Abstract
Plague is an exotic vector-borne disease caused by the bacterium Yersinia pestis that causes mortality rates approaching 100% in black-tailed prairie dogs (Cynomys ludovicianus). We mapped the perimeter of the active portions of black-tailed prairie dog colonies annually between 1999 and 2005 at four prairie dog colony complexes in areas with a history of plague, as well as at two complexes that were located outside the distribution of plague at the time of mapping and had therefore never been affected by the disease. We hypothesized that the presence of plague would significantly reduce overall black-tailed prairie dog colony area, reduce the sizes of colonies on these landscapes, and increase nearest-neighbor distances between colonies. Within the region historically affected by plague, individual colonies were smaller, nearest-neighbor distances were greater, and the proportion of potential habitat occupied by active prairie dog colonies was smaller than at plague-free sites. Populations that endured plague were composed of fewer large colonies (>100 ha) than populations that were historically plague free. We suggest that these differences among sites in colony size and isolation may slow recolonization after extirpation. At the same time, greater intercolony distances may also reduce intercolony transmission of pathogens. Reduced transmission among smaller and more distant colonies may ultimately enhance long-term prairie dog population persistence in areas where plague is present.
Key Words: Connectivity, Cynomys ludovicianus, Epizootic, Fragmentation, Population regulation, Yersinia pestis
Introduction
Plague, caused by the bacterium Yersinia pestis, has occurred in black-tailed prairie dogs (Cynomys ludovicianus) at least since 1945, when plague-positive fleas (Oropsilla hirsuta) were first recorded from black-tailed prairie dog burrows in western Kansas (Public Health Service records cited in Cully et al. 2000). Apparent epizootic die-offs of black-tailed prairie dogs were reported from near Denver, Colorado, and in west Texas at around the same time (Ecke and Johnson 1952). Yersinia pestis is an exotic pathogen in the prairie dog system, and its impacts on populations of all four U.S. species are well known (Barnes 1982, Miller and Cully 2001, Gage and Kosoy 2005) with population declines at individual colonies ranging between 85% and 100% (Barnes 1982, 1993, Cully et al. 1997, Cully and Williams 2001, Antolin et al. 2002), and overall declines at mesoscale colony complexes (40,000–250,000 ha) from 80% to 95% (Cully and Johnson 2005).
Plague decreases the size and number of colonies, thereby creating larger distances between neighboring colonies and may cause the locations of colonies to change (Augustine et al. 2008). Moreover, past research indicates that plague is more likely to occur in large colonies than in small colonies (Cully and Williams 2001, Lomolino and Smith 2001, Collinge et al. 2005, Snäll et al. 2008). Because of the recurrent nature of sylvatic plague and because it decimates prairie dog colonies, it appears unlikely that prairie dog populations within the range of plague will attain preplague levels or be as abundant as in areas without plague (Antolin et al. 2002, Cully et al. 2006). We tested these assertions by comparing the distribution and area of prairie dog colonies from several areas within the range of plague to other areas that were historically free of plague. This study was designed to determine if empirical data supported a conceptual model based on black-tailed prairie dog colony distributions at Cimarron National Grassland, Kansas (Cully and Williams 2001). This model suggests that plague epizootics spread much more easily among colonies once a certain area and density of prairie dog colonies is present on the landscape. At low densities of small colonies, prairie dog colony area in a complex can grow until a density is reached that may allow an epizootic to spread among colonies, again reducing colony size and colony density, and thus regulating the prairie dog metapopulation size on that landscape. In this paper we compare colony sizes and spatial distributions of active colony area among five National Grasslands within the geographic range of plague with two areas that, at the time of observation, had no previous records of plague. We hypothesized that in the absence of plague, (1) individual colonies would be larger, (2) intercolony distances would be shorter, (3) overall colony density would be higher, and (4) colonies would cover a larger portion of suitable potential habitat on the landscape than at sites that lie within the historical range of plague.
We made three major assumptions when interpreting observed results. First, given that colonies are local populations and patches of occupied habitat, we assumed that colony area and straight-line distance to the nearest-neighboring colony are reasonable surrogates for population size and connectivity, respectively. Second, we assumed that changes in the colony area reflect changes in prairie dog population size. If colony area grows consistently at a high rate for several years at multiple colonies, it is fair to assume that the population is growing even if population growth is not consistently proportional to change in area. Third, we assumed that plague transmission between colonies increases as a function of density of colonies (inverse of intercolony distance) and individual colony sizes (large colonies are expected to produce more migrants and be exposed to more plague propagules than small colonies). If plague is transmitted among colonies by dispersing prairie dogs carrying infected fleas, then large colonies should produce and receive more dispersing prairie dogs than small colonies. Likewise, if predators are responsible for intercolony transmission by carrying infected prairie dog fleas, colony size should be important in that large colonies can be expected to attract larger numbers of predators than small colonies. In contrast, if prairie dogs contract plague from other rodent species that function as maintenance hosts, individual colony epizootics may or may not be independent of colony size and rather may depend on the presence of plague in the enzootic species at the location of the prairie dog colony. Colonies close together would still be expected to have a higher probability of acquiring plague if at least one colony becomes infected. With these assumptions in mind, we mapped prairie dog colonies to investigate the potential for the bacterial pathogen, Y. pestis, to limit the size and spatial distribution of black-tailed prairie dog populations at sites within versus outside the geographic range of this pathogen in the western United States.
