Abstract
Microdialysis is an in vivo sampling technique that permits the quantification of various substances (e.g., neurotransmitters, peptides, electrolytes) in blood and tissue. It is also used to infuse substances into the brain and spinal cord. This unit describes methods for the construction and stereotaxic implantation of microdialysis probes into discrete brain regions of the rat and mouse. Procedures for the conduct of conventional and quantitative microdialysis experiments in the awake and anesthetized rodent are also provided.
Keywords: microdialysis probe construction, stereotaxic surgery, liquid swivel, dialysate sample, concentric dialysis probe
INTRODUCTION
Microdialysis is an established sampling technique for the in vivo measurement of a variety of substances in both blood and tissue. These include neurotransmitters and neuropeptides, enzyme activity, and electrolytes, as well as various hormones and pharmaceutical agents. More recently, microdialysis has been used to infuse exogenous as well as endogenous substances into the brain and spinal cord. In microdialysis, a semipermeable dialysis membrane is introduced into the fluid or tissue compartment to be sampled and perfused with physiological fluid. As a result of passive diffusion, molecules migrate across the membrane along their concentration gradient. Molecules found in high concentrations within the tissue compartment migrate across the membrane into the dialysis tubing where they can be collected for subsequent quantification (unit 7.4), whereas molecules found in high concentrations within the membrane diffuse outward into the surrounding tissue compartment.
A microdialysis probe consists of a tubular dialysis membrane with inlet and outlet tubes for perfusion and sample collection, respectively (see unit 7.1 for an overview of microdialysis). During the microdialysis experiment, the inside of the membrane is perfused with fluid and the outside of the membrane is in direct contact with the compartment to be sampled. The recovery of substances from the compartment depends on the length and molecular weight cutoff of the membrane as well as the composition and rate of flow of the perfusate. Microdialysis probes can be divided into four types on the basis of their geometry: concentric, side-by side, U-shaped, and horizontal. Each design has advantages and disadvantages. The horizontal probe, which is relatively easy to construct, permits bilateral sampling and has been used most extensively in studies of the spinal cord. It cannot be applied to ventral structures within the brain, and the lack of a rigid guide cannula restricts the time interval between implantation and subsequent experimentation (i.e., ≤1 week). Furthermore, the surgical procedures for implantation are relatively complex. In contrast, the concentric and U-shaped probes can be used in combination with a guide cannula, which can be inserted into an animal some time prior to the actual commencement of experiments (up to 2 months). The surgical procedure is straightforward and can be completed within 20 min.
A variety of dialysis membrane types are available. They differ with regard to size, molecular weight cutoff, and chemical composition. Studies (Maidment et al., 1989; Hsiao et al., 1990; Kendrick, 1990) examining the effects of membrane composition on analyte recovery have shown that the in vitro recoveries of various analytes differ depending on the type of membrane employed. Although the in vivo recovery of low-molecular-weight substances was affected to a much lesser degree, this may not be the case with larger molecules or those that are “sticky” (e.g., peptides). Therefore, these results should be considered when choosing a probe design (also see unit 7.1). This unit describes construction of concentric and side-by-side microdialysis probes (Robinson and Whishaw, 1988; Pettit and Justice, 1991), which differ with respect to size and inflow/outflow tube compositions (see Basic Protocol 1 and Alternate Protocol 1), as well as a modification of a horizontal probe (Imperato and Di Chiara, 1984; Zetterstrom et al., 1984; also see Alternate Protocol 2). Probe/cannula assemblies of varying dimensions can also be obtained from commercial sources (see Background Information). Although they are, in some cases, more expensive than the handmade types, they are standardized with regard to in vitro recovery and can be reused.
This unit also covers methods for surgically implanting microdialysis probes in rats (see Basic Protocol 2 and Alternate Protocol 3) and mice (see Basic Protocol 3) and for conducting microdialysis in vitro (see Basic Protocol 4) and in vivo in rodents (see Basic Protocol 5). For microdialysis in nonhuman primates, see unit 7.3. Finally, procedures are provided for carrying out quantitative microdialysis techniques (see Support Protocols 1 and 2; see unit 7.4 for HPLC detection of neurotransmitters).
PREPARATION OF DIALYSIS PROBE/GUIDE CANNULA ASSEMBLY: CONSTRUCTION OF A CONCENTRIC PROBE (DESIGN 1)
This protocol describes an interlocking dialysis assembly consisting of an epoxied inflowoutflow 36-G section and a cannula/dialysis section with a concentric dialysis probe. The assembly has been designed to twist apart, enabling reuse. The assembly also locks in place and does not require external adhesives to secure it during the actual experiment. For preparing dialysis probe/guide cannula assemblies using glass instead of stainless steel, see Alternate Protocol 1; for preparing assemblies with a horizontal dialysis probe, see Alternate Protocol 2.
A number of probes are made at one time because ~10% will be discarded due to leakage or breakage. The authors typically prepare probes no more than 4 days prior to intended use.
Materials
Epoxy, rapid drying
70% ethanol
36-G stainless steel tubing
Razor blades
Jeweler's pliers
Fine forceps
Dremel tool (optional; Small Parts)
Dissecting microscope
Sandpaper (optional)
Dialysis fiber (regenerated cellulose hollow fiber; Fisher)
Stainless steel tubing or pin small enough to fit inside dialysis fiber
Microbore tubing to fit over 28-G internal guide cannula (Plastics One)
28-G internal cannula (Plastics One)
Sonicator
40°C oven
Vernier calipers
29-G stainless steel tubing (optional)
Additional reagents and equipment for perfusing probes in vitro (see Basic Protocol 4)
Prepare probe materials
Cut 36-G stainless steel tubing into 33-mm lengths by scoring with a razor blade. Grasp the tube (relative to score marks) distally with fine jeweler's pliers and proximally with fine forceps. Gently bend the tube back and forth until it breaks (a dremel tool with cutting disk can be used for this purpose). Examine tube with dissecting microscope. Remove rough edges and/or burrs by using a dremel tool or by gently rubbing with sandpaper.
- Prepare dialysis fiber bundle according to manufacturer's instructions. Cut one fiber to a length two to four times greater than that of the dialyzing surface to have sufficient material to work with. Gently grasp the cut fiber with forceps and dip one end into a small bead of epoxy. Insert stainless steel tubing or pin, which will serve as a holder, into the epoxied end. Dry the membrane/pin assembly.
- The ends of the fiber bundle can be taped to cardboard while drying. Store the dried bundle in a container at room temperature (shelf life is 6 months to 1 year or longer). Fibers should be handled only with forceps.
Cut microbore tubing into ~40-mm lengths. Clean the 36-G stainless steel tubing and 28-G internal cannula by sonicating in 70% ethanol. Dry in a 40°C oven.
Construct probe
- 4. Using a dissecting microscope and forceps, insert 3 to 4 mm of the microbore tubing over the top of the 28-G internal cannula (stretch tubing using forceps). Bend the tubing to form a right angle to the top of the cannula and pierce with a pin by inserting the pin into the lumen of the microbore tubing at an angle and allowing the tip to enter the internal cannula. Insert the 36-G tubing into the microbore tube via the pinhole used to introduce the microbore tubing over the internal cannula. Thread the tubing through the internal cannula until it protrudes the desired length from the cannula tip (e.g., if the active length of the membrane will be 2.0 mm, then the desired length will be 2.0 mm). Check length using vernier calipers.
- Optionally, a 29-G stainless steel tube can be inserted over the 36-G tubing to protect the assembly during storage.
5. Place a large bead of epoxy over the microbore tubing so that the 36-G entry point is fully covered. Allow assembly to dry.
6. Cut the dialysis membrane to a length 0.5 mm longer than that desired for the active surface. Measure from the epoxied end. Spread a bead of epoxy around the dialysis membrane at a point ~0.2 mm above the desired active area to form an O ring.
7. After the epoxy has dried, gently insert the dialysis membrane into the internal cannula so that the O ring forms a loose seal. Apply a small bead of epoxy between the internal cannula and the O ring. Allow to dry. Using a razor blade, cut the membrane from the pin (~0.2 mm from the bubble).
8. Apply an additional coat of epoxy to the epoxied tip. Inspect assembly under a dissecting microscope for nicks and to ensure that the desired length of the dialysis membrane is free from epoxy.
9. Perfuse the probe prior to use to test for leaks and to assess probe recovery or probe efficiency (see Basic Protocol 4).
10. To recycle the dialysis assembly, remove the dialysis membrane from the assembly by holding the probe at the epoxied end with one hand while twisting the internal guide assembly with the other (this separates the probe into two sections). Insert a thin wire into the internal cannula/dialysis section to dislodge the membrane. Clean the internal cannula with 70% ethanol and then reinsert the 36-G inflow/outflow section as described in steps 4 and 5. Apply epoxy to reseal the connection. Insert a newly prepared membrane into the assembly.
CONSTRUCTION OF A SIDE-BY-SIDE PROBE (DESIGN 2)
This protocol describes a dialysis probe assembly that employs silicate glass instead of stainless steel tubing (see Basic Protocol 1) for the inflow/outflow lines (Fig. 7.2.1). Although the assembly is typically used only once, all materials except the membrane can be recycled, thus reducing cost.
Figure 7.2.1.
Diagram of side-by-side microdialysis probe described in Alternate Protocol 1 (Design 2) for in vivo preparations in which the microdialysis probe is inserted through a guide cannula in the rat. (A) Two pieces of fused silica tubing are threaded through a connector that is fitted with a short piece of Silastic tubing attached to the inlet tubing and a guide cannula of the appropriate length. The lengths of fused silica tubing exiting the cannula are adjusted so that the tips of the outflow and inflow tubing are separated by the distance that defines the active area of the membrane. Further, the two pieces of silica are placed so that the tip of the outflow tubing is flush with the end of the guide cannula. (B) Enlarged view: Once the silica is properly positioned, superglue is applied to the Silastic tubing to permanently fix the silica in place. The guide cannula is then removed and saved for later implantation into the rat. The final step in constructing the dialysis end of the probe (refer to panel A) involves slipping a tube of dialysis membrane over the end of the two pieces of silica until the sealed end of the dialysis tubing is flush with the inflow silica tubing. The membrane is then attached to the silica with polyimide sealing resin, and all areas of the membrane not intended to support dialysis are sealed with a thin coating of the resin. To complete the probe for in vivo use, the length of silica tubing that runs from the connector to the pump is covered with Teflon tubing, and the junction of the connector, Silastic tubing, and Teflon tubing are stabilized by covering the area with a small tube (e.g., made from a cut-off syringe cap) and filling with fast-drying epoxy. Adapted from Wages et al., 1986.
