Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections are predominantly those affecting skin and soft tissues. Although progress has been made, our knowledge of the molecules that contribute to the pathogenesis of CA-MRSA skin infections is incomplete. Here we tested the hypothesis that alpha-hemolysin (Hla) contributes to severity of USA300 skin infections in mice and determined whether vaccination against Hla reduces disease severity. Compared with wild-type USA300 and Newman strains, isogenic hla-negative (Δhla) strains caused significantly smaller skin lesions in a mouse infection model. Moreover, infection with wild-type strains produced dermonecrotic skin lesions, whereas there was little or no dermonecrosis in mice infected with Δhla strains. Passive immunization with Hla-specific antisera or active immunization with a non-toxigenic form of Hla significantly reduced the size of skin lesions caused by USA300 and prevented dermonecrosis. We conclude Hla is a potential target for therapeutics or vaccines designed to moderate severe S. aureus skin infections.
Keywords: alpha-hemolysin, MRSA, skin infection, Staphylococcus aureus, vaccine
Introduction
S. aureus is a leading cause of infection in hospitals as well as in the community. Community-associated methicillin-resistant S. aureus (CA-MRSA) strains typically cause infections in otherwise healthy individuals, and therefore, may be considered as highly virulent. This notion is supported by data from animal infection models, in which prominent CA-MRSA strains such as USA300 are more virulent than traditional hospital-associated strains [1, 2]. The enhanced virulence phenotype of USA300 is likely due in part to relatively high expression of virulence factors such as phenol soluble modulins (PSMs) and alpha-hemolysin (α-hemolysin, α-toxin, Hla) [1]. Hla is a secreted pore-forming toxin that has cytolytic activity toward a variety of host cell types, including human keratinocytes, epithelial cells, and lymphocytes [3-8]. Expression of Hla is regulated at least in part by the two-component agr and saeR/S signal transduction systems [9].
Hla is lethal to animals, especially rodents and rabbits [10], and S. aureus strains deficient in hla have significantly reduced virulence in animal infection models [6, 11-18]. Despite this previous work, the role of Hla in the pathogenesis of CA-MRSA infections was unknown until recently. Using USA300 and USA400 wild-type and isogenic hla-negative mutant (Δhla) strains, Bubeck Wardenburg et al. demonstrated that Hla is essential for pathogenesis in a mouse model of CA-MRSA pneumonia [6, 13]. Subsequent studies showed that vaccination against Hla protects mice from lethal USA300 or USA400 pneumonia [6, 13]. More recent studies by Bartlett et al. demonstrated that Hla elicits production of CXC chemokines by host cells during experimental S. aureus pneumonia, thereby promoting severe lung inflammation [19].
Inasmuch as CA-MRSA infections are primarily those affecting the skin and soft tissues, we determined the role played by Hla in a mouse model of USA300 skin infection, and in turn, tested whether a vaccine approach directed at Hla moderated disease severity.
Methods
S. aureus strains and culture conditions
S. aureus strains used in this study were characterized previously [13]. To prepare inocula, S. aureus were cultured to mid-exponential phase of growth, washed twice in Dulbecco's phosphate buffered saline (DPBS), and diluted in DPBS to the appropriate concentration. Inocula were stored on ice until used.
Mouse skin infection model
We used a previously described mouse skin infection model [2, 20, 21]. Animal experiments were performed in accordance with a protocol approved by the IACUC at Rocky Mountain Laboratories, NIAID, NIH. Shaved female Balb/c mice were anesthetized with isoflurane and inoculated by subcutaneous injection in the right flank with 1 × 107 S. aureus in 50 μL of DPBS. Mice were weighed before inoculation and mass and abscess formation were monitored at 24-h intervals for 14 days. The size abscesses was calculated using a standard formula for area [A = (π/2) × l × w], as described previously [2, 21, 22]. Dermonecrosis was scored as present or absent (+ or −). Fifteen mice were used for each treatment (bacterial strain) or control group unless indicated otherwise.
Histological examination of mouse tissues
Mouse skin was harvested on day 1, 2, and 3 post inoculation and fixed in 10% neutral-buffered formalin for 48 h. Fixed tissues were dehydrated in a graded series of ethanol, cleared in xylene, infiltrated and embedded in Paraplast Extra (Thermo Fisher Scientific) following a routine 12-h schedule in a VIP Tissue Tek processor (Sakura). Tissue blocks were sectioned at 5 μm and slides stained with hematoxylin-eosin. Tissue samples were evaluated by an experienced veterinary pathologist (D.J.G.).
