Abstract
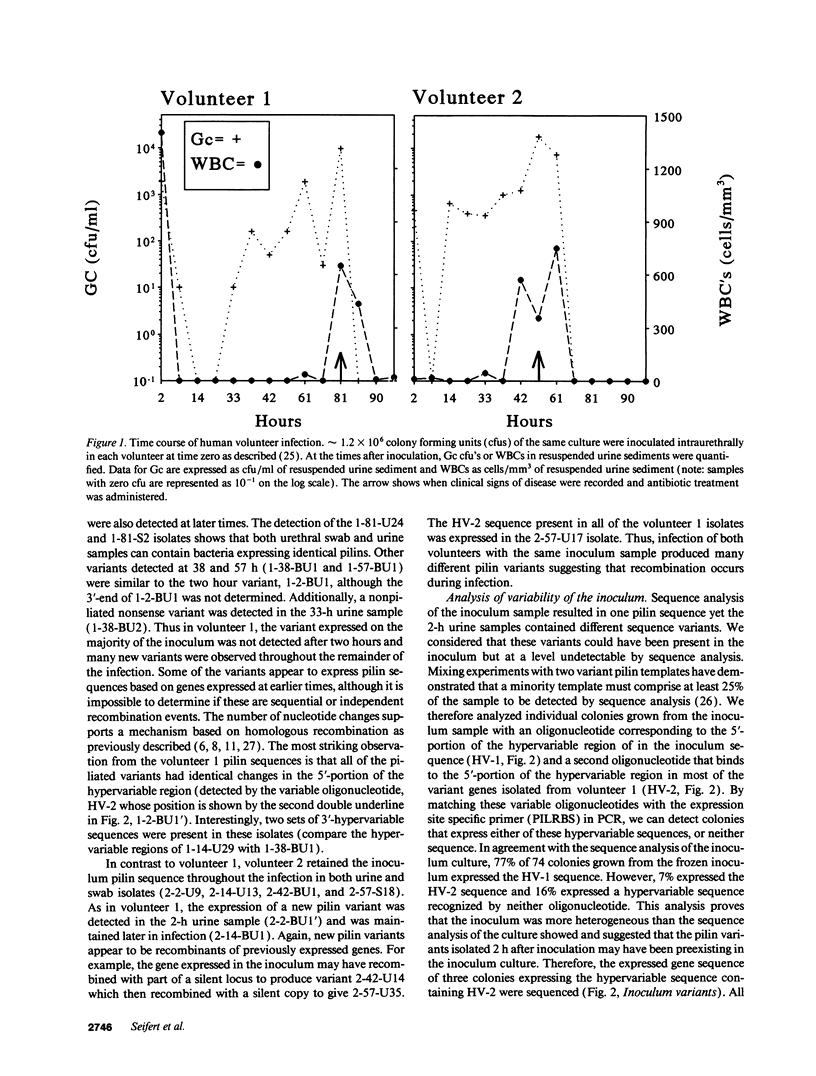
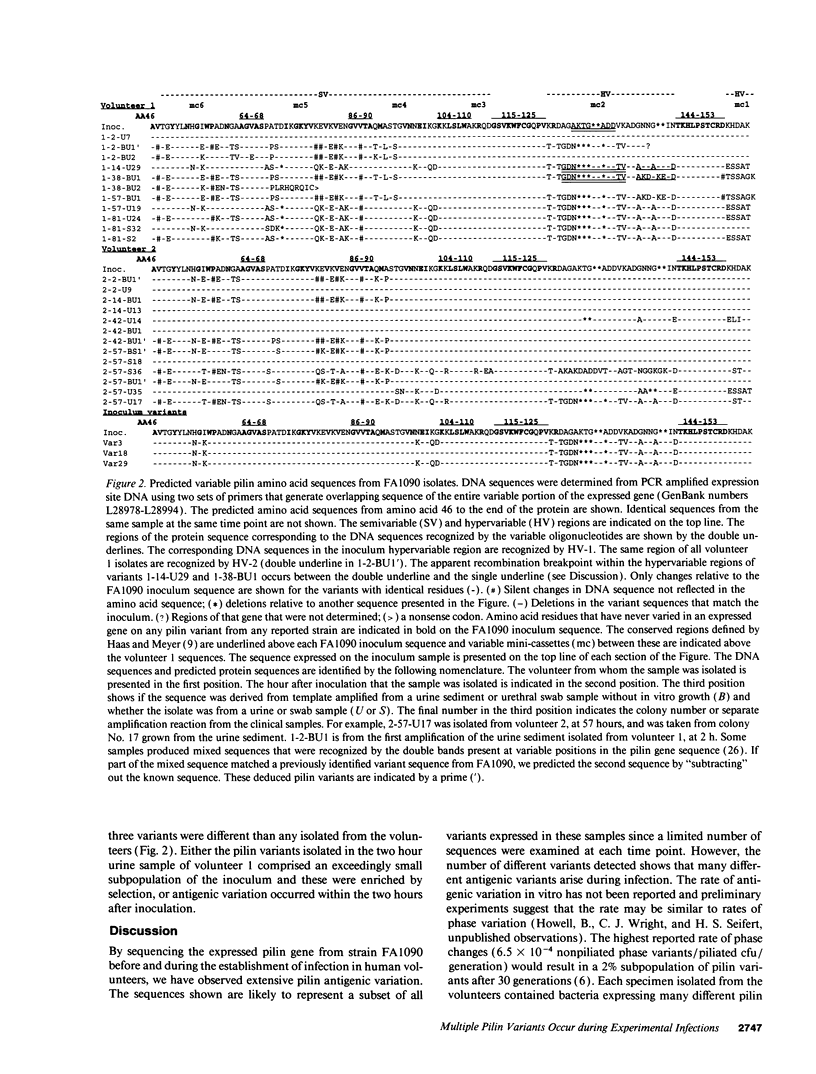
Gonococcal pilin variation is thought to allow immune evasion and change the adherence properties of the pilus. We have examined the process of pilin antigenic variation in human volunteers inoculated with strain FA1090. Our data show that pilin variation occurred throughout the process of infection, that at each time sampled after inoculation multiple pilin variants were present, and that later pilin variants appear to be recombinants between previously expressed genes and the silent storage pilin copies. Thus, during infection a large repertoire of proteins are available to the population to help avoid immune responses, to provide pili with varying functions, and to transmit to a new host.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström S., Robbins K., Koomey J. M., Swanson J. Piliation control mechanisms in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3890–3894. doi: 10.1073/pnas.83.11.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Cannon J. G., Jerse A. E., Charniga L. M., Isbey S. F., Whicker L. G. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994 Mar;169(3):532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- Gibbs C. P., Reimann B. Y., Schultz E., Kaufmann A., Haas R., Meyer T. F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989 Apr 20;338(6217):651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- Haas R., Meyer T. F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986 Jan 17;44(1):107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- Haas R., Schwarz H., Meyer T. F. Release of soluble pilin antigen coupled with gene conversion in Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9079–9083. doi: 10.1073/pnas.84.24.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Veit S., Meyer T. F. Silent pilin genes of Neisseria gonorrhoeae MS11 and the occurrence of related hypervariant sequences among other gonococcal isolates. Mol Microbiol. 1992 Jan;6(2):197–208. doi: 10.1111/j.1365-2958.1992.tb02001.x. [DOI] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Taylor-Robinson D., McGee Z. A. Species specificity of attachment and damage to oviduct mucosa by Neisseria gonorrhoeae. Infect Immun. 1977 Dec;18(3):833–839. doi: 10.1128/iai.18.3.833-839.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. C., Chung R. C., Deal C. D., Boslego J. W., Sadoff J. C., Wood S. W., Brinton C. C., Jr, Tramont E. C. Human immunization with Pgh 3-2 gonococcal pilus results in cross-reactive antibody to the cyanogen bromide fragment-2 of pilin. J Infect Dis. 1991 Jan;163(1):128–134. doi: 10.1093/infdis/163.1.128. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey M., Gotschlich E. C., Robbins K., Bergström S., Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987 Nov;117(3):391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., Watt P. J. Effect of anti-pilus antibodies on survival of gonococci within guinea pig subcutaneous chambers. Infect Immun. 1982 Oct;38(1):27–30. doi: 10.1128/iai.38.1.27-30.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Melly M. A., Gregg C. R., McGee Z. A. Studies of toxicity of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):423–431. doi: 10.1093/infdis/143.3.423. [DOI] [PubMed] [Google Scholar]
