Abstract
Skeletal muscle–derived stem cells (MDSCs) are able to differentiate into cardiomyocytes (CMs). However, it remains to be investigated whether differentiated CMs contract similar to native CMs. Here, we developed a three-dimensional collagen gel bioreactor (3DGB) that induces a working CM phenotype from MDSCs, and the contractile properties are directly measured as an engineered cardiac tissue. Neonate rat MDSCs were isolated from hind-leg muscles via the preplate technique. Isolated MDSCs were approximately 60% positive to Sca-1 and negative to CD34, CD45, or c-kit antigens. We sorted Sca-1(−) MDSCs and constructed MDSC-3DGBs by mixing MDSCs with acid soluble rat tail collagen type-I and matrix factors. MDSC-3DGB exhibited spontaneous cyclic contraction by culture day 7. MDSC-3DGB expressed cardiac-specific genes and proteins. Histological assessment revealed that cardiac-specific troponin-T and -I expressed in a typical striation pattern and connexin-43 was expressed similar to the native fetal ventricular papillary muscle. β-Adrenergic stimulation increased MDSC-3DGB spontaneous beat frequency. MDSC-3DGB generated contractile force and intracellular calcium ion transients similar to engineered cardiac tissue from native cardiac cells. Results suggest that MDSC-3DGB induces a working CM phenotype in MDSCs and is a useful 3D culture system to directly assess the contractile properties of differentiated CMs in vitro.
Introduction
Limited cardiomyocyte (CM) proliferative capacity is a major barrier to myocardial regeneration and the restoration of contractile function of injured postnatal myocardium. Various strategies to restore postnatal CM proliferation and myocardial regeneration are under investigation, including strategies for the repair and regeneration of damaged myocardium using allogeneic and autologous cell and tissue grafts.1 Studies suggest that fetal, finitely proliferating CMs display the best cell survival, functional integration, and sustained cardiac recovery, and thus could be an optimal cell type for cardiac repair.2,3 However, the use of fetal heart cells is contraindicated for clinical use. Stem cells provide an alternative solution, and a range of cell types have been employed in cellular cardiomyoplasty strategies, including bone marrow–derived stromal and stem cells, fibroblasts, skeletal myoblasts, mesenchymal stem cells, embryonic stem cells, and resident cardiac stem cells.4 However, despite some promising results,5 the rate of CM differentiation from transplanted stem cells remains insufficient to fully recover the recipient myocardial function.2,4, 6–8 Therefore, a preferred strategy for cellular cardiomyoplasty might be the delivery of progenitor/stem cell–derived CMs, rather than undifferentiated cells, into injured myocardial tissue.9
Skeletal muscle–derived stem cells (MDSCs) are a somatic stem cell population obtained from skeletal muscle specimens in animals and humans that can be readily expanded in vitro and then transplanted as an autologous graft.8,10,11 MDSCs are multipotent and have been shown to differentiate along skeletal and smooth muscle, bone, tendon, nerve, endothelial, and hematopoietic lineages.10,12,13 Previous studies, including our own work, have shown that MDSCs, isolated using variations of a modified preplate technique, can differentiate into CMs or cells with cardiac phenotypes and can facilitate cardiac repair.8,14–20 However, none of the previous studies investigated whether differentiated MDSC-derived CMs generate contractile force similar to the native CMs. In addition, undifferentiated MDSCs or MDSCs preconditioned with chemical reagents were used to evaluate in vivo CM differentiation from MDSCs, not transplanted MDSC-derived CMs in animal models of injured myocardium.1,5 Our recent studies have also shown that the rate of CM differentiation of transplanted MDSCs within acute myocardial infarction model is not sufficient to replace injured CMs, and improvement of recipient cardiac function by MDSC transplantation is due to combined effects of myogenic differentiation of transplanted MDSC, angiogenesis, stimulation of recipient CM proliferation, and reduction of recipient CM apoptosis.8,20 Therefore, it remains unclear whether differentiated CM phenotypic cells replace recipient dead CMs and have contractile function that improves recipient cardiac function.