Passive immunization
Rabbit polyclonal Hla-specific antisera was generated using purified HlaH35L as an immunogen as described previously [6]. Female Balb/c mice (∼7 wks of age) received 100 μl of rabbit pre-immune serum or polyclonal Hla-specific rabbit antisera via i.p. injection 4 h before S. aureus challenge and 2 days after S. aureus challenge. Animal weight and abscess formation were monitored once per day for 14 days as noted above. Passive immunization with rabbit anti-Hla sera typically results in circulating rabbit antibody titers of ∼1:500, as detected by ELISA, which was shown previously to protect against the effects of staphylococcal alpha-hemolysin [6].
Active immunization
Female Balb/c mice (4 wks of age) were administered 20 μg of endotoxin free GST-HlaH35L in complete Freund's adjuvant (CFA, Difco Laboratories, Detroit, MI) via intramuscular (i.m.) injection, followed by a boost with 20 μg of endotoxin free GST-HlaH35L in incomplete Freund's adjuvant (IFA, Difco Laboratories) 10 days later. Animals were challenged with S. aureus 21 days after the initial immunization and monitored once per day for 14 days. Sera were collected on day 20 (24 hours prior to infection) to assess Hla-specific antibody titer by ELISA.
Anti-Hla ELISA
Blood was obtained from 5 control and 10 infected mice via submandibular bleeding as described by Golde et al. [23]. Mouse serum antibody titers were determined by ELISA using MaxiSorp microtiter plates (ThermoFisher Scientific) coated with 1 μg/ml HlaH35L as described [6]. Antibody reactivity to Hla was detected with horseradish peroxidase-conjugated secondary antibodies and Opti-EIA (BD Biosciences) using a microplate spectrophotometer (GENios, Tecan).
Immunoblot analysis
S. aureus strains were cultured to early stationary phase of growth or overnight (late stationary phase of growth) and culture supernatants were harvested by centrifugation. Exoproteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed with anti-Hla rabbit IgG (Sigma, St. Louis, MO), pre-immune rabbit sera or anti-Hla rabbit sera. Proteins were detected using donkey anti-rabbit secondary antibodies conjugated to horseradish peroxidase (GE Healthcare, Piscataway, NJ) and enhanced chemiluminescence (SuperSignal West Pico Detection kit, Pierce, Rockford, IL).
Statistical analyses
Statistics for abscess/lesion area were performed using a one-way analysis of variance (ANOVA) with a Dunnett's posttest (figure 1C) or 2-way ANOVA and Bonferroni's posttest as indicated. Student's t-test or one-way ANOVA and Dunnett's posttest were used to compare the number of mice with dermonecrosis over the course of 14 days (aggregate analysis). Results are expressed as mean ± standard error of the mean (SEM) unless otherwise indicated. Analyses were done using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, California) or SigmaPlot2001 for Windows version 7.0 (SPSS Inc., Chicago, IL).
