Abstract
The chronic inflammatory bowel diseases are characterized by aberrant innate and adaptive immune responses to commensal luminal bacteria. In both human IBD and in experimental models of colitis there is an increased expression of the enzyme indoleamine 2,3 dioxygenase (IDO). IDO expression has the capacity to exert antimicrobial effects and dampen adaptive immune responses. In the murine TNBS model of colitis, inhibition of this enzyme leads to worsened disease severity suggesting that IDO acts as a natural break in limiting colitis. In this investigation we show that induction of IDO-1 by a TLR-9 agonist, immunostimulatory-DNA (ISS-DNA), critically contributes to its colitis limiting capacities. ISS-DNA induces intestinal expression of IDO-1, but not the recently described paralog enzyme IDO-2. This induction occurred in both epithelial cells and in subsets of CD11c+ and CD11b+ cells of the lamina propria which also increase after ISS-ODN. Signaling required for intestinal IDO-1 induction involves interferon dependent pathways, as IDO-1 was not induced in STAT-1 knockout mice. Using both the TNBS and DSS models of colitis we show the importance of IDO-1s induction in limiting colitis severity. The clinical parameters and histologic correlates of colitis in these models were improved by administration of the TLR-9 agonist; however, when the function of IDO is inhibited, the colitis limiting effects of ISS-ODN were abrogated. These findings support the possibility that targeted induction of IDO-1 is an approach deserving further investigation as a therapeutic strategy for diseases of intestinal inflammation.
Keywords: Rodent; Intestinal Inflammation; Indoleamine 2,3 Dioxygenase; Tolerance
Introduction
Indoleamine 2,3 dioxygenase-1 (IDO-1) is a metabolically active enzyme with the capacity to promote immune tolerance and exert anti-microbial effects.(1–3) IDO-1, and its recently discovered paralog IDO-2 (collectively referred to here as IDO), function to catalyze the initial and rate limiting step of tryptophan degradation via the kynurenine pathway.(4, 5) Until recently most studies have examined IDO-1, which is expressed in various cell types including professional antigen presenting cells (APCs) as well as fibroblasts, trophoblasts and epithelial cells. IDO-2’s expression pattern is distinct from IDO-1 but both are expressed in dendritic cells.(4, 6)
IDO expression supports localized depletion of tryptophan, the rarest of essential amino acids, while its kynurenine based metabolites are produced in excess. These changes to the microenvironment lead to important immunomodulatory effects, in part via activation of GCN2-kinase.(7) These changes include the inhibition of T-cell replication, induction of T-cell apoptosis and the support of regulatory T-cell differentiation and suppressor function activation.(8) Additionally they create a hostile environment for invading microbes including numerous bacteria, viruses and intracellular parasites.(1) These important functions have led to evaluation of the role of IDO-1 in autoimmune and inflammatory conditions.(9) The physiologic relevance of IDO-1 induction has already been demonstrated in models of pulmonary disease where upregulation of IDO-1 via TLR9 agonists was shown to inhibit of pathogen growth and lessen asthma severity.(10, 11)
Similar to the lungs, the luminal surface of the mammalian gastrointestinal tract continually interacts with foreign antigens including microbial products from both commensal and pathogenic bacteria. In health, the mucosal immune system is geared towards tolerance and limits inflammatory responses to these antigens. In genetically predisposed individuals, when tolerance to commensal bacteria is lost, chronic inflammatory bowel disease (IBD) may develop. The in vivo mechanisms mediating immune tolerance is an area of active investigation and involves complex interactions between professional APCs, epithelial cells and cells of the adaptive immune system. IDO-1 is a key molecule mediating tolerance by linking the innate and adaptive immune responses in other some systems.(2) IDO may also play an important role in gut mucosal defense and act as a natural brake to intestinal inflammatory responses.
The gut is a site of high IDO-1 expression in the basal state, and its expression is increased in the setting of intestinal inflammation. (12, 13) Biopsies from patients with an inflammatory bowel disease (IBD) have IDO-1 levels that are significantly increased compared with biopsies from healthy individuals. In IBD the increased IDO-1 expression is apparent in both epithelial and mononuclear cells in areas of active disease, particularly near sites of ulceration.(13–16) Similarly, in animal models of IBD IDO-1 enzyme expression is upregulated.(13, 17) In the TNBS (trinitrobenzene sulfonic acid) colitis model, IDO expression and functionality is increased over baseline, particularly so in professional antigen presenting cells (APCs) of the lamina propria.(17) Administration of a specific IDO inhibitor, 1-methyl tryptophan (1-mT), significantly augmented the normal inflammatory response to TNBS suggesting that IDO plays an important role in down-regulating Th1 responses within the intestinal tract. These observations suggest that increasing IDO expression within the intestine may have the therapeutic capacity to abrogate colitis.
To address whether IDO upregulation lessens colitis severity, we examined the effect of a known IDO inducing agent, immunostimulatory oligodeoxynucleotide (ISS-ODN), on two experimental models of colitis, TNBS and DSS (dextran sodium sulfate). Here demonstrate that administration of an anti-colitic dose of ISS-ODN induces interferon dependent expression of IDO-1, but not IDO-2, throughout the intestinal tract in both professional antigen presenting cells of the lamina propria as well as intestinal epithelial cells. Furthermore, we show that IDO-1s induction is critical to the anti-colitic effects of ISS-ODN in both TNBS and DSS colitis in that co-administration of a specific IDO inhibitor negates the beneficial effects of this TLR9 agonist.
Materials and Methods
Oligodeoxynucleotides
Oligonucleotides were purchased as RP-HPLC purified, LPS-free, single-stranded, phosphothioate oligodeoxynucleotides (Trilink, San Diego, CA). The immunostimulatory-ODNs (ISS-ODN) and the control or mutated (M-ODN) used were synthesized to match those found previously to be effective or ineffective respectively at limiting colitis and are type B CpG ligands which bind TLR9.(18) The sequence for the ISS-ODN was 5-TGACTGTGAACGTTCGAGATGA-3 and the M-ODN sequence was 5-TGACTGTGTTCCTTAGAGATGA-3.
Induction of colitis and administration of pharmacologic agents
8 week old female SJL/J, C57B/6J or BALB/c mice weighing ~18g were purchased from the Jackson Laboratory (Bar Harbor, ME). All animals were maintained at a controlled temperature and light/dark cycle in a specific pathogen free facility at Washington University School of Medicine. Animal procedures and protocols were conducted in accordance with the institutional review board at Washington University School of Medicine. To induce colitis, we administered an enema containing 0.5 mg or 0.7 mg of TNBS (Trinitrobenzene sulfonic acid Sigma, St. Louis, MO) in 35% ethanol via a flexible 3.5 Fr catheter inserted 4 cm proximal to the anus as previously described (17). For the recurrent TNBS model, mice initially received a 0.4 mg TNBS enema to reduce early deaths, followed by a 0.5 mg TNBS enema at day 7 similar to previously described techniques for chronic TNBS colitis.(19, 20) In the DSS colitis model, a 2.0% aqueous solution of dextran sodium sulfate (TdB Consultancy, Upsula, Sweden) was passed through a 0.22-µm cellulose acetate filter then administered to mice for 7 days as drinking water. 10 ug of the ISS-DNA or the Control-ODN was injected i.p. prior to each administration of TNBS or DSS. Pellets containing slow release 1-methyl Tryptophan (1-mT) (Innovative Research of America, Sarasota, Florida) were surgically inserted under the dorsal skin of certain mice at the time of TNBS administration also as previously described and released 1-mT at a rate of 0.9mg/hour (17). To assess morphological and histological differences and to obtain tissues for analysis surviving mice were sacrificed at the times detailed with each result.
Morphological and histological analysis
The colon was removed from its mesentery to the pelvic brim by blunt dissection. The colon was opened longitudinally along the mesenteric attachment and pinned flat so that the mucosal surface could be examined. The entire length of the colon was pinned out, then in 10% formalin and transferred to 70% ethanol before being processed for embedding in paraffin. Five µm serial sections were prepared and stained with hematoxylin and eosin for histologic grading. Methods for scoring TNBS treated mice for morphology and histology have been described previously and are based on the systems described respectively by Colon et al and Fuss et al. (17, 21, 22) For DSS treated mice a previously described 12 point scoring system was used which evaluated colonic epithelial damage (0–6 points) and inflammatory infiltrate separately assigned to the mucosa (0–3), submucosa (0–2), and muscular layer and serosa (0–1).(23) Scoring was performed in a blinded fashion independently by two of the authors (MAC, EB).