Methods
Colony spatial data
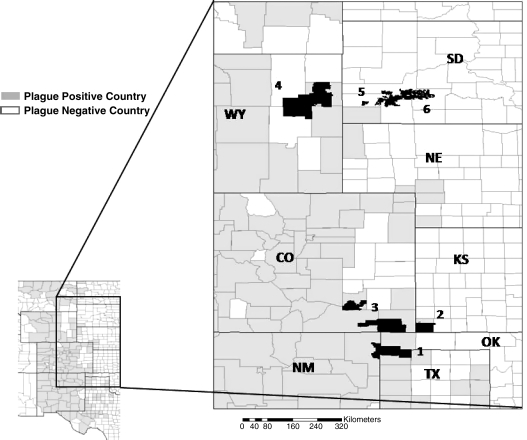
We assessed the impacts of sylvatic plague on black-tailed prairie dog populations by monitoring colonies on public land holdings for 6 years. We surveyed 815 prairie dog colonies on approximately 900,000 ha of public land on the western Great Plains at six complexes of black-tailed prairie dog colonies (Fig. 1). Four areas were located on the United States Department of Agriculture Forest Service property within the current distribution of plague, referred to hereafter as “plague sites.” Plague sites included: (1) Cimarron National Grassland in Kansas, (2) Comanche National Grassland in Colorado, (3) Kiowa and Rita Blanca National Grasslands at the intersection of Texas, Oklahoma, and New Mexico, and (4) Thunder Basin National Grassland in Wyoming, referred to hereafter as Cimarron, Comanche, Kiowa/Rita Blanca, and Thunder Basin. Each of the plague sites has experienced plague during the past 10 years.
FIG. 1.
Location of six study sites. Plague sites include: (1) Kiowa and Rita Blanca National Grasslands, Texas (TX), Oklahoma (OK), and New Mexico (NM); (2) Cimarron National Grassland, Kansas (KS); (3) Comanche National Grassland, Colorado (CO) showing the two management units, Carizo (south) and Timpas (north); and (4) Thunder Basin, Wyoming (WY). Historically plague-free sites located beyond the current distribution of plague include: (5) Wind Cave National Park, South Dakota (SD); and (6) Badlands National Park and Conata Basin, Buffalo Gap National Grassland, SD.
Two additional complexes were selected to represent “plague-free sites.” Plague-free sites were (5) Wind Cave National Park, South Dakota, and (6) Badlands National Park and the Conata Basin, adjacent to Badlands National Park within Buffalo Gap National Grasslands, South Dakota. These plague-free sites are referred to hereafter as Wind Cave and Badlands/Conata Basin.
Although Thunder Basin experienced a plague epizootic in 2000–2001, it is not clear whether epizootics had occurred there prior to 2000. There were no records of plague in the core area of the grassland where most colonies occurred prior to the epizootic in 2000–2001 (Tim Byer, personal communication). However, there was at least one documentation of plague in prairie dogs from the eastern edge of the grassland in 1994 (USDA, Forest Service 2002), and there appeared to be epizootics, which were not confirmed, on nearby ranches during the 1980s (Dean Biggins, personal communication). Because we were unsure of the history of plague at Thunder Basin, we used two approaches. First, we included a separate analysis of 2001 data as a plague-free site. (Please see below for mapping methods and dates.) This was done to test whether the colony distribution at Thunder Basin in 2001 was similar to the plague-free sites. Second, we combined the 2001 data with data from 2002 to 2004 and analyzed them the same as data from the plague sites. Plague may have occurred at Thunder Basin, but a sufficiently long time ago that colonies had time to grow to large size. An important variable for which we have limited data is the frequency with which plague occurs at a site.
Management practices at all study sites were similar. No study site has been actively poisoned for prairie dog control for more than 10 years prior to mapping. Recreational shooting of prairie dogs has been allowed and continues at Cimarron, Kiowa/Rita Blanca, and portions of Thunder Basin. Shooting was not permitted at Comanche, Badlands/Conata Basin, or Wind Cave. Prairie dog shooting may depress colony annual growth rates below the 30–50% that we typically observed, but except at the smallest colonies, it is unlikely to cause the extreme population reductions or localization (spatial clumping) of survivors that could be confused with the effects of plague (Reeve and Vosburgh 2006, Pauli and Buskirk 2007). Cattle (Bos taurus) or bison (Bison bison) grazed all areas.
Colony mapping
We mapped prairie dog colonies in 1999 at Cimarron, Comanche, and Kiowa/Rita Blanca, and again between late May and early October 2001–2005. At Thunder Basin, we mapped colonies between June and August 2002–2004. Colonies were mapped by park or grassland staff at Badlands/Conata Basin in 2005, Wind Cave in 2003, and Thunder Basin in 2001. Prairie dog colonies were mapped on the publicly held portions of each park or grassland, using a hand-held Trimble GeoExplorer3* GPS unit (Trimble, Sunnydale, CA) set to obtain positional readings every second. Colony boundaries were delineated using an all-terrain vehicle. All study sites had inclusions of private land where we do not have data on the presence of colonies. It is possible that colonies present on inholdings could reduce intercolony distances, but our impression was that this would be a minor factor. Where we were aware of colonies on private land, they were generally small.
During 2001, Grassland staff mapped prairie dog colonies at Thunder Basin. Most large colonies present before the plague epizootic had small residual populations after the epizootic when mapped in 2001. Those colonies where no survivors were found were not mapped, but if survivors were seen, even on a small portion of the colony, the full previous extent of the colony where burrows could be found was mapped. We used the 2001 data as a conservative estimate of the preepizootic extent of colony acreage.
