Abstract
Background
We report local recurrence (LR) after breast-conserving surgery and radiation (BCS + RT) for ductal carcinoma in situ (DCIS) to determine outcomes for patients aged ≤ 40 years compared with older women.
Methods
The study included 440 women with DCIS treated from 1978 to 2007. All patients received whole-breast radiotherapy with a boost in 95% of cases. Demographics, characteristics, surgical and adjuvant treatments were analyzed for an effect on LR.
Results
Median age was 56.5 years with 24 patients aged ≤ 40. Median DCIS size was 0.8 cm. Re-excision was required in 62% of patients, and in 75% of those aged ≤ 40. Tamoxifen was used in 22%, but only 1 patient aged ≤ 40. Median follow-up was 6.8 years. Actuarial LR was 7% (95% confidence interval of 4–11%) at 10 years and 8% (5–14%) at 15 years. There was no difference in LR by age (p=0.76).
Conclusions
The long-term risk of LR after BCS + RT for DCIS is low, even in patients ≤ 40 years. This may be due to patient selection for small size, high utilization of re-excision and radiation boost. Young age may be a smaller contributor to LR risk in DCIS than previously suggested.
Keywords: Breast cancer, radiation therapy, DCIS, young age
INTRODUCTION
Ductal carcinoma in situ (DCIS) of the breast has increased in the United States from 3% of new breast cancer cases in 1980 to greater than 20% in 2003 (1). This corresponded to an increase in the age-adjusted rate of DCIS from 2.4 to 27.7 per 100,000 women between 1981 and 2001 after the advent of the screening mammography (2–5). Mastectomy was initially the standard of care for DCIS but breast-conserving surgery and radiation (BCS + RT) therapy are now also standard practice for many of these patients (2, 6–9) . The use of tamoxifen for DCIS has also increased after the reporting of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 (10–12) which demonstrated that 5 years of Tamoxifen is effective in reducing the risk of invasive or non-invasive ipsilateral and contralateral tumors after radiation.
Proper management of DCIS is critical to prevent local recurrence (LR) and progression to invasive breast cancer, as 50% of recurrent tumors are invasive (13). Previously identified predictors of LR after BCS + RT include margin status, size of DCIS, nuclear grade, necrosis, mammographic presentation, histologic subtype, volume of excised specimens and distance from the nipple (14–18) .
The importance of age in the management of DCIS is controversial with no consensus on its impact or on the definition of “young”. Young patient age in DCIS has been variously defined as </= 35, </=40, or </=45 years of age and the risk of LR after DCIS in young patients has been reported to be approximately 25–30% (19–22). This is significantly higher than the long term LR risk of approximately 15% in other studies (23, 24) consisting predominantly of women over 40. One explanation may be the presence of adverse pathologic features in younger patients (25) though treatment-related factors that appear to be of particular importance in younger women include margin status and use of a boost (21, 26). We therefore reviewed the long-term results with BCS + RT in young patients with DCIS with a special attention to the interaction of age with other known prognostic factors for LR.
METHODS
There were 573 patients with DCIS seen at the Fox Chase Cancer Center with a diagnosis of DCIS between September 1998 and October 2007. The study population consists of 440 consecutive women with DCIS treated with BCS + RT during that interval. Inclusion criteria consisted of clinically node negative patients with DCIS (AJCC 6th Ed. Stage 0) (27), treated with a completed course of radiation at Fox Chase Cancer Center. A prospectively collected Institutional Review Board-approved, Health Insurance Portability and Acountability Act-compliant institutional database that tracks breast cancer patients for demographics, tumor characterstics, and treatment related information was reviewed. Patients were excluded if they were male, had microinvasion, underwent mastectomy, had a diagnosis of Paget’s disease of the nipple, or underwent conservative surgery without radiation.
All patients underwent a history and physical examination, baseline mammogram and pathologic diagnosis consisting of fine needle aspiration, core needle biopsy or excisional biopsy. A definitive surgical excision was performed in all patients with or without a re-excision when needed for surgical margins. All outside pathology slides were reviewed at Fox Chase Cancer Center at the time of initial radiotherapy consultation. All patients underwent postoperative radiation. The radiotherapy technique consisted of treatment to the entire breast to a dose of 46 –50 Gy delivered in 1.8 – 2 Gy fractions over a period of 4.5–5 weeks. The median whole breast dose was 50 Gy. A 6 MV linear accelerator was used for majority of patients. Women with large breasts or separation greater than 21–22 cm were often treated with higher energy photons (10 or 15 MV) in 33% of cases. A boost to the primary site and scar was used in 95.7% of patients, almost all with electrons (or 192Ir and external beam) for an additional 10 – 18 Gy.
Study end points including LR, regional recurrence, distant metastasis, and overall survival were analyzed based upon established risk factors. Patient characteristics included age at diagnosis, menopausal status, race, and family history of breast cancer. Tumor characteristics included primary tumor location, pathological tumor size, hormonal receptor status, histological type, grade and necrosis. Treatment characteristics included use of re-excision, radiotherapy dose, fractionation schedule, use of and type of boost, and use of tamoxifen. Kaplan-Meier analysis was used for estimates of LR and survival. The log-rank test was used to assess the significance between variables. All the statistical analyses were performed in statistical package for the SAS version 9.0 (SAS Institute, Cary, NC). Statistically significant differences were confirmed by p values of ≤ 0.05.