- Nicolson I. J., Perry A. C., Virji M., Heckels J. E., Saunders J. R. Localization of antibody-binding sites by sequence analysis of cloned pilin genes from Neisseria gonorrhoeae. J Gen Microbiol. 1987 Apr;133(4):825–833. doi: 10.1099/00221287-133-4-825. [DOI] [PubMed] [Google Scholar]
- Norlander L., Davies J., Norqvist A., Normark S. Genetic basis for colonial variation in Neisseria gonorrhoeae. J Bacteriol. 1979 Jun;138(3):762–769. doi: 10.1128/jb.138.3.762-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T., van Putten J. P., Gibbs C. P., Haas R., Meyer T. F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992 Nov;6(22):3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Segal E., Hagblom P., Seifert H. S., So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Ajioka R. S., Marchal C., Sparling P. F., So M. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature. 1988 Nov 24;336(6197):392–395. doi: 10.1038/336392a0. [DOI] [PubMed] [Google Scholar]
- Stephens D. S. Gonococcal and meningococcal pathogenesis as defined by human cell, cell culture, and organ culture assays. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S104–S111. doi: 10.1128/cmr.2.suppl.s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A., Melly M. A., Hoffman L. H., Gregg C. R. Attachment of pathogenic Neisseria to human mucosal surfaces: role in pathogenesis. Infection. 1982;10(3):192–195. doi: 10.1007/BF01640777. [DOI] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Barrera O., Robbins K., Corwin D. Pilus- gonococcal variants. Evidence for multiple forms of piliation control. J Exp Med. 1985 Aug 1;162(2):729–744. doi: 10.1084/jem.162.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Morrison S., Barrera O., Hill S. Piliation changes in transformation-defective gonococci. J Exp Med. 1990 Jun 1;171(6):2131–2139. doi: 10.1084/jem.171.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Corwin D., Boslego J., Ciak J., Blake M., Koomey J. M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987 May 1;165(5):1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Hodge W. C., Gilbreath M. J., Ciak J. Differences in attachment antigens of gonococci in reinfection. J Lab Clin Med. 1979 May;93(5):730–735. [PubMed] [Google Scholar]
- Virji M., Everson J. S., Lambden P. R. Effect of anti-pilus antisera on virulence of variants of Neisseria gonorrhoeae for cultured epithelial cells. J Gen Microbiol. 1982 May;128(5):1095–1100. doi: 10.1099/00221287-128-5-1095. [DOI] [PubMed] [Google Scholar]
- Wright C. J., Jerse A. E., Cohen M. S., Cannon J. G., Seifert H. S. Nonrepresentative PCR amplification of variable gene sequences in clinical specimens containing dilute, complex mixtures of microorganisms. J Clin Microbiol. 1994 Feb;32(2):464–468. doi: 10.1128/jcm.32.2.464-468.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]