To investigate year-to-year changes in colony size, only the areas of each colony actively occupied by prairie dogs were mapped, except at Thunder Basin in 2001 as described above. Active colonies were identified by visually and audibly locating prairie dogs. The boundary of the active area was determined by signs of recent digging on and near burrow mounds, the presence of fresh prairie dog scat, and clipped vegetation indicating foraging activity or the characteristic “mowing” that prairie dogs undertake to enhance visibility on the colony (Hoogland 1995). Because plague is the only disease known to cause extensive die-offs among prairie dogs (Barnes 1993), we assumed that a die-off or the loss of a significant and contiguous portion of a colony was due to plague. We attempted to validate this assumption whenever a die-off was observed. Fleas were collected from burrows and sent to the Centers for Disease Control and Prevention, Division of Vector-borne Infectious Diseases, Ft. Collins, Colorado, where they were identified to species and screened for the presence of Y. pestis.
Spatial analyses
All spatial data were differentially corrected using Trimble Pathfinder software (Trimble) and information from base stations in Elkhart, KS, and Casper, WY (Trimble). Corrected GPS data on colony boundaries were incorporated into a geographic information system (Enviromental Systems Research Institute (ESRI), ArcView 3.2; projected to NAD 1927) to quantify two spatial characteristics for each colony in each year of the study: colony area (ha) and distance to the nearest-neighboring colony (m). Nearest-neighbor (edge-to-edge) distances were calculated using the Nearest Feature ESRI ArcScript (J. Jenness, www.jennessent.com/arcview/arcview_extensions.htm). Occasionally, colonies were located close (<75 m) to neighboring colonies. We treated colonies located <75 m apart as a single colony.
Following die-offs of very large colonies (>1000 ha) at Thunder Basin, small surviving or recolonized areas remained within the former colony boundaries. This resulted in smaller nearest-neighbor distances than occurred between the large colonies mapped prior to plague; the mean nearest-neighbor distance decreased, despite the reduction in overall colony area. This process of reestablishment at multiple locations within the area of a former single colony does not allow an appropriate test of our hypothesis regarding connectivity, so nearest-neighbor distances of colonies at Thunder Basin were not included in the comparison of nearest-neighbor distances between plague and plague-free sites. This was not a problem at other plague sites, where colonies did not attain large size.
Statistical analyses
Colony area and nearest-neighbor data were not normally distributed (as checked with Wilks–Shapiro test) and were log transformed. To determine whether (log-transformed) colony areas and nearest-neighbor distances varied among years within sites, we used one-way analysis of variance (ANOVA) assuming a significance (alpha) level of 0.05 (because colonies were only mapped once at the plague-free sites, repeated measures ANOVA was not possible). The Waller–Duncan K-ratio t-test was used to identify which years differed at each site (SAS v 9.1, SAS Institute, Inc., Cary, NC).
We asked if the distribution of colony sizes differed between study sites, and most importantly, between plague and plague-free sites. To test the hypothesis that colony sizes would be larger in plague-free areas, we used data from each site during the year when colony area was largest (for a conservative test) to calculate the frequency distributions of colonies in seven size categories (0–5, 5–10, 10–25, 25–50, 50–100, 100–250, and >250 ha). Size categories were not equal because of the large variation in colony size at all sites. At all sites, there were a large number of colonies in small size categories; however, these colonies contributed a small proportion of the total amount of colony area at each site. For example, at Badlands/Conata Basin 140 of the 303 colonies were <5 ha, and these contributed only 2% of the total colony area. Conventional frequency distributions (count per category) do not address the contribution of colonies of a given size category to the total area. To address this we calculated the percent of total colony area contained in each size category. The sum of all categories at each site is thus 100. We used the Cochran–Mantel–Haenszel chi-square test to identify differences in colony size distributions among sites (Stokes et al. 1995). This test allows for the comparison of multiple categories, and unlike Pearson chi-square, small counts in a few cells do not invalidate the test as long as the overall sample is large (Stokes et al. 1995). A Bonferroni correction was applied to pair-wise comparisons between sites (α/n; n = 21; 0.05/21; α = 0.002).
We estimated the proportion of area of each plague or plague-free site that was occupied by active prairie dog colonies by dividing the occupied area by the total area of potential prairie dog habitat at each park or grassland. Potential habitat area figures were obtained from agency biologists for Cimarron, Comanche, and Thunder Basin National Grasslands and Wind Cave and Badlands National Parks and from the Grassland Management Plan published on the website for Kiowa Rita Blanca National Grasslands. Generally, potential habitat occurred on loamy soils with slopes <10%, with vegetation dominated by grasses. Variable densities of shrubs may have been present. The mean and maximum proportion of potential habitat occupied over all years mapped at sites within the range of plague were compared using ANOVA in program R (R Development Core Team 2006) with data supplied by Wind Cave National Park for 2003 and Badlands National Park and the Wall Ranger District of Buffalo Gap National Grassland in 2005. The proportion of potental habitat at each site occupied by prairie dog colonies was arcsin square root transformed prior to analyses.