The effects of various biomechanical stimuli on multipotent stem cells have been investigated to elucidate their roles in CM induction and differentiation. Previous studies have shown that cell–cell interactions and specific culture conditions are often necessary prerequisites for efficient CM differentiation.21–23 The culture of multipotent stem cells in aggregate spheres has been shown to facilitate cell–cell coupling, increase differentiation capacity, modify cellular metabolism, modulate the response to therapeutic agents,23–25 and stimulate the synthesis and release of extracellular matrix (ECM) constituents.26 Studies also suggest that three-dimensional (3D) in vitro culture conditions may be optimal for donor CM preparation,2,3,27 and that tissue-engineered cardiac tissue constructs provide the requisite 3D environment for efficient cell survival, functional integration, and sustained cardiac recovery.27,28
Thus, the objective of the present study was to develop a culture method that induces CM differentiation from MDSCs and facilitates myocardial tissue formation that enables us to directly evaluate the contractile properties of MDSC-derived CMs in vitro. We tested the hypothesis that the combined MDSC-aggregate formation and 3D collagen gel bioreactor can induce MDSCs to differentiate into cells with a CM phenotype and that the differentiated CMs form a working 3D cardiac like tissue in vitro. We determined that MDSC-aggregate formation followed by 3D collagen gel bioreactor (3DGB) culture succeeded in generating cells with an immature CM phenotype mimicking the native fetal myocardium. Thus, our results suggest that MDSC-3DGB is a useful 3D culture system to directly assess the contractile properties of differentiated CMs from MDSCs in vitro.
Materials and Methods
MDSC isolation
MDSCs were isolated from neonatal Lewis rat hind-leg muscles using an established preplate technique.10–12,29–31 Briefly, five postnatal day-3 rat pups were euthanized with 5% isoflurane anesthesia followed by cervical truncation. Gastrocnemius muscles were excised, minced in HBSS, and enzymatically digested. Briefly, tissue slurry underwent sequential incubation of collagnease XI (0.2% collagenase XI), dispase (2.4 U/mL), and 2 × trypsin. Isolated cells then underwent sequential preplating (24-h interval between each preplate) until the sixth preplate phase31 in standard MDSC growth medium containing high-glucose Dulbecco's modified Eagle's medium w/l-glutamine (Invitrogen, Carlsbad, CA), 10% horse serum (Invitrogen), 10% fetal bovine serum (FBS; Invitrogen), 0.5% chick embryo extract (US Biological, Swampscott, MA), and 1% antibiotic–antimycotic solution (Invitrogen).26,31,32 Our research protocol followed the National Institutes of Health (NIH) guidelines for animal care and was approved by the University of Pittsburgh's Institutional Animal Care and Use Committee and the Children's Hospital of Pittsburgh Animal Research Care Committee.
Isolated rat MDSCs were passaged 15 times and expanded to obtain enough cell number (at least 20 million cells per mL).12,29 Fluorescent-activated cell sorting (FACS, FACS Aria; BD Biosciences, San Jose, CA) revealed that cultured rat MDSCs were approximately 60% positive to Sca-1 antigen (BD Biosciences), and negative to CD34 (BD Biosciences), CD45 (BD Biosciences), and c-kit (BD Biosciences) antigens (Supplemental Fig. S1, available online at www.liebertonline.com/ten; Table 1). Zuba-Surma et al. have shown that Sca-1(−) skeletal muscle stem cells are “inherently predisposed to undergo cardiac differentiation.15” Thus, we sorted Sca-1(−) MDSCs that were expanded in standard two-dimensional (2D) flasks for 48 h to reach a cell number of 3 million. These Sca-1(−) MDSCs maintained no Sca-1 expression throughout culture (Supplemental Fig. S2, available online at www.liebertonline.com).
Table 1.
Fluorescent-Activated Cell Sorting Analysis of Unsorted Muscle-Derived Stem Cells and Muscle-Derived Stem Cell-3DGB
| % Positive | |
|---|---|
| MDSCs | |
| Sca-1 | 57.3 ± 2.8 (n = 4) |
| CD34 | 0.1 ± 0.0 (n = 4) |
| CD45 | 0.0 ± 0.0 (n = 2) |
| c-kit | 0.1 ± 0.1 (n = 2) |
| MDSC-3DGB | |
| cTn-T | 18.6 ± 2.5 (n = 3) |
Data are mean ± standard error.
n, number of FACS sets. For MDSCs, 10 million cells/set and for MDSC-3DGB, 6 MDSC-3DGBs/set.