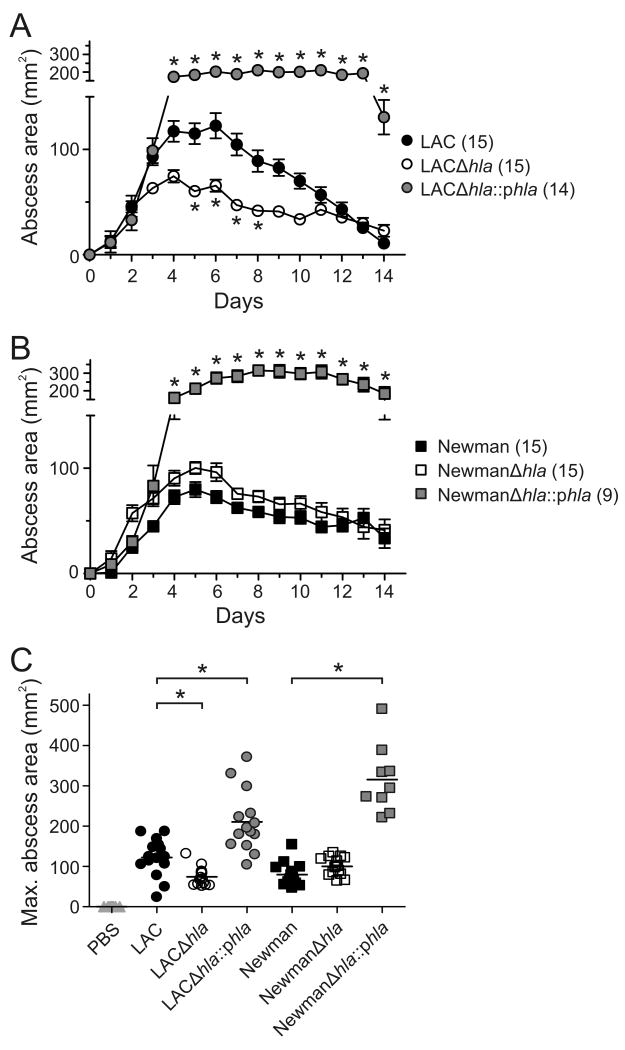
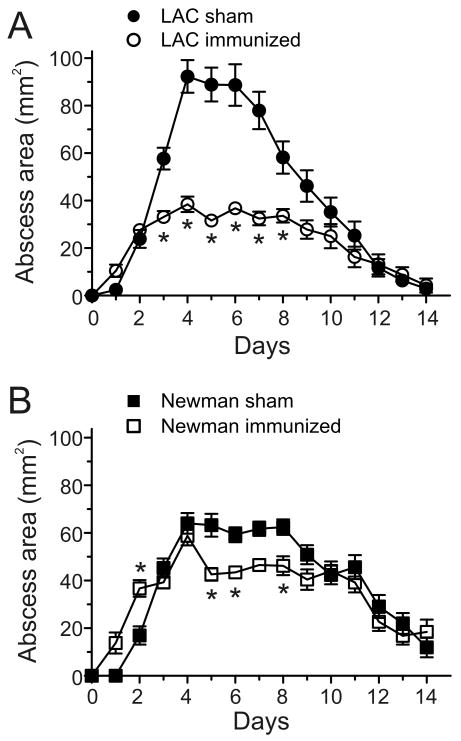
Figure 1.
hla contributes to the pathogenesis of USA300 skin infections. Results are the mean +/- SEM for all groups; (n) = number of mice per group. A and B, Mouse abscess size was monitored once per day after subcutaneous infection with 1 × 107 of the indicated bacteria. *, p < 0.05 versus wild-type LAC or Newman strains using a two-way ANOVA and Bonferroni's posttest. C, Size of abscesses at maximum. *, p < 0.05 using an ANOVA and Dunnett's posttest.
Results
Expression of hla enhances virulence in skin and soft-tissue infections
As a first step toward understanding the role played by hla in USA300 skin infections, we compared skin abscess formation in mice following infection with LAC (USA300) or Newman (non-USA300) wild-type, isogenic hla-negative mutant (Δhla), and Δhla-complemented mutant strains (Δhla∷phla) (figure 1). Infection of mice with LAC or Newman caused skin abscess or lesions that reached maximum size by day 5 or 6 (figure 1A and B). Mice infected with LACΔhla had significantly smaller lesions than animals infected with the wild-type strain (figure 1A and C). Unexpectedly, there was no difference in lesion size between Newman wild-type or Δhla-infected mice (figure 1B and C). Animals infected with the complemented mutant strain (LACΔhla∷phla) had abscesses that were similar in appearance to those infected with the wild-type strain, but lesions were significantly larger and took much longer to resolve by comparison (figure 1A-C). LACΔhla∷phla and NewmanΔhla∷phla expressed more Hla compared to the wild-type strains (reference [6] and data not shown), thereby providing an explanation for the larger abscess size observed in the mice infected with complemented mutant strains (figure 1). Consistent with differences in abscess size between wild-type and Δhla-infected mice, animals infected with wild-type or complemented mutant strains in general lost more body mass over the course of the experiment than those infected with Δhla strains (data not shown).