Additional Materials (also see Basic Protocol 1)
Polyimide resin
Superglue
Push-pull perfusion connector to fit 26-G guide cannula (Plastics One)
26-G guide cannula, concave
24-G Silastic tubing
Fused silica tubing (100-μm o.d., 0.40-μm i.d.; Polymicro Technologies)
Teflon tubing
Tape
Plastic syringe caps
26-G needle
Needle with plastic hub
Additional reagents and equipment for perfusing probes in vitro (see Basic Protocol 4)
Prepare probe materials
- One day prior to probe construction, cut dialysis fibers into 10- to 15-mm lengths. Seal one end with polyimide resin (or epoxy) and let dry for 24 hr (see Basic Protocol 1, step 2).
- The resin plug should be as small as possible to avoid adding extra length or width to the probe.
Modify push-pull perfusion connector by removing all but the central tube. Attach connector to 26-G guide cannula. Fit a small piece of 24-G Silastic tubing onto the connector inlet tube.
- Cut a length of fused silica tubing to be used as the inflow tube. Cut a second length of tubing ~10 cm shorter than the first (outflow tube). Thread both lengths of silica tubing through the connector/cannula.
- The tubing should be long enough to extend from the head of the animal to the top of the cage and out from there to a pump or collection vial. The length can be as much as 1 m, depending on the animal and pump setup. Alternatively, the inflow line may be shorter, terminating at a swivel at the top of the cage, in which case the length could be ~30 cm. The length should be kept as short as possible since increasing length will increase the dead volume.
- It is recommended that the two lines be cut to different lengths so that during construction there is no confusion as to which is inflow/outflow. The inflow tube should be the minimum length needed for the actual perfusion setup.
Construct probe
- 4. While viewing probe with a dissecting microscope, adjust the length of silica tubing protruding from the cannula so that the longest piece of silica (inflow) extends (from the tip of the cannula) the desired length of the active membrane (e.g., 2.0 mm), making sure that the shorter piece (outlet) of silica is flush with the tip of the guide cannula. Secure silica into the Silastic tubing with superglue. Avoid applying glue to the stainless steel cannula, to enable reuse of the connector. Remove guide cannula.
- The desired length of the membrane will depend on the desired active surface of the probe. The length for rat caudate is typically 2.0 to 3.0 mm, whereas a 1.0-mm length is used for the ventral tegmental area.
- For ease of measuring, the dissecting microscope should be modified so that one eyepiece has millimeter markings. In this case, the focal distance employed should not be varied since this will alter the measured distance. Alternatively, a ruler can be inserted under the microscope.
5a. For use with anesthetized animal: Cover silica tubing with Teflon tubing and secure with tape at Silastic tubing interface. Leave 2.5 cm of distal inflow line exposed.
- 5b. For freely moving preparation: Fit Teflon tubing over inflow and outflow lines. Allow 2.5 cm of silica tubing to protrude from the Teflon tubing so that it can be inserted into the syringe pump (inflow) or collection vial (outflow). Fit plastic syringe cap, cut to form a tube, over the connector/Silastic tubing connection at a 45° angle. Fill cut off syringe cap with epoxy so that the Teflon tubing and Silastic tubing joint as well as the Silastic tubing/connector tubing joints are coated. Avoid applying epoxy to plastic components of the connector.
- The tubing setup described here serves to protect the silica from the strain of motions made by a conscious animal. It is critical that the epoxied area include the metal prong of the connector so that the finished product cannot move when tugged. This will reduce breakage when the animal is connected to a spring or other swivel system.
- 6. Using a dissecting microscope, carefully fit the dialysis membrane (from step 1) onto the two ends of silica tubing protruding from the connector. Seal membrane/silica joint with polyimide resin (or epoxy). Using a 26-G needle as a spatula, coat the silica shaft, silica/membrane joint, and the nondiffusible length of the membrane. Leave the desired length of active membrane free of resin.
- Use a dissecting microscope to make sure that there are no glue or resin droplets that would impede insertion into the metal cannula.
7. Attach a plastic-hubbed needle to the inflow line. Thread 2.5 cm of silica tubing, which extends from the Teflon tubing into a 26-G needle. Apply epoxy to the needle/silica joint and slide the two pieces back and forth several times to move epoxy into the needle shaft. Finally, slide the silica tubing through the needle and into the Teflon tubing, making sure not to glue shut the opening of the silica tubing. Let dry.
- 8. Prior to use, remove any remaining glycerol from dialysis membrane by soaking in 70% ethanol for ~5 min. Perfuse the probe for several minutes with distilled water or perfusion fluid (flow rate 2 μl/min) and check for leaks or air bubbles inside the membrane.
- Preparation of a loop-type probe (Ungerstedt et al., 1982) is achieved with minor modification of the concentric probe protocols described in Basic Protocol 1 and herein. Dow 50 cellulose tubing (o.d. 0.25 mm) and 23-G stainless steel tubing can be employed. Both ends of the cellulose tubing are glued inside the stainless steel cannula, and the tubing is folded into a loop. Prior to surgical implantation, a stylet is inserted into the loop to add rigidity.
CONSTRUCTION OF A HORIZONTAL PROBE
Figure 7.2.2 shows the arrangement of a horizontal probe for in vivo dialysis in the rat. This type of probe can be used for bilateral sampling of discrete nuclei in the brain and, with minor modification, for blood and peripheral tissue. Because of its flexibility and the lack of a rigid guide cannula, it is well suited for dialysis studies in spinal cord.
Figure 7.2.2.
Schematic of a horizontal dialysis probe prepared as described in Alternate Protocol 2. The probe is implanted in the rat brain so that the active length of dialysis membrane is located within the area of interest. Epoxy is used to prevent sampling from areas surrounding the active length of the probe. The methods used for implantation of the probe are detailed in Alternate Protocol 3.
Additional Materials (also see Basic Protocol 1)
Dialysis fiber (340-μm o.d.; Amicon)
Tungsten wire (125-μm diameter, prestraightened)
Stainless steel tube (0.65-mm o.d.; Small Parts)
Waterproof pen
Stereotaxic atlas
Additional reagents and equipment for perfusing probes in vitro (see Basic Protocol 4)
- Cut dialysis fiber to desired length, avoiding excessive handling of fiber, which will close pores.
- The desired length is calculated by determining the width of the structure to be sampled as well as the distance to the skull.
Insert tungsten wire into fiber so that one end of the wire extends ~2 mm beyond one end of the dialysis fiber (this provides rigid support of the fiber). Bevel ends of wire with sandpaper to ease insertion.
Secure other end of fiber inside stainless steel tube (cut to 1 cm in length) so that 3.0 mm extends over the dialysis fiber. Crimp slightly so that fiber and wire are resistant to pulling. Glue fiber to outer metal tube with epoxy.
- Measure ~10 mm from the tip of the wire and mark with a waterproof pen (during surgery, the wire will be pulled until this mark rests at the temporal bone). Using a stereotaxic atlas, calculate the distance between the temporal bone and the areas to be dialyzed. Using this distance, epoxy the fiber, leaving areas to be dialyzed unexposed.
- Exact measurement of this distance is critical for ensuring that only the region of interest is sampled. Stereotaxic atlases are available for rat (Konig and Klippel, 1963; Pellegrino and Cushman, 1979; Paxinos and Watson, 1986), mouse (Slotnick and Leonard, 1975), and monkey (Snider and Lee, 1961).
Allow fiber to dry. Check for leaks or tears using a dissecting microscope (see Basic Protocol 4). Store dust-free and undisturbed.
IMPLANTATION AND TETHERING OF DIALYSIS PROBE/GUIDE CANNULA ASSEMBLY IN THE RAT: IMPLANTATION OF CONCENTRIC OR LOOP PROBE/GUIDE ASSEMBLIES
The brain is well suited for microdialysis because the blood-brain barrier prevents the rapid exchange of most hydrophilic substances between the brain interstitial space and the vascular compartment. For short- or long-term measurements of neurochemicals in the conscious animal, a guide cannula is typically implanted above the region of interest. This provides support and protection of the more delicate dialysis probe and a means to introduce the probe without the trauma of a just-completed surgery. Prior to experiments, the probe is inserted into the guide cannula (see Basic Protocols 4 and 5). For studies employing an anesthetized preparation, the microdialysis probe is either fixed to the skull after implantation or inserted via a guide cannula that has been fixed to the skull. Due to trauma resulting from the introduction of the probe or the probe/cannula assembly into the tissue, the probe is left in situ for some hours prior to the commencement of experiments (see Basic Protocol 5). During this period, the probe is perfused with physiological fluid. For an illustration of the general setup, see Figure 7.2.3.
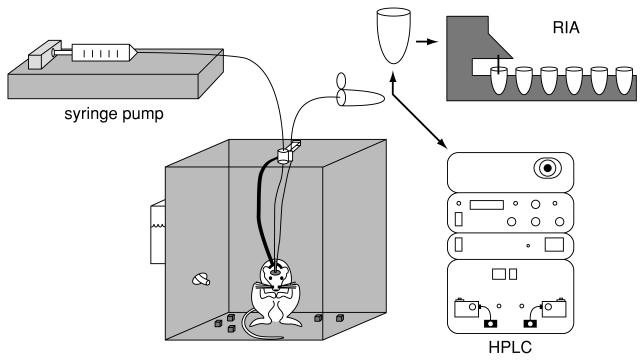
Figure 7.2.3.
Sequence of steps in performing microdialysis in the rat. The microdialysis method involves constructing a microdialysis probe that can be implanted surgically into tissue (e.g., brain tissue). The probe consists of an inflow and outflow tube separated by a tube made of dialysis membrane (not visible in this figure). Perfusate, typically artificial cerebrospinal fluid, is pushed through the probe at a slow flow rate (<3 μl/min), and, through the process of diffusion across the dialysis membrane, analytes in the extracellular space are collected into the perfusate and then pushed through the outflow tubing into a collection vial (microcentrifuge tube). The collected sample is then analyzed by whatever method is most appropriate for detection of the particular analyte(s) under study (e.g., HPLC or RIA; see units 7.4 & 7.5). Different detection methods will require different amounts of sample handling.
A number of reports have been published in which the microdialysis probe, alone, is implanted 24 to 48 hr prior to experiments (Adell and Artigas, 1998; Zapata et al., 1998; Harte and O'Connor, 2005). Since the membrane is in contact with tissue, drying of the membrane within this period does not appear to occur and, thus, perfusion is not necessary until some hours prior to the experiment.