FACS, fluorescent-activated cell sorting; MDSC, muscle-derived stem cells; cTn-T, cardiac troponin-T; 3DGB, 3D collagen gel bioreactor.
MDSC-3D collagen gel bioreactor (MDSC-3DGB) construction
Expanded MDSCs on the 2D flask were trypsinized using a 0.05% trypsin–EDTA solution (Invitrogen), and the cell suspension was cultured on a 100-mm-diameter suspension culture dish (Corning, Lowell, MA) for 24 h at 37°C using a gyrating shaker (50 rotations/min) to form 50- to 70-μm-diameter MDSC aggregates (MDSC-aggregate, 330–350 cells/aggregate) under standard MDSC growth medium. Acid-soluble rat tail collagen type-I solution (pH 3; Sigma, St. Louis, MO) was neutralized with alkali buffer (0.2 M NaHCO3, 0.2 M HEPES, and 0.1 M NaOH) on ice. Matrigel (13% of total volume; BD Biosciences) was then added, and the cell suspension and matrix solution mixed to reach a final collagen type-I concentration of 0.67 mg/mL. Approximately 200 μL of the cell/matrix mixture was poured into the 20-mm-long × 2-mm-wide cylindrical cast of a Flexcell Tissue Train collagen type-I–coated silicone membrane culture plate (FX4000TT; Flexcell International, Hillsborough, NC) and incubated for 120 min (37°C, 5% CO2)32 to form a cylindrical MDSC-3DGB. Each MDSC-3DGB was cultured in a 5% FBS containing growth medium. We compared the efficiency of CM induction from culture day 7 MDSC-3DGBs to (1) freshly formed MDSC-aggregates after 24 h rotation culture in standard culture medium; (2) two-dimensional MDSC culture (2D-MDSC) at culture day 7 on rat tail collagen type-I (Sigma)–coated tissue culture plates (Corning) with 5% FBS and Matrigel (17% of total volume, a concentration equivalent to 3D culture, dissolved in cell suspension at the beginning of culture; BD Biosciences); or (3) three-dimensional MDSC culture (3D-MDSC) at culture day 7 in which the MDSCs were embedded into collagen gel without MDSC-aggregate formation. We also constructed engineered cardiac tissue from gestational day 14 fetal cardiac cells (EFCT) or neonatal day 1 cardiac tissue (ENCT) to investigate whether MDSC-3DGB contractile properties mimic engineered cardiac tissue from native immature cardiac cells. For EFCT construction, pregnant mothers were anesthetized using 3% isoflurane inhalation with 100% oxygen and hysterectomy was performed. Immediately after hysterectomy, the mother was euthanized by induced asystole under 5% isoflurane anesthesia. The excised uteri were transferred to a sterilized Petri dish filled with cold phosphate-buffered saline (PBS) buffer and 1% antibiotic–antimycotic solution (Invitrogen), the fetuses were excised by hysterotomy, and the fetal hearts were harvested. Isolated cells were preplated for 1 h and then cultured on a gyratory shaker (50 rotations/min) for 24 h to reaggregate viable CMs for the cell suspension for 3D construction. For ENCT construction, neonatal day 1 rat pups were euthanized by cervical truncation under 5% inhaled isoflurane with 100% oxygen, and the ventricular tissue was excised and pooled. Great vessels and atrium were removed from each heart, and ventricular tissue was collected and pooled. Pooled ventricles were then enzymatically digested by 2 mg/mL of collagenase type-II followed by 0.05% trypsin–EDTA solution (Invitrogen). Isolated cells were preplated for 1 h and then cultured on a gyratory shaker (50 rotations/min) for 24 h to reaggregate viable CMs for the cell suspension for each construct. Engineered cardiac tissue construction was the same as MDSC-3DGB, and the constructed EFCT or ENCT was cultured with 10% FBS containing growth medium for 7 days.