RESULTS
Patient characteristics are listed in Table 1. The median age was 56.5 years (range 31–91 years). There were 24 patients ≤ 40 years of age (5.5%), 169 patients were 41–54, 169 patients were 55–69 and 78 patients were over 70 years of age. 294 patients (66.7%) were postmenopausal, 119 (27.1%) were premenopausal and 27 (6.2%) were perimenopausal. African-Americans comprised 7.3% while 90.4% were white and others were 2.5%. Positive family history of breast cancer was seen in 168 patients (38.3%), 105 with a history in one relative and 62 with more than 2 affected relatives. In 377 patients (85.9%) the diagnosis was made based on mammogram only, in 4.8% by only physical examination, 9.3% by both mammogram and physical examination. The most common mammographic finding was presence of microcalcifications without associated mass (N=336, 76.5%). 43 patients had mass without calcifications and in 30 patients both mass and microcalcifications was noticed. In 54.9% the tumor was located in the outer quadrant, 17.5% inner quadrant, 21.4% central and in 6.4% subareolar region.
Table 1.
Patient and tumor related characteristics of Mammographically detected DCIS
| No. of. Patients (%) | |
|---|---|
| Total | 440 |
| Age (years) Median | 56.5 |
| < 41 | 24 (5.5) |
| 41–54 | 169 (38.5) |
| 55–69 | 169 (38.5) |
| > 70 | 78 (17.8) |
| Menopausal status | |
| Pre | 119 (27.1) |
| Peri | 27 (6.2) |
| Post | 294 (67) |
| Race | |
| White | 397 (90.4) |
| Black | 32 (7.3) |
| Others | 11 (2.5) |
| Family history | |
| Negative | 18 (4.1) |
| Positive | 168 (38.3) |
| 1 relative | 105 (62.9) |
| >2 relatives | 62 (37.1) |
| Mammographic findings | |
| Calcification only | 336 (76.5) |
| Mass | 43 (9.8) |
| Mass and calcifications | 30 (6.8) |
| Location primary | |
| Outer | 241 (54.9) |
| Inner | 77 (17.5) |
| Central | 94 (21.4) |
| Subareolar | 28 (6.4) |
| Method of detection | |
| Mammogram + PE | 41 (9.3 ) |
| Mammogram only | 377 (85.9) |
| PE only | 21 (4.8) |
| Other (MRI/CT) | 1 (0.2) |
Abbreviations: DICS= Ductal carcinoma in situ; PE= Physical examination; MRI= Magnetic resonance imaging; CT: Computed tomography.
DCIS characteristics are shown in Table 2. The predominant histological pattern was mixed in 36.9%, comedo in 16.9%, cribriform in 15.5%, solid in 9.3%, papillary in 3.4%, micropapillary in 3.2% and not reported in 14.6%. Nuclear grade was assessed in 281 patients; 63.86% (9.1% Grade 1, 23.7% Grade 2, 31.2% Grade 3). Necrosis was recorded as present in 162. The median tumor size was 8 mm (range 0.1–5.0 mm). 77 patients were both ER and PR positive (17.5%) and 24 were ER/PR positive (5.5%) and negative in 32 patients (7.3%). LCIS was seen in 36 (8.2%) patients.
Table 2.
Tumor related characteristics
| No. of. Patients (%) | |
|---|---|
| Path size primary | |
| Median tumor size | 0.8 cm |
| Tumor size range | 0.1–5.0 cm |
| Histology | |
| Comedo | 74 (16.9) |
| Papillary | 15 (3.4) |
| Micropapillary | 14 (3.2) |
| Cribriform | 68 (15.5) |
| Solid | 41 (9.3) |
| Mixed | 162 (36.9) |
| Unknown | 64 (14..6) |
| Grade of tumor | |
| Grade 1 | 40 (9.1) |
| Grade 2 | 104 (23.7) |
| Grade 3 | 137 (31.2) |
| Unknown | 159 (36.2) |
| Necrosis | |
| Yes | 162 (36.9) |
| No | 9 (2.1) |
| Unknown | 269 (61.3) |
| Nodal status | |
| Nx | 378 (86.1) |
| N0 | 62 (14.1) |
| Hormone Receptors | |
| Both ER/PR positive | 77 (17.5) |
| Both ER/PR negative | 32 (7.3) |
| ER/PR positive | 24 (5.5) |
| ER/PR unknown | 307 (69.9) |
| LCIS | |
| Positive | 36 (8.2) |
| Negative | 404 (92) |
| Unknown | 0 (0.0) |
Abbreviations: ER= Estrogen receptor; PR= Progesterone receptor; LCIS= Lobular carcinoma in situ.
Treatment-related characteristics are shown in Table 3. A re-excision was performed in 61.7% overall, and in 75% for patients age ≤ 40. The final margins of re-excision were negative (>2 mm) for 342 patients (77.9%), positive for 13 patients (3%), close in 49 (11.2%), LCIS only in 6 (1.4%) and unknown in 30 (7%). 62 patients underwent axillary dissection, all of which were negative. A post biopsy mammogram was obtained prior to initiation of radiation in 240 patients (54.7%). In 45 patients there were residual microcalcification, and it was negative in 195 patients. 23/45 patients with residual microcalcification had reexcision, and 22 did not. 198 patients did not have a postbiopsy mammogram and in 2 patients it was unknown. Tamoxifen was given in 96 patients (21.9%).