Results
Plague occurrences
During this study, we observed prairie dog die-offs due to plague at Thunder Basin, Kiowa/Rita Blanca, Comanche, and Cimarron. There has been limited evidence of plague at Thunder Basin since 2001 (subsequently documented in mice or fleas in 2002 and 2004: Pauli et al. 2006, Thiagarajan et al. 2008). Colony area increased at Thunder Basin at a rate of 50%/year during 2002–2004 (Table 1); however, the largest colonies were still considerably smaller than they were when mapped in 2001 (<250 ha vs. >1000 ha). We first documented plague at Kiowa/Rita Blanca in 2002, where the severity and extent of colony die-off increased each year until 2005. Ten colonies were extirpated during 2002 and 2003 and despite this loss, total colony area on the grassland continued to increase. Plague was more widespread at Kiowa/Rita Blanca in 2004 and 2005, affecting 23 and 16 colonies, respectively, and reducing the active area of individual infected colonies by >90% annually. These dramatic reductions in colony area caused an overall reduction in colony area of >33% annually at Kiowa/Rita Blanca in 2004 and 2005 (Table 1). Colony extirpation as a result of plague was documented at Cimarron between 1997 and 2000 (Cully et al. 2000, Cully, unpublished data), and again in 2005. Between 2001 and 2005, colony area grew at an annual rate of 22% at Cimarron. At Comanche, there was an extensive die-off in 1995–1996. There were no subsequent die-offs noted until 2005, when epizootics reduced nine colonies near the center of the Carrizo Unit. Colony area grew at Comanche at an annual rate of 40% from 2001 to 2005.
Table 1.
Colony Area and Nearest-Neighbor Distances of Black-Tailed Prairie Dog Colonies at Plague and Plague-Free Sites
| Study site | Year | Habitat area (ha) | No. of colonies | Area (ha) | % Coverage | Mean area (ha) | SE area (ha) | Grouping area | Mean NN (m) | SE NN (m) | Grouping NN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plague-free sites | |||||||||||
| BC | 2004a | 63,000 | 303 | 11,561 | 18.35 | 38 | 12.54 | 768 | 57.80 | ||
| WC | 2003a | 3468 | 19 | 756 | 21.8 | 39 | 16.92 | 863 | 189.90 | ||
| Plague present | |||||||||||
| CIM | 2001 | 34,174 | 49 | 1068 | 3.13 | 20 | 3.90 | A | 1538 | 183.87 | |
| 2002 | 54 | 1344 | 3.93 | 24 | 4.22 | AB | 1332 | 155.46 | |||
| 2003 | 57 | 1622 | 4.75 | 28 | 4.79 | AB | 1308 | 143.63 | |||
| 2004 | 54 | 2280 | 6.67 | 42 | 7.01 | B | 1271 | 142.16 | |||
| 2005 | 51 | 2342 | 6.85 | 45 | 7.65 | B | 1319 | 148.43 | |||
| Mean | 1557 | 4.56 | 29 | 1405 | |||||||
| COM | 1999a | 155,016 | 78 | 799 | 0.52 | 10 | 1.40 | A | 2260 | 219.67 | A |
| 2001 | 93 | 1757 | 1.13 | 18 | 2.12 | B | 2267 | 190.07 | A | ||
| 2002 | 105 | 2497 | 1.61 | 23 | 3.02 | BC | 2219 | 191.30 | A | ||
| 2003 | 111 | 2680 | 1.73 | 24 | 2.64 | BC | 1975 | 187.51 | B | ||
| 2004 | 125 | 4810 | 3.10 | 40 | 4.37 | C | 1711 | 166.57 | B | ||
| 2005 | 119 | 6323 | 4.08 | 46 | 6.13 | C | 1329 | 151.70 | C | ||
| Mean | 3144 | 2.03 | 27 | 1960 | |||||||
| KRB | 1999a | 40,749 | 38 | 696 | 1.71 | 18 | 4.31 | A | 3016 | 935.84 | |
| 2001 | 44 | 1663 | 4.08 | 38 | 5.36 | B | 2050 | 331.39 | |||
| 2002 | 64 | 2186 | 5.36 | 34 | 4.94 | B | 1815 | 221.64 | |||
| 2003 | 65 | 2740 | 6.72 | 42 | 6.16 | B | 1724 | 240.07 | |||
| 2004 | 71 | 1809 | 4.44 | 25 | 4.99 | A | 1481 | 199.32 | |||
| 2005 | 71 | 1236 | 3.03 | 17 | 4.48 | C | 1328 | 185.27 | |||
| Mean | 1721 | 4.22 | 29 | 1902 | |||||||
| TB | 2001 | 157,852 | 96 | 9296 | 5.89 | 95 | 25.59 | A | 1664 | 174.22 | |
| 2002 | 130 | 1750 | 1.11 | 13 | 3.11 | B | 1563 | 164.03 | |||
| 2003 | 135 | 2278 | 1.44 | 17 | 2.94 | C | 1597 | 163.01 | |||
| 2004 | 146 | 3847 | 2.44 | 26 | 4.06 | C | 1425 | 160.73 | |||
| Mean | 4292 | 2.72 | 38 | 1562 | |||||||
At plague sites, bold for the year indicates that plague was active that year. Grouping indicates years that shared letters were not significantly different. If no letters are present for a site, then mean area or nearest neighbor (NN) distance did not differ among years.
Data supplied by area staff.
BC, Badlands/Conata Basin; WC, Wind Cave; CIM, Cimarron; COM, Comanche; KRB, Kiowa and Rita Blanca; TB, Thunder Basin.