This protocol describes the techniques used for stereotaxic implantation of guide cannula assemblies into discrete regions of the rat brain. Tethering systems for the freely moving animal are also covered. The procedures described are those approved by the National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) Animal Care and Use Committee. For methods of inserting horizontal probes, see Alternate Protocol 3; for implantations in other species, see Basic Protocol 3 for the mouse and unit 7.3 for nonhuman primates.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Materials
Rat
Sodium pentobarbital
Atropine sulfate
Betadine disinfectant (povidone-iodine)
75% ethanol
Hydrogen peroxide (optional)
Dental acrylic (e.g., Plastics One)
Stereotaxic atlas for rat
Stereotaxic frame equipped with carrier for guide cannula or dialysis probe/guide assembly (David Kopf Instruments, Stoelting, or Harvard Instruments)
Shaver
Guide cannula assembly (e.g., David Kopf Instruments, Stoelting, or Harvard Instruments)/dialysis probe (see Basic Protocol 1 or Alternate Protocol 1)
Scalpel
Serrefine forceps or hemostats
Q-tips or cotton swabs
Spatula
Dental or hand drill
Jeweler's forceps and screwdriver
3/16-in. bone screws, 0 to 80 stainless steel (Small Parts, Plastics One)
Heating blanket and thermometer
Liquid swivel and tether (see Background Information)
NOTE: All surgical instruments must be sterile and aseptic procedures should be used unless otherwise specified.
Set stereotaxic positioning
- Using a stereotaxic atlas, determine stereotaxic coordinates for the target brain region by using either the midpoint of the interaural line as the reference point (determined prior to insertion of the rat into the stereotaxic frame; see substep a, below) or the bony landmarks on the skull (bregma), which is done after the anesthetized animal has been inserted into the stereotaxic frame (see substep b, below).
- A stereotaxic atlas provides coordinates for the anterior-posterior (AP), lateral (L), and dorsal-ventral (DV) planes. Accurate placement depends on adherence to the reference points used for atlas preparation. Depending on the atlas employed, the position of the head on the incisor bar may be either above or below the interaural line. In addition, brain size may vary depending on strain, age, or sex. Pilot studies should be conducted to validate or adjust the coordinates if necessary.
- To determine the interaural line: Before preparing or mounting the rat, push the ear bars of the stereotaxic frame together symmetrically so that the tip of the guide cannula or probe rests between the tips of the ear bar. Note that the AP, L, and DV scales of the instrument are defined as the instrument zero. Add or subtract the instrument readings from the respective coordinates obtained from the atlas to determine the coordinates to be used for implantation.
- To use bregma as the reference point: Anesthetize rat, mount in the stereotaxic apparatus, and expose the skull (steps 2 to 8, below). Lower the cannula so that it is centered on the bregma (midline intersection of the coronal and sagittal sutures). Read the AP, L, and DV coordinates from the scale and add or subtract from the stereotaxic coordinates.
- The use of bregma facilitates the correction of lateral and DV coordinates. Furthermore, since the measurements are obtained after the rat is mounted in the instrument, correct mounting of the animal is not as critical as when the interaural reference is used (substep a, above). It is, however, imperative that the rat be positioned in the ear bars so that the head pivots in the DV but not lateral planes.
- Prior to surgery, autoclave or chemically sterilize all surgical instruments. If the probe is to be implanted, check for leaks and air bubbles. Make a final check to ensure that the active length of the probe extends the appropriate distance from the guide cannula tip.
- Gently restrain the rat and inject intraperitoneally (i.p.) with sodium pentobarbital (male, 60 mg/kg; female, 40 mg/kg; 1.0 ml/kg). Administer atropine sulfate (0.05 mg/kg) to relieve respiratory distress. Check level of anesthesia by observing breathing (should be abdominal) and test pain reflexes by pinching footpad with forceps. If animal still responds after 10 min, administer a supplemental dose of anesthetic (e.g., 0.01 ml/250-g male rat; 0.005 ml/female rat).
- Other anesthetics can be used.
- Shave head of animal prior to mounting in the stereotaxic instrument.
- To mount the animal in the ear bars of the stereotaxic apparatus, lock one of the ear bars at the ~5-mm position. Loosely grasp the head, neck, and upper body of the rat from above with one hand and with a free hand maneuver one ear bar into the auditory meatus. Move the ear up and down while gently pushing the ear bar until a popping sound is heard, indicating insertion to the tympanic membrane. Keeping the animal in the same position and maintaining pressure so that the position of the animal in the first ear bar is not altered, gently insert the second ear bar. Loosen bar to enable movement in both the DV and L planes. Gently push the second ear bar into the auditory canal while moving it up and down. When no further movement is possible, lock the ear bar in place.
- Some institutional animal care and use committees object to the rupturing of the tympanic membrane as it causes unnecessary distress to the animal. To avoid this, the use of blunted ear bars has become commonplace in recent years, although their use may result in somewhat less accurate positioning of the animal. This is mainly a factor when using the interaural plane as a reference. As discussed above, the use of bregma as a reference point allows for correction of slight differences in animal positioning.
- If the animal is correctly positioned, only movement in the DV plane should be possible.
- Read the coordinates of each ear bar and then center the animal between the bars. First, loosen the fittings holding each ear bar in place, while maintaining the ear bar placement manually. Applying equal pressure to both ear bars, move the bars together to adjust the ear bar coordinates. Retighten the fittings to secure placement of the ear bars. Next, gently pinch the mouth of the rat open and insert the upper incisors over incisor bar. Adjust nose clamp.
Perform surgical procedure
7. Disinfect the head using Betadine and 75% ethanol and Betadine again. Make a midline incision using the point of the scalpel blade to pierce the skin. After piercing the skin, move the blade parallel to the skin so that the whole cutting surface of the blade is used. Make the incision with one stroke and cut to the skull. Retract skin with serrefine forceps or hemostats.
- 8. With Q-tips or cotton swabs, scrape away fascia and connective tissue. Dry the entire area with a cotton sponge or Q-tips. Identify bregma (intersection of the coronal suture with midline) and lambda (intersection of the occipital suture with the midline). Check that bregma and lambda are in the same plane; this is called the horizontal or flat skull position and most brain atlases are constructed using this plane. For checking lambda and bregma plane, obtain the ventral coordinate using a sylet or needle mounted in an electrode holder. If necessary, release the nose clamp and adjust the incisor bar height until lambda and bregma are in the same plane.
- Dilute hydrogen peroxide can be applied to facilitate drying of the skull and aid visualization of bregma. This step is critical for the proper adherence of dental acrylic to the skull at the end of this surgical procedure.
Implant the guide/probe assembly
- 9. Place dental or hand drill with a burr sized appropriately for the diameter of the guide cannula or probe assembly on an electrode carrier or manipulator that has been modified to accommodate the width of drill. Adjust AP and L coordinates (step 1, substep a or b). Drill until the dura is punctured, using an up-and-down motion.
- The size of the drill bit should be that which will allow lowering of the cannula into the brain without catching the bone. Exercise caution in drilling so that the meninges and underlying brain tissue are not damaged.
- If a hand drill is to be used, first mark the stereotaxic coordinates by inking a stainless steel stylet that has been mounted into an electrode carrier. The burr should be calibrated to extend only through to the dura.
- Bleeding can be reduced by several methods, including application of pressure with gauze or a Q-tip, cauterization, or application of a gelatin sponge (Gelfoam, Upjohn).
- 10. Drill three additional holes around the cannula/probe hole; drill screw holes far enough so as not to interfere with cannula insertion. Using jeweler's forceps and a screwdriver, insert 3/16-in. bone screws into the holes.
- The screws serve to anchor the cannula/probe assembly when dental acrylic is applied. The screws should be rigid but should not extend to the dura.
11. Attach the guide cannula (precut so that it will not extend over the active length of the membrane) or probe into the stereotaxic holder and straighten. Center the cannula over the drill hole. Slowly lower into the target region until the cannula or probe base just rests on the skull.
- 12. Remove skin retractors and dry skull again if necessary.
- A dry skull is critical for proper adhesion of the dental acrylic to the bone.
- 13. Apply dental acrylic to the base of the guide cannula or probe, the three screws, and the exposed skull. Pour the powder onto and over the skull and add the liquid component drop by drop until the mixture is wet but not loose. Make sure the acrylic extends to the muscle. Gently place the skin over the acrylic so that it will adhere to it when the acrylic is dry. For anchoring a tether, also embed the flat head of a slotted screw as far as possible from the cannula and screws.
- In the authors' experience, when this technique is employed no sutures or staples are necessary.
14. Allow acrylic to dry and carefully remove the holder. Smooth any jagged edges by applying additional dental acrylic.
- 15. If an anesthetized animal is to be used, either keep the animal in the stereotaxic apparatus or remove it. Use a heating blanket and thermometer to maintain body temperature. If a guide cannula has been implanted, insert the perfused probe and commence experiments when neuronal release of neurotransmitter has been demonstrated (see Basic Protocols 4 and 5). If experiments will commence at a later time, place the animal in its cage and provide warmth until consciousness is regained. House animals individually to avoid damage to the implant unless a protective dummy cannula (that cannot be gnawed by other rats) is employed.
- For the freely moving preparation, the authors recommend use of the head block tethering system designed by Instech (see suppliers appendix). It is composed of a stainless steel slotted flat screw to which a protective spring is attached. The inflow and outflow tubes can be taped onto or inserted through the center of the spring. A tether clamp enables attachment of the head mount system to a liquid swivel and balancing arm. If this system is to be used, the flat head of a slotted screw must be embedded in dental acrylic in step 13. The flat end of the screw is held firmly in place on top of the skull (a guide carrier placed over the screw can be used for this purpose), positioned as far as possible from the cannula and screws, prior to the application of the dental cement. The dental acrylic is then placed around the cannula, bone screws, and tether screws.
- Other tethering systems (both commercial and laboratory-made) are available. Several companies (e.g., Alice King Chatham Medical Arts, CMA/Microdialysis, Harvard Apparatus, Spalding Medical Products; see suppliers appendix) offer a balance arm and liquid swivel connected via a wire guide and spring clamp to a collar that is inserted over the neck prior to insertion of the probe. Probe inflow and outflow tubing are secured to the wire guide, which pulls the liquid swivel/balance arm (see Fig. 7.2.3).
IMPLANTATION OF A HORIZONTAL PROBE
When using the horizontal probe prepared in Alternate Protocol 2, a different implantation procedure must be used. The protocol below describes implantation of this kind of probe in the rat brain.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Additional Materials (also see Basic Protocol 2)
Saline
1× artificial cerebrospinal fluid (aCSF; see recipe) or Ringer's solution
Epoxy
Electrode carriers, modified to position probe in lateral axis
Horizontal dialysis probe (see Alternate Protocol 2)
Stereotaxic ear bars (David Kopf Instruments), modified to raise position of the head in the apparatus
Sutures
Gauze
2.5-mm drill bit
22-G stainless steel tubing (cannula) and removable plastic caps (e.g., Plastics One)
Polyethylene (PE) tubing to fit 22-G tubing
Additional reagents and equipment for perfusing probes in vitro (see Basic Protocol 4)
NOTE: All surgical instruments must be sterile and procedures are to be performed in a sterile manner unless otherwise specified.