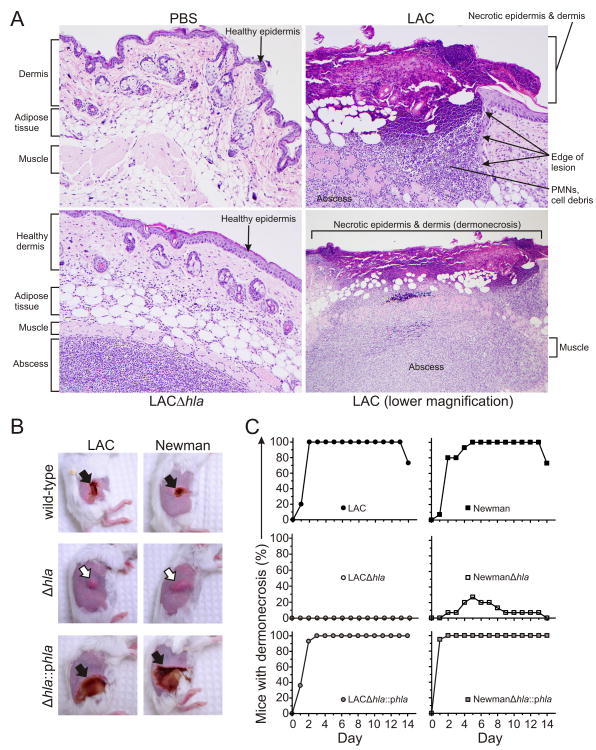
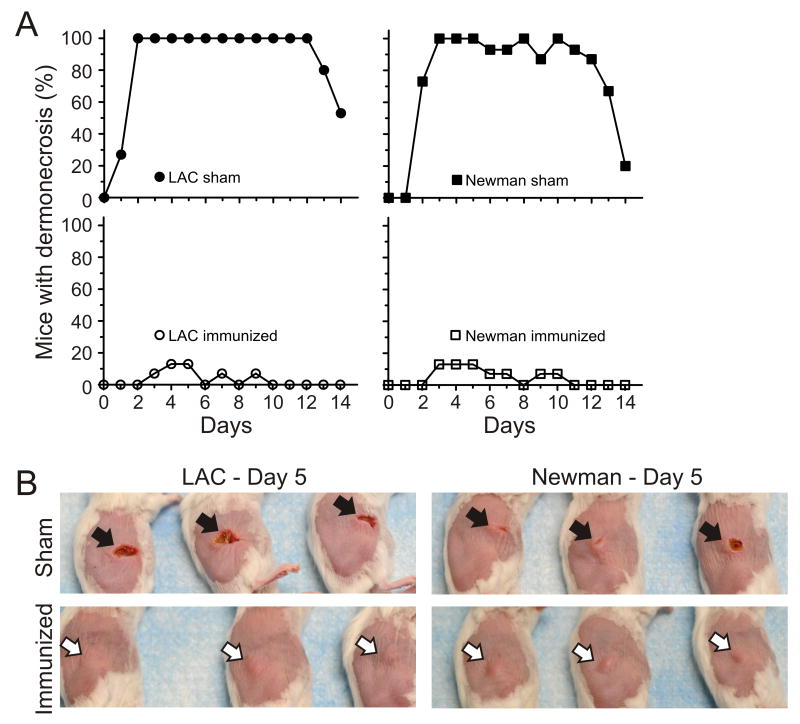
S. aureus skin infections in this animal model manifested as either raised abscesses/lesions or relatively flat dermonecrotic lesions (figure 2A and B). One of the more notable differences in lesion phenotype between wild-type and Δhla-infected mice was in the formation of dermonecrotic skin lesions (figure 2A and B). Mice infected with wild-type strains had dermonecrotic lesions, whereas those infected with LACΔhla and NewmanΔhla had lesions with little or no dermonecrosis (figure 2B and C). Dermonecrosis was defined by necrosis of the epidermis and dermis, and significant numbers of PMNs and cell debris adjacent to this region (figure 2A). This finding was especially striking in mice infected with complemented mutant strains (figure 2B and C). Taken together, these data demonstrate that Hla contributes to the severity of USA300 skin infections.