Real-time–polymerase chain reaction
Total RNA was prepared using Trizol solution (Invitrogen) and treated with TURBO DNA-free kit (Ambion, Austin, TX). A cardiac α-actin primer was designed using Primer-3 (forward 5′–3′ GCCCTGGATTTTGAGAATGA; reverse 5′–3′ CCTTTTGCATACGATCAGCA; product size of 289 bp). Other primers whose target genes were Nkx2.5, GATA4, α- and β-cardiac myosin heavy chains (MHCs), and connexin-43 (Cx-43) were obtained from Qiagen (Valencia, CA) Quanti-Tect Primer Assay with the target fragment sizes approximately 100 bp. One step real-time (RT) was performed with a total volume of 1 μg RNA in a total volume of 25 μL that used MuLy (Roches, Pleasanton, CA) with the following program: 42°C 15 min, 99°C 5 min, 5°C 5 min, one cycle. cDNA (1 μL) was used for polymerase chain reaction (PCR) that used the following program: 94°C 2 min, 95°C 50 s, 58°C 30 s, 72°C 1 min, 35 cycles 72°C 7-min extension. For normalization of RT-PCR results, β-actin was used as an internal control. All PCR products were confirmed by University of Pittsburgh DNA Sequence Core Facilities, performed by Eppendorf Mastercycles. All RT-PCR assays were completed in triplicate (total n = 18 MDSC-3DGBs).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
Whole cell lysates were prepared from native adult and gestational day 20 fetal hearts (n = 6) and gastrocnemius muscle tissue (n = 6), MDSC-3DGB tissue (n = 18), 3D-MDSC (n = 18), and MDSC-aggregate (n = 6 culture plates) pooled populations, and separated by sodium dodecyl sulfate–-polyacrylamide gel electrophoresis (7.5% separating gel; Bio-Rad Laboratories, Hercules, CA). Immunoblotting was carried out using routine protocols. Each lane contained 20 μg of total protein. Mouse monoclonal β-actin antibody (Abcam, Cambridge, MA), mouse monoclonal cardiac troponin-T (cTn-T; Abcam), mouse monoclonal anti-Cx-43 (Abcam), and mouse monoclonal cardiac troponin-I (cTn-I; Abcam) were observed with IR-Dye 800 donkey anti-mouse secondary antibody (Rockland Immunochemicals, Gilbertsville, PA). All proteins were observed using an infrared Western blot imaging system (Odyssey; LI-COR Biosciences, Lincoln, NE). Immunoblots were performed in triplicate and quantified using densitometry and an expression ratio was calculated (Odyssey, LI-COR Biosciences).
Confocal microscopy
Three-dimensional tissue constructs and fetal ventricular samples were fixed with 4% paraformaldehyde/PBS for 15 min and embedded in the 13% polyacrylamide gel. One hundred and fifty-micrometer-thick sections were made using a vibratory microtome (Vibratome-1000; Vibrotome.com, St. Louis, MO).32 2D-MDSC samples were fixed with 4% paraformaldehyde/PBS for 5 min. Sections or 2D-MDSC samples were permeabilized with 0.1% Triton X-100 for 30 or 5 min, respectively, and stained for mouse monoclonal anti-cTn-T (Abcam), cTn-I (Abcam), α-sarcomeric actinin (Sigma), or Cx-43 (Abcam) primary antibodies and Alexa Fluor 488, Alexa Fluor 647, or Alexa Fluor 594 secondary antibodies (Invitrogen). We reconstructed 3D projection images from stacks of z-axis optical scans using a standard laser confocal microscopy system (FV1000; Olympus, Tokyo, Japan) and Scion Image software (Scion, Frederick, MD).32 The composite 3D projection images were further processed using Adobe Photoshop software (Adobe, San Jose, CA).
Spontaneous beating activity
Culture day 7 MDSC-3DGBs (n = 8) were imaged at two different regions/construct using a digital video microscopy system and Scion Image Software with a CG-7 frame-grabber board (Scion) to determine baseline spontaneous beat frequency. MDSC-3DGBs were then treated with 2 μM of the nonselective β-adrenergic receptor agonist, isoproterenol (ISP), or 1 mM of the nonselective sodium and calcium ion channel inhibitor, cadmium chloride. Five minutes after treatment, MDSC-3DGBs were imaged again, and were then incubated with fresh growth media for 15 min and re-imaged to determine a posttreatment baseline.