Prepare horizontal probe and rat for stereotaxic implantation
Mount an electrode carrier that has been modified to manipulate a probe in the lateral axis onto the stereotaxic apparatus. Fix the stainless steel portion of the dialysis probe to the carrier so that it extends laterally. Straighten the probe and then carefully remove carrier.
Anesthetize rat and mount in the stereotaxic instrument (see Basic Protocol 2, steps 1 to 6). Use modified ear bars that elevate the head above the stereotaxic instrument to enable clear access and insertion of the probe through the temporal bone. Disinfect and expose skull according to Basic Protocol 2; make incision and expose skull and temporal bones by retracting the skin, muscle, and fascia.
Separate temporal muscle from the bone. Use sutures to tie off muscle, thereby reducing bleeding. Pack the muscle with saline-saturated gauze and keep moist throughout surgery.
Drill holes (using a 2.5-mm drill bit) bilaterally in the temporal bones at a depth appropriate for the target structure (determined by the stereotaxic atlas employed). Puncture dura.
Implant horizontal probe
- 5. Position probe carrier on stereotaxic arm with the tungsten wire pointed at the exposed dura. Slowly introduce the probe tip into the brain until ~10 mm of the tip extends through the temporal bone on the other side (e.g., use mark as reference point; see Alternate Protocol 2, step 4).
- Probe insertion is best done using a second electrode carrier mounted on the other arm of the stereotaxic apparatus. This carrier should be modified so that putty or bone wax can be inserted onto it. The putty or bone wax on the carrier is positioned so that the stylet will penetrate it as it exits the skull. The putty is then used to pull the probe through the brain to the 10-mm mark. This is accomplished by loosening the first carrier to enable use of the second carrier.
6. Once the probe is positioned, tighten the first electrode carrier. Disconnect the tungsten wire from the probe by clipping the wire. Gently retract the remainder of the wire.
- 7. Check probe for patency by attaching PE tubing to the steel cannula and perfusing the probe with 1× aCSF or Ringer's solution (see Basic Protocol 4).
- The perfusate fluid should exit the tube. Lack of fluid exiting the tube indicates a break, leak, or occlusion of the probe.
- When perfusing, make sure that the probe is firmly attached to the electrode holder so that the fiber will not be dislodged from its position in the brain.
8. Glue the free end of the probe into the 22-G stainless steel cannula tubing using epoxy. Bend tubing to allow it to rest comfortably around the skull. Check probe patency again using PE tubing (as in step 7).
- 9. Cement the stainless steel tubes on either side of the probe to the bone with dental acrylic. Insert bone screws into the skull to aid anchoring of the cannula or probe assembly. Apply dental acrylic over skull.
- It is essential that the dental acrylic not damage the probe. To prevent damage, the exposed fiber can be placed in a protective covering (e.g., PE tubing or a pipet tip).
10. Free temporal muscle from its retracted position and suture the skin around the headpiece assembly. Cover the ends of the cannula with removable plastic caps until dialysis studies commence.
SURGICAL IMPLANTATION OF DIALYSIS PROBE/GUIDE CANNULA ASSEMBLY IN THE MOUSE
The development of transgenic and antisense technologies has generated considerable interest in monitoring the effects of such manipulations on brain neurochemistry. Techniques for the implantation and long-term maintenance of cannula/probe assemblies in the mouse are available. This protocol describes the surgical procedures involved for studies conducted in the anesthetized mouse. Although implantation of a commercially available cannula/guide assembly is described, the probe designs described in Basic Protocol 1 and Alternate Protocol 1 can also be used. A tethering system for studies in the freely moving mouse is also described.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Materials
Anesthetic: urethane (nonsurvival), sodium pentobarbital (survival)
Bonding resin (dental acrylic, Denmat)
Shaver
Stereotaxic frame (David Kopf Instruments, Stoelting) adapted for use with small rodents (e.g., Kopf mouse adapter set)
Stage to elevate body of mouse to level of ear bars (e.g., inverted pipet-tip box)
Blunt forceps
Instruments for small animal surgery
Dental or hand (Dremel) drill
Miniature pin vice
Guide cannula (CMA Microdialysis, Plastics One)/probe assembly
Stereotaxic atlas for mouse (e.g., Slotnick and Leonard, 1975; Paxinos and Franklin, 2001)
Electrode carrier (David Kopf Instruments, Stoelting)
Slotted peg for tether attachment
Additional reagents and equipment for probe implantation in the rat (see Basic Protocol 2)
NOTE: All surgical instruments must be sterile and procedures are to be performed in an aseptic manner unless otherwise specified.
Position mouse in stereotaxic apparatus
- Anesthetize mouse. Shave top of head.
- Urethane is a nonsurvival anesthetic with a 4- to 8-hr duration of action.
- Sodium pentobarbital (70 to 80 mg/kg, i.p.), which results in a surgical level of anesthesia for ~30 to 40 min, can be used for survival surgeries. The righting reflex is typically regained within 1 hr. Gas anesethesia can be used for surgery or for supplementation of pentobarbital.
- To insert mouse into stereotaxic frame equipped with Kopf mouse adapter, first place the head of the mouse into the serrated ear cups that replace the standard ear bars. Lock one ear cup so that it is stationary and place the temporal mandibular joint of the mouse firmly against it. Move the second ear cup onto the opposite joint and tighten the ear cup fittings.
- The mouse should be level with the bars, and the head should not pull to one side or the other. The head should pivot up and down but not from side to side.
- For positioning and subsequent surgery, the animal must be elevated. The platform should be small enough so as to not interfere with the incisor bar. The authors typically use a pipet-tip box for this purpose.
- Normal rat ear bars can be successfully used instead of the serrated ear cups, although extreme care must be taken not to crush the mouse skull.
- Using a pair of blunt forceps, insert the upper incisors over the incisor bar and gently close nose bar by screwing it lightly in place.
- Too much pressure on the nose will prevent the mouse from breathing properly. Therefore, it is essential to screw the nose bar lightly.
Perform surgery and implant guide/probe assembly
4. Make a midline incision to expose the skull. Scrape the skull clean of fascia. Make sure the skull is dry so that bony landmarks can be clearly visualized (see Basic Protocol 2, step 8).
- 5. Adjust head to achieve a flat skull position by rotating the head using the movable cheek and nose bar until the DV coordinates of bregma and lambda are within 0.1 mm.
- The mouse has three bony sutures. The middle suture (bregma) is used to determine the vertical zero coordinate. See Basic Protocol 2 for explanation of stereotaxic coordinates.
6. Use a correction factor for determining stereotaxic coordinates. For each animal, divide the actual distance between lambda and bregma by the lambda-bregma distance of the stereotaxic atlas (3.6 mm; Slotnick and Leonard, 1975; Matochik et al., 1994). Multiply the correction factor by the atlas AP, L, and DV coordinates to correct the coordinates.
7. Once the coordinates have been marked on the skull, drill a hole using a dental drill or Dremel tool held in a miniature pin vice.
- 8. Place a prestraightened guide cannula into the electrode holder and lower it into the target region. Apply Denmat bonding resin (dental acrylic) around the cannula shaft, connecting it to the bone surface. Once the acrylic is dry, carefully remove the cannula holder. Secure the cannula in place with additional dental acrylic (e.g., Denmat). For anchoring a tethering system (see step 9b), also attach the small peg from the system with dental acrylic.
- The use of Denmat increases implant longevity and the guide cannula can, if required by the experimental design, be implanted 4 to 6 weeks prior to microdialysis experiments.
- 9a. For anesthetized mouse: Insert probe and perfuse (see Basic Protocol 5) for a minimum of 2 hr.
- Work in the authors' laboratory has shown that, in the case of dopamine, tetrodotoxin-dependent release of neurotransmitter can be demonstrated after 2 hr of microdialysis perfusion (Thompson et al., 1996).
9b. For freely moving mouse: Anchor the tether by inserting a 0.010-in. looped wire into the small peg and clamping it to a 25-G liquid swivel and balancing arm. Lock the wire into the peg by placing a wire sleeve over it.
MICRODIALYSIS IN VITRO
There are several instances in which it is desirable to use microdialysis to collect analyte from a solution ex vivo. The most common use is for calibrating the microdialysis probe (see Basic Protocol 1) by determining an in vitro measure of probe efficiency. The measurement of probe efficiency is then used to assure that the integrity of the probe remains constant over repeated use, and that probe efficiency is constant over a range of analyte concentrations (i.e., probe efficiency should be concentration-independent).
Another common use for in vitro assays is to evaluate the microdialysis procedure that will be applied in vivo and/or to evaluate the impact of particular changes in the microdialysis procedure on relative recovery. This is particularly useful if the substance to be collected has not previously been sampled by microdialysis. In vitro microdialysis is far less expensive and time-consuming than in vivo assays and, therefore, is a good way to test the influence of various microdialysis parameters on probe efficiency (Thompson et al., 1995).
Recent studies (Chefer et al., 2006) indicate that in vitro probe characterization is especially important for assessing changes in DA uptake using quantitative microdialysis methods because the in vitro extraction fraction (Ed) value obtained under well-stirred conditions delineates the upper limit for Ed in vivo (see unit 7.1 for an overview of in vitro and in vivo recovery). Few microdialysis studies have actually directly compared the in vitro and in vivo characteristics of the same microdialysis probes. Although highly time consuming, it is desirable since interpretation of the results of quantitative micro-dialysis is aided by careful characterization of the probe in vitro.
Finally, the in vitro method can be applied to ex vivo tissue preparations to provide estimates of interstitial analyte concentrations.
Materials
Analyte of interest (e.g., neurotransmitter, peptide or drug; usually in powder form) Perfusate, e.g., 1× artificial cerebrospinal fluid (aCSF; see recipe) or Ringer's solution (see unit 7.1 for discussion of perfusates)
37°C platform shaking incubator
Microsyringe pump and glass Luer-Lock gas-tight microsyringes (Hamilton)
Microdialysis probe (see Basic Protocol 1 and Alternate Protocols 1 and 2)
Dissecting microscope
Collection vials (e.g., 0.2-ml microcentrifuge tubes)
Prepare in vitro test solution by making standard dilutions of the analyte of interest in the perfusate and spiking the test solution with a known concentration of analyte.
- Pour test solution into appropriate container (at least 50-ml) and incubate for the duration of the experiment at 37°C with shaking.