Figure 2.
hla promotes dermonecrosis. A, Representative histological sections showing normal mouse skin tissue (PBS), mouse USA300 abscess with dermonecrosis (LAC), and an hla-deficient USA300 abscess without dermonecrosis (LACΔhla). Mice were inoculated s.c. with either PBS, LAC, or LACΔhla as described in Methods and skin was harvested on day 3. Original magnification of images labeled as PBS, LAC and LACΔhla is ×200; that for LAC (lower magnification) is ×100. B, Representative mouse skin lesions (day 5). Black arrows indicate dermonecrosis and white arrows indicate abscess formation without dermonecrosis. C, Percentage of mice per group that had dermonecrosis on each day. p < 0.001 for mice infected with either LAC or Newman wild-type or complemented mutant strains versus Δhla strains over the 14-day time course. Data were analyzed using a one-way ANOVA and Dunnett's posttest.
Passive immunization with Hla-specific antisera reduces skin lesion size and severity
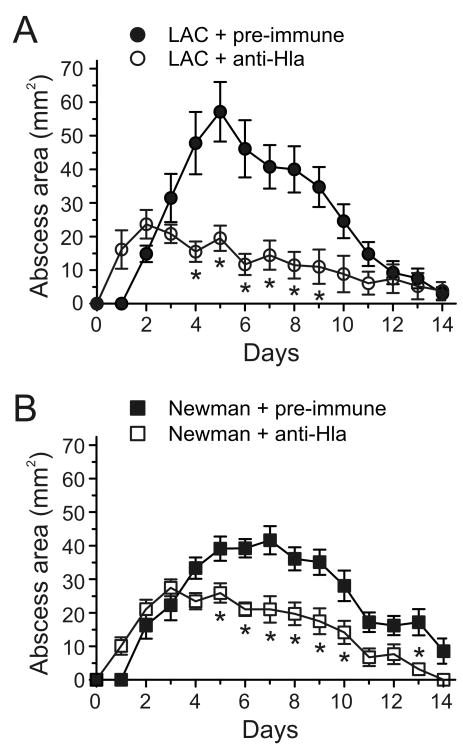
Inasmuch as Hla contributed to disease severity in the mouse skin infection model, we next determined if passive immunization with rabbit antisera directed against Hla would moderate severity of skin disease in infected mice. Previous studies demonstrated that intraperitoneal injection of Hla-specific rabbit antisera protects mice from lethal S. aureus pneumonia [6]. Therefore, we used this vaccination model to test whether passive immunization against Hla prevents or moderates severe skin infection. Skin lesions of mice infected with LAC or Newman were significantly smaller following passive immunization with Hla-specific rabbit antisera compared to those of mice that received pre-immune sera (figure 3A and B). Moreover, the clinical course of disease in immunized mice is altered, as the peak lesion size is reached by day 2, followed by gradual resolution of the abscess by days 12-14 (figure 3A and B). In contrast, mice treated with pre-immune sera prior to infection with LAC suffer progressive disease until day 5, after which resolution occurs by days 12-14 (figure 3A). Similar results were seen for mice infected with strain Newman (figure 3B). Immunized mice infected with LAC also lost less weight than those given pre-immune sera (data not shown).
Figure 3.
Passive immunization with Hla-specific rabbit anti-sera (anti-Hla) reduces size of lesions caused by USA300 or Newman. A and B, Mice received 100 μl of pre-immune rabbit sera (Pre-immune) or Hla-specific rabbit anti-sera (anti-Hla) 4 h before subcutaneous infection with 1 × 107 with LAC or Newman and on day 2 post-infection. Results are the mean +/- SEM for all groups; n = 15 mice per group. *, p < 0.05 versus wild-type LAC or Newman strains using a two-way ANOVA and Bonferroni's posttest.