Western blotting for IDO
Cell and tissue lysates were prepared from whole tissue or from the respective cell layers. Protein concentrations were determined using BCA protein assay (Pierce). Samples were then subjected to electophoresis on a 10% Tris-HCL ready gel (Bio-Rad Hercules, CA) and transferred onto Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA). Blocking was with 5% non-fat dry milk in TBS. IDO-1 antibodies were a polyclonal rabbit anti-mouse IDO antibody (17) or monoclonal rat anti-mouse IDO Ab (BioLegend, SanDiego, Ca). The IDO-1 antibodies were tested for specificity by confirming they did not bind to recombinant IDO-2 protein. The mouse anti-IDO-2 antibody was used as previously described (4). Phospho-specific STAT1 (pY701) was from BD Biosciences (SanJose, CA). Antigen detection was achieved using horseradish peroxidase linked secondary antibodies and the enhanced chemiluminescence system (Amersham Pharmacia Biotech, UK). The membranes were then stripped and re-probed for β Actin to confirm equal protein concentrations (Santa Cruz Biotechnology). Alternatively, a membrane from a simultaneously run gel with equal loading volumes was probed for β Actin.
Real-time PCR
Primers were designed for multiple genes using Primer Express Software (Applied Biosystems, Foster City, CA)and synthesized by Integrated DNA Technologies (Coralville, IA). The following sequences were used: IDO-1 (5’- CGGACTGAGAGGACACAGGTTAC; 5’-ACACATACGCCATGGTGATGTAC), GAPDH (5’-TGCACCACCAACTGCTTAG; 5’-GATGCAGGGATGATGTTC), IDO-2 (5’-CCCAGAAGGACCGTTGGAA; 5’-GCCCCGCAGGCTCTCT) TNFα (5'- GAC CCT CAG ACT CAG ATC ATC TTC T; 5'-CCA CTT GGT GGT TTG CTA CGA), IFNγ (5' - AGG CCA TCA GCA ACA ACA TAA GCG; 5' - TGG GTT GTT GAC CTC AAA CTT GGC), IFNα (5' - TGC TTT CCT GAT GAC CCT GCT AGT; 5' - ATC CCA AGC AGC AGA TGA GTC CTT), IL-6 (5' - CCA GAA ACC GCT ATG AAG TTC CT; 5' - CAC CAG CAT CAG TCC CAA GA - 3'), IL-10 (5’ - CCC TTT GCT ATG GTG TCC TT; 5' - TGG TTT CTC TTC CCA AGA CC), IL12p40 (5' - GGG TGT CCA GGC ACA TCA G; 5' - GCG ACA GGG AAG AGG AGA GA), CD11b (5' - ATC CTG TAC CAC TCA TTG TGG GCA; 5' - TCA TCA TGT CCT TGT ACT GCC GCT), CD11c (5' - TCG TTG GCC TCT AAC GAG CTT TCT; 5' AGG ATA ACA TGG AAG CAC GGA CCA), TGFβ (5' - AGT GTG ACC TGG AGT TTC GGA GAT; 5' -TTG CCC TGA GGA CTT TCT TGA CCT), IL1β (5' - TCA GGC AGG CAG TAT CAC TCA; 5' - GGA AGG TCC ACG GGA AAG AC). Total RNA was isolated from homogenized whole tissues or cell layers using Trizol per manufacturer’s directions (Invitrogen, Carlsbad, CA). Spectrophotometry was used to measure mRNA concentrations. RT-PCR was performed using the iScript One-Step RT-PCR kit (Bio Rad, Hercules, CA) on the MyIQ i-Cycler RT-PCR detection system (Bio-Rad). Each sample was run in triplicate and reported as fold change after normalization against GAPDH. The PCR products were validated by melting point analysis.
Immunofluoresence
Fresh-frozen intestinal sections were prepared by freezing tissues in TISSUETEK O.C.T Compound (Miles, Elkhart, IN). Sections were cut at 6 microns, allowed to warm to room temperature, fixed in a 1:1 mixture of methanol and acetone for 20 minutes, and then washed in water followed by phosphate buffered saline (PBS). Endogenous peroxidases were quenched by incubation in 3% H202. An Avidin/Biotin blocking kit (Vector Burlingame, Ca) was used and followed by blocking with 1% BSA in PBS with M.O.M (Mouse On Mouse; Vector Laboratories) when a murine primary antibody was used. Primary antibodies used for IDO-1 staining was the rabbit anti-mouse IDO antibody (17). The IDO-2 antibody was the same as used for the WBs. Other primary antibodies included biotin anti-CD11c and anti-CD11b (eBiosciences), mouse anti-IDO-2 (4), and mouse anti-cytokeratin 18 (Chemicon International). Secondary antibodies bound to the described chromagens were from Jackson Immunoresearch (West Grove, PA). Vectashield with DAPI was used for nuclear counterstaining and sections were viewed with a Zeiss Axiovert 200 with AxioCam MRm camera.
Determination of MPO activity
Colon tissues were opened longitudinally and a lengthwise portion was taken from the anal verge to the ascending colon and weighed (~50 mg). This was homogenized in hexadecyltrimethyl ammonium bromide (0.5%) in 50 mmol/l phos-phate buffer, pH 7.0. The homogenate was sonicated for 10 seconds, frozen and thawed 3 times, and centrifuged for 15 minutes. An aliquot of the supernatant was used for determination of enzyme activity as described (18).
Isolation of lamina propria mononuclear cells and epithelial cell layers
The intestines were removed from mice, flushed with cold PBS, opened along the mesenteric border and then washed again in cold PBS. The epithelial cell layer was removed by incubation in calcium and magnesium free HBSS containing 5mM EDTA at 37°C, then preserved for RNA or protein isolation. The lamina propria cell layer was isolated as previously described. (24).
Statistical Analysis
Morphological and histological data was assessed using Student’s t-test. Survival data was assessed using a Chi square test. Real-time PCR data and weights are expressed as mean ± SEM. Significance of fold increase in mRNA expression over control was assessed using a Student’s t test.
Results
ISS-ODN induces IDO-1, but not IDO-2 throughout the intestinal tract
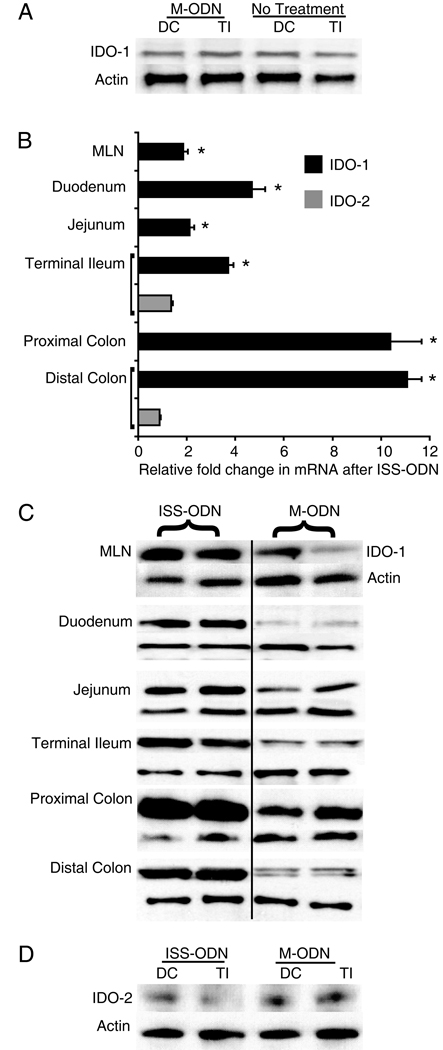
Certain ISS-ODN sequences are protective in experimental colitis models whereas M-ODN’s, mutated versions of the ISS-ODN’s, do not offer effective protection from colitis.(18) In determining whether IDO-1 expression contributes to the anti-colitic effect of ISS-ODN, we first sought to assess whether the same sequences, used at the same colitis preventing dosage, could induce intestinal IDO. ISS-ODN (10 µg), M-ODN (10 µg) or PBS was administered via intraperitoneal injection and tissues were harvested for RNA or protein. Time course experiments led to the choice of the 36 hour time point for RNA and 72 hours for protein. Small intestine and colon tissue from M-ODN treated mice had no induction of IDO-1 protein over PBS treated mice (figure 1A). Fold changes in IDO-1 mRNA also did not increase significantly (Colon 1.10±0.64 p=0.89; SI 1.13±0.15 p=0.79). However, when compared to M-ODN treatment, ISS-ODN administration led to a significant increase in IDO-1 protein and mRNA in both the colon (average 10.7 fold increase) and in the small intestine (average 3.5 fold increase) (figure 1B,C). Additionally, ISS-DNA significantly increased IDO-1 in the mesenteric lymph nodes (MLNs) (1.9±0.2), though to a lesser degree than in the intestinal tissue.
Figure 1.
Intestinal tract IDO-1, but not IDO-2, is induced by ISS-ODN. 10 µg of either the ISS-ODN or the M-ODN was injected i.p. and the tissues were harvested at 72 hours for protein. Compared to control (untreated mice), the administration of M-ODN does not lead to induction of IDO-1 in either the colon or the small intestine (A). However, the injection of ISS-ODN leads to a significant induction of IDO-1 mRNA and protein throughout the intestinal tract and MLNs when compared to control mice injected with M-ODN (B,C). Analysis of the same samples did not show an increase in IDO-2 protein after ISS-ODN (D). All experiments consisted of at least 4 mice/group and were repeated at least twice. Two representative samples are shown in each western blot for figure 1C. RT-PCR data is shown as fold change relative to control ± SEM. * represents statistical significance of p≤0.05.