- The size of the container should be large relative to the probe to avoid damaging the probe and to provide an “infinite” source of analyte in solution relative to what is removed by microdialysis.
- Prepare microsyringe pump. Set flow rate and secure syringe and needle containing perfusate onto the pump. Turn pump on and ensure that all air is out of the needle tip and hub. Leave pump on until the probe is attached.
- A typical syringe pump holds two syringes. Each dialysis probe requires a minimum of one syringe. If more than two to three solutions are to be infused (e.g., aCSF, aCSF + drug 1, and aCSF + drug 2), then several pumps will be required.
- Prepare probe. Attach a connector to the pump end of the inflow line of the probe so that there is an airtight connection between the probe and the syringe on the pump.
- Commercial probes (see Background Information) are typically packed along with instructions on conditioning the probe prior to initial use. Most dialysis fibers are packed in glycerol to improve shelf life, and the glycerol must be removed before use. Usual removal of glycerol is to soak probes for 2 to 3 min in 70% ethanol. Home-made probes may require similar treatment, although much of the glycerol is removed during initial handling of the probe during construction. A brief soak in ethanol, however, may help to remove oils or dust that may have accumulated on the surface during handling.
- Fill another syringe with perfusate and attach it to the probe via the connector just added.
- On hand-made probes, the connector may be a needle already attached to the inflow line.
- Watching the membrane tip of the probe under a dissecting microscope, slowly push perfusate through the probe. Observe the membrane filling up with perfusate and note the flow of perfusate out of the outflow lines. Check for leaks and air bubbles. Once the probe has been adequately filled and is free of air bubbles, attach it to the syringe pump.
- If leaks are noted, discard the probe. Air bubbles may be dislodged by gently prodding the probe while continuing to apply the same amount of pressure. Another strategy is to shake the probe gently, in much the same way one shakes down a mercury thermometer. Do not apply too much pressure since ultrafiltration (“sweating”) can occur.
- If fluid does not appear within the membrane, check for leaks along the inflow line. If no leaks are visible, cut off a small piece of the inflow line from the top (pump end) as sometimes this end becomes blocked while building the probe or attaching the inflow line to the connector. If air bubbles return to the membrane whenever pressure is decreased, there may be a block in the outflow line. Check the outflow line integrity and try cutting off the tip of the outflow line to resolve the problem. Avoid introducing air to the inflow line when attaching the probe to the microsyringe pump by carefully filling the connector with perfusate. Use great care when tightening the inflow line-syringe connection. Too much pressure can rupture the membrane.
- Probe holder stands are commercially available but can also be constructed from standard laboratory equipment.
Place probe in the middle of the test solution container. Make sure that it does not accidentally come in contact with the container walls as this decreases the surface available for dialysis and leads to erroneous results.
- Collect samples into collection vials immediately and continuously, as desired.
- A period of time may be necessary before a steady state of recovery is achieved. Information regarding equilibration time can be obtained by simply collecting several samples until a constant concentration of analyte is achieved across consecutive dialysate samples. This information will be a useful guide for in vivo studies. Remember to also take samples from the test solution to validate the actual beaker concentration.
- Store samples, standards, and test solution samples for later quantification (e.g., by HPLC-EC CE-LF; see unit 7.4).
- The precise storage method will depend on the nature of the analyte to be assayed. In most cases, samples, standards, and test solutions are frozen at −80°C immediately following collection. Samples should be quantified as soon as possible after collection to optimize detection. Although freezing may retard analyte loss, long-term storage may increase the number of contaminants in a sample (e.g., substances may leach out of the collection vials into the sample) and decrease analyte resolution.
- Using the analytical method of choice, quantify the concentration of analyte in the samples and in the test solution. Calculate probe efficiency as the percent relative recovery of the analyte collected from the samples according to the following equation:
100 where Csample and Csolution are the concentration of analyte in the sample and test solution, respectively.
IN VIVO MICRODIALYSIS
This protocol describes the preparation and steps necessary to collect samples from conscious or anesthetized animals. It is presumed that prior to carrying out this procedure, the investigator has made several decisions regarding microdialysis parameters (e.g., flow rate and perfusate composition) suitable for the particular experimental design (see unit 7.1). This protocol describes the conventional microdialysis method in which the dependent measure is dialysate levels of an analyte under study. Support Protocol 1 describes the application of a quantitative microdialysis method that allows the investigator to obtain estimations of extracellular concentration during steady-state and transient conditions.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Materials
Perfusate: e.g., 1× artificial cerebrospinal fluid (aCSF; see recipe)
Rat or mouse with implanted dummy cannula (see Basic Protocol 2 or 3)
Large flasks
Microsyringe pump and glass Luer-Lock gas-tight microsyringes (Hamilton)
Liquid swivel (see Background Information)
Microdialysis probe (see Basic Protocol 1 or Alternate Protocol 1)
Collection vials
Testing cage
Dissecting microscope
NOTE: All surgical instruments must be sterile and procedures are to be performed in an aseptic manner unless otherwise specified.
Prepare microdialysis equipment
Prepare perfusate in large clean flask.
Prepare microsyringe pumps (see Basic Protocol 4, step 3), fill syringes with perfusate, and attach syringes.
- Prepare probe. Be sure the inflow and outflow lines are long enough to reach the pump (inflow) and provide easy and unobtrusive removal of the sample (outflow). Check that the inflow and outflow lines are protected against strain and torque, as well as chewing, by means of a tether and liquid swivel system mounted onto the testing cage (see Basic Protocols 2 and 3). Attach a connector to the pump end and/or swivel end of the inflow line of the microdialysis probe so that the inflow line can be connected to the syringe on the pump or to the swivel.
- If conscious animals are being studied, connect the inflow line directly to a swivel. Use another piece of tubing to connect the swivel to the syringe pump.
Attach inflow line from the syringe pump to the swivel and ensure good perfusate flow through the swivel (see Basic Protocol 4, step 6). Determine dead volume of the tubing (e.g., volume of solution contained in the final length of tubing to be used) since the lag time between introduction of a substance to the perfusate and its entrance into the dialysis probe should be taken into account during sample collection.
- Attach inflow tube of the probe to the syringe pump or swivel. Place probe securely into a vial filled with distilled water or perfusate until ready to insert the probe into the animal. After the probe is connected to the syringe pump or swivel, allow some time to assure proper flow.
- Initially set the flow rate higher (i.e., twice as high) than that to be used during experiments to make sure the perfusate flows freely through all probes.
- Adjust pump to appropriate flow rate and collect perfusate in a collection vial to check that all probes are providing the same and appropriate volume.
- The volume can be determined by weighing the collection vial before and after a “collection” period to determine the dialysate weight (assume that 1 mg of dialysate equals 1 μl of dialysate).
Prepare test animal
- 7. Remove the dummy cannula from rat or mouse guide cannula and slowly insert the probe into the animal. For nonanesthetized animals, hold the animal in one hand while inserting the probe into the guide cannula.
- Remember that the probe is tethered to the testing cage, so the investigator must stand in close proximity to the cage to insert the probe (there is a tendency to drift backward while focusing on insertion of the probe into a small animal).
- The procedure should take <1 min, and there should be very little resistance offered by the animal. If necessary, the animal may be wrapped in a towel to help in handling.
- Alternatively, the animal can be lightly anesthetized with an inhalational anesthetic (isoflurane) that will render the animal unconscious for 1 to 2 min. As consciousness is regained, however, the animal will be extremely excited, and the investigator should take care that the inflow and outflow lines do not become damaged.
- 8. After the probe is inserted, attach the tether to the animal and place it in the testing cage. For nonanesthetized animals, be sure the testing cage contains the equipment necessary to tether the animal safely. For anesthetized animals, monitor body temperature to ensure that it is maintained during the experiment.
- It is critical that the animal is accustomed to the cage and tether system as well as to being handled. This will reduce the chances of the animal moving its head and damaging the probe and also reduces stress of the animal and investigator during the procedure.
- When testing hypotheses about behavior, the design of the testing cage and the location of the microdialysis probe outlet line require some consideration so that samples can be removed without confounding concurrent behavioral measurements. Ideally, sample collection is “on-line,” with collection directly into the analytical device used to quantitate the analyte under study (e.g., outflow is directly to sample loop of HPLC or autoinjector for derivatization; also see unit 7.4). Alternatively, the outflow line must exit the testing cage in such a way that the experimenter can remove the collection vial without disturbing the animal.
- Following probe implantation, allow recovery of the tissue from the physical damage rendered by probe implantation.
- Most studies in rats have allowed an equilibration period of 2 hr in anesthetized preparations before beginning sample collection (Benveniste and Huttemeier, 1990; Parsons and Justice, 1994). In a conscious animal, the standard equilibration time is typically 6 to 10 hr. When a long equilibration is employed, the flow rate of perfusate can be reduced until 1 to 2 hr prior to sample collection.
Collect and analyze samples
- 10. Collect samples, prepare standards and standard dilutions, and perform experimental manipulations (e.g., inject drug or begin behavioral test).
- Samples can be obtained continuously.
- 11. Remove and store samples immediately following their collection to help prevent sample degradation or evaporation.
- Keep in mind that sample volume is typically small and samples are subject to evaporation. Therefore, as a matter of standard procedure, seal collection vials quickly.
12. Quantify using analytical method of choice (unit 7.4).
- 13. Evaluate data (see Support Protocols 1 and 2). Demonstrate that baseline samples were at steady state by determining that basal dialysate levels remain constant over several collection periods.
- The first time a particular microdialysis procedure is applied, the investigator should demonstrate that the dialysate levels of analyte were in fact of neuronal origin. This is accomplished by observing the result of co-perfusion of substances that specifically modify neuronal release of the analyte (e.g., tetrodotoxin, K+, Ca2+ chelators). Statistical analysis can be performed on the dialysate levels or, after transformation of the data, on the percent change in dialysate levels. The average basal dialysate level, determined from the last three consecutive samples prior to drug administration or behavioral manipulation of the animal is typically used as the baseline for the transformation of data to a percentage of baseline. The latter procedure is frequently adopted to evaluate the effect of a behavioral or pharmacological intervention over time. In this case, the transformation is expected to reduce the number of repeated measures (increases the power of the statistical test) and may reduce within-group variability. Whenever the data are transformed, it is important to compare and then report group baseline dialysate levels. Significant differences in baseline dialysate levels need to be addressed, particularly when a difference was not predicted by the hypothesis. Area under the curve (AUC) values can also be determined and are useful in determining the effects of a manipulation in cases where basal dialysate levels differ between treatment groups.