Colony size
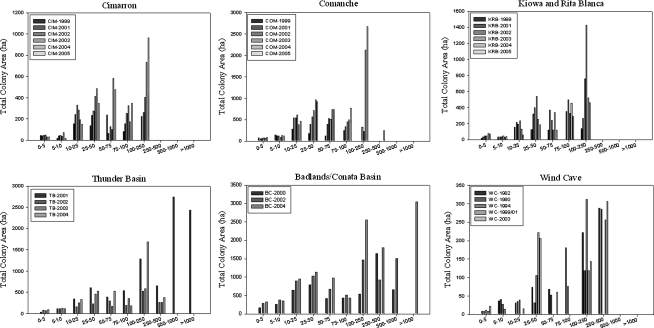
At sites with a history of plague, maximum and mean colony sizes were smaller than at sites with no history of plague and at Thunder Basin in 2001 (Table 1, Fig. 2). Mean colony size differed between years at all plague sites (p < 0.0001; Table 1). Significant differences in colony size among years at all plague sites reflect annual colony growth in the absence of plague and colony collapse in the presence of plague.
FIG. 2.
Distribution of black-tailed prairie dog colony sizes at plague and plague-free sites. Areas with no history of plague (Badlands/Conata Basin and Wind Cave), as well as Thunder Basin in 2001, have colonies distributed throughout the range of colony sizes including >250 ha. With the exception of one colony at Thunder Basin after the 2001 epizootic, and one colony at Comanche in 2005, colony complexes with a history of plague (Cimarron, Comanche, Kiowa/Rita Blanca, and Thunder Basin 2002–2004) have all their colony area in colonies of 250 ha or less.
Colony size distributions
The area of prairie dog colonies in large size classes differed between plague and plague-free sites (Table 2, Fig. 2). Large colonies (>500 ha) occurred at the two plague-free sites when mapped, and at Thunder Basin in 2001. Among the plague sites, one colony at Thunder Basin was >250 ha in 2002, but it collapsed by 2003 and another colony reached 376 ha in 2004. After 10 years (1995–2005) there were three colonies at Comanche that had grown to ∼250 ha, but they were decimated by plague during 2006–2007 (USDA, Forest Service, unpublished records). At the other sites, there was not adequate time for colonies to reach such large size before being reduced by plague. The colony size distribution was similar for all pair-wise comparisons of Cimarron, Comanche, Kiowa/Rita Blanca, and Thunder Basin postplague (Table 2). Likewise, size distributions of colonies at the plague-free sites, Badlands/Conata Basin, Wind Cave, and Thunder Basin 2001 were not significantly different. With Bonferroni correction, Wind Cave differed significantly from KRB, but not from the other plague sites. Wind Cave was similar to Badlands/Conata Basin and Thunder Basin 2001. In general, colonies at plague sites were smaller and further apart than at plague-free sites, even after several years of postplague colony growth.
Table 2.
Pair-Wise Comparisons of Proportion of Colony Area Contributed to Each Site by Size Categories Using Cochran–Mantel–Haenszel Chi-Square Test
| KRB | CIM | COM | TB-2001 | TB | WC | BC | |
|---|---|---|---|---|---|---|---|
| KRB | – | ||||||
| CIM | 0.09 | – | |||||
| COM | 0.35 | 0.10 | – | ||||
| TB-2001 | 14.64a | 13.86a | 12.25a | – | |||
| TB | 0.04 | 0.24 | 0.54 | 13.80a | – | ||
| WC | 9.58a | 8.85 | 7.27 | 1.31 | 9.01 | – | |
| BC | 15.43a | 14.78a | 12.89a | 0.05 | 14.28a | 1.01 | – |
Denotes significant difference with Bonferroni correction (α = 0.002).
Proportion of area occupied
The proportion of potential habitat occupied by black-tailed prairie dog colonies (Table 1) at their maximum extent in the plague areas was less than at the plague-free areas (F(1,4) = 75.02, p = 0.0010). The proportion of potential habitat occupied by black-tailed prairie dogs in plague-free areas when mapped was 18.35% at Badlands/Conata Basin and 21.8% at Wind Cave (Table 1). Among the plague areas, Cimarron had the highest proportion of area occupied (6.85%). Cimarron, Comanche (4.08), Kiowa/Rita Blanca (6.72), and Thunder Basin (5.94) were similar to each other. Occupied area at Cimarron and Comanche was maximized in 2005, and Thunder Basin following plague, in 2004. Plague epizootics were identified at Cimarron and Comanche in 2005 (see above) and continued through 2008 (USDA Forest Service, unpublished records). Plague occurred at nine colonies in 2004 at Thunder Basin.
Nearest-neighbor distances
Mean nearest-neighbor distances were greater at Cimarron (1405 m), Comanche (1960 m), and Kiowa/Rita Blanca (1902 m) than at Badlands/Conata Basin (768 m), or Wind Cave (863 m; p = 0.001). Nearest-neighbor distances varied among years at Comanche (p < 0.0001).
Discussion
There were strong and significant differences in colony size distributions, proportion of potential habitat area occupied, nearest-neighbor distances, and presence of large colonies between black-tailed prairie dog colony complexes in areas with and without a history of plague. Comparisons of populations that have not been exposed to Y. pestis, populations that have a history of plague exposure, and populations that have recently experienced epizootics provided a unique opportunity to quantify the immediate and long-term effects of sylvatic plague on the spatial structure of black-tailed prairie dog colonies. In the absence of plague, colonies might have continued to grow, but at Cimarron and Comanche (2005–2008), and Kiowa/Rita Blanca (2002–2005), plague reduced or eliminated all large colonies. We have not analyzed data after 2004 at Thunder Basin.