A paralog to the originally identified tryptophan catabolizing enzyme IDO (which is now referred to as IDO-1) was recently identified.(4, 5) Metz and colleagues had demonstrated that by western blot the IDO-2 protein was slightly smaller than IDO-1. (4) This raised the question of which intestinal IDO was upregulated by ISS-ODN. We first sought to determine the specificity of our IDO-1 antibodies and found that neither of our IDO-1 antibodies detected recombinant IDO-2 protein (data not shown). Having established the specificity of our reagents for IDO-1, we next sought to determine if IDO-2 is also induced by ISS-ODN. RT-PCR using primers specific for IDO-2 did not detect any changes in mRNA expression after administration of ISS-ODN (figure 1B, gray bars). Finally, with high protein loading concentrations (50 ug/well) a distinct band for IDO-2 was identified in colonic and small intestinal tissues; however, the band was faint and showed no discernable difference in treatment verses control samples (figure 1D). Additionally, relative to our prior findings that IDO-1 is increased in TNBS colitis, we examined colonic tissue from mice with TNBS colitis and found no change in IDO-2 mRNA (avg 0.8 ± 0.22 fold change vs control, p=NS) or IDO-2 protein expression over controls. Together these findings suggest that only IDO-1 and not IDO-2 expression is upregulated by ISS-ODN and TNBS colitis.
Localization of intestinal IDO-1 expression after ISS-ODN
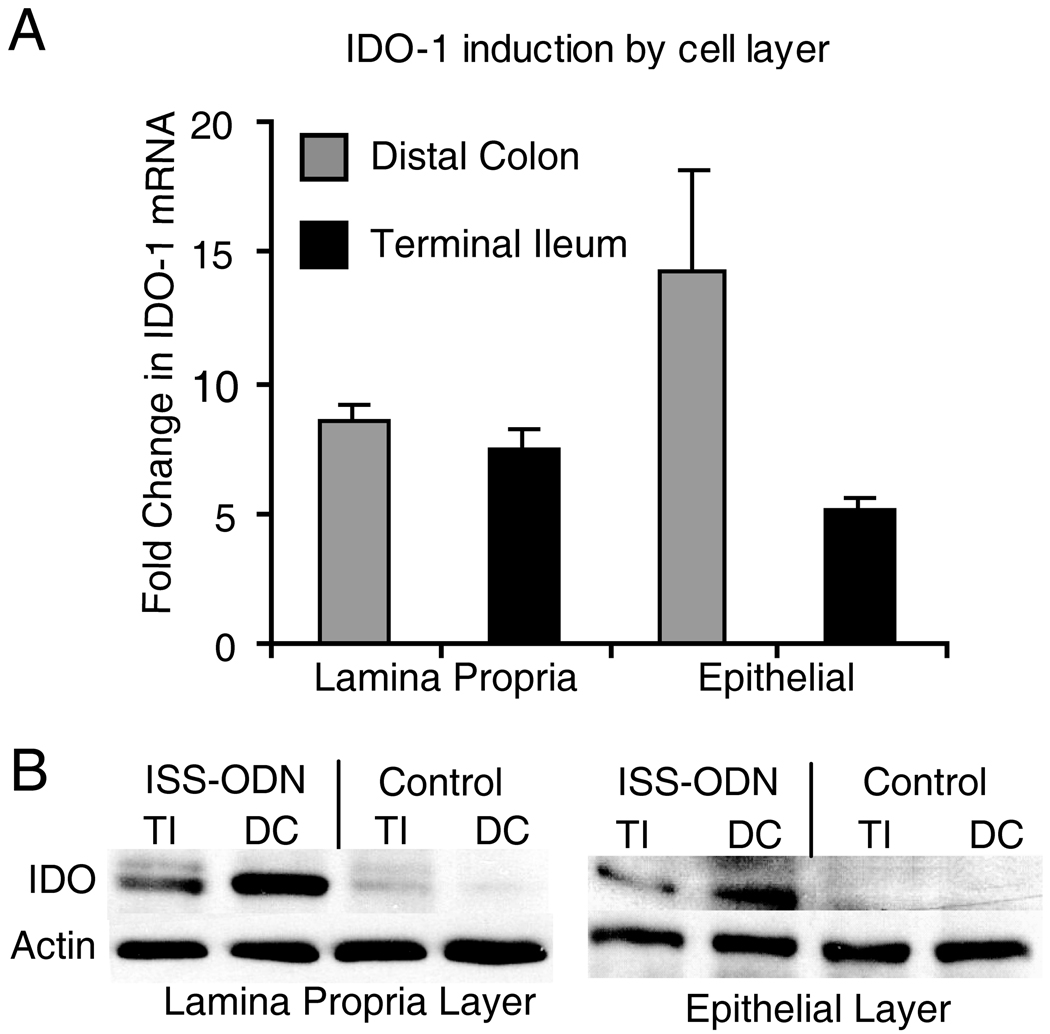
Having confirmed the induction of IDO-1 by ISS-ODN in whole intestinal protein and mRNA extracts, we sought to determine which tissue layers and cells types demonstrated the increase in IDO-1 expression. Previous studies focused on the importance of IDO-1 expression in immune regulatory cells, specifically dendritic cell populations.(2, 3) However, non-professional APC’s have also been shown to express IDO-1 with physiologic relevance in other organ systems.(10) After injection of ISS-ODN or M-ODN, whole colon and small intestine sections were flash frozen or processed for isolation of the epithelial and lamina propria layers. RT-PCR and western blotting showed that after ISS-ODN injection IDO-1 was upregulated in both lamina propria and the epithelial cell layers (figure 2A,B). In these experiments, the average induction of IDO-1 ranged from 5 to 14 fold over baseline within epithelial and lamina propria cell layers. The fold increases in IDO-1 induced by ISS-ODN were similar in the distal colon and terminal ileum. Moreover, the fold increases in IDO-1 were similar in the lamina propria and epithelial layers in both organs. The differential level of IDO expression between either the ileal or colonic layer types or between epithelial and lamina propria layers within the colon or small intestine was not statistically significant.
Figure 2.
ISS-ODN administration leads to increased expression of IDO-1 in both lamina propria and epithelial cells layers. 10 µg of the ISS-ODN or the control M-ODN was injected i.p. into SJL/J mice and the tissues were harvested at 36 hours for RNA or 72 hours for protein. The tissue was processed to isolate the epithelial cell layer (via CMF-HBSS + EDTA) and the lamina propria cell layer (via Dispase/Collagenase digestion). Induction of IDO-1 mRNA by ISS-ODN is shown in each cell layer from both the colon and small intestine (A). An increase in IDO-1 protein is confirmed on this western blot for two representative samples from isolated cell layers from the colon and small intestine (B). This experiment was repeated twice with 2 mice in each group.
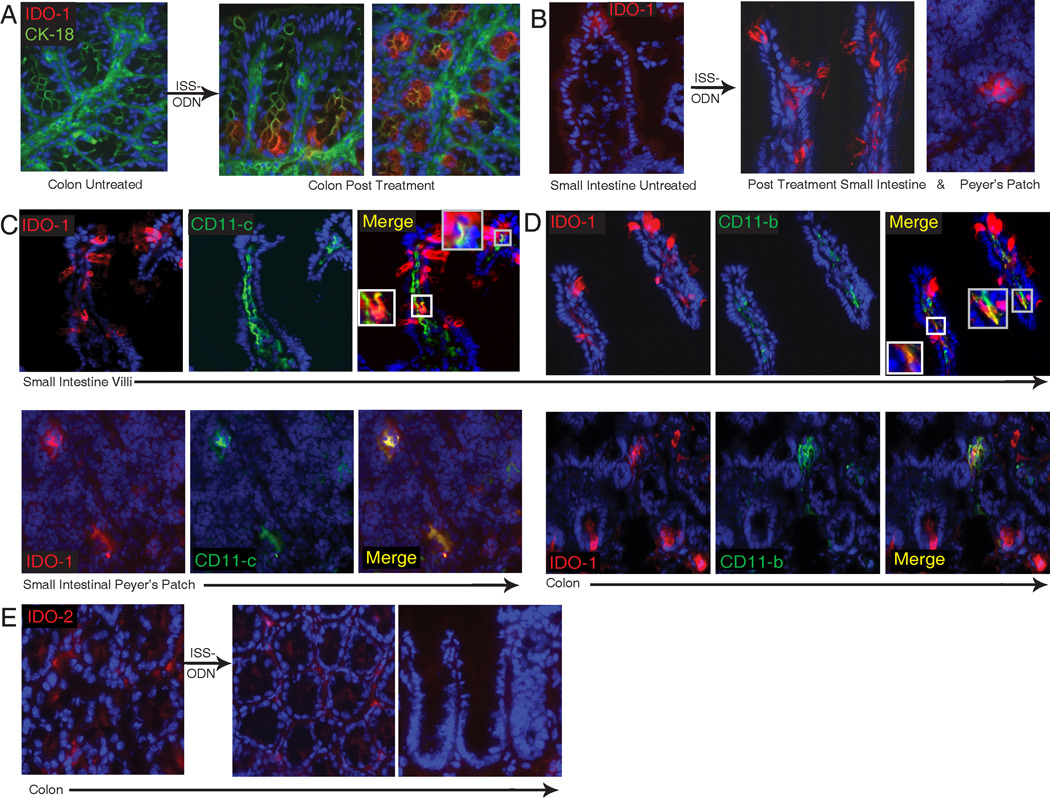
To further delineate where IDO-1 was expressed after ISS-ODN exposure we used immunofluorescence to examine frozen sections of the colon and small intestine. Only faint IDO-1 staining was detected in the control treated tissues (Figure 3A & B; left image), whereas samples from ISS-ODN treated mice demonstrated intense staining for IDO-1 (figure 3A,B; images to right). Cytokeratin-18 costaining (FITC) was used to assist in delineating the detection in colonic epithelial cells. In the colon IDO-1 staining was strongest in the epithelial cells at the crypt base. In contrast, in the small intestine staining was strongest in goblet cells and lamina propria cells of the villi. IDO-1 staining was also detected in select cells of small intestinal Peyer’s patches (Figure 3B far right image).