IN VIVO DETERMINATION OF EXTRACELLULAR CONCENTRATION
This protocol describes collection of data using the technique of quantitative microdialysis. Quantitative microdialysis, in contrast to conventional dialysis, provides an unbiased estimate of extracellular concentration (see unit 7.1 for principles of microdialysis). Two experimental methods have been developed to provide an unbiased estimate of extracellular concentration that is independent of changes of in vivo probe efficiency (Jacobson et al., 1985; Lonnroth et al., 1987, 1989). Both methods are based on the assumption that the tissue levels of the analyte of interest are at steady state over the time during which these methods are applied. Only one method, put forth by Lonnroth and referred to as the difference method, is presented here. This method is particularly powerful since it has also been adapted for non-steady-state conditions (Olson and Justice, 1993). See unit 7.1 for additional methods.
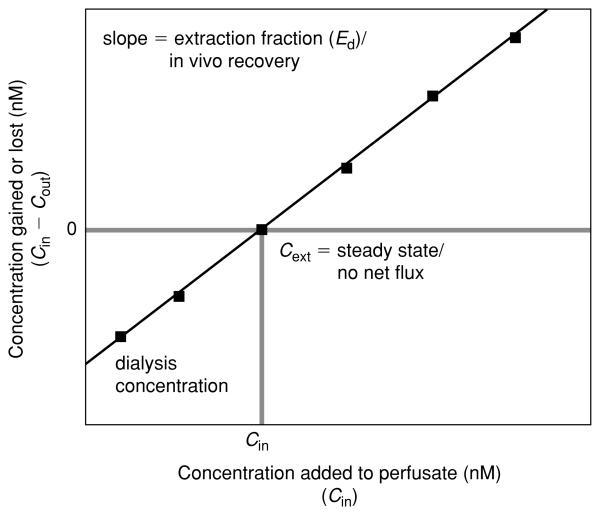
The difference method for steady-state conditions involves: (1) perfusing several known concentrations of the analyte of interest (Cin) through the microdialysis probe; (2) measuring the net gain or loss of analyte from the perfusate following dialysis (Cin – Cout); (3) performing a linear regression of the difference in dialysate concentration (Cin – Cout) against the starting concentration of analyte (Cin); and then (4) using the resultant – linear equation to calculate the starting concentration necessary to produce no net change in the dialysate concentration (Cin – Cout = 0; see Support Protocol 2). The calculated concentration at which no net flux of analyte occurs during dialysis is the estimated extracellular concentration (Cext). The dependent measure for each animal is the estimated extracellular concentration of analyte.
The difference method is based on two assumptions. First, the actual extracellular concentration remains constant throughout the application of the various perfusate concentrations (Cin). Second, it assumes that a straight line best describes the relationship between Cin – Cout and Cin. The latter assumption is roughly evaluated by noting the coefficient of determination (r2), which is the power of Cin to predict Cin – Cout in a linear relationship. A more complete evaluation of the second assumption can be determined by assessing statistically the goodness of fit of a linear versus curvilinear line on a set of data (n > 1). The first assumption restricts the application of this method to those studies in which the experimental manipulation occurred before the microdialysis experiment and only basal extracellular concentration is evaluated (e.g., comparison of basal extracellular levels between transgenic mouse lines). Alternatively, the experimental manipulation produces a long-term steady-state change in neurochemistry (e.g., continuous drug infusion) so that the entire microdialysis procedure can be carried out before and after the experimental manipulation.
A modification of the difference method has been developed that enables its application during transient conditions (e.g., immediately following a pharmacological manipulation; Olson and Justice, 1993; Chefer et al., 2003). Each animal is perfused with only one concentration of analyte for the entire experiment. Treatment (e.g., drug injection) occurs during the experiment, and the dependent measure for each animal is the gain or loss of analyte from the dialysate (e.g., dialysate difference, Cin – Cout). Different animals receive a different Cin, and the relationship between Cin – Cout and Cin (from which extracellular concentration is calculated) is determined after data from all animals are collected. Several methods for statistically testing hypotheses regarding differences in the extracellular concentration between groups or over time are discussed in Support Protocol 2. (Only the estimate of extracellular concentration is independent of recovery; the difference score is affected by variations in recovery, and thus a simple group by group comparison of the difference score is no better than comparing straight dialysate levels.) One drawback of the transient design is that for statistical analysis of group differences, the number of subjects needed is three to four times larger than under the steady-state design.
Materials
Perfusate, e.g., 1× artificial cerebrospinal fluid (aCSF; see recipe) or Ringer's solution
Analyte of interest
- Prepare 10 ml each of three or more solutions of perfusate spiked with varying concentrations of the analyte of interest.
- The concentrations used should straddle the expected extracellular concentration, so that the extracellular concentration will be interpolated from a verifiable linear region. Moreover, it is frequently of interest to collect samples during the 0 nM analyte perfusate condition to compare the data to that generated from a conventional microdialysis experiment. This can be achieved by including a 0 nM analyte solution either as an additional perfusate concentration or as one of the three perfusate concentrations to be used.
Prepare microdialysis equipment and test animals through equilibration (see Basic Protocol 5, steps 2 through 9).
- Under steady-state conditions, change the perfusate to that containing one concentration of the analyte of interest. Allow time for equilibration of the new perfusate to occur (e.g., >5 min depending on the diffusional characteristics of the analyte) and then collect dialysate samples, in duplicate if possible.
- A pilot study may be necessary to demonstrate that sufficient time is allowed for equilibration. This can be verified by showing that there are no systematic variations in dialysate levels between two or more samples collected at each concentration of analyte added to the perfusate.
Replace the perfusate with one containing a different concentration of the analyte and repeat the equilibration/collection procedure. Repeat until samples have been collected for at least three different perfusate concentrations of the analyte.
- Under transient conditions, assign each animal to a particular perfusate concentration of the analyte, and begin perfusing this concentration at the start of the experiment (after a suitable probe equilibration period; see Basic Protocol 5, step 9; the animal will receive this perfusate for the entire experiment).
- It may be necessary to provide fresh perfusate at regular intervals to assure that the analyte does not degrade within the syringe.
Store dialysate sample, standards, and representative samples of each perfusate Cin.
Quantitate dialysate levels of the analyte (see Basic Protocol 4, step 10).
Evaluate data by deriving Cext (see Support Protocol 2).
DETERMINING EXTRACELLULAR CONCENTRATION (Cext)
This support protocol describes analysis of data from the quantitative dialysis experiments detailed in Support Protocol 1.
Determination of Cext under steady-state conditions
At the end of a steady-state experiment, each animal will have dialysate levels (Cout) for each of three or more different starting perfusate concentrations (Cin) of the analyte of interest. To determine the extracellular concentration (Cext), the Cin is regressed against the gain or loss of the analyte during dialysis (Cin – Cout). The resultant linear regression equation can be resolved to determine the Cext. Taking the general form for a linear regression: y = mx + b, where y is the dependent measure (Cin – Cout), x is an independent variable (Cin), m is the slope, and b is the y-intercept. Rearranging the equation to solve for x when y equals zero yields Cext [Cin(x) when Cin – Cout(y) equals zero], xy = 0 = −b/m.
A graphical resolution (see Fig. 7.2.4) can also be used by plotting Cin – Cout (y axis) against Cin (x axis) and drawing the best fit line through each point. The x value at the intersection of the regression line with y = 0 is the Cext. These calculations are carried out for each animal to determine the estimated Cext values.
Figure 7.2.4.
Representative plot of data derived from the difference (no net flux) method of dialysis. Various concentrations (Cin) of the analyte are infused into the dialysis probe, and the amount of analyte gained or lost during dialysis (Cin – Cout) is determined. In a plot of Cin versus Cin – Cout, the y-intercept is equivalent to the dialysate concentration obtained in a conventional microdialysis experiment. The estimated extracellular concentration (Cext) is derived by finding the x-axis value (DA concentration) corresponding to y = 0 from the regression line. The slope of the linear regression is equal to the extraction fraction (Ed). See unit 7.1 for detailed discussion.
Determination of Cext under transient conditions
At the end of a transient experiment, the net gain or loss of analyte from the dialysate (Cin – Cout) can be determined for each subject at each time point. The Cin for each animal is known (manipulated independent variable). A linear regression analysis of Cin versus Cin – Cout of data from all animals in each experimental group will yield an estimate for extracellular concentration at each time point. Follow the same graphical or mathematical procedure described above for the calculation of extracellular concentration under steady-state conditions. The dependent measure for each animal is the difference between the starting concentration of the analyte and the dialysate concentration of the analyte. A calculation of Cext can only be made on a group of data, and thus, for each group, only a single estimate of Cext is obtained. It is possible to propagate the error around the slope and y-intercept to obtain a conservative estimate of the error surrounding Cext. A possible alternative application might be to subdivide the animals in each experimental group into smaller matched groups of three or four. The single dependent measure of Cext would then be calculated from this small cohort. The group statistic would then be made on four or more Cext values obtained from each smaller subgroup.
Group comparisons (or pre- versus post-treatment comparisons) can be made by analysis of covariance (ANCOVA), where the Cin is a manipulated covariate. However, the proper application of ANCOVA demands that the regression lines between groups or times lie in parallel. The slope defined by the linear regression of the data is, in fact, the in vivo extraction fraction (Ed) or in vivo relative recovery (see unit 7.1). Unfortunately, it is very possible that the lines will not be parallel, particularly when relative recovery is low (<50%). In this case, the data could be transformed to force parallelism of the slopes prior to analysis by covariance. Alternatively, the cohort method described above would simplify the statistical analysis to the type described for steady-state conditions.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see appendix 2a; for suppliers, see suppliers appendix.
Artificial cerebrospinal fluid (aCSF, rat)
10× stock solution
0.284 g Na2HPO4 (20 mM final)
0.952 g MgCl2 (10 mM final)
0.133 g CaCl2 (12 mM final)
0.201 g KCl (27 mM final)
8.470 g NaCl (1.45 M final)
H2O to 100 ml
Filter sterilize, adjust to pH 7.4 if necessary with 1 N NaOH or phosphoric acid, and store up to 1 month at 4°C.
Working solution
Dilute the stock solution 1:10, filter sterilize, and adjust to pH 7.4 prior to the start of an experiment.
A typical microdialysis experiment in rats requires 100 to 200 ml perfusate.
If the analyte of interest is susceptible to oxidation, as is the case of monoamine neuro-transmitters, it is useful to include an antioxidant in the aCSF. The authors typically use ascorbic acid (0.25 mM). This is especially useful in quantitative microdialysis studies when the analyte included in the perfusate may be sitting in the syringes for a period of time. In this case, any loss of analyte to oxidation may confound Cin – Cout calculations.
