Abstract
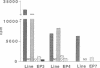
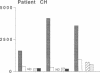
Graves' ophthalmopathy is an autoimmune condition characterized by T cell infiltration of the retrobulbar tissue. Phenotypic and functional analysis of these infiltrating cells may provide insight into the pathogenesis of the disease. IL-2-responsive cells were therefore grown out of the retrobulbar tissue from two patients with severe Graves' ophthalmopathy undergoing orbital decompression surgery, and six T cell lines were established and characterized. They consisted predominantly of CD8 + CD45RO+ cells and secreted IL-4, IFN-gamma, and IL-10 upon activation. When screened for their antigen reactivity, all lines proliferated in response to stimulation with autologous retrobulbar fibroblasts in an HLA class I-restricted manner, but did not recognize autologous peripheral blood mononuclear cells, crude eye muscle extract, allogeneic cells, or purified protein derivate of Mycobacterium tuberculosis. In contrast, PBMC from the same patients responded readily to purified protein derivate of Mycobacterium tuberculosis and allogeneic PBMC, but did not recognize autologous fibroblasts. Interestingly, only one of the six retrobulbar T cell lines displayed cytotoxicity towards its specific target cell population. These results suggest that the retrobulbar fibroblasts are a major T cell target in Graves' ophthalmopathy. Pronounced cytokine production in the absence of target cell cytotoxicity may explain fibroblast proliferation, glycosaminoglycan secretion, and secondary eye muscle enlargement in this condition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson S., Holcombe M., Kendall-Taylor P. Ophthalmopathic immunoglobulin in patients with Graves' ophthalmopathy. Lancet. 1984 Aug 18;2(8399):374–376. doi: 10.1016/s0140-6736(84)90543-9. [DOI] [PubMed] [Google Scholar]
- Bahn R. S., Gorman C. A., Johnson C. M., Smith T. J. Presence of antibodies in the sera of patients with Graves' disease recognizing a 23 kilodalton fibroblast protein. J Clin Endocrinol Metab. 1989 Sep;69(3):622–628. doi: 10.1210/jcem-69-3-622. [DOI] [PubMed] [Google Scholar]
- Ciampolillo A., Marini V., Mirakian R., Buscema M., Schulz T., Pujol-Borrell R., Bottazzo G. F. Retrovirus-like sequences in Graves' disease: implications for human autoimmunity. Lancet. 1989 May 20;1(8647):1096–1100. doi: 10.1016/s0140-6736(89)92382-9. [DOI] [PubMed] [Google Scholar]
- FALCONER M. A., ALEXANDER W. S. Experiences with malignant exophthalmos. Relationship of the condition to thyrotoxicosis and to the pituitary thyrotropic hormone. Br J Ophthalmol. 1951 May;35(5):253–283. doi: 10.1136/bjo.35.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fells P. Thyroid-associated eye disease: clinical management. Lancet. 1991 Jul 6;338(8758):29–32. doi: 10.1016/0140-6736(91)90014-g. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Derfler K., Kassal H., Knapp W., Krisch K., Liszka K., Smyth P. P., Waldhäusl W. Immunological features of nonimmunogenic hyperthyroidism. J Clin Endocrinol Metab. 1985 Jan;60(1):150–155. doi: 10.1210/jcem-60-1-150. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Londei M., Greenall C., Pirich K., Kassal H., Waldhäusl W., Feldmann M. Pathogenetic relevance of HLA class II expressing thyroid follicular cells in nontoxic Goiter and in Graves' disease. J Clin Invest. 1988 May;81(5):1608–1614. doi: 10.1172/JCI113495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993 Jan;23(1):10–17. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Gorman C. A., Bahn R. S. Modulation of HLA-DR expression on retroocular fibroblasts from patients with active thyroid-related ophthalmopathy: in vitro effects of agents used in the management of hyperthyroidism and ophthalmopathy. Exp Clin Endocrinol. 1991 May;97(2-3):206–211. doi: 10.1055/s-0029-1211066. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Wenzel B. E., Gorman C. A., Bahn R. S. Detection, cellular localization, and modulation of heat shock proteins in cultured fibroblasts from patients with extrathyroidal manifestations of Graves' disease. J Clin Endocrinol Metab. 1991 Oct;73(4):739–745. doi: 10.1210/jcem-73-4-739. [DOI] [PubMed] [Google Scholar]
- Holter W., Majdic O., Kalthoff F. S., Knapp W. Regulation of interleukin-4 production in human mononuclear cells. Eur J Immunol. 1992 Oct;22(10):2765–2767. doi: 10.1002/eji.1830221047. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977 Jul 25;252(14):4777–4785. [PubMed] [Google Scholar]
- Hsu D. H., Moore K. W., Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int Immunol. 1992 May;4(5):563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- Kadlubowski M., Irvine W. J., Rowland A. C. Anti-muscle antibodies in Graves' ophthalmopathy. J Clin Lab Immunol. 1987 Nov;24(3):105–111. [PubMed] [Google Scholar]