Figure 3.
Cellular localization of IDO after ISS-ODN. Colon and small intestinal sections from control (M-ODN treated) mice shows faint staining for IDO-1 (red) (A,B; first image in each). Cytokeritin 18 (green) staining is used on colon sections to highlight structure. Dapi was used for nuclear staining. 72 hours after ISS-ODN administration IDO staining becomes markedly more positive in both epithelial cells (particularly at the base of the colonic crypts) as well as within the lamina propria and Peyer’s patches (A,B; images to right of arrow in each). Dual staining of frozen sections shows IDO-1 co-staining in some, but not all cells with CD11c (C) and CD11b (D), markers for dendritic cells and macrophages respectively. Staining for IDO-2 (red) was performed on the same control and ISS-ODN treated tissues. Faintly positive cells were identified in the lamina propria with no difference identified between the treated and untreated tissues (E).
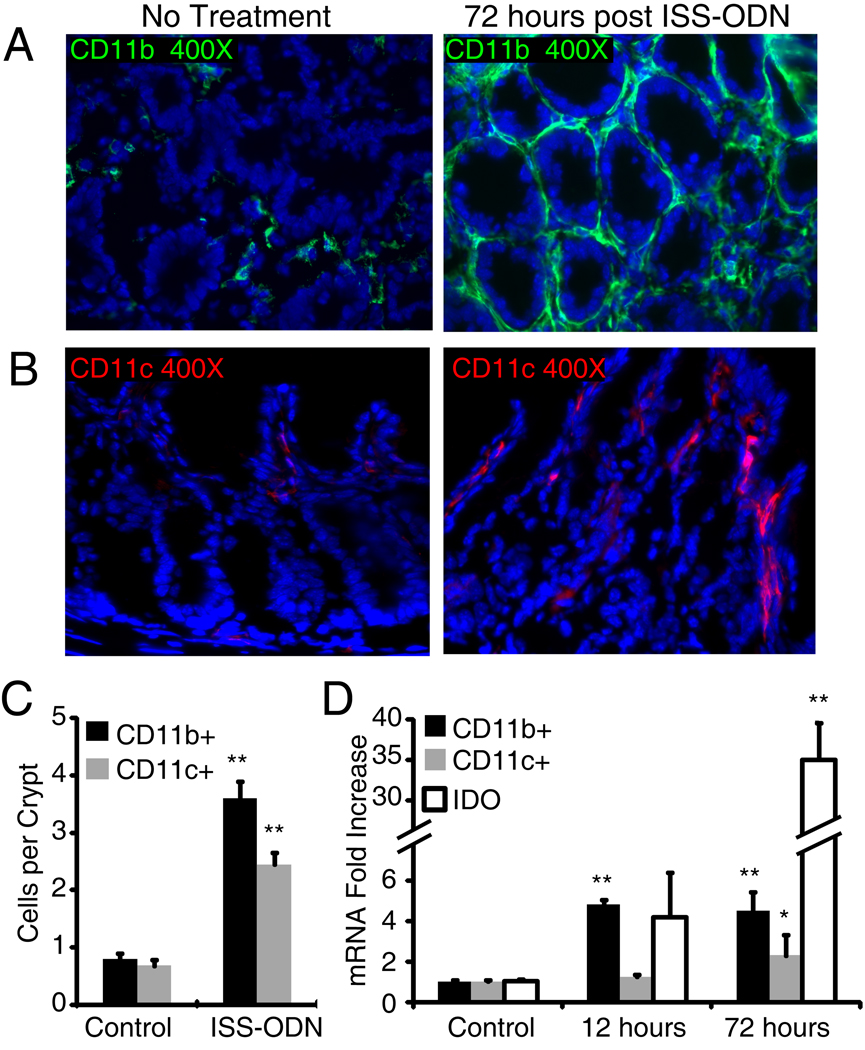
Given the previously identified physiologic importance of IDO’s induction in dendritic cells and macrophages, we co-stained for IDO-1 and CD11c or CD11b to determine if ISS-ODN’s was also inducing IDO-1 in these cell types (figure 3C,D).(2, 3, 25) In the small intestine there were numerous CD11c+ cells in the lamina propria cells of the villus. A few of these CD11c+ cells costained for IDO-1 suggesting that ISS-ODN induces IDO expression in a fraction of dendritic cells. There were also a small number of CD11b+ cells in the villus lamina propria. Some, but not all of these CD11b+ cells also expressed IDO-1. After the administration of ISS-ODN, most of the IDO-1 positive cells in the colon are epithelial cells at the base of the crypts, but there are also CD11b+ and CD11c+ cells that co-express IDO-1. Moreover, we found that the administration of ISS-ODN is positively associated with an quantitative increase in both CD11b+ and CD11c+ cells (figure 4A–D).
Figure 4.
CD11b and CD11c expressing cells increase after ISS-ODN administration. WT mice were administered one (12 hours prior) or two doses (12 and 72 hours prior) of ISS-ODN (20 µg). Immunoflorescence on frozen sections showed an increase in colon lamina propria CD11b and CD11c expressing cells (A,B). The number of positive staining cells in twenty crypts of three colonic cross sections from three different mice per group was counted. The average number of both cell types per crypt was increased (C). qRT-PCR showed a significant increased in only CD11b at 12 hours, but a significant increase in CD11b, CD11c and IDO-1 mRNA was apparent at 72 hours (D). n=4 mice/group repeated twice. * p<0.05, **p<0.01
Immunofluorescence for IDO-2 in tissue from untreated mice showed very faint staining in lamina propria cells of the colon without significant epithelial cell staining (Figure 3E, left image). Staining for IDO-2 did not appear to be increased after ISS-ODN treatment as it had with IDO-1 (Figure 3E, middle and right image). Thus, the immunofluorescence staining supports our findings that IDO-1 expression is upregulated by ISS-ODN in both intestinal lamina propria mononuclear cells and epithelial cells.