Unfortunately, there is no consensus on the type of perfusion buffer that should be used. Most laboratories employ a modified Ringer's solution: KCl (2.7 to 4 mM), NaCl (130 to 145 mM), CaCl (1.2 to 2.4 mM), and MgCl (1 to 2 mM). Increased KCl and/or CaCl concentrations are often used to achieve higher dialysate concentrations of those analytes for which basal dialysate levels are extremely low and close to the limit of sensitivity of the analytical technique. Importantly, however, increasing the concentrations of these ions evokes neurotransmitter release. Therefore, basal neurotransmitter overflow is not assessed. Furthermore, since release is stimulated, homeostatic mechanisms controlling extracellular neurotransmitter levels (i.e., auto- and hetero-receptors, transporters, etc.) may be altered. Another strategy used to artificially increase neurotransmitter levels if they are below detection limits is to include a reuptake inhibitor (for 5-HT, for example) or a cholinesterase inhibitor (typically neostigmine) in the case of acetylcholine. In all these cases, the same caveats concerning alterations in basal homeostasis apply. The aCSF recipe included in this unit is not buffered. Some laboratories include a phosphate buffer. The use of CO2/bicarbonate buffer is discouraged since most tubing is gas permeable, and that, together with the slow perfusion flow rates may lead to CO2 evaporation and alkalinization of the solution, which in turn may result in the precipitation of calcium and magnesium bicarbonate salts. Finally, some authors include glucose (5 to 10 mM). However, its inclusion facilitates bacterial growth in the tubing and swivels so thorough cleaning of the system is necessary after each experiment. If glucose or ascorbic acid is used, these must be added fresh daily to avoid bacterial contamination or oxidation of the ascorbic acid.
COMMENTARY
Background Information
The conduct of in vivo microdialysis experiments in the rodent requires: (1) the selection and construction of the probe/guide cannula assembly; (2) implantation of the assembly into the region to be dialyzed; (3) preparation of the animal and probe for dialysis sampling; (4) selection of an appropriate dialysis method; and (5) storage and quantification (unit 7.4)of dialysate samples. Attention to the details of each of these facets is critical for the achievement of accurate and reproducible results.
Probe/guide cannula assemblies
Points to consider when selecting a probe/guide assembly include the region to be sampled, the duration of the experiment, and the analyte to be measured.
The size of the area to be dialyzed determines the length of the active membrane. An active length of 2.0 to 3.0 mm is typically used for the rat nucleus accumbens, whereas the same length, if used in the mouse, would result in sampling from both the nucleus accumbens and caudate. The width of the structure to be sampled is also an important consideration when selecting the guide cannula assembly. Larger-gauge guide cannulas and probes can be safely used in large structures (e.g., caudate) of the rat. However, for smaller structures such as the ventral tegmental area of the rat or the caudate of the mouse striatum, smaller-diameter assemblies are required. The location and accessibility of the region to be sampled is also important in choosing probe type. The horizontal-type probe, which lacks a rigid guide cannula, is optimal for studies in the spinal cord of a conscious animal. In contrast, the concentric, side-by-side, or loop design should be used for deeper structures in brain, since the surgical procedures required for implantation would result in less damage to overlying muscle than that for implantation of the horizontal probe.
The time interval between animal preparation and subsequent dialysis can influence the choice of assembly type. Guide cannulas can be implanted weeks prior to the commencement of sampling, whereas implantation of the horizontal probe or of other probes that will not be used in conjunction with a guide cannula require that experiments be performed shortly after surgery. Thus, in studies assessing long-term affects of drugs, a guide cannula assembly is critical.
As discussed in unit 7.1, a variety of membrane materials are available for dialysis studies. These include regenerated cellulose, Cuprophan, polyacrylonitrile/sodium methallysulfonate copolymer, and polycarbonate-ether. Membrane composition has been shown to affect the in vitro recovery of several substances (Hsiao et al., 1990; Kendrick, 1990). Therefore, the analyte to be sampled should be considered when selecting a dialysis membrane.
Finally, assemblies can be made in the laboratory or purchased. Probes are commercially available from such suppliers as Applied Neuroscience, Bioanalytical Systems, CMA/Microdialysis, ESA, and Metallhantering AB, Bioanalytical Systems (see suppliers appendix). Self-made assemblies are often less expensive then commercially available ones if an inexpensive source of labor (e.g., students) is available. However, sufficient undisturbed space in a laboratory is required. Furthermore, training and practice is required for the construction of probes, and a number of probes will subsequently be discarded because of leakage. Commercially available probes are expensive but can be reused several times. It is often possible to salvage guide cannulas by chipping away dental acrylic or dissolving it with appropriate organic solvents (e.g., acetone for the CMA 10 guide cannula), somewhat reducing cost.
Implantation of cannula/probe assemblies
Accurate and reproducible implantation into the area to be sampled is crucial for micro-dialysis experiments. Several stereotaxic atlases for the rodent brain are available (see Literature Cited) and these should be used in initial pilot studies determining the actual stereotaxic coordinates to be used. Stereotaxic coordinates, however, can differ depending upon the strain, age, and sex of an animal. Therefore, extensive piloting of sites and verification by histology is necessary.
It is essential that the guide cannula be rigid and adhere firmly to the skull. The guide cannula can be maintained in the rat for some months following implantation. However, in the case of the mouse, where the use of screws is limited by the size and softness of skull, long-term use of the assembly may be hard to achieve.
In the case of freely moving preparations, the type of tethering systems available should also be considered. If a head mount system is to be employed, it is necessary that this be attached to the skull at the time of assembly implantation.
Finally, when performing surgery in the rodent, sterile conditions should be maintained. Instruments and guide cannulas should be sterilized. Masks and gloves should be worn. Animals should be kept warm until they recover from surgery and postoperative medications should be given as needed.
Preparation of the animal for dialysis
Dialysis studies can be conducted in the anesthetized or conscious animal. Each procedure has its advantages and limitations. The anesthetized preparation does not require the use of a tethering system and experiments can be conducted as soon as neuronal release of transmitter can be demonstrated. The type and depth of anesthesia as well as body temperature can, however, affect the release of several neurotransmitters. Therefore, these issues must be considered when choosing the type of preparation to be used.
Studies in the conscious animal allow for simultaneous monitoring of behavior and are not compromised by the use of anesthesia. However, a tethering system is essential for protection of the probe and movement of the animal in a test cage. Such systems are commercially available from a number of companies (see suppliers appendix). All tethering systems are designed to enable free movement of the animal while protecting the inflow and outflow tubes of the probe from tension. They consist of a liquid swivel, a swivel-mounting device, and a spring or wire that attaches the swivel to the animal. A variety of liquid swivel and tethering systems are available. The swivel, which rotates to keep the tubing from tangling, is a critical component of any microdialysis experiment. The type of swivel used depends on the weight of the animal and a lighter-weight swivel must be used for the mouse. Single- and dual-channel swivels are available from a variety of sources and dead volume can vary. The amount of dead volume is an important factor to consider in pharmacokinetic or time-course studies. The dual swivel is used in those experiments in which simultaneous sampling of two structures is required or when using an intravenous infusion line. The composition of the liquid swivel can vary and the analyte to be sampled should be considered when determining whether a quartz-lined swivel is necessary. Quartz-lined swivels reduce dead volume and may prevent oxidation of some neurotransmitters. The swivel is essential for studies in the awake animal and proper maintenance (e.g., washing) is critical. Swivel mounts enable attachment of the swivel to the test cage. Counterbalanced lever arms, which permit movement, as well as a simple rod design, are commercially available. Protocols for preparing less expensive swivels and mounting systems in the laboratory are also available (Blair et al., 1980).
Probe preparation
The preparations for insertion of a probe vary according to the type used. The dialysis membrane is usually supplied in glycerol, which prevents it from drying out. Conditioning of all probes, whether they are made in the laboratory or obtained commercially, is necessary prior to use. Failure to condition can compromise subsequent analyte quantification. It is also imperative that the probe be checked under a dissecting scope for leaks and air bubbles prior to insertion (see Basic Protocol 4). The presence of either will ruin a dialysis experiment.
Calibration of the probe is also essential. Pilot in vitro recovery studies should be conducted to determine the flow rate of the perfusate that yields optimal quantification of the analyte under study. In vitro recovery data also provide important information as to probe efficiency and whether the integrity of the probe remains constant over repeated use.
Inflow and outflow lines are often overlooked when designing a dialysis experiment. The dead space of the lines should be determined and length of the inlet and outlet lines kept as short as possible to minimize time artifacts.
Sample collection and storage
The composition of the perfusate is critical for dialysis experiments and will vary according to the experimental design and analyte to be quantified (see unit 7.1). Artificial cerebrospinal fluid (aCSF; see recipe) with ascorbate is often used for quantification of amines, whereas the addition of neostigmine has been used in the majority of studies assessing acetylcholine levels (see unit 7.4).
Analyte stability during collection of samples can profoundly affect subsequent quantification. Catecholamines and indoleamines oxidize rapidly and should be frozen if not analyzed on-line. Although the presence of antioxidants in the perfusate or collection vial retards degradation, samples should be frozen at −80°C if >12 hr will elapse between sampling and quantification. The physiochemical properties of the analyte should be considered when selecting collection vials and storage methods. Although polyethylene vials are appropriate for the storage of catecholamines, amino acids, or acetylcholine, opioid peptides and other sticky analytes often adhere to the walls of certain plastic or glass vials. Therefore, when developing a protocol for a less-studied analyte, in vitro experiments should be undertaken to determine the appropriate material for sample collection and storage.
Dialysis method
In initial studies, it is essential to demonstrate neuronal release of neurotransmitters and peptides. Insertion of the probe results in damage to tissue and blood vessels; therefore dialysate samples collected immediately following insertion may be both neuronal and non-neuronal in origin. Glia is also a source of several neurotransmitters and can also be a source of analyte. Typically, neuronal origin can be assessed by infusing tetrodotoxin via the probe. If the analyte contained in dialysate samples represents neuronal release, this manipulation should result in a substantial decrease of analyte. Varying the composition of the perfusate (i.e., concentration of potassium and calcium) also provides information as to the source of the analyte obtained in dialysates. Once neuronal release of analyte is determined, experiments can begin.
In a conventional dialysis experiment, a series of dialysis samples is first obtained for determination of basal analyte levels. Repeated samples are collected until stable baseline levels are achieved (~1 hr). Depending upon the experimental protocol, the probe can then be used for sampling as well as for drug delivery. A liquid switch can be used for delivery of different perfusates into the region dialyzed. With practice, this can also be done manually. Following completion of the experimental manipulation to be performed (i.e., injection of drug), samples are again collected for quantification of analyte levels.