As we predicted, sites with no history of plague maintained more total colony area in large colonies, and colonies covered a larger percentage of potential habitat on the landscape, at least in part because colonies in these areas were not periodically reduced by plague as they were in areas where plague was active. In areas where plague occurred, affected colonies were reduced by >90%, and because plague often affected multiple colonies and many colonies were extirpated or reduced to small sizes, the overall footprint of black-tailed prairie dogs on the landscape was reduced. Periodic reemergence of plague may be responsible for the result that, with one exception at Thunder Basin after 2001, the largest colony size within plague areas was ∼250 ha, whereas some colonies were >500 ha within all plague-free areas. At Badlands/Conata Basin and at Thunder Basin in 2001, the largest colonies were >1000 ha (Fig. 2).
Where plague was present, the proportion of potential habitat occupied by prairie dog colonies, as well as the colony size distributions, was a function of the time since the last epizootic. In the absence of visible plague, between 2001 and 2005, colonies grew at an average annual rate of 22% at Cimarron and 40% at Comanche. At Thunder Basin, from 2002 to 2004, colony area grew at 50% annually. If these rates could be sustained, following a plague epizootic that reduced colony area by 95%, it would require 15 years at Cimarron, 9 years at Comanche, and 7 years at Thunder Basin for total colony area to be restored to the preepizootic area. This may be an especially important point for Thunder Basin, which lies within the geographic range of plague, but where plague has not been recorded (at least in the core colony area) for more than 10 years prior to the plague outbreak that occurred there in 2000–2001. Before that outbreak, colony area and the colony size distribution were similar to the plague-free sites. Where colony growth is slower and where plague occurs more frequently, colony area and colony sizes can be expected to be smaller.
Where plague occurs, the smaller sizes of colonies and the greater distances between colonies result in greater colony isolation, which likely lowers the probability of recolonization following a plague event. These changes in colony distribution may adversely affect the viability of prairie dogs, although they may also be beneficial for prairie dogs (see below), but the reduction of habitat area and increased habitat fragmentation for other, prairie dog–dependent species, such as black-footed ferrets (Mustela nigripes) or burrowing owls (Athene cunicularia), may result in additional serious conservation problems (Desmond et al. 2000, Lockhart et al. 2000).
Increased distances between colonies and decreased colony size may, however, lessen the impacts of subsequent plague epizootics. In the Oklahoma panhandle, in the presence of plague, the most isolated prairie dog colonies had the highest probability of persistence (Lomolino and Smith 2001). These results and ours suggest an intuitive explanation for the nonintuitive but general observation that species in decline are lost first within the more populous, central portions of their ranges (Lomolino and Channell 1995, Channell and Lomolino 2000). This pattern of range decline, which results in remnant populations located at the periphery of a species' former range, is general to many geographic regions and taxonomic groups (Channell and Lomolino 2000). Previous explanations for this pattern of range decline implicated “contagious” processes associated with human activity, such as habitat degradation and effects of introduced species (Channell and Lomolino 2000). We suggest that disease may be responsible for inverting the distribution of certain species. Disease, introduced or exacerbated by human activities, may spread more easily through the central portion of a species range, where there is higher connectivity among individuals and populations. Peripheral populations may remain unaffected by virtue of their relative isolation from this contagious process. This pattern has been reported at a local scale for Gunnison's prairie dogs (C. gunnisoni: Cully et al. 1997).
That infectious diseases may disrupt the distribution of species, especially where populations enjoy high connectivity, would seem to support the argument that connectivity increases the probability of disease transmission and possible extinction (Hess 1994, 1996). Isolated populations are still susceptible to disease, and without some connectivity among colonies, extirpated colonies cannot be recolonized following an epizootic (McCallum and Dobson 1995, 2002, Gog et al. 2002, Wagner et al. 2006). We have little information on the underlying disease dynamics of reservoir hosts and are unable to positively identify routes or sources of transmission among colonies. We can caution, however, that disease and other stressors may combine to thin and perhaps eradicate species where they were once common.
Our observations indicate that in areas where plague is present, highly connected complexes of large prairie dog colonies may actually be more susceptible to decline because the spread of plague is enhanced by increased connectivity among colonies. In these situations, effective conservation strategies may include the identification and monitoring of stepping stone colonies, which could be dusted for fleas, or eliminated in the presence of plague to reduce connectivity across large landscapes. This is the opposite of the usual perception that it is beneficial to population stability to maintain high levels of connectivity. Although fragmentation is often perceived as having a detrimental effect on populations, the disruption of dispersal of infectious animals among colonies, with the intent to control the spread of contagious disease, may be beneficial (Simberloff and Cox 1987, Saunders et al. 1991), especially with virulent exotic pathogens such as Y. pestis in North America. This argument assumes that plague is dispersed by prairie dogs. It may not apply if plague is carried between colonies by carnivores or raptors, which may be able to traverse long distances.
At the four plague sites, prairie dog abundance was less than at the plague-free sites. At least at Thunder Basin in 2001, we documented that abundance based on colony size distribution had the potential to duplicate than at the plague-free sites. At Comanche, colony area mapped in 1995 at 2186 ha was reduced by a plague epizootic during 1995–1996 (Augustine et al. 2008). Mapping did not resume there until 1999 when colony area was 799 ha, and subsequently grew at an annual rate of 40% to 6323 ha in 2005. (These figures are greater than reported by Augustine et al. [2008], because we include data from the Timpas Unit of the grassland which were not included in their analysis.) In the absence of the 2005 plague epizootic, it would have taken only one more year for colony distributions to nearly equal that observed in Thunder Basin in 2001. Although there may be differences in the distribution and quality of potential habitat among our sites, data from Thunder Basin and Comanche demonstrate that colony area could be substantially greater than what we observe in the presence of plague. The length of the epizootic interval—for which we have few data—appears to be a key variable governing the extent to which plague limits black-tailed prairie dog colony area below the potential carrying capacity.