Gut IDO-1 expression after ISS-ODN is associated with interferon induction and STAT1 dependent signaling
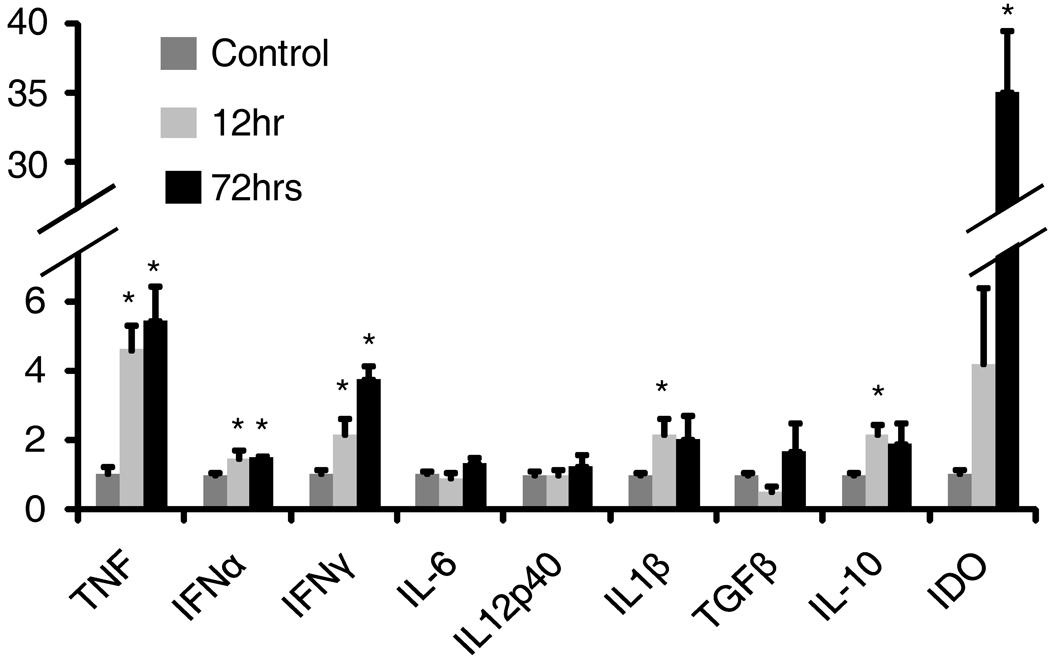
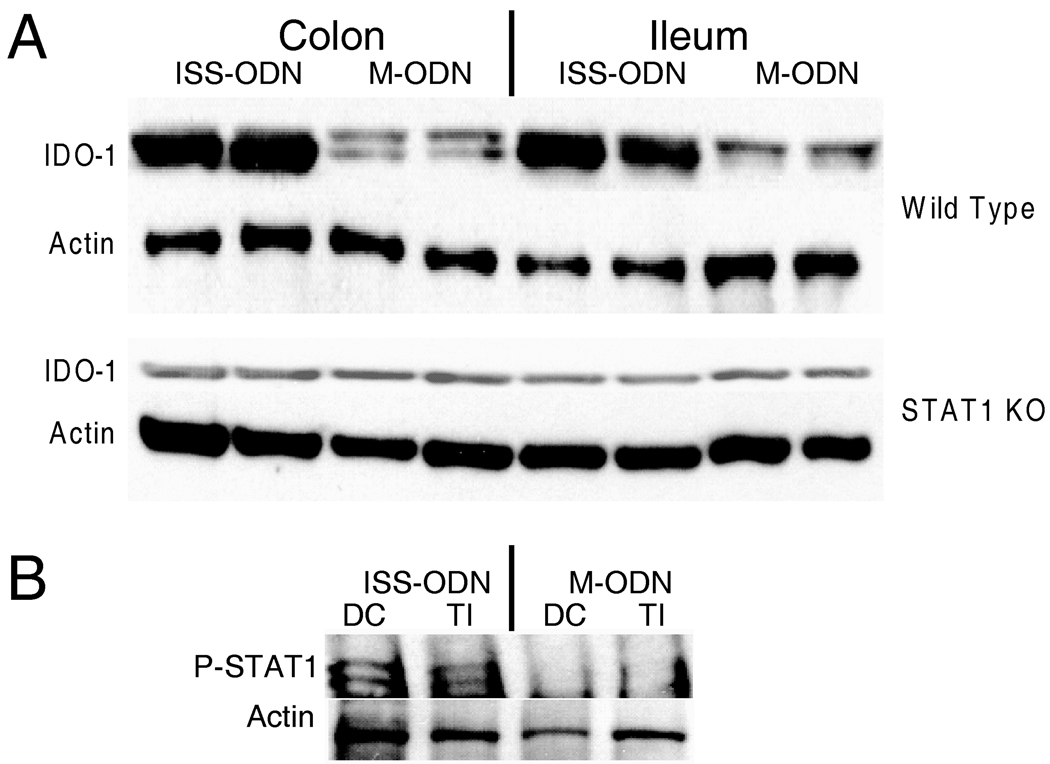
IDO-1 expression has been shown to be increased by cytokines including TNF-α and IL-1 as well as TLR ligands including LPS.(2) Both type I and type II interferons also induce IDO-1.(2, 3, 10) Similarly, both type I and type II interferon’s have been implicated as mediators of the physiological effects of TLR-9 agonists.(3, 10, 23, 26) STAT1 is a downstream signaling molecule for both type I and type II IFN’s (27, 28). We evaluated cytokines expression by mRNA at 12 and 72 hours after ISS-ODN and found TNFα, IFNα and IFNγ all to be significantly increased (figure 5). After 72 hours dosing ISS-ODN twice to maximize detection, IDO-1 expression was high at 35 fold over untreated mice. Then to determine if, within the intestinal tract, ISS-ODN’s upregulation of IDO was dependent on IFN signaling we employed the use of STAT1 KO mice. These STAT1 KO mice were compared to background matched C57/B6 WT controls. After ISS-ODN, IDO-1 expression was increased in both the colon and small intestine of the WT, but not the STAT1 KO mice (figure 6A). RT-PCR confirmed these findings (data not shown). Using the intestinal samples from the WT mice we also used western blotting to confirm that ISS-ODN administration lead to activation of STAT-1 through phosphorylation (figure 6B)
Figure 5.
Colon cytokine expression changes associated with ISS-ODN. Mice were administered one (12 hours prior) or two doses (12 and 72 hours prior) of ISS-ODN (20 µg) and tissues were harvested and analyzed by qRT-PCR for changes in cytokine expression. Cytokines known to induce IDO-1 including TNFα, IFNγ, and IFNα were significantly increased at both time points. IL-1B and IL-10 were also significantly induced at the 12, but not the 72 hour time point. n=4 mice/group repeated twice. *p<0.05
Figure 6.
ISS-ODN induces IDO-1 in a STAT-1 dependent manner. Age matched WT C57Bl/6 mice and STAT-1 KO mice on a C57Bl/6 background were given 10 ug ISS-ODN or control M-ODN. IDO-1 expression increases in both the small intestine and colon of WT C57B/6 mice but not in the STAT1 KO mice (A). Two representative mice are shown for each condition. In the WT mice, activation of STAT-1 signaling by phosphorylation is shown on this western blot (B).
Clinical and histologic benefits offered by ISS-ODN’s in TNBS colitis are abrogated by concurrent IDO inhibition
Having demonstrated that doses of ISS-ODN that prevent colitis also significantly increase intestinal IDO-1, a known tolerance promoting enzyme; we next wanted to determine if IDO-1 upregulation contributed to the beneficial effects of ISS-ODN on TNBS colitis. To address this question, we concurrently administered ISS-ODN and 1-mT, a specific IDO inhibitor (10, 17, 25, 29–31), and then followed clinical and pathology parameters known to be altered in TNBS colitis.(17, 29)
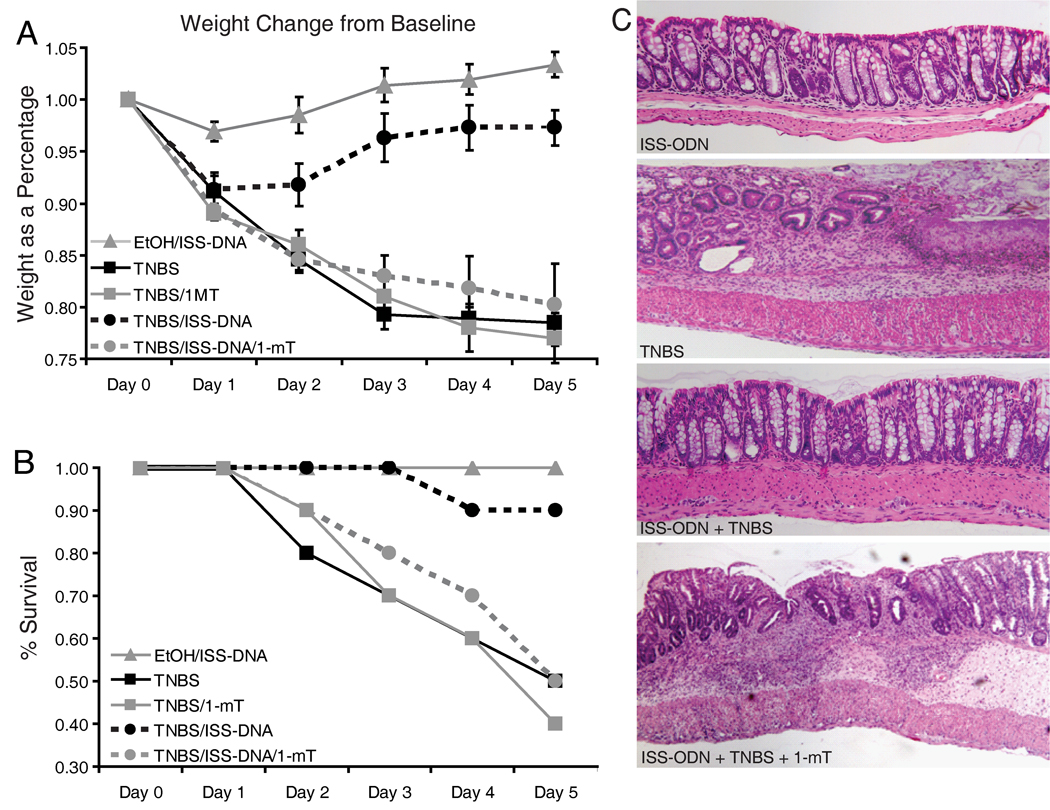
Mice were given ISS-ODN, and subsequently a slow release tablet of 1-mT was inserted under their dorsal skin at the time of TNBS enema. All mice lost weight by day one, but by day two the mice receiving ISS-ODN along with either the TNBS or ethanol (control) enema began regaining weight (figure 7A). The mice receiving TNBS alone or in combination with 1-mT, with or without concurrent ISS-ODN, all continued to lose weight. At day five, weight loss in the ISS-ODN treated group was significantly less than in the TNBS alone group (Table 1). However, weight loss in mice receiving the ISS-ODN along with the IDO inhibitor was significantly greater than mice in the ISS-ODN/TNBS group, but not statistically different than the group receiving TNBS alone. Similarly, survival experiments performed using a higher dose TNBS enema confirmed that ISS-ODN lessened mortality; and this survival benefit was negated by concurrent IDO inhibition (figure 7B).