Mechanical testing
The passive and active force of MDSC-3DGB (n = 7), 3D-MDSC (n = 6), and EFCT (n = 7) constructs was measured as previously described.32 In brief, each construct was transferred from the Flexcell culture dish to the perfusion chamber of the muscle testing station containing a cold (25°C) calcium-free Ringer solution composed of (in mM): 135 NaCl, 4.0 KCl, 10 Trizma-HCl, 8.3 Trizma-base, and 11.0 glucose, and gassed with 95% O2/5% CO2 (pH 7.4). One end of the construct was attached to a force transducer (model 403A; Aurora Scientific, Aurora, Canada) and the other end to a length controller mounted on a micromanipulator using 10-0 mono-filament nylon sutures. The buffer within the perfusion chamber (1.5 mL total chamber volume) was then replaced with a warmed Ringer solution buffer (37°C, containing 2 mM Ca2+) and perfused at a rate of 1 mL/min. The construct was field-stimulated (1 Hz, 4 ms, 50–100 V, rectangular pulses) using a stimulator (Harvard Apparatus, Holliston, MA). The longitudinal length of the construct was increased in 5% increments up to a 15% elongation from original length (L0.15). The external diameters of the construct were recorded at each stretch increment using a digital video microscopy system (Model KPD-50; Hitachi, Tokyo, Japan and Scion Image Software with a CG-7 frame-grabber board; Scion).
Intracellular free calcium ion transient recording
MDSC-3DGBs (n = 3) or ENCTs (n = 5) were loaded for 30 min at 25°C with Fura 2-AM (Invitrogen) at a final concentration of 5 μM within a custom-built muscle chamber equipped for the simultaneous measurement of force (force transducer 403A, high-speed length controller model 22C and 322C, digital control system Series 600A; Aurora Scientific) and intracellular free calcium ([Ca2+]i) recording using fluorescent probes and IonOptix hardware and software (IonOptix, Milton, MA). The muscle chamber was perfused with 37°C Ringer solution at a rate of 1 mL/min. MDSC-3DGBs were field-stimulated (1 Hz, 5 ms, 50 V; Harvard Apparatus), and Fura-2 fluorescence was recorded at a sampling rate of 100 Hz by alternately illuminating the preparation with light of 340- and 380-nm wavelength while measuring fluorescence at 510 nm. Acquired data were stored for offline analysis. To characterize [Ca2+]i transients, the maximal ratio (F340/F380) and minimal ratio (F340/F380) for 10 successive transients were calculated and averaged. We also determined [Ca2+]i transients at L0.15, and pacing frequencies of 1 to 6 Hz.
Statistical analysis
Data are expressed as mean ± standard error. One-factor analysis of variance was used to compare the protein analysis and spontaneous beat frequency among experimental groups. Two-factor repeated analysis of variance was performed to compare the active stress–length relations among experimental groups. We performed a Tukey post hoc test to determine individual differences between experimental groups. Statistical significance was defined by a value of p < 0.05. All calculations were performed using SigmaStat (Systat Software, Point Richmond, CA).
Results
CM phenotypic cell differentiation within MDSC-3DGB
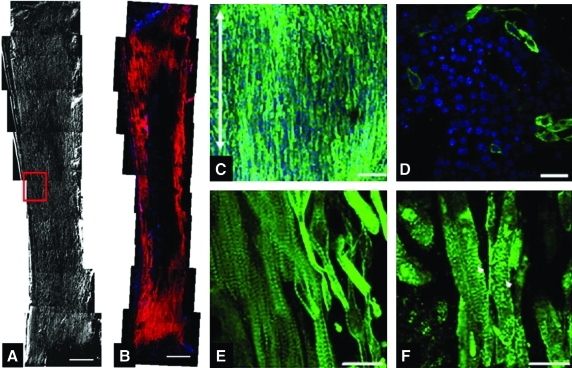
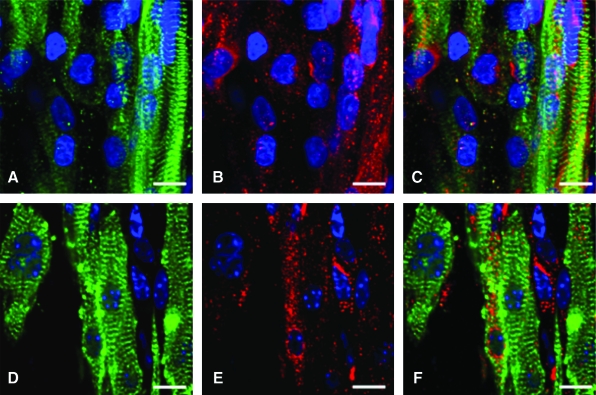