Table 3.
Treatment related characteristics
| No. of. Patients (%) | |
|---|---|
| Re-excision | |
| Yes | 271 (61.7) |
| No | 169 (38.5) |
| Final margin status | |
| Negative | 342 (77.9) |
| Close | 49 (11.2) |
| Positive | 13 (3) |
| LCIS only | 6 (1.4) |
| Unknown | 30 (7) |
| Radiotherapy | |
| Total dose primary (median dose), tangents | 5000 cGy (200–5040) |
| Boost , median dose-range | 1000 (1000–1800) |
| Yes | 420 (95.7) |
| No | 20 (4.6) |
| Post Bx Mammogram | |
| Yes | 240 (54.7) |
| No | 198 (45.1) |
| Unknown | 2 (0.5) |
| Adjuvant treatment | |
| Tamoxifen | 96 (21.9) |
| No systemic therapy | 342 (77.9) |
| Chemotherapy only | 1 (0.2) |
| Chemotherapy/Tamoxifen | 1 (0.2) |
Abbreviations: cGy= Centi gray; LCIS= Lobular carcinoma in situ; Bx= Biopsy.
The median follow up was 6.8 years (range 0.2–24). Patterns of recurrence are shown in Table 4. There were 22 local recurrences (5%), two with simultaneous regional nodal recurrence. None of the patients had distant metastases at the time of LR. 7/22 (32%) of the local recurrences were invasive cancers. The interval to LR was 71 months (range: 7–247 months).
Table 4.
Pattern of failures
| No. of. Patients (%) | |
|---|---|
| First failures | |
| None | 406 (92.5) |
| Local Recurrence (LR) | 22 (5) |
| Local only | 20 (91) |
| Local + Regional | 2 (9) |
| Local + Distant | 0 (0.0) |
| Distant Recurrence | 10 (2.3) |
| Contralateral Breast Cancer | 72 (16.4) |
| Contra prior to XRT | 43 (59.7) |
| Contra after XRT | 25 (34.7) |
| Contra simultaneous | 4 (5.6) |
Abbreviations: LR= Local recurrence; XRT= External beam radiation therapy.
Pathologic size did not appear to correlate with the risk of LR. The median size of tumor in patients without LR was 0.8 cm (range: 0.1–5 cm) compared to with LR 0.7 cm (range: 0.4 – 2.4 cm), (p=0.5). There was no apparent correlation between architectural pattern and the risk of a LR. 1 out of 74 of these patients with comedo histology recurred, 2/68 with cribriform histology, compared to 18 of 296 with non-comedo/cribriform histology (5 LR were in patients in whose histologic subtype was not recorded). Re-excision was required in 271 patients (61.7%) either for a therapeutic resection after excisional biopsy or due to margin status. 102 of these patients had a focally positive reexcision margin, 160 had a negative margin, and 9 had LCIS. 15 LR occurred in patients who had a reexcision (5.5%, 15/271) and 5 had distant metastases. 8 LR occurred in patients whose reexcision demonstrated no residual tumor, and there were 7 LR in the setting of a positive reexcision. There were 14 LR and one locoregional recurrence in 198 patients who had no postbiopsy mammogram, compared with 3 patients of 240 with a postbiopsy mammogram who had LR.
10 patients (2.3%) ultimately developed distant metastases. 8 of these patients died of disease and 2 patients were alive with disease (one had 79 months, second had 2 months of followup). The 10 and 15 year actuarial cause-specific survival are 98% and 93%. 39 patients died of other causes at a median of 9.9 years after their treatment. The 10- year and 15- year actuarial overall survival rates are 93% and 92% respectively. 74 patients (16.2%) developed contralateral breast cancer. 44 patients received prior radiation therapy, 26 developed after radiotherapy and 4 had simultaneous contralateral tumors. The median time to develop contralateral breast tumor in 26 patients was 66.5 months (range: 3–196 months). The histology of the contralateral primary was invasive ductal cancer and DCIS. 60/74 patients were alive without disease. 39 contralateral breast cancer occurred in patients with a positive family history and 35 had negative family history. 19/74 patients were treated with prior tamoxifen and in 4/26 who developed contralateral tumor, tamoxifen was used.
Actuarial outcome for breast recurrence is presented in Table 5. The 10 year and 15 year actuarial rates of LR for all patients are 7% (4–11) and 8% (5–14) respectively (Fig. 1). There was no difference in LR by age (p=0.76), as shown in Figure 2. The LR rates at 15 years were 10% (3–35%) in patient’s ≤ 40 years, 7% (3–16%) 41–54, 11% (5–23%) 55–69, and 4% (1–15%) =70 years. There were no significant differences in 15 year LR in patients with close 3% (0.5–22), positive 9% (1–49), or negative 10 (6–17) margins (0.43). Post-biopsy mammogram (p=0.18), and use of tamoxifen (p=0.48) had no effect on LR. In the subgroup of patients with close or positive margins, 10-year LR with tamoxifen (n=13) was 20% (3–80) compared with 3% (0.4–19) without tamoxifen (n=49) (p=0.51).