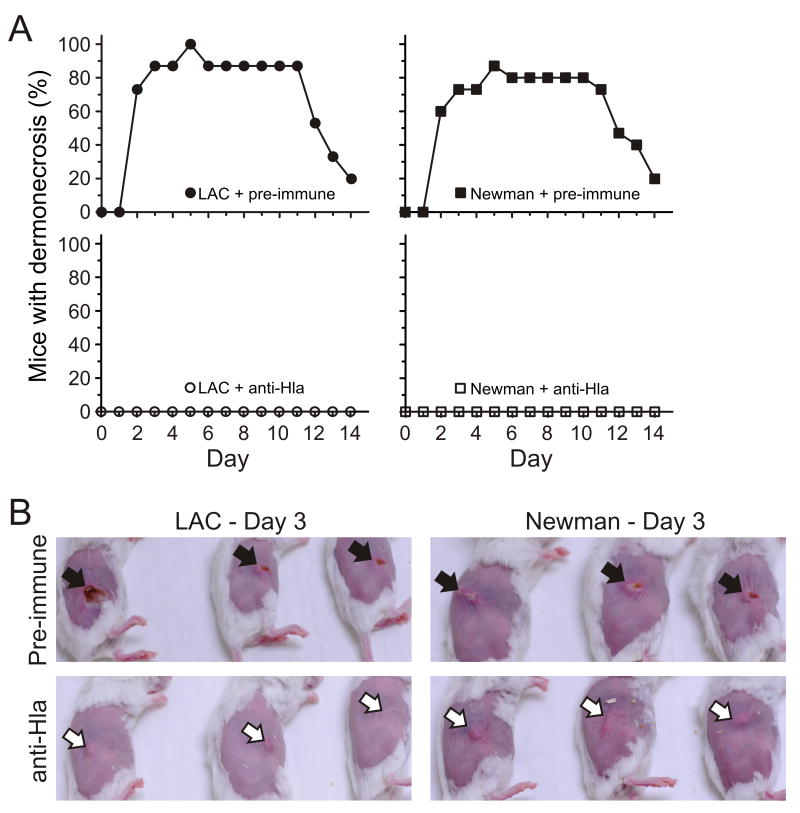
Mice that received Hla-specific rabbit antisera either failed to develop dermonecrotic lesions following infection with either LAC or strain Newman or the area of dermonecrosis was limited (figure 4). Abscesses or lesions of wild-type infected mice given Hla-specific antisera developed skin lesions comparable in appearance and size to those infected with Δhla strains in the absence of passive vaccination (compare figure 2 and figure 4). We note that rabbit pre-immune serum contains naturally occurring antibodies specific for S. aureus, and therefore, skin disease caused by wild-type strains in the presence of pre-immune serum was less severe compared to that in untreated animals (compare figure 2B and figure 4B).
Figure 4.
Passive immunization with Hla-specific rabbit anti-sera prevents dermonecrosis. A, Percentage of mice per group that had dermonecrosis on each day. p < 0.0001 for mice administered pre-immune versus anti-Hla serum following infection with either LAC or Newman over the 14-day time course. B, Representative skin lesions of mice on day 3 for each of the treatment conditions. Passive immunization was performed as described in Methods and the legend of figure 3. Pre-immune rabbit sera (Pre-immune); Hla-specific rabbit anti-sera (anti-Hla). Black arrows indicate dermonecrosis and white arrows indicate abscess formation without dermonecrosis.
Immunization with HlaH35L moderates severity of USA300 skin infections
Previous studies have shown that a non-cytolytic mutant form of Hla (HlaH35L) can be used as protective immunogen against S. aureus infections in animal infection models [6, 17]. To determine whether active immunization with HlaH35L protects mice from severe S. aureus skin infections, mice were immunized i.m. with HlaH35L 21 days prior to infection with LAC or Newman. Mice immunized with HlaH35L produced antibodies specific for the toxin as determined by ELISA (half-maximal anti-Hla antibody titers were 1:491 ± 49 and 1:596 ± 87 for each of two groups of 5 immunized mice tested, whereas anti-Hla was undetectable in 5 unimmunized mice). Correspondingly, S. aureus abscess size was reduced significantly in mice immunized with HlaH35L (figure 5A and B). The modest or limited protective effect observed in mice infected with strain Newman may reflect a limited contribution of Hla to severity of skin disease caused by this strain (see figure 1B). In addition, there was little or no dermonecrosis in infected mice that had been immunized (figure 6), demonstrating that active immunization with HlaH35L moderates severity of S. aureus skin infections.
Figure 5.
Active immunization with HlaH35L decreases size of abscesses caused by USA300 or Newman. Mice were injected i.m. with CFA + DPBS at 4 wks of age followed by IFA + DPBS 10 days later (sham) or CFA + HlaH35L at 4 wks of age and IFA + HlaH35L 10 days later (immunized). A and B, Abscess formation was monitored once per day after subcutaneous infection with 1 × 107 of the indicated bacteria 21 days after primary immunization. Results are the mean +/- SEM and n = 15 mice per group. *, p < 0.05 versus wild-type LAC or Newman strains using a two-way ANOVA and Bonferroni's posttest.