Before embedding MDSC-aggregates or MDSCs in 3D culture, we assessed the cellular proliferation activity of MDSC-aggregates. MDSC-aggregate formation significantly decreased MDSC cellular proliferation (16.6 ± 4.5% [n = 3 experimental sets, p < 0.05]) versus standard 2D culture (36.1 ± 2.8% [n = 3]) (Supplemental Fig. S3, available online at www.liebertonline.com). When MDSC-aggregates were cultured under standard 2D conditions, MDSC-aggregates attached to the culture dish bottom within an hour and MDSC-aggregates had completely disappeared and MDSCs were randomly oriented similar to standard 2D-MDSC culture at 24 h. MDSC-aggregates expanded within each 3DGB and the majority of cells aligned along the construct longitudinal axis forming a muscle-like tissue (Fig. 1A), and the cells were noted to spontaneously beat by culture day 5, which was observed in all MDSC-3DGBs. The cells within 3D-MDSC (without MDSC-aggregate formation) expanded along the construct longitudinal axis by culture day 3, which was faster than MDSC-aggregates placed in 3DGB. However, we noted a lower incidence of spontaneous beating cells at culture day 7 within the 3D-MDSC constructs (6 of 36 constructs or 17%), which was substantially lower than the spontaneous beating rate of MDSC-3DGB constructs (48 of 48 constructs or 100%, p < 0.05 by Fisher exact test). Synchronous tissue contraction of MDSC-3DGB was observed by culture day 7 (Supplemental Movie, available online at www.liebertonline.com). Histological assessment revealed that MDSC-3DGB contained cells with cardiac-specific protein cTn-T organized in a striated pattern (Fig. 1C), whereas the cTn-T–positive cells in 2D-MDSC were not organized in a clear striated pattern (Fig. 1D). MDSC-3DGB also expressed the cardiac-specific protein cTn-I organized in a striated pattern similar to cTn-T (Fig. 1E, F), whereas cTn-I was negative in 2D-MDSC. FACS analysis based on cTn-T expression revealed that the fraction of cTn-T–positive cells from culture day 7 MDSC-3DGB was 18.6 ± 2.5% (from six MDSC-3DGBs in each FACS analysis, three independent sets of pooled MDSC-3DGBs) (Supplemental Fig. S4, available online at www.liebertonline.com and Table 1). Cx-43 was expressed in cTn-T–positive cells within MDSC-3DGB, and the Cx-43 expression pattern of cTn-T–positive cells was not typical to mature adult CMs, but was similar to CMs of gestational day 20 native fetal left ventricular papillary muscle (Fig. 2).
FIG. 1.
Histologic analysis of MDSC-3D collagen gel bioreactor (3DGB). (A) Phase contract image of culture day 7 MDSC-3DGB. Scale bar indicates 500 μm. Red box indicates the area of the tissue where high-magnification images are taken. (B) α-Sarcomeric actinin expression in MDSC-3DGB. Scale bar indicates 500 μm. (C) Cardiac troponin-T (cTn-T) expressed in oriented cells of MDSC-3DGB. Blue staining (DAPI) indicates nuclei. Scale bar indicates 50 μm. The white double-head arrow indicates MDSC-3DGB longitudinal axis. (D) cTn-T expression of MDSC-2D. Scale bar indicates 20 μm. (E) cTn-T expression of MDSC-3DGB was a typical striated muscle pattern at a higher magnification. Scale bar indicates 20 μm. (F) Cardiac troponin-I (cTn-I) also expressed in a typical striated pattern (white arrowheads). Scale bar indicates 20 μm. 2D, two-dimensional; MDSC, muscle-derived stem cell; DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertonline.com/ten.
FIG. 2.
Native fetal left ventricular papillary muscle at gestational day 20 stained for (A) α-sarcomeric actinin (green). (B) Gap junction protein Cx-43 (red), and (C) merged. Culture day 7 MDSC-3DGB stained for (D) α-sarcomeric actinin (green). (E) Gap junction protein Cx-43 (red), and (F) merged. Cx-43 expression of MDSC-3DGB was similar to gestational day 20 fetal left ventricular papillary muscle Cx-43 expression. Blue staining (DAPI) indicates nuclei. Scale bar indicates 10 μm. Cx-43, connexin-43. Color images available online at www.liebertonline.com/ten.
