google Scholar]
- 30.Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, Berman NG. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- 31.Meyer JS, Quach M, Thornby J, Chowdhury M, Huang J. MRI identifies MCI subtypes: vascular versus neurodegenerative. J Neurol Sci. 2005;230:121–129. doi: 10.1016/j.jns.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, Levey AS, Folstein MF, Sarnak MJ. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53:438–447. doi: 10.1053/j.ajkd.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleck C, Janz A, Schweitzer F, Karge E, Schwertfeger M, Stein G. Serum concentrations of asymmetric (ADMA) and symmetric (SDMA) dimethylarginine in renal failure patients. Kidney Int Suppl. 2001;78:S14–S18. doi: 10.1046/j.1523-1755.2001.59780014.x. [DOI] [PubMed] [Google Scholar]
- 34.Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, Paul RH, Jefferson AL, Haley AP, Cohen RA. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38:308–312. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int. 2001;59:407–414. doi: 10.1046/j.1523-1755.2001.059002407.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiss MF, Erhard P, Kader-Attia FA, Wu YC, Deoreo PB, Araki A, Glomb MA, Monnier VM. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int. 2000;57:2571–2585. doi: 10.1046/j.1523-1755.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- 37.Raj DS, Choudhury D, Welbourne TC, Levi M. Advanced glycation end products: a nephrologist's perspective. Am J Kidney Dis. 2000;35:365–380. doi: 10.1016/s0272-6386(00)70189-2. [DOI] [PubMed] [Google Scholar]
- 38.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 39.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab. 2007;27:1861–1869. doi: 10.1038/sj.jcbfm.9600478. [DOI] [PubMed] [Google Scholar]
- 40.Seliger SL, Longstreth WT, Jr, Katz R, Manolio T, Fried LF, Shlipak M, Stehman-Breen CO, Newman A, Sarnak M, Gillen DL, Bleyer A, Siscovick DS. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]