It is important to note that dialysis levels obtained from a conventional microdialysis experiment do not provide a direct measure of extracellular levels. Changes in dialysate concentration may be due to alterations in analyte clearance, release, or extracellular concentration. Data from a conventional microdialysis study does not permit resolution of whether a change in dialysate levels is due to an alteration in one or more of these processes (also see unit 7.1). In addition, a pharmacological treatment could increase both clearance and release of a neurotransmitter, resulting in no net change in dialysate levels. However, the conclusion that the treatment failed to affect the activity of that neurotransmitter system would be erroneous. This issue should be kept in mind whenever reviewing the results of dialysis studies.
Quantitative microdialysis techniques (e.g., difference method; see Support Protocol 1 and unit 7.1) permit assessment of changes in extracellular levels of an analyte as well as in vivo probe recovery or extraction fraction (which in turn provides information as to the effects of a particular manipulation on clearance mechanisms). As such, they are powerful techniques for characterizing changes in neurotransmitter dynamics that occur in response to a particular manipulation. These studies are, however, time-consuming and require good organizational skills. Varying concentrations of the analyte to be measured are infused via the probe and care must be taken to ensure that equilibration periods are adequate and that the perfusate is prepared and switched correctly. A single steady-state experiment typically takes a whole day.
Critical Parameters and Troubleshooting
In constructing the probe, it is essential that the membrane be prepared according to the manufacturer's directions and maintained in a clean, dry environment. It must be conditioned and tested prior to use. In vitro recoveries should be similar from probe to probe. Once the probe is attached to the pump, flow should be constant and should yield an appropriate volume (relative to the rate of perfusion). Lower volumes can indicate the presence of leaks or air bubbles in the membrane or lines. If leaks are noted, the probe should be discarded. Air bubbles can be dislodged by gently prodding the probe while continuing to apply the same amount of pressure. If there is no perfusate flow once the probe is connected to the pump and no leaks are noted, a small piece of the inflow line nearest to the pump can be cut. Alternatively, the probe can be gently shaken in the same way that one shakes down a mercury thermometer.
It is essential that the dead volumes of the swivel, inflow and outflow tubes be determined since dead space will introduce a time lag.
Stereotaxic surgery requires that the animal be firmly mounted into the stereotaxic instrument. No horizontal movement of the head should occur. The presence of such indicates improper mounting of the animal. Ear bars may, in the case of the rat, require tightening or reinsertion. Mounting the mouse in the stereotaxic instrument requires some practice even for investigators with extensive rat experience. It is critical that the head be restrained; yet this is often only accomplished by applying too much pressure on the nose bar, which, in turn, results in a loss of breathing.
Probe insertion requires a steady hand since it is easy to damage the delicate membrane. Lack of outflow, after probe insertion, is often due to occlusion of the outflow tubing. This can be overcome by cutting the tubing. If this does not result in flow, the system (i.e., tubing and pump connectors) should be checked for leaks. If flow still does not occur, the probe is probably at fault. If such is the case, the probe should be removed and inspected for leaks or tears. It is possible to reinsert the probe; however, an ample amount of time must then elapse before sampling can begin.
Animals are often maintained in the chamber used for microdialysis experiments for ≥12 hr. Therefore, food and water should be made available. However, food and water consumption can affect dialysate levels of certain neurochemicals and this should be tested and controlled for if necessary.
It is often necessary to change perfusates during experiments. Each perfusate switch can result in the introduction of air bubbles into the probe (resulting in a decrease in the active membrane area). Therefore, care must be taken when performing this step. Liquid switches are available for this purpose but they are expensive. Manual switching of the perfusate is possible; however, practice is necessary. Reattachment of the needle to a new syringe can result in momentary pressure fluctuations that will affect perfusion rate and thus analyte recovery. Practice can be obtained by conducting an in vitro experiment.
Time Considerations
Probes are usually constructed in batches and 1 day or half of a day must be dedicated to probe construction. Surgical implantation of a single guide cannula in the rat can be accomplished within 30 min provided that all instruments have already been prepared. The induction of anesthesia and placement of the animal into the stereotaxic apparatus requires 5 to 10 min. The rate-limiting step is drying of the dental acrylic and the amount of time required for this step depends on the type of acrylic employed.
Probe implantation in the mouse typically requires more time (i.e., 30 to 40 min) than in the rat due to the extra care required for the insertion of the animal in the apparatus and defining stereotaxic zero.
Implantation of the horizontal probe can require 1 hr or more depending upon the location of the structure to be sampled. Muscle must be tied back and/or sutured and the probe must be carefully pulled through the brain tissue and out of the skull. The presence of leaks must be tested for at several stages of the surgery.
The duration of the microdialysis study will vary depending upon the experimental protocol. If an anesthetized preparation is used, experiments usually commence within 2 to 3 hr after insertion of the probe (when neuronal release of analyte can be demonstrated). Preparation for the experiment (standards and dilutions, perfusate, and pump) can require as long as a half day depending upon whether a conventional or quantitative study is planned. When a conscious preparation is used, 2 to 3 hr is required for the preparation of liquid swivels, probe insertion, and tethering of four rats. This is most often done the evening before the actual experiment. It is helpful to check the animals some hours after initial setup (and reconnect the tethers, if necessary). The length of the actual experiment should not exceed 48 hr, since gliosis will occur along the membrane, reducing the area of tissue accessible to the membrane.
Literature Cited
- Adell A, Artigas F. A microdialysis study of the in vivo release of 5-HT in the median raphe nucleus of the rat. Br. J. Pharmacol. 1998;125:1361–1367. doi: 10.1038/sj.bjp.0702206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Huttemeier PC. Microdialysis: Theory and application. Prog. Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Blair R, Fishman B, Amit Z, Weeks JR. A simple double channel swivel for infusions of fluids into unrestrained animals. Pharmacol. Biochem. Behav. 1980;12:463–466. doi: 10.1016/0091-3057(80)90055-6. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: Quantitative microdialysis in rats under transient conditions. J. Neurosci. 2003;23:3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zapata A, Shippenberg TS, Bungay P. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J. Neurosci. Meth. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Harte M, O'Connor WT. Evidence for a selective prefrontal cortical GABA(B) receptor-mediated inhibition of glutamate release in the ventral tegmental area: A dual probe microdialysis study in the awake rat. Neuroscience. 2005;130:215–222. doi: 10.1016/j.neuroscience.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Hsiao JK, Ball BA, Morrison PF, Mefford IN, Bungay P. Effects of different semipermeable membranes on in vitro and in vivo performance of microdialysis probes. J. Neurochem. 1990;54:1449–1452. doi: 10.1111/j.1471-4159.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Trans-striatal dialysis coupled to reverse-phase high performance liquid chromatography with electro-chemical detection: A new method for study of the in vivo release of endogenous dopamine and metabolites. J. Pharmacol. Exp. Ther. 1984;4:966–977. doi: 10.1523/JNEUROSCI.04-04-00966.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson I, Sandberg M, Hamberger A. Mass transfer in brain dialysis devices—A new method for the estimation of extracellular amino acids concentration. J. Neurosci. Meth. 1985;15:263–268. doi: 10.1016/0165-0270(85)90107-4. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Microdialysis measurement of in vivo neuropeptide release. J. Neurosci. Meth. 1990;34:35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Konig JF, Klippel RA. The Rat Brain: A Stereotaxic Atlas of the Forebrain and Lower Parts of the Brain Stem. Williams and Wilkins; Baltimore: 1963. [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am. J. Physiol. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Fredholm BB, Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. Am.J.Physiol. 1989;256:E250–E255. doi: 10.1152/ajpendo.1989.256.2.E250. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Microdialysis of extracellular endogenous opioid peptides from rat brain in vivo. Neuroscience. 1989;33:549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Sipos ML, Nyby JG, Barfield RJ. Intracranial androgenic activation of male-typical behaviors in house mice: Motivation vs. performance. Behav. Brain Res. 1994;60:141–149. doi: 10.1016/0166-4328(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Olson RJ, Justice JB., Jr. Quantitative microdialysis under transient conditions. Anal. Chem. 1993;65:1017–1025. doi: 10.1021/ac00056a012. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr. Quantitative approaches to in vivo brain microdialysis. Crit. Rev. Neurobiol. 1994;8:189–220. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Pellegrino L, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. Plenum; New York: 1979. [Google Scholar]
- Pettit HO, Justice JB. Procedures for microdialysis with smallbore HPLC. In: Robinson TE, Justice JB Jr., editors. Microdialysis in the Neurosciences. Elsevier Science Publishing; New York: 1991. pp. 117–154. [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: A microdialysis study in a freely moving rat. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Leonard CM. A Stereotaxic Atlas of the Albino Mouse Forebrain. Government Printing Office; Rockville, Maryland: 1975. (DHEW Publication (ADM) 75-100, U.S). [Google Scholar]
- Snider RS, Lee JC. A Stereotaxic Atlas of the Monkey Brain (Macaca mulatta) University of Chicago Press; Toronto: 1961. [Google Scholar]
- Thompson AC, Justice JB, Jr., McDonald JK. Quantitative microdialysis of neuropeptide Y. J. Neurosci. Meth. 1995;60:189–198. doi: 10.1016/0165-0270(95)00012-j. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Matochik JA, Acri JB, Pani AK, Shippenberg TS. Characterization of striatal dopamine in the mouse using conventional and quantitative microdialysis methods. In: Gonzalez-Mora JL, Berges R, Mas M, editors. Seventh International Conference on In Vivo Methods. University of Laguja; Santa Cruz de Tenerife, Spain: 1996. p. 21. [Google Scholar]
- Ungerstedt U, Herrera-Marchintz M, Jungnelius U, Stahle L, Tossman U, Zetterstrom T. Dopamine synaptic mechanisms reflected in studies combining behavioral recordings and brain dialysis. In: Kotisaka M, editor. Advances in Dopamine Research. Pergamon Press; Elmsford, N.Y.: 1982. pp. 219–231. [Google Scholar]
- Wages SA, Church WH, Justice JB. Sampling considerations for on-line microbore liquid chromatography of brain dialysis. Anal. Chem. 1986;58:1649–1656. doi: 10.1021/ac00121a012. [DOI] [PubMed] [Google Scholar]
- Zapata A, Capdevila JL, Trollas R. Region-specific and calcium-dependent increase in dialysate choline levels by NMDA. J. Neurosci. 1998;18:3597–3605. doi: 10.1523/JNEUROSCI.18-10-03597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterstrom T, Sharp T, Marsden CA, Ungerstedt U. Effects of neuroleptic drugs on striatal dopamine release and metabolism in the awake rat: Studies by intracerebral dialysis. Eur. J. Pharmacol. 1984;106:27–37. doi: 10.1016/0014-2999(84)90674-5. [DOI] [PubMed] [Google Scholar]