Table 5.
Local recurrence rates by Univariate analysis
| Variable | Group | 5 year LR (%) | 10 year LR (%) | 15 Year LR (%) | P value |
|---|---|---|---|---|---|
| Overall | 3 (1–5) | 7 (4–11) | 8 (5–14) | ||
| Age | 0.76 | ||||
| ≤ 40 | 5 (1–31) | 10 (3–35) | 10 (3–35) | ||
| 41–54 | 2 (0.4–7) | 7 (3–16) | 7 (3–16) | ||
| 55–69 | 3 (1–8) | 6 (3–14) | 11 (5–23) | ||
| > 70 | 4 (1–16) | 4 (1–15) | 4 (1–16) | ||
| Margin status | 0.43 | ||||
| Negative | 3 (1–6) | 7 (4–12) | 10 (6–17) | ||
| Close | 3 (0.5–22) | 3 (0.5–22) | 3 (0.5–22) | ||
| Positive | 9 (1–49) | 9 (1–49) | 9 (1–49) | ||
| Tamoxifen | 0.48 | ||||
| No | 2 (1–5) | 6 (4–11) | 8 (5–14) | ||
| Yes | 7 (2–21) | 7 (2–21) | 7 (2–21) | ||
| Post Bx mammo | 0.18 | ||||
| No | 4 (2–10) | 9 (4–17) | 12 (7–13) | ||
| Yes | 2 (1–5) | 4 (3–10) | 4 (3–10) | ||
| Mammo finding | 0.05 | ||||
| Calcification only | 2 (1–5) | 6 (3–11) | 6 (3–11) | ||
| Mass only | 7 (2–25) | 7 (2–25) | 7 (2–25) | ||
| Both | 0 (0–0) | 6 (1–33) | 6 (1–33) | ||
| Margin & Tamoxifen | 0.51 | ||||
| N=2/49 | Close/positive margin, No Tam | 3 (0.4–19) | 3 (0.4–19) | 3 (0.4–19) | |
| N= 1/13 | Close/positive margin, Tam | 20 (3–80) | 20 (3–80) | 20 (3–80) | |
| Negative margin | 3 (1–6) | 7 (4–12) | 10 (6–17) |
Abbreviations: LR= Local recurrence; Mammo= Mammography; Bx= Biopsy; Tam= Tamoxifen.
Figure 1.
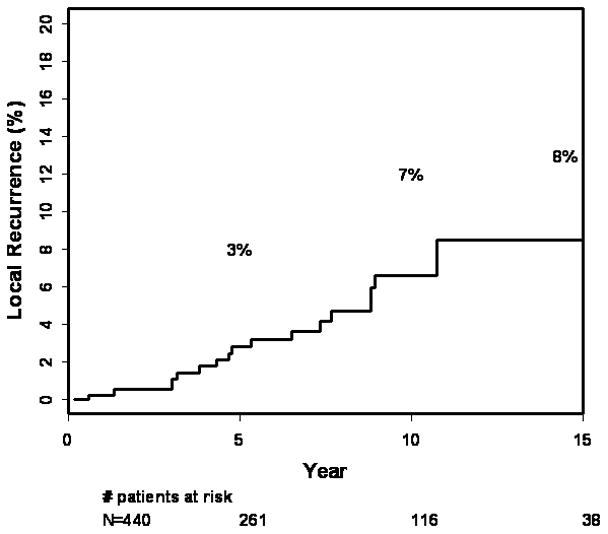
15-year rates of local recurrence for 440 patients with DCIS
Figure 2.
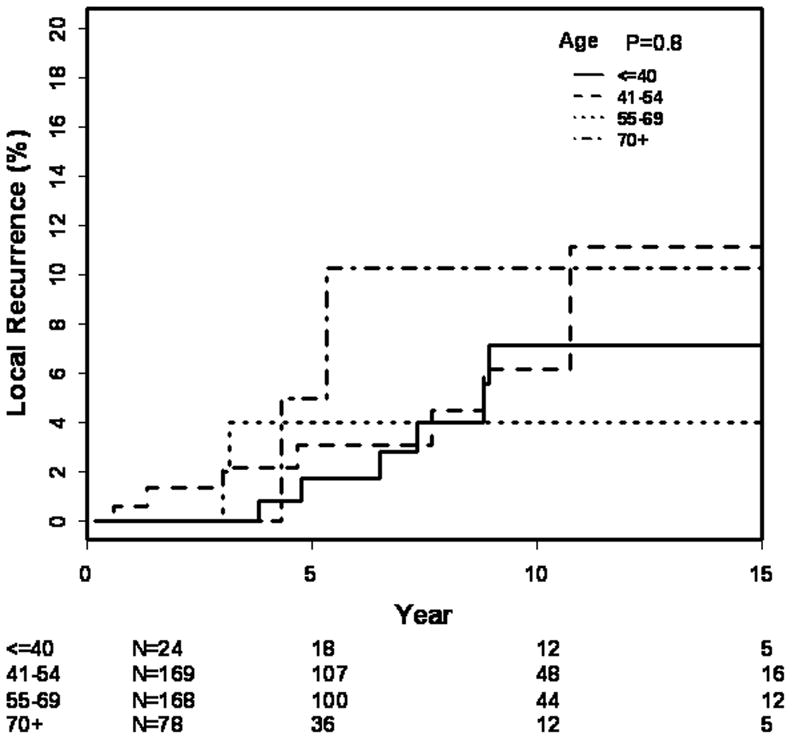
15-year rates of local recurrence by age
There were 21 patients who developed an invasive LR recurrence underwent mastectomy alone without nodal staging and one had local excision only. The histology of recurrence was colloid carcinoma (4 patients), invasive ductal and ductal carcinoma in situ (7 patients), DCIS only (9 patients), invasive lobular in patient and unknown in one patient. The size of recurrence was 0.9 cm (range: 0.3–2.5 cm). All the recurrences were detected mammographically, and 4 also had a palpable mass, 2 had bloody nipple discharge. The median interval to recurrence was 71 months (range: 7 to 247 months). Ten of 22 LR were in a separate quadrant from the primary. At the time of mastectomy, 9 patients had negative axillary dissection and 12 patients had no axillary dissection. One patient received chemotherapy and one patient had both adjuvant chemotherapy and tamoxifen. With a median followup of 62 months years after salvage mastectomy, 18 of 22 patients remain alive without evidence of disease, 1 died of disease and 3 died without evidence of disease.