- Kawai H., Saito M., Takagi M., Tsuchihashi T., Arii Y., Kondo A., Iwasa M., Hirose T., Hizawa K., Saito S. Hashimoto's thyroiditis in HTLV-I carriers. Intern Med. 1992 Oct;31(10):1213–1216. doi: 10.2169/internalmedicine.31.1213. [DOI] [PubMed] [Google Scholar]
- Kodama K., Sikorska H., Bandy-Dafoe P., Bayly R., Wall J. R. Demonstration of a circulating autoantibody against a soluble eye-muscle antigen in Graves' ophthalmopathy. Lancet. 1982 Dec 18;2(8312):1353–1356. doi: 10.1016/s0140-6736(82)91267-3. [DOI] [PubMed] [Google Scholar]
- Kroll A. J., Kuwabara T. Dysthyroid ocular myopathy. Anatomy, histology, and electron microscopy. Arch Ophthalmol. 1966 Aug;76(2):244–247. doi: 10.1001/archopht.1966.03850010246017. [DOI] [PubMed] [Google Scholar]
- Lagaye S., Vexiau P., Morozov V., Saal F., Gony J., Poorters A. M., Loosfeld-Poirot Y., Hors J., Cathelineau G., Emanoil-Ravier R. Detection of HTLV-1 gag related sequences in leucocyte DNA from patients with polyendocrinopathies (Basedow-Graves' disease and insulin-dependent diabetes). C R Acad Sci III. 1991;312(7):309–315. [PubMed] [Google Scholar]
- Londei M., Grubeck-Loebenstein B., de Berardinis P., Greenall C., Feldmann M. Efficient propagation and cloning of human T cells in the absence of antigen by means of OKT3, interleukin 2, and antigen-presenting cells. Scand J Immunol. 1988 Jan;27(1):35–46. doi: 10.1111/j.1365-3083.1988.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Maggi E., Del Prete G., Macchia D., Parronchi P., Tiri A., Chrétien I., Ricci M., Romagnani S. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur J Immunol. 1988 Jul;18(7):1045–1050. doi: 10.1002/eji.1830180712. [DOI] [PubMed] [Google Scholar]
- Munro R. E., Lamki L., Row V. V., Volpé R. Cell-mediated immunity in the exophthalmos of Graves' disease as demonstrated by the migration inhibition factor (MIF) test. J Clin Endocrinol Metab. 1973 Aug;37(2):286–292. doi: 10.1210/jcem-37-2-286. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98(4):279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Rotella C. M., Zonefrati R., Toccafondi R., Valente W. A., Kohn L. D. Ability of monoclonal antibodies to the thyrotropin receptor to increase collagen synthesis in human fibroblasts: an assay which appears to measure exophthalmogenic immunoglobulins in Graves' sera. J Clin Endocrinol Metab. 1986 Feb;62(2):357–367. doi: 10.1210/jcem-62-2-357. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Salvi M., Miller A., Wall J. R. Human orbital tissue and thyroid membranes express a 64 kDa protein which is recognized by autoantibodies in the serum of patients with thyroid-associated ophthalmopathy. FEBS Lett. 1988 May 9;232(1):135–139. doi: 10.1016/0014-5793(88)80402-2. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Bahn R. S., Gorman C. A., Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991 May;72(5):1169–1171. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- Spits H., de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99(1):8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- Tas M., de Haan-Meulman M., Kabel P. J., Drexhage H. A. Defects in monocyte polarization and dendritic cell clustering in patients with Graves' disease. A putative role for a non-specific immunoregulatory factor related to retroviral p15E. Clin Endocrinol (Oxf) 1991 Jun;34(6):441–448. doi: 10.1111/j.1365-2265.1991.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Trieb K., Sztankay A., Hermann M., Gratzl R., Szabo J., Jindal S., Grubeck-Loebenstein B. Do heat shock proteins play a role in Graves' disease? Heat shock protein-specific T-cells from Graves' disease thyroids do not recognize thyroid epithelial cells. J Clin Endocrinol Metab. 1993 Aug;77(2):528–535. doi: 10.1210/jcem.77.2.8345059. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Gatter K. C., Fells P., Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989 Feb;75(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Fells P., Shine B. T and B cell reactivity to extraocular and skeletal muscle in Graves' ophthalmopathy. Br J Ophthalmol. 1989 May;73(5):323–327. doi: 10.1136/bjo.73.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P. Thyroid-associated eye disease: pathophysiology. Lancet. 1991 Jul 6;338(8758):25–28. doi: 10.1016/0140-6736(91)90013-f. [DOI] [PubMed] [Google Scholar]
- Wick G., Grubeck-Loebenstein B., Trieb K., Kalischnig G., Aguzzi A. Human foamy virus antigens in thyroid tissue of Graves' disease patients. Int Arch Allergy Immunol. 1992;99(1):153–156. doi: 10.1159/000236350. [DOI] [PubMed] [Google Scholar]
- Wick G., Trieb K., Aguzzi A., Recheis H., Anderl H., Grubeck-Loebenstein B. Possible role of human foamy virus in Graves' disease. Intervirology. 1993;35(1-4):101–107. doi: 10.1159/000150300. [DOI] [PubMed] [Google Scholar]
- de Carli M., D'Elios M. M., Mariotti S., Marcocci C., Pinchera A., Ricci M., Romagnani S., del Prete G. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metab. 1993 Nov;77(5):1120–1124. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]