Figure 7.
The clinical, histologic and morphologic benefits offered by ISS-ODN prior to TNBS colitis are abrogated by concurrent IDO inhibition. ISS-ODN was offered at 10 µg IP prior to TNBS or ethanol enema. The 1-mT slow release pellet was inserted concurrently with the TNBS enema. N=10 in each group. Relative weight changes from baseline weight are graphed as mean change ± SEM (A). TNBS enema was at 0.5 mg/mouse. Survival curve of mice with the same treatment groups is shown (B), where TNBS was administered at 0.7 mg/mouse. Distal colon sections were harvested 5 days after treatment and representative sections are shown at 100X stained with H&E (C).
Table I.
Day 5 Weight and Mortality Comparison
| Mortality | P value | % Δ in Weight | P value | |
|---|---|---|---|---|
| A) ISS-ODN + EtOH enema | 0/10 | + 3 ± 1.2 | ||
| B) TNBS | 5/10 | 0.02 vs EtOH | − 22 ± 1.7 | 1.3 × 10−9 vs EtOH |
| C) TNBS + 1-mT | 6/10 | NS vs TNBS | − 23 ± 2.4 | 0.68 vs TNBS |
| D) TNBS + ISS-ODN | 1/10 | 0.07 vs TNBS | − 3 ± 1.7 | 6.4 × 10−7 vs TNBS |
| E) TNBS + ISS-ODN + 1-mT | 5/10 | 0.07 vs TNBS + ISS-ODN N.S. vs TNBS |
− 20 ± 3.9 | 0.001 vs TNBS + ISS-ODN 0.71 vs TNBS |
NOTE: 10 mice/group. This table numerically summarizes day 5 of the data presented in figure 5 and provides statistical analysis using the Fisher’s exact test for mortality and Student’s t-test for weight loss.
Colons were harvested from the mice after day five of the weight loss experiments and were compared for histology and morphology. Mirroring the weight loss and mortality results, mice who received ISS-ODN prior to a single TNBS enema had lower histologic and morphologic scores; however, this benefit was lost in the mice who received the 1-mT pellet for concurrent IDO inhibition (figure 7C, table 2). TNBS colitis has most frequently been used as an acute colitis model in which mice received only one TNBS enema.(18, 29) However, use of a second TNBS administration increases T-cell mediated disease, while repeated dosing (up to 8 times) leads to chronic inflammation with IL-13 triggered tissue fibrosis.(19, 20) Since a key function of IDO-1 is to downregulate T-cell responses, we compared the same groups previously tested when TNBS enema was administered twice. The ISS-ODN was administered on day 0 and 7, while the 1-mT pellet was administered on the day before the second enema performed on day 7. The results were similar to the single TNBS administration where ISS-ODN reduced colitis severity, but the effect was abrogated by inhibition of IDO-1 with 1-mT (Table III). There was no significant differences in final weights between groups; however, colon lengths were significant longer in the TNBS + ISS-ODN groups than the TNBS alone or with co-administration of ISS-ODN and 1-mT or 1-mT alone (9.5 cm vs 8.9cm and 9.0 cm respectively, p<0.01). These findings suggest that the beneficial effects of ISS-ODN in both single and recurrent TNBS colitis involved an IDO-1 dependent mechanism.
Table II.
Histology and morphology scores for acute phase after TNBS enema
| Histology | P value | Morphology | P value | |
|---|---|---|---|---|
| TNBS | 5.5 ± 0.4 | 3.9 ± 0.3 | ||
| TNBS + ISS-ODN | 2.9 ± 0.5 | 0.001 vs TNBS | 1.7 ± 0.3 | 0.001 vs TNBS |
| TNBS+ 1-mT | 6.5 ± 0.4 | 0.038 vs TNBS | 4.4 ± 0.2 | 0.05 vs TNBS |
|
TNBS + ISS-ODN + 1-mT |
5.2 ± 0.5 | 0.004 vs TNBS + ISS-ODN 0.62 vs TNBS |
3.3 ± 0.3 | 0.006 vs TNBS + ISS-ODN 0.19 vs TNBS |
NOTE: Day 5 after single TNBS enema to SJL/J mice
Table III.
Histology and morphology scores after recurrent TNBS treatment
| Histology | P value | Morphology | P value | |
|---|---|---|---|---|
| TNBS | 3.8 ± 0.51 | 2.3 ± 0.19 | ||
| TNBS + ISS-ODN | 2.7 ± 0.39 | 0.16 vs TNBS | 1.4 ± 0.29 | 0.041 vs TNBS |
| TNBS+ 1-mT | 4.9 ± 0.55 | 0.048 vs TNBS | 2.6 ± 0.22 | 0.33 vs TNBS |
|
TNBS + ISS-ODN + 1-mT |
4.4 ± 0.32 | 0.018 vs TNBS + ISS-ODN 0.44 vs TNBS |
2.3 ± 0.15 | 0.046 vs TNBS + ISS-ODN 0.81 vs TNBS |
NOTE: Day 13 after first TNBS enema and Day 6 after second TNBS enema to BALB/c mice. 5 mice per group performed twice.
IDO-1 induction by ISS-ODN is critical to limiting severity of DSS colitis
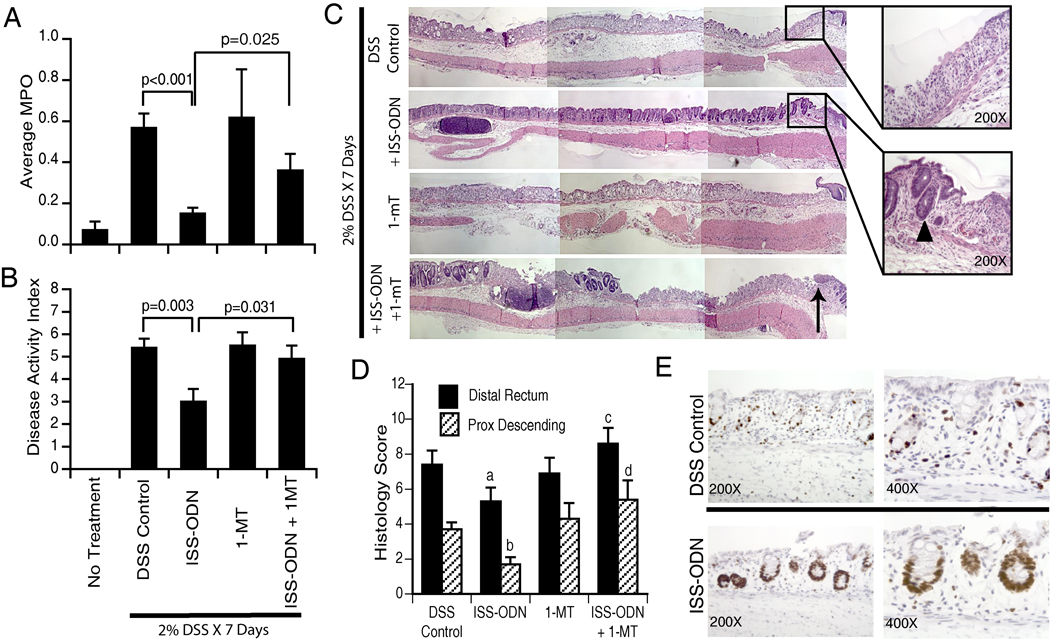
ISS-ODN has also been shown to be effective in limiting disease severity in non T-cell mediated DSS colitis model.(18, 23) Recognizing that IDO-1’s most examined immunologic function is its ability to downregulate T-cell responses, we sought examine the possibility that induction of IDO-1 could also be critical to the ISS-ODN’s effects in the DSS model. A similarly designed experiment to the TNBS model evaluated DSS colitis at 7 days with ISS-ODN being tested with or without IDO-1 inhibition (figure 8). ISS-ODN administration lessened DSS disease severity as evidenced by lower myeloperoxidase (MPO) activity, disease activity indexes (DAI) and histology scores (figure 8A,B,D). Regenerative crypts and epithelial proliferation were also more apparent in these mice as evidenced on H&E and Ki-67 staining (figure 8 C, E). Administration of the IDO inhibitor 1-mT alone did not significantly worsen any of these measured parameters compared to DSS alone. However, 1-mT blockade of IDO significantly abrogated the beneficial effects of ISS-ODN on all of these parameters. The results from this second model of colitis strengthen the case for IDO-1 induction by ISS-ODN being critical for its anti-colitic properties. Furthermore, the efficacy of IDO-1 induction in the acute DSS model suggests that this enzyme’s colitis preventing properties are not limited to its ability to dampen T-cell responses.
Figure 8.