DISCUSSION
The prevention of LR (invasive and noninvasive) is a primary goal of the treatment for DCIS. Women with DCIS can experience serious concerns and psychological distress as women with invasive cancer despite their excellent prognosis with near 100% five-year disease-specific survival rates (28). Although DCIS was originally detected predominantly by physical examination as a mass (29), screening and treatment for DCIS have changed as mammographic screening detects smaller areas of nonpalpable DCIS amenable to BCS. BCS + RT has now become a standard option to mastectomy, with adjuvant tamoxifen or aromatase inhibitors postoperatively to reduce the incidence of recurrence (9, 10, 15, 30–32).
There are several randomized trials proving that the addition of radiation therapy decreases LR rates. National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B-17 (30), conducted by Fisher et al compared lumpectomy with lumpectomy plus radiation, demonstrating reduction in invasive and noninvasive LR at 8 years of followup (non-invasive LR 13.4% to 8.2%; invasive LR 13.4% to 3.9%). The cumulative incidence of LR of any type was reduced from 26.8% to 12.1% with the addition of radiation (absolute recurrence rate at 5 years: 21% in control arm and 10% in radiotherapy arm). In the European Organisation for Research and Treatment of Cancer (EORTC) 10853 trial (15, 24) by Bijker et al, with the addition of RT, the risk of LR reduced by 47% at 10 year follow up (absolute recurrence rate at 4 years: 16% and 9% respectively). The disease-free survival rate was 74% in patients treated with BCS alone compared with 85% in women treated with BCS + RT. In the United Kingdom Coordinating Committee on Cancer Research (UKCCCR) trial (31), at a median follow up of 52.6 months, the LR rates were 4.8% with RT and 13.7% without RT. There was absolute reduction in risk of LR of 8.9% in the RT arm. Age is thought to possibly have contributed to the lower rates in the UKCCR trial as the cohort was slightly older by comparison, with 90.5% of patients having been ≥50 years of age, although this is by no means certain. In an international collaborative multi-institutional study reported by Solin et al (21), 422 patients with DCIS were treated prospectively with BCS + RT. The 10 year rate of LR was 31% in patients aged ≤ 39 years, 13% in 40–49 year age, 8% for 50–59 years and 6% for age ≥ 60 years (p= 0.0001). Patient age ≤ 39 years and positive margins of resection were independently associated with increased risk of LR (p= 0.0006 and p= 0.023, respectively).
Younger patient age has been associated in retrospective studies with a higher risk of LR compared to older women when treated with BCS and RT (Table 6). Silverstein et al (14, 18) felt the need to modify the original Van Nuys prognostic index by adding age (14) due to this association. The relative LR rate was 2.3 in patients 40–60 years of age compared with 3.2 in patients <40 years (p = 0.001). The disease-free survival was statistically significant for age (p ≤ 0.01) along with other predictive factors such as tumor size, margin width and pathologic classification. In a retrospective review by Van Zee et al (33), at median follow up of 74 months, there were 33 local recurrences (crude rate, 21%) in young women. The actuarial LR rate was 16% at 6 years and the actuarial 6-year LR rates were 10.8%, 14%, and 47.2% in patients aged = 70, 40–69 and <40 years, respectively (p = 0.047). Radiotherapy reduced the risk of LR for each age subgroup. In patients ≤ 39 years, the 6 year actuarial rate of LR in patients with BCS alone was 59% compared to 39% in those treated with RT, while for patients 40–69, the rates were 19% and 8%, and for ≥ 70 years, the rates were 14% and 0% respectively. Vicini et al (20) reported that younger patients have greater risk of LR independent of other risk factors (5 and 10 year recurrence rates were 21%, 26% in <45 years and 7%, 9% in >45 yr). Jhingran et al (34) reported an actuarial rate of LR-free survival at 5 and 10 years of 96% and 88% at 63 months. There was no difference in incidence of true recurrence/marginal miss at 5 and 10 years in younger patients ( ≤ 40 years: 5%, 5%) compared with older patients (≥ 40 years: 3%, 4%), p= 0.39. Analysis of 259 patients of DCIS by Di Saverio et al (35) showed that age was not found to be significant factor influencing the LR rates. At a mean follow up 130 months, LR was seen in 21 patients (8%). The actuarial rates at 10 years were 89.3%, 90.7% and 88% for each group according to age < 40, 40–40, and > 60 years, respectively (p=NS).