Population regulation implies that populations are limited to a level below which they would attain in the absence of control, but are allowed to expand if the level drops below some threshold. Cully and Williams (2001) described prairie dog colony growth in the presence of plague at Cimarron during the 1990s when colonies were small and relatively isolated from one another. During 5 years without plague, 2001–2005, rapid colony growth occurred, and, when plague reappeared, total colony area declined from 2342 ha in 2005 to 541 ha in 2008 (Cimarron National Grassland, unpublished records). At Comanche, where colonies grew steadily from 1999 to 2005, plague returned in 2005 and reduced colony area during 2006–2008 from 6323 ha in 2005 to 1975 ha in 2008 (Comanche National Grassland, unpublished records). Plague had similar impacts at Kiowa/Rita Blanca and Thunder Basin when it appeared after an unknown interval of colony growth in the absence of plague. At the scale of the National Grasslands, it is clear that plague limits black-tailed prairie dog population size.
Black-tailed prairie dog colony spatial characteristics differed in areas with a history of plague from areas where plague has not been reported. We found that in areas with plague, mean colony sizes were smaller, the maximum sizes of colonies were smaller, and distances between colonies were greater. As a result, the proportion of potential habitat occupied by prairie dogs was less in areas with plague. These results indicate that prairie dogs are less abundant and may provide less benefit to dependent species in areas with plague. In contrast, because of the larger colonies and shorter nearest-neighbor distances outside the range of plague, these important areas may be at greater risk if plague does enter, perhaps as a result of changing climate.
Our results also indicate that plague may start by infecting small colonies and small numbers of colonies, as at Kiowa Rita Blanca in 2002–2003 and Cimarron 2005, before spreading more widely. With close monitoring of prairie dog colonies, early detection of plague may offer opportunities to intervene to slow or stop the epizootic by dusting burrows, application of vaccines to reduce susceptibility (when they become available), or perhaps other methods. Plague has devastating effects on prairie dogs, but the potential for management is improving.
Footnotes
Mention of trade marks or commercial products does not imply endorsement by the U.S. Government.
Acknowledgments
We thank David Augustine, Stephanie Shively, and Tom Peters—Comanche National Grassland; Andy Chappell, Geri Mason, and Joe Hartman— Cimarron National Grassland; Dan Garcia and Nancy Walls—Kiowa/Rita Blanca National Grassland; Tim Byer and Cristi Lockman—Thunder Basin National Grassland; Barbara Menchau and Dan Roddey—Wind Cave National Park; Doug Albertson, Greg Schroeder, and Brian Kenner—Badlands National Park and Buffalo Gap National Grassland; and especially the private landowners across our study sites for their cooperation. We thank Robert Marsh, John Kraft, and Justin Kretzer for field assistance, and Chris Frey and Bala Thiagarajan for their valuable comments. Clint Boal, the Cully/Sandercock lab group, Paul Stapp, Dean Biggins, and two anonymous reviewers of an earlier manuscript offered many valuable suggestions. Financial support for this research was provided by the National Center for Environmental Research, Science to Achieve Results Program of the United States Environmental Protection Agency (R82909101-0), the National Science Foundation and National Institutes of Health joint program in Ecology and Infectious Diseases (DEB-0224328), United States Geological Survey, United States Forest Service, Kansas Department of Wildlife and Parks, the Division of Biology at Kansas State University, and the Kansas Cooperative Fish and Wildlife Research Unit.
Disclosure Statement
No competing financial interests exist.
References
- Antolin MF. Gober P. Luce B. Biggins DE, et al. The influence of Sylvatic plague on North American wildlife at the landscape level with special emphasis on black-footed ferret and prairie dog conservation. Trans N Am Wildl Nat Resour Conf. 2002;67:104–127. [Google Scholar]
- Augustine DJ. Matchett MR. Toombs TP. Cully JF, Jr., et al. Spatiotemporal dynamics of black-tailed prairie dogs affected by plague. Landsc Ecol. 2008;23:255–267. [Google Scholar]
- Barnes AM. Surveillance and control of bubonic plague in the United States. Symp Zool Soc Lond. 1982;50:237–270. [Google Scholar]
- Barnes AM. A review of plague and its relevance to prairie dog populations and the black-footed ferret. In: Oldemeyer JL, editor; Biggins DE, editor; Miller BJ, editor. Management of Prairie Dog Complexes for the Reintroduction of the Black-Footed Ferret. Washington, DC: Biological Report 13; 1993. pp. 28–37. [Google Scholar]
- Channell R. Lomolino MV. Dynamic biogeography and conservation of endangered species. Nature. 2000;403:84–86. doi: 10.1038/47487. [DOI] [PubMed] [Google Scholar]