Cardiac-specific gene and protein expression
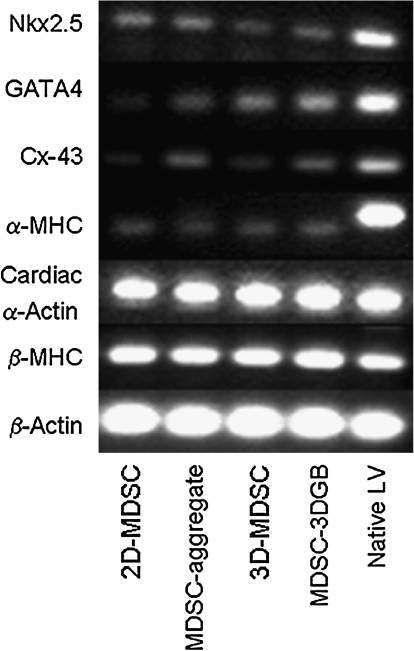
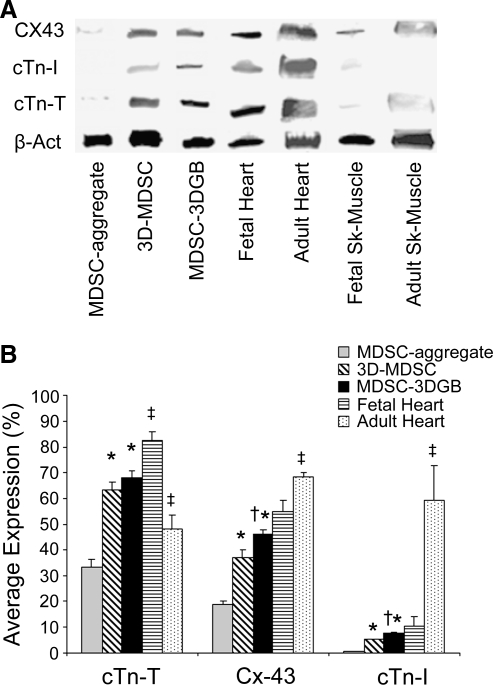
Cardiac-specific genes were expressed in culture day 7 MDSCs, regardless of the culture condition (Fig. 3), suggesting that 7 days of culture can trigger cardiac gene expression of rat MDSCs. Western blots showed that both MDSC-aggregates and 3D-MDSC and MDSC-3DGB groups expressed cTn-T and Cx-43 proteins (Fig. 4A). Native tissue protein expression shows that developmental stage alters cardiac-specific protein expression in both heart and skeletal muscle. Maturation causes cardiac-specific protein expression to increase in heart muscle and remain unchanged in skeletal muscle. MDSC-3DGB had similar protein expression to gestational day 20 fetal heart (Fig. 4A). Densitometry analysis of each protein normalized to β-actin expression demonstrated that 3D culture (MDSC-3DGB and 3D-MDSC groups) was associated with higher cTn-T, Cx-43, and cTn-I expression versus MDSC-aggregate culture (p < 0.05). Within 3D culture groups, we noted that both cTn-I and Cx-43 expression levels were higher in the MDSC-3DGB group than in the 3D-MDSC group (p < 0.05) (Fig. 4B). Notably, MDSC-3DGB had similar cTn-I and Cx-43 expression levels as well as a cTn-T/cTn-I ratio (9.1 ± 0.4 [n = 3]) to gestational day 20 fetal heart (9.3 ± 1.7 [n = 3]), which was significantly different compared to adult ventricular tissue (0.8 ± 0.1 [n = 4, p < 0.05]) cTn-T/cTn-I ratio as well as cTn-I and Cx-43 expression levels (p < 0.05) (Fig. 4B). These data indicate that while MDSCs express cardiac-specific genes at 7 days in culture, regardless of culture method, the cardiac-specific protein expression profiles differed with culture method; specifically, MDSC-aggregate formation followed by 3DGB culture synergistically increased induction of cells with an immature CM phenotype.
FIG. 3.