Table 6.
Table showing risk of recurrence after WE+XRT in DCIS
| Author | Year | No. of. patients | Age | IBTR | Time interval |
|---|---|---|---|---|---|
| Fowble | 1997 | 8 | ≤40 | 25% | 5 years |
| Fisher | 1999 | 137 | ≤49 | 15% | 8 years |
| Van Zee | 1999 | 15 | < 40 | 33% | 6 years |
| Vicini | 2000 | 31 | < 45 | 24% | 10 years |
| Szeli-Stevens | 2000 | 44 | < 50 | 9.1% | 8.7 years |
| Vicini | 2002 | 31 | < 45 | 21% | 7 years |
| Solin | 2001 | 31 | < 40 | 31% | 10 year |
| Rodrigues | 2002 | 9 | ≤35 | 0 % | 8 years |
| Cutuli | 2002 | 8 | < 40 | 29% | 7 years |
| Jhingran | 2002 | 20 | ≤40 | 5% | 5 years |
| Omlin | 2006 | 108 | ≤39 | 28% | 10 years |
| Bijker | 2006 | 65 | ≤40 | 34% | 10 years |
| Di Saverio | 2008 | 22 | < 40 | 10.7, 0.3, 12% | 10 years |
Abbreviations: WE= Wide excision; XRT= External beam radiation therapy; DCIS= Ductal carcinoma in situ; IBTR= Ipsilateral Breast Tumor Recurrence.
The cause of the association of young age with LR may be due to association with other adverse pathologic factors. A literature review by Vicini and Recht (25) studied the influence of age at diagnosis on characteristics and outcomes for women with DCIS. Younger patients contained grade 3 tumors, those of comedo subtype and those having higher maximum distance of intraductal spread through the breast, with higher residual tumor burdens when compared with the older patients. Younger patients treated with BCS + RT had a significantly higher rate of LR than older patients especially for invasive LR. There was inconsistency among different studies in defining the age limit for younger women ranging from ≤ 35 or ≤ 50 years. They concluded that this age group requires careful attention to selection and surgical technique but young age should not be a contraindication to BCS + RT.
The potential limitations of our study include that the data is from a single institution and a retrospective analysis. Our study of younger patients age ≤ 40 years is limited by small numbers. We did not find a significant difference by age in LR, but to detect such a difference would require an extremely large cohort. Patients with mastectomy are not included in the analysis. There may have been other factors of patient selection at work as well, such as preference for mastectomy in patients who were tested for BRCA positivity. We did not see a statistical difference in LR by use of post-biopsy mammograms, although as a negative margin was defined as 2 mm and not tumor on ink, the effect of postexcisional mammography may have been minimized. Despite this, we still advocate re-imaging of a patient prior to radiation to confirm that all calcifications have been completely removed for assurance of the adequacy of resection. Similarly, with widely varying opinions on the definition of a clear margin (36) and without prospective randomized data comparing the impact of margins on DCIS LR, we continue to recommend re-excision when margins or positive or close, as defined by our institutional standard.
The use of a boost in DCIS was standard in our experience, although it does not have the same level of randomized evidence as in invasive breast cancer. The total radiotherapy dose used in all randomized trials of DCIS was 50 Gy to the whole breast. A boost to the tumor bed was not considered in these studies. There was an emphasis on boost in a subset of patients especially young patients aged ≤ 40 years in a retrospective study by Omlin et al (26) to estimate the effect of boost radiotherapy on LR. The disease-free survival at 10 years was 46% for patients without radiotherapy, 72% for those with radiotherapy without boost and 86% for those given radiotherapy and boost (p <0.0001). The LR-free rates for patients aged 39 years or younger was 63% compared to 81% for those aged 40–45 years (Hazard ratio: 1.00, p= 0.010). The routine use of a boost in our study may have contributed to the low risk of LR seen in our young patients.