- Collinge SK. Johnson WC. Ray C. Matchett R, et al. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landsc Ecol. 2005;20:941–955. [Google Scholar]
- Cully JF., Jr. Barnes AM. Quan TJ. Maupin G. Dynamics of plague in a Gunnison's prairie dog complex from New Mexico. J Wildl Dis. 1997;33:706–719. doi: 10.7589/0090-3558-33.4.706. [DOI] [PubMed] [Google Scholar]
- Cully JF. and Johnson TL. Unpublished annual report: A summary of black-tailed prairie dog abundance and occurrence of sylvatic plague. On file at Comanche National Grassland, Springfield, Colorado. 2005:19p. [Google Scholar]
- Cully JF., Jr. Biggins DE. Seery DB. Conservation of prairie dogs in areas with plague. In: Hoogland JL, editor. Conservation of the Black-Tailed Prairie Dog. Washington, DC: Island Press; 2006. pp. 157–168. [Google Scholar]
- Cully JF., Jr. Carter LG. Gage KL. New records of sylvatic plague in Kansas. J Wildl Dis. 2000;36:389–392. doi: 10.7589/0090-3558-36.2.389. [DOI] [PubMed] [Google Scholar]
- Cully JF., Jr. Williams ES. Interspecific comparisons of sylvatic plague in prairie dogs. J Mammal. 2001;82:894–905. [Google Scholar]
- Desmond MJ. Savidge JA. Eskridge KM. Correlations between burrowing owl and black-tailed prairie dog declines: a 7-year analysis. J Wildl Manag. 2000;64:1067–1075. [Google Scholar]
- Ecke DH. Johnson CW. Plague in Colorado and Texas. Public Health Serv Monogr. 1952;6:1–37. [PubMed] [Google Scholar]
- Gage KL. and Kosoy MY. Natural history of the plague: Perspectives from more than a century of research. Annual Review of Entomology. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- Gog J. Woodroffe R. Swinton J. Disease in endangered metapopulations: the importance of alternative hosts. Proc R Soc Lond Biol Sci Ser. 2002;269:671–676. doi: 10.1098/rspb.2001.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess GR. Conservation corridors and contagious disease: a cautionary note. Conserv Biol. 1994;8:256–262. [Google Scholar]
- Hess GR. Disease in metapopulation models: implications for conservation. Ecology. 1996;77:1617–1632. [Google Scholar]
- Hoogland JL. The black-tailed prairie dog. University of Chicago Press; Chicago, IL: 1995. [Google Scholar]
- Lockhart M. Marinari P. Gober P. Ferrets home on the range. Endangered Spec Bull. 2000;25:16–17. [Google Scholar]
- Lomolino MV. Channell R. Splendid isolation: patterns of geographic range collapse in endangered mammals. J Mammal. 1995;76:335–347. [Google Scholar]
- Lomolino MV. Smith GA. Dynamic biogeography of prairie dog (Cynomys ludovicianus) towns near the edge of their range. J Mammal. 2001;82:937–945. [Google Scholar]
- McCallum HI. Dobson AP. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- McCallum HI. Dobson AP. Disease, habitat fragmentation and conservation. Proc R Soc Lond. 2002;269:2041–2049. doi: 10.1098/rspb.2002.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD. Cully JF., Jr. Conservation of black-tailed prairie dogs (Cynomys ludovicianus) J Mammal. 2001;82:889–893. [Google Scholar]
- Pauli JN. Buskirk SW. Risk-disturbance overrides density dependence in a hunted colonial rodent, the black-tailed prairie dog Cynomys ludovicianus. J Appl Ecol. 2007;44:1219–1230. [Google Scholar]
- Pauli JN. Buskirk SW. Williams ES. Edwards WH. A plague epizootic in the black-tailed prairie dog. J Wildl Dis. 2006;42:74–80. doi: 10.7589/0090-3558-42.1.74. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Reeve AF.Vosburgh TC.Recreational shooting of prairie dogsHoogland, JH,Conservation of the Black-Tailed Prairie Dog Washington, DC: Island Press; 2006139–156. [Google Scholar]
- Saunders DA. Hobbs RJ. Margules CR. Biological consequences of ecosystem fragmentation: a review. Conserv Biol. 1991;5:18–32. [Google Scholar]
- Simberloff D. Cox J. Consequences and costs of conservation corridors. Conserv Biol. 1987;1:63–71. [Google Scholar]
- Snäll T. O'Hara RB. Ray C. Collinge SK. Climate driven spatial dynamics of plague among prairie dog colonies. Am Nat. 2008;171:238–248. doi: 10.1086/525051. [DOI] [PubMed] [Google Scholar]
- Stokes ME. Davis CS. Koch GG. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute; 1995. [Google Scholar]
- Thiagarajan B. Bai Y. Gage KL. Cully JF., Jr. Prevelance of Yersinia pestis in rodents and fleas associated with black-tailed prairie dogs (Cynomys ludovicianus) at Thunder Basin National Grassland, Wyoming. J Wildl Dis. 2008;44:731–736. doi: 10.7589/0090-3558-44.3.731. [DOI] [PubMed] [Google Scholar]
- [USDA] United States Department of Agriculture Forest Service. Supplemental Information Report to the proposed northern Great Plains plans revision Final Environmental Impact Statement and 2001 revision Thunder Basin National Grassland Plan. 2002. www.fs.fed.us/r2/mbr/projects/forestplans/thunderbasin/pdfdoc/supplements/prairie_dog_SIR_Jan_14_02.pdf www.fs.fed.us/r2/mbr/projects/forestplans/thunderbasin/pdfdoc/supplements/prairie_dog_SIR_Jan_14_02.pdf
- Wagner DM. Drickamer LC. Krpata DM. Allender CJ, et al. Persistence of Gunnison's prairie dog colonies in Arizona. Biol Conserv. 2006;130:331–339. [Google Scholar]