IDO-1 induction is key to ISS-ODN’s protection against DSS colitis. 8 week old C57B/6 mice were offered 2.0% DSS in drinking water. On the day DSS was started, mice received 10 µg of ISS-ODN and/or 1-mT as listed. N=5 mice/group and the experiment was repeated 3 times with averaged composite values being shown in the figure. On day 7 disease activity index scores were compiled and the colons were subsequently harvested to test myeloperoxidase activity and fixed for histology evaluation of the distal rectum and proximal descending colon. The MPO activity (A), DAI (B), and colitis severity (C,D) were all significantly lower in the mice treated with ISS-ODN. No difference was found if 1-mT was administered alone. However, the addition of 1-mT eliminated the protective effect of each parameter which was improved by ISS-ODN. Representative histology sections (40X paired images) are shown in the bottom panel with the arrow marking the anorectal junction. Regenerative crypts (arrowhead (C)) with proliferating epithelial cells by Ki-67 staining (E) were more prevalent in the distal rectum of ISS-ODN treated mice. Similarly, this effect was lost with 1-mT co-administration. (p-values for histology: a=0.039 and b=0.001 vs DSS controls. c=0.003 and d=0.004 vs DSS + ISS-ODN treated groups)
Discussion
In the mammalian gastrointestinal tract, the innate immune system has a key function to recognize and determine appropriate responses to luminal antigens and microbial products (32). Some of the genes encoding for proteins which regulate the innate immune response pathways are susceptibility genes for the development of the chronic inflammatory bowel diseases (33). Toll-like receptors (TLRs), a class of transmembrane pattern recognition receptors, are an important part of the innate immune system and are widely expressed by various cell types in the GI mucosa (32). Upon activation, TLRs initiate signaling to induce antimicrobial effector pathways as well as to control adaptive immune responses. IDO, an enzyme which regulates tryptophan catabolism, is a downstream mediator of TLR signaling known to exhibit both antimicrobial effects and effects on the adaptive immune response (1–3). IDO induction in vivo has been associated with downregulation of models of acute and chronic inflammation. In models of pulmonary disease upregulation of IDO-1 by a TLR9 agonist inhibits pathogen growth and lessens asthma severity (10, 11). In the inflammatory bowel disorders, where tolerance is lost and aberrant intestinal immune responses prevail, it is critical to understand the complex signaling pathways for the innate immune interaction with adaptive immunity (34, 35).
Roles for the activation, antagonism and deletion of TLRs have been examined in experimental models of colitis (35). Activation of certain TLRs (TLR2/3/4/9) exerts anti-colitic effects, particularly when the agonists are administered prior to the induction of colitis (36–39). Specific sequences of immunostimulatory oligodeoxynucleotides (ISS-ODNs), which are TLR9 agonists, lessen the severity of colitis models including DSS, TNBS and DNBS colitis and the colitis seen in IL10−/− mice (18). The beneficial effects exerted by some probiotic bacteria in these colitis models have been shown to be mediated by the ability of their DNA to initiate TLR signaling (40). Type 1 interferons play a key role in mediating the protective effects of ISS-ODN in DSS induced colitis (23). ISS-ODN increases IFN-α/β levels in the serum and culture media from isolated splenocytes; moreover, co-administration of IFN-α/β blocking antibody negates the benefits of ISS-ODN in DSS colitis (23).
Although ISS-ODN is known to lessen the severity of TNBS colitis, DSS colitis and the colitis associated with IL-10 deficiency, the potential role of IDO-1 induction as the mechanism for the beneficial effects of ISS-ODN in these models has not been explored. In this study we found that ISS-ODN increased IDO-1 expression both in colonic epithelial cells and in CD11b+ and CD11c+ cells in the lamina propria. The TNBS colitis model involves both the innate and adaptive mucosal immune responses and is associated with a robust Th1-mediated response which driven by activated T-cells (41). Since IDO-1 was found to be expressed by cells which link the innate and adaptive immune responses, we sought to determine if increased expression of IDO-1 prior to colitis induction would reduce disease severity. Intraperitoneal ISS-ODN, prior to the TNBS enema, did result in a decrease of colitis severity; moreover, the beneficial effects of ISS-ODN were blocked by the co-administration of 1-mT, suggesting that the beneficial effects were mediated through the induction of IDO-1. The effect of 1-mT administration alone also worsened disease severity, though less dramatically than previously observed (17), a finding which may be explained by variability in TNBS stock or mouse vendor (29, 42). All experiments here were performed with age/sex matched mouse groups from the same vendor.
TNBS colitis is commonly evaluated within 7 days after the first rectal dose (29). A second dose of TNBS still results in a Th-1 response; however, dosing greater than 3 times leads to an IL-13 triggered TGF-B dependent tissue fibrosis with a concurrent decline in Th1 cytokines (19, 43). In recognition of IDO’s role in dampening T-cell responses, we also evaluated ISS-ODN in recurrent model of TNBS. The results were similar, though less dramatic, than with the single administration, a finding which may be explained by the reduced TNBS dose so as to avoid excessive mortality. IDO-1 expression by CD11b and CD11c positive antigen presenting cells is associated with inhibition of effector T-cell activation and downregulation of the immune response (2, 3, 44). The overall decrease in severity of this Th-1 mediated colitis after administration of ISS-ODN, an agent that induces IDO-1 in dendritic cells, fits with this model.
ISS-ODN induced IDO-1 expression in colonic epithelial cells which was greatest at the crypt base. While it is not clear why IDO-1 was selectively expressed in these epithelial cells, it is possible that TLR9 is selectively expressed in these epithelial cell populations or that IFN signaling is differentially regulated in these cells compared with other epithelial cells. It is also possible that a paracrine, rather than an epithelial cell autonomous mechanism mediates the induction of IDO-1. The ISS-ODN mediated induction of IDO-1 in colonic epithelial cells raised the question of whether the induction of IDO-1 expression in epithelial cells might also affect intestinal inflammation. To address this issue intraperitoneal ISS-ODN was given prior to oral DSS. The colitis induced by DSS is T-cell independent and is thought to be a product of epithelial injury induced by DSS (45, 46). In DSS colitis, IDO-1 has been reported to be upregulated in colonic epithelial cells (47), particularly in those at the crypt base (48). ISS-ODN lessens the severity of DSS colitis and similarly to the TNBS model, this effect is blocked by 1-mT. These results raise the possibility that the induction of epithelial IDO-1 protects the animal from DSS induced colitis. It is possible that the protective effects of IDO-1 induction in the DSS model may be mediated indirectly by IDO-1 induced in lamina propria mononuclear cells; however, the induction of IDO-1 in colonic crypt epithelial cells in the area of greatest epithelial proliferation suggests that the epithelial IDO may be involved in the protective effects of ISS-ODN in the DSS colitis model.
Together the results from the DSS and TNBS models suggest that IDO-1 in experimental colitis has multiple roles. The induction of IDO-1 in epithelial cells at the colonic crypt base may limit the initial acute changes associated with epithelial disruption by enabling epithelial proliferation through limiting bacterial invasion and perhaps direct pro-proliferative effects. The DSS and acute stages of the TNBS model best demonstrate this. Expression of IDO-1 within APCs of the lamina propria may then limit the progression of adaptive immune responses driving the subsequent perpetuation of colitis.
Type 1 interferons play a key role in mediating the protective effects of ISS-ODN in DSS colitis (23). In wild type mice ISS-DNA leads to phosphorylation of intestinal STAT1, a member of the signal trasducers and activators of transcription family activated by IFN binding (figure 6B). Mice lacking STAT1 signaling did not upregulate IDO-1 after ISS-ODN exposure. This complements the previous observation that STAT1−/− mice developed a more severe form of TNBS colitis than WT mice (17). While signaling of ISS-ODN through IFN-α/β may be critical to the preventive effects in the DSS model, there may be other beneficial effects mediated through IFN-γ which is a more potent stimulus for IDO induction (49). Both Type 1 (α/β) and Type II (γ) IFN’s have been shown to signal through STAT1 and can lead to increased levels of IDO-1 (27, 28).
Administration of ISS-ODN results in an increase in the number of CD11b and CD11c positive cells as assessed by immunohistochemistry. The CD11b positive cells were in the lamina propria surrounding the crypts whereas the CD11c positive cells were also in the lamina propria but more associated with the surface epithelium. ISS-ODN also results in an ~5 fold increase in CD11b mRNA and a 2 fold increase in CD11c mRNA as assessed by qRT-PCR. The increases in CD11b and CD11c may represent a combination of increased cell expression of CD11b and CD11c, the proliferation of CD11b and CD11c positive cells, and/or the infiltration of monocytes that differentiate into CD11b and CD11c positive cells. The increase in IDO-1 expression is significantly higher than each, at ~35 fold, suggesting that the increase in CD11b and c positive cells is not alone responsible for the increased IDO-1 levels.