In our study, Tamoxifen was given in 95 patients (21.6%) but we did not detect a significant difference in LR. Prospective data is mixed regarding the magnitude of the benefit of tamoxifen in the setting of DCIS. Receptor expression is important in DCIS for predicting the patients who will benefit from the addition of tamoxifen in the adjuvant setting (10, 37–39). Estrogen receptors (ER) are highly expressed in 50–60% of patients with DCIS. The levels of ER expression are higher in DCIS lesions with less aggressive histologic features, such as low grade, increasing differentiation and absence of necrosis, than in those with more aggressive features (13). In 2003, the St Gallen International Consensus Panel endorsed the use of tamoxifen in hormone- receptor positive DCIS (40) and the role of tamoxifen has been evaluated in different series (10, 11, 13, 31, 38, 39, 41). The use of tamoxifen has increased from 24% to 46% in a study by Yen (11) et al before and after the year 1999. 60% were offered tamoxifen and 54% chose to take the drug. In another study by Naklis (38) et al, tamoxifen was offered to 91% of DCIS patients and the accepted by 63%. In the NSABP B-24 study (10), (Fisher et al) patients were randomized to treatment with lumpectomy, RT, and placebo or lumpectomy, RT, and tamoxifen and there was reduction in breast cancer events at 5 years (13.4% vs. 8.2%, p= 0.0009). Younger patients (≤ 49 years) were at higher risk for LR than older patients (LR were 16% vs 6.5% in placebo group, 11% vs 5.2% with Tamoxifen, p= 0.09). Tamoxifen administration resulted in a 38% reduction in LR in women younger than 50 years and a 22% reduction in women older than 50 years. There was little benefit with the addition of tamoxifen in the UKCCCR trial (31). The differnce was not significant statistically on subgroup analysis (10% in observation arm, 6% with tamoxifen alone, 1% in RT alone and 1% with RT and tamoxifen). In patients ≤ 50 years, the LR rates were 18% in those treated with tamoxifen and 26% without tamoxifen (p= 0.19). Tamoxifen also did not significantly reduce the incidence of LR in the United Kingdom/Australia New Zealand ramdomized trial (31).
CONCLUSIONS
We report very low risks of LR after BCS + RT for DCIS, even in young patients ≤ 40 years. There was no apparent difference in LR rates by age, margins, or use of tamoxifen. This may be due to strict patient selection for size and margins, a high utilization of re-excision, and radiation boost. This data may better allow teams of mutispecialty physicians to communicate risks and benefits of breast conservation with their patients, particularly those of young age. Study of newer predictors of biologic behaviour may be helpful in assessing which of these patients are at increased risk for progression to invasive cancer.
Table 7.
Risk of recurrence after WE+XRT+Tamoxifen in DCIS
| Author | Year | No. of. patients | Age | IBTR | Time interval |
|---|---|---|---|---|---|
| Fisher | 1999 | 602 | ≤ 49 | 13.2% | 6 years |
| Houghton | 2003 | 160 | < 50 | 13% | 4.4 years |
Abbreviations: As in table 6.
Acknowledgments
The authors thank Cindy Rosser for her collection and management of the data for the study population.
Footnotes
Presented in part at the scientific session of the 50th annual meeting of the American Society for Therapeutic Radiology and Oncology in Boston, MA, September 21-25, 2008.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Ernster VL, Barclay J, Kerlikowske K, et al. Incidence of and treatment for ductal carcinoma in situ of the breast. Jama. 1996;275:913–918. [PubMed] [Google Scholar]
- 3.Sumner WE, 3rd, Koniaris LG, Snell SE, et al. Results of 23,810 cases of ductal carcinoma-in-situ. Ann Surg Oncol. 2007;14:1638–1643. doi: 10.1245/s10434-006-9316-1. [DOI] [PubMed] [Google Scholar]
- 4.Freedman GM, Anderson PR, Goldstein LJ, et al. Routine mammography is associated with earlier stage disease and greater eligibility for breast conservation in breast carcinoma patients age 40 years and older. Cancer. 2003;98:918–925. doi: 10.1002/cncr.11605. [DOI] [PubMed] [Google Scholar]
- 5.Tabar L, Chen HH, Fagerberg G, et al. Recent results from the Swedish Two-County Trial: the effects of age, histologic type, and mode of detection on the efficacy of breast cancer screening. J Natl Cancer Inst Monogr. 1997:43–47. doi: 10.1093/jncimono/1997.22.43. [DOI] [PubMed] [Google Scholar]
- 6.Bleicher RJ, Abrahamse P, Hawley ST, et al. The influence of age on the breast surgery decision-making process. Ann Surg Oncol. 2008;15:854–862. doi: 10.1245/s10434-007-9708-x. [DOI] [PubMed] [Google Scholar]
- 7.Baxter NN, Virnig BA, Durham SB, et al. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96:443–448. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 8.Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. 2001;19:2254–2262. doi: 10.1200/JCO.2001.19.8.2254. [DOI] [PubMed] [Google Scholar]
- 9.Fisher ER, Leeming R, Anderson S, et al. Conservative management of intraductal carcinoma (DCIS) of the breast. Collaborating NSABP investigators. J Surg Oncol. 1991;47:139–147. doi: 10.1002/jso.2930470302. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 11.Yen TW, Kuerer HM, Ottesen RA, et al. Impact of randomized clinical trial results in the national comprehensive cancer network on the use of tamoxifen after breast surgery for ductal carcinoma in situ. J Clin Oncol. 2007;25:3251–3258. doi: 10.1200/JCO.2006.10.2699. [DOI] [PubMed] [Google Scholar]
- 12.Yen TW, Hunt KK, Mirza NQ, et al. Physician recommendations regarding tamoxifen and patient utilization of tamoxifen after surgery for ductal carcinoma in situ. Cancer. 2004;100:942–949. doi: 10.1002/cncr.20085. [DOI] [PubMed] [Google Scholar]