Figure 6.
Active immunization with HlaH35L prevents dermonecrosis caused by USA300 or Newman skin infections. A, Percentage of mice per group that had dermonecrosis on each day. Immunization is described in the legend of figure 5. p < 0.0001 for sham versus immunized mice following infection with either LAC or Newman over the 14-day time course. B, Representative mouse skin lesions, day 5. Black arrows indicate dermonecrosis and white arrows indicate abscess formation without dermonecrosis.
Discussion
USA300 is the leading cause of community-associated bacterial infections in the United States [24]. In addition, USA300, which is epidemic in the United States, appears to have enhanced virulence compared to traditional hospital-associated MRSA strains [1, 25]. Although the pathogen can cause severe or fatal invasive disease [26, 27], the vast majority of USA300 infections are those of skin and soft tissue [28, 29]. There has been an intense effort to better understand mechanisms of USA300 virulence and transmission (reviewed in refs. [30-33]). Recent work indicates Hla is produced by USA300 at relatively high levels in vitro [34], and studies in animal models revealed a prominent role for this secreted toxin in the pathogenesis of USA300 pneumonia [6, 13]. However, the contribution of Hla to the severity of USA300 skin infections had not been evaluated until now.
Herein we demonstrate that Hla contributes significantly to the severity of USA300 skin infections in a mouse model. Compared with strain Newman, the contribution of Hla to disease severity was more pronounced in mice infected with USA300 (LAC) (figure 1 and figure 3). LAC is a widely used clinical isolate representative of the USA300 epidemic clone [12, 25], whereas Newman is a methicillin-susceptible S. aureus strain originally isolated from a case of secondarily infected osteomyelitis in 1952 [35]. Newman expresses less Hla compared to LAC (ref. [6] and data not shown), and consistent with this observation, deletion of Hla from Newman minimally changed the size of skin lesions (figure 1B). These data are in accordance with the recent studies of Li et al. [1], which indicate that differential expression of virulence molecules rather than presence or absence in the core genome dictates (at least in part) differences in virulence among S. aureus strains. Thus, the findings here suggest factors other than Hla in strain Newman play a more prominent role in the pathogenesis of skin infections, whereas the toxin is a major determinant of severity in USA300 skin infections. It is also evident that multiple S. aureus molecules contribute to skin infections in general, since deletion or neutralization of Hla did not completely ablate formation of abscesses.
Previous studies have not attempted to correlate expression of Hla with severity of skin infections. However, production of Hla was recently shown to correlate with refractory S. aureus skin colonization in patients with atopic dermatitis [36]. Inasmuch as hla is present in the genome of many S. aureus strains, it is likely the toxin contributes to severity of human S. aureus infection, a notion that has been confirmed in animal infection models [6, 11, 13, 14, 17, 37]. Although the mouse skin infection model cannot mimic all of the features of human skin infection, such as the size of inoculum, the primary readouts of our mouse skin infection model (skin necrosis and abscess size) are two parameters used to classify severe human skin and soft tissue infections [38]. Moreover, USA300 is known to cause necrotizing skin and soft tissue infections in humans [26]. Therefore, it is reasonable to conclude that the mouse skin infection model described herein can be used as a general or rudimentary approximation of S. aureus skin infection in humans.
There is also little information on anti-Hla antibody titers in human skin infections. In 1962, Lack and Towers reported that only 48% of patients with proven S. aureus infection had relatively high anti-Hla antibody titers [39]. More notably, anti-Hla antibody titers did not consistently increase in patients with S. aureus infections, and when they did increase following acute infection, antibody levels then decreased rapidly over time [39]. These early observations suggest humans may lack or have limited protection against Hla, albeit the anamnestic antibody response to Hla should be robust in individuals re-infected with Hla-producing S. aureus. The passive and active immunization data presented here suggest Hla is a potential target for vaccines or therapeutics designed to moderate the severity of S. aureus skin infections. Inasmuch as Hla is a core genome-encoded toxin present in virtually all CA-MRSA strains, a therapeutic approach directed at Hla would be relatively broad in scope.