Administration of ISS-ODN resulted in increased cytokine production as assessed by qRT-PCR of mRNA from the distal colon. Two doses of intraperitoneal ISS-ODN resulted in a >5 fold increase in TNFα and a ~4 fold increase in IFNγ, cytokines associated with Th1 responses. ISS-ODN also induced an ~2 fold increase in IFNα. The increases in IFNγ and IFNα are likely to be involved in the STAT-1 dependent induction of IDO-1 by ISS-ODN. Our results show that among cytokines and enzymes which favor tolerance that the fold changes in IL-10 and TGF-B were more modest than IDO-1. Increases in intestinal IL-10 as well as IL-6, IL-12 and COX2 at alternate time points after ISS-ODN administration have also been reported, though these experiments found the prostaglandin pathway not to be an important mediator of ISS-ODN’s anti-inflammatory properties (18, 23).
In addition to assessing the role of IDO-1 in intestinal inflammation we also studied the expression of IDO-2. This is the first report on IDO-2 in the gastrointestinal tract and among the first to distinguish between and describe the individualized physiologic effects of the two IDO enzymes (4, 50, 51). IDO-1 is the more widely expressed and more thoroughly investigative of the two enzymes, both of which function as the initial and rate limiting steps in tryptophan metabolism. Roles for IDO-1 have been found in autoimmune, allergic, malignant and infectious diseases (1, 9, 10, 52). IDO-2 has only recently been described and seems to be highly expressed in the kidney, epididymis and liver, but has also been found to be expressed in dendritic cells and tumors, including those of the GI tract (4–6, 50, 51). The functional significance of IDO-2 is still being investigated. IDO-1 and IDO-2 are differentially regulated; whereas IDO-2 was detected at low levels of the colon and intestine, its expression was not increased by ISS-ODN. This finding supports previous reports that IDO-2 expression is less responsive to IFNγ than is IDO-1 (4, 5). The activity of IDO-2 can be suppressed by 1-mT; in light of our prior studies, we cannot exclude the possibility that IDO-2 activity may be contributing to the natural brake tryptophan metabolism puts on intestinal inflammation (17). However, given its significantly greater expression and activity level, we propose that IDO-1 rather than IDO-2 exerts the greater impact on control of colitis.
IDO-1 expression is increased in animal models of colitis and human IBD. Increased IDO-1 expression is a component of the innate immune system’s effort to control microbial invasion and dampen or modulate the adaptive immune response. IDO-1 activity promotes depletion of the rarest of essential amino acids, tryptophan, in the microenvironment and leads to the generation of toxic and immune modulating kynurenine metabolites. These effects have been shown to result in inhibition of T-cell replication, induction of T-cell apoptosis, differentiation and suppressor function.
The studies described here show that a TLR9 agonist induces IDO-1 and that this induction is critical in its ability to decrease severity of both a T-cell dependent and T-cell independent model of colitis. Furthermore, in addition to IDO-1 expression by professional antigen presenting cells, these studies raise the possibility that IDO-1 induction in epithelial cells may also contribute to limiting colitis severity. Together these findings suggest possibility that pharmacologic upregulation of IDO-1 may be an approach deserving further investigation for the induction and maintenance of remission in human IBD.
Acknowledgments
We thank Mickey Hyun and Ameet Thaker for technical assistance and Dr Ling Zheng for technical guidance.
This work was supported by the Crohn’s and Colitis Foundation of America and National Institutes of Health Grants DK075713 (WFS & RDN), DK064798 (RDN) and The Washington University DDRCC grant P30 DK52574 (MAC). MAC is the recipient of career development award from the Crohn’s and Colitis Foundation of America and a DDRCC pilot and feasibility award.
Abbreviations used in this paper
- 1-mT
1-methyl-tryptophan
- ISS-ODN
immunostimulatory oligodeoxynucleotide
- M-ODN
mutated oligodeoxynucleotide
- IBD
inflammatory bowel disease
- TNBS
Trinitrobenzene sulfonic acid
- DSS
Dextran Sodium Sulfate
Footnotes
Disclosures
The authors have no financial conflict of interest
References
- 1.MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 3.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 4.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 5.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 9.Penberthy WT. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Curr Drug Metab. 2007;8:245–266. doi: 10.2174/138920007780362545. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T, Rao SP, Takabayashi K, Van Uden JH, Kornbluth RS, Baird SM, Taylor MW, Carson DA, Catanzaro A, Raz E. Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated by indoleamine 2,3-dioxygenase. Infect Immun. 2001;69:6156–6164. doi: 10.1128/IAI.69.10.6156-6164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayaishi O. Properties and function of indoleamine 2,3-dioxygenase. J Biochem. 1976;79:13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinande L, Demetter P, Perez-Novo C, Waeytens A, Taildeman J, Rottiers I, Rottiers P, De Vos M, Cuvelier CA. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol. 2008;21:289–295. doi: 10.1177/039463200802100205. [DOI] [PubMed] [Google Scholar]
- 14.Barcelo-Batllori S, Andre M, Servis C, Levy N, Takikawa O, Michetti P, Reymond M, Felley-Bosco E. Proteomic analysis of cytokine induced proteins in human intestinal epithelial cells: implications for inflammatory bowel diseases. Proteomics. 2002;2:551–560. doi: 10.1002/1615-9861(200205)2:5<551::AID-PROT551>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Dieckgraefe BK, Stenson WF, Korzenik JR, Swanson PE, Harrington CA. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics. 2000;4:1–11. doi: 10.1152/physiolgenomics.2000.4.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, Fuchs D, Brandacher G, Winkler C, Geboes K, Rutgeerts P, Tilg H. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428–1441. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- 19.Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A, Strober W. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 20.Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Colon AL, Madrigal JL, Menchen LA, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Stress increases susceptibility to oxidative/nitrosative mucosal damage in an experimental model of colitis in rats. Dig Dis Sci. 2004;49:1713–1721. doi: 10.1023/b:ddas.0000043391.64073.e4. [DOI] [PubMed] [Google Scholar]
- 22.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 23.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newberry RD, McDonough JS, Stenson WF, Lorenz RG. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: directing the tone of the intestinal immune response. J Immunol. 2001;166:4465–4472. doi: 10.4049/jimmunol.166.7.4465. [DOI] [PubMed] [Google Scholar]
- 25.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sainathan SK, Hanna EM, Gong Q, Bishnupuri KS, Luo Q, Colonna M, White FV, Croze E, Houchen C, Anant S, Dieckgraefe BK. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmerer JM, Lesinski GB, Radmacher MD, Ruppert A, Carson WE., 3rd STAT1-dependent and STAT1-independent gene expression in murine immune cells following stimulation with interferon-alpha. Cancer Immunol Immunother. 2007;56:1845–1852. doi: 10.1007/s00262-007-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-gamma. Curr Top Microbiol Immunol. 2007;316:119–154. doi: 10.1007/978-3-540-71329-6_7. [DOI] [PubMed] [Google Scholar]
- 29.te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995–999. doi: 10.1097/01.mib.0000227817.54969.5e. [DOI] [PubMed] [Google Scholar]
- 30.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 31.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 32.Stenson WF. Toll-like receptors and intestinal epithelial repair. Curr Opin Gastroenterol. 2008;24:103–107. doi: 10.1097/MOG.0b013e3282f44a2a. [DOI] [PubMed] [Google Scholar]
- 33.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 34.Cario E. Therapeutic impact of toll-like receptors on inflammatory bowel diseases: a multiple-edged sword. Inflamm Bowel Dis. 2008;14:411–421. doi: 10.1002/ibd.20294. [DOI] [PubMed] [Google Scholar]
- 35.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 36.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obermeier F, Dunger N, Strauch UG, Grunwald N, Herfarth H, Scholmerich J, Falk W. Contrasting activity of cytosin-guanosin dinucleotide oligonucleotides in mice with experimental colitis. Clin Exp Immunol. 2003;134:217–224. doi: 10.1046/j.1365-2249.2003.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856–864. doi: 10.1002/ibd.20142. [DOI] [PubMed] [Google Scholar]
- 40.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 41.van Lierop PP, de Haar C, Lindenbergh-Kortleve DJ, Simons-Oosterhuis Y, van Rijt LS, Lambrecht BN, Escher JC, Samsom JN, Nieuwenhuis EE. T-cell regulation of neutrophil infiltrate at the early stages of a murine colitis model. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.21073. [DOI] [PubMed] [Google Scholar]
- 42.Pohlmann A, Tilling LC, Robinson A, Woolmer O, McCleary S, Kruidenier L, Warnock LC, Lewis HD, Hobson AR, James MF. Progression and variability of TNBS colitis-associated inflammation in rats assessed by contrast-enhanced and T2-weighted MRI. Inflamm Bowel Dis. 2009;15:534–545. doi: 10.1002/ibd.20800. [DOI] [PubMed] [Google Scholar]
- 43.Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. J Clin Invest. 2005;115:3057–3071. doi: 10.1172/JCI24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 45.Troy AE, Zaph C, Du Y, Taylor BC, Guild KJ, Hunter CA, Saris CJ, Artis D. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 47.Mizoguchi E, Xavier RJ, Reinecker HC, Uchino H, Bhan AK, Podolsky DK, Mizoguchi A. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology. 2003;125:148–161. doi: 10.1016/s0016-5085(03)00665-6. [DOI] [PubMed] [Google Scholar]
- 48.Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 50.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 51.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]