- 13.Leonard GD, Swain SM. Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst. 2004;96:906–920. doi: 10.1093/jnci/djh164. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186:337–343. doi: 10.1016/s0002-9610(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 15.Bijker N, Peterse JL, Duchateau L, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 16.Sahoo S, Recant WM, Jaskowiak N, et al. Defining negative margins in DCIS patients treated with breast conservation therapy: The University of Chicago experience. Breast J. 2005;11:242–247. doi: 10.1111/j.1075-122X.2005.21617.x. [DOI] [PubMed] [Google Scholar]
- 17.Bijker N, Peterse JL, Duchateau L, et al. Histological type and marker expression of the primary tumour compared with its local recurrence after breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2001;84:539–544. doi: 10.1054/bjoc.2000.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverstein MJ, Lagios MD, Craig PH, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77:2267–2274. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2267::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Fowble B, Hanlon AL, Fein DA, et al. Results of conservative surgery and radiation for mammographically detected ductal carcinoma in situ (DCIS) Int J Radiat Oncol Biol Phys. 1997;38:949–957. doi: 10.1016/s0360-3016(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 20.Vicini FA, Kestin LL, Goldstein NS, et al. Impact of young age on outcome in patients with ductal carcinoma-in-situ treated with breast-conserving therapy. J Clin Oncol. 2000;18:296–306. doi: 10.1200/JCO.2000.18.2.296. [DOI] [PubMed] [Google Scholar]
- 21.Solin LJ, Fourquet A, Vicini FA, et al. Mammographically detected ductal carcinoma in situ of the breast treated with breast-conserving surgery and definitive breast irradiation: long-term outcome and prognostic significance of patient age and margin status. Int J Radiat Oncol Biol Phys. 2001;50:991–1002. doi: 10.1016/s0360-3016(01)01517-6. [DOI] [PubMed] [Google Scholar]
- 22.Cutuli B, Cohen-Solalle Nir C, de Lafontan B, et al. Breast-conserving therapy for ductal carcinoma in situ of the breast: the French Cancer Centers' experience. Int J Radiat Oncol Biol Phys. 2002;53:868–879. doi: 10.1016/s0360-3016(02)02834-1. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 25.Vicini FA, Recht A. Age at diagnosis and outcome for women with ductal carcinoma-in-situ of the breast: a critical review of the literature. J Clin Oncol. 2002;20:2736–2744. doi: 10.1200/JCO.2002.07.137. [DOI] [PubMed] [Google Scholar]
- 26.Omlin A, Amichetti M, Azria D, et al. Boost radiotherapy in young women with ductal carcinoma in situ: a multicentre, retrospective study of the Rare Cancer Network. Lancet Oncol. 2006;7:652–656. doi: 10.1016/S1470-2045(06)70765-3. [DOI] [PubMed] [Google Scholar]
- 27.American Joint Committee on Cancer. Breast. In: Greene FL, Balch CM, Page DL, et al., editors. AJCC Cancer Staging Manual. 6. New York: Springer; 2002. pp. 257–282. [Google Scholar]
- 28.Rakovitch E, Franssen E, Kim J, et al. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003;77:285–293. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- 29.Winchester DP, Jeske JM, Goldschmidt RA. The diagnosis and management of ductal carcinoma in-situ of the breast. CA - A cancer journal for clinicians. 2000;50:184–200. doi: 10.3322/canjclin.50.3.184. [DOI] [PubMed] [Google Scholar]
- 30.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol. 1998;16:441–452. doi: 10.1200/JCO.1998.16.2.441. [DOI] [PubMed] [Google Scholar]
- 31.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 32.Smith GL, Smith BD, Haffty BG. Rationalization and regionalization of treatment for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys. 2006;65:1397–1403. doi: 10.1016/j.ijrobp.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Van Zee KJ, Liberman L, Samli B, et al. Long term follow-up of women with ductal carcinoma in situ treated with breast-conserving surgery: the effect of age. Cancer. 1999;86:1757–1767. doi: 10.1002/(sici)1097-0142(19991101)86:9<1757::aid-cncr18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Jhingran A, Kim JS, Buchholz TA, et al. Age as a predictor of outcome for women with DCIS treated with breast-conserving surgery and radiation: The University of Texas M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 2002;54:804–809. doi: 10.1016/s0360-3016(02)02988-7. [DOI] [PubMed] [Google Scholar]
- 35.Di Saverio S, Catena F, Santini D, et al. 259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: a retrospective review with long term follow up. Breast Cancer Res Treat. 2008;109:405–416. doi: 10.1007/s10549-007-9668-7. [DOI] [PubMed] [Google Scholar]
- 36.Taghian A, Mohiuddin M, Jagsi R, et al. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241:629–639. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kepple J, Henry-Tillman RS, Klimberg VS, et al. The receptor expression pattern in ductal carcinoma in situ predicts recurrence. Am J Surg. 2006;192:68–71. doi: 10.1016/j.amjsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Nakhlis F, Lazarus L, Hou N, et al. Tamoxifen use in patients with ductal carcinoma in situ and T1a/b N0 invasive carcinoma. J Am Coll Surg. 2005;201:688–694. doi: 10.1016/j.jamcollsurg.2005.06.195. [DOI] [PubMed] [Google Scholar]
- 39.Hird RB, Chang A, Cimmino V, et al. Impact of estrogen receptor expression and other clinicopathologic features on tamoxifen use in ductal carcinoma in situ. Cancer. 2006;106:2113–2118. doi: 10.1002/cncr.21873. [DOI] [PubMed] [Google Scholar]
- 40.Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 41.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]