Acknowledgments
We thank Anita Mora, Austin Athman, and Gary Hettrick (RML, Hamilton, MT) for help with photography and Ralph Larson and Laura Talley (RML, Hamilton, MT) for assistance with mouse immunizations.
Financial Support: This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.B.W. and O.S. acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153).
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:5883–8. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 3.Walev I, Martin E, Jonas D, et al. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–9. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thelestam M, Mollby R, Wadstrom T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973;8:938–46. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezepchuk YV, Leung DY, Middleton MH, Bina P, Reiser R, Norris DA. Staphylococcal toxins and protein A differentially induce cytotoxicity and release of tumor necrosis factor-alpha from human keratinocytes. J Invest Dermatol. 1996;107:603–9. doi: 10.1111/1523-1747.ep12583377. [DOI] [PubMed] [Google Scholar]
- 6.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valeva A, Walev I, Pinkernell M, et al. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci U S A. 1997;94:11607–11. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hocke AC, Temmesfeld-Wollbrueck B, Schmeck B, et al. Perturbation of endothelial junction proteins by Staphylococcus aureus alpha-toxin: inhibition of endothelial gap formation by adrenomedullin. Histochem Cell Biol. 2006;126:305–16. doi: 10.1007/s00418-006-0174-5. [DOI] [PubMed] [Google Scholar]
- 9.Xiong YQ, Willard J, Yeaman MR, Cheung AL, Bayer AS. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J Infect Dis. 2006;194:1267–75. doi: 10.1086/508210. [DOI] [PubMed] [Google Scholar]
- 10.Bernheimer AW. Staphylococcal alpha toxin. Ann N Y Acad Sci. 1965;128:112–23. doi: 10.1111/j.1749-6632.1965.tb11633.x. [DOI] [PubMed] [Google Scholar]
- 11.O'Reilly M, de Azavedo JC, Kennedy S, Foster TJ. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986;1:125–38. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy AD, Otto M, Braughton KR, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A. 2008;105:1327–32. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 14.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–4. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel AH, Nowlan P, Weavers ED, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–10. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramley AJ, Patel AH, O'Reilly M, Foster R, Foster TJ. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–94. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzies BE, Kernodle DS. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun. 1996;64:1839–41. doi: 10.1128/iai.64.5.1839-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElroy MC, Harty HR, Hosford GE, Boylan GM, Pittet JF, Foster TJ. Alpha-toxin damages the air-blood barrier of the lung in a rat model of Staphylococcus aureus-induced pneumonia. Infect Immun. 1999;67:5541–4. doi: 10.1128/iai.67.10.5541-5544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–35. doi: 10.1086/592758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with gram-positive cocci. Infect Immun. 1992;60:2636–40. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunce C, Wheeler L, Reed G, Musser J, Barg N. Murine model of cutaneous infection with gram-positive cocci. Infect Immun. 1992;60:2636–40. doi: 10.1128/iai.60.7.2636-2640.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 24.Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis. 2005;11:928–30. doi: 10.3201/eid1106.040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voyich JM, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–19. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 26.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 27.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–7. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 28.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–91. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi SD, DeLeo FR. An update on community-associated MRSA virulence. Curr Opin Pharmacol. 2009 doi: 10.1016/j.coph.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 31.DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest. 2009;119:2464–74. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23:17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burlak C, Hammer CH, Robinson MA, et al. Global analysis of community-associated methicillin-resistant Staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection. Cell Microbiol. 2007;9:1172–90. doi: 10.1111/j.1462-5822.2006.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 36.Wichmann K, Uter W, Weiss J, et al. Isolation of alpha-toxin-producing Staphylococcus aureus from the skin of highly sensitized adult patients with severe atopic dermatitis. Br J Dermatol. 2009;161:300–5. doi: 10.1111/j.1365-2133.2009.09229.x. [DOI] [PubMed] [Google Scholar]
- 37.Menzies BE, Kernodle DS. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun. 1994;62:1843–7. doi: 10.1128/iai.62.5.1843-1847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am. 2009;23:571–91. doi: 10.1016/j.idc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Lack CH, Towers AG. Serological tests for staphylococcal infection. Br Med J. 1962;2:1227–31. doi: 10.1136/bmj.2.5314.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]