Cardiac-specific mRNA expression. Lane 1, 2D-MDSC evaluated after 7 days in culture; lane 2, MDSC-aggregate evaluated after 24 h rotation culture; lane 3, 3D-MDSC (without MDSC-aggregate formation) evaluated 7 days after tissue construction; lane 4, MDSC-3DGB evaluated 7 days after tissue construction; lane 5, adult rat ventricular tissue.
FIG. 4.
Cardiac-specific protein quantification. (A) Representative Western blot analysis of lane 1, MDSC-aggregate evaluated after 24 h rotation culture; lane 2, 3D-MDSC (without MDSC-aggregate formation) evaluated 7 days after tissue formation; lane 3, MDSC-3DGB evaluated 7 days after tissue formation; lane 4, gestational day 20 fetal rat ventricular tissue; lane 5, twelve-week-old adult rat ventricular tissue; lane 6, gestational day 20 fetal rat lower leg skeletal muscle; lane 7, twelve-week-old adult rat gastrocnemius muscle. (B) Densitometric data normalized to β-actin expression (average expression vs. β-actin, %). *p < 0.05 versus MDSC-aggregate culture normalized expression. †p < 0.05 versus 3D-MDSC culture normalized expression. ‡p < 0.05 versus MDSC-3DGB culture normalized expression. Each lane contains 20 μg of protein per sample, and experiments were repeated in triplicate.
Chronotropic effects of ISP and cadmium chloride on MDSC-3DGB
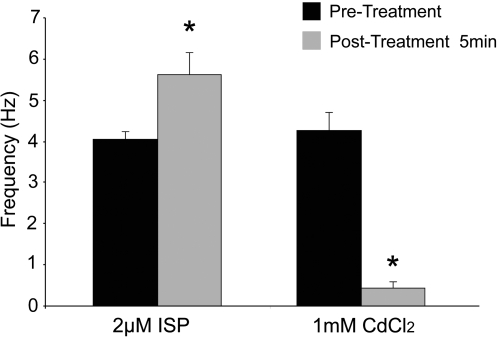
Culture day 7 MDSC-3DGBs were treated with ISP or cadmium chloride. Five minutes after treatment, ISP increased MDSC-3DGB spontaneous beat frequency (5.62 ± 0.54 Hz [n = 8, p < 0.05]) versus pretreatment (4.05 ± 0.20 Hz [n = 8]), whereas cadmium chloride suppressed spontaneous beating activity (0.44 ± 0.16 Hz [n = 8, p < 0.05]) compared to pretreatment (4.27 ± 0.44 Hz [n = 8]) (Fig. 5). No changes in pretreatment baseline spontaneous beat frequency and posttreatment baseline spontaneous beat frequency were noted indicating reversible effects. These chronotropic responses do not occur in twitching mature skeletal muscle or skeletal myotubes.16
FIG. 5.
Effects of ISP and CdCl2 treatment on spontaneous beating activity of MDSC-3DGB. Culture day 7 MDSC-3DGBs treated with 2 μM ISP for 5 min increased spontaneous beat frequency. MDSC-3DGBs treated with 1 mM CdCl2 for 5 min decreased spontaneous beating. *p < 0.05 versus pretreatment. ISP, isoproterenol; CdCl2, cadmium chloride.
Contractile properties of 3D MDSC culture
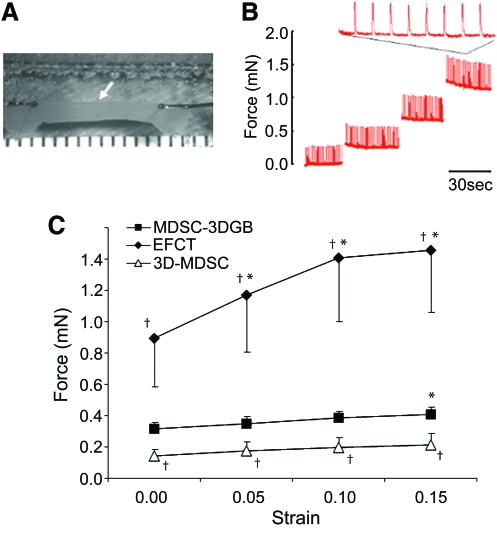
Upon field stimulation, both 3D-MDSC and MDSC-3DGB generated contractile force. MDSC-3DGB generated contractile force similar to engineered cardiac tissue from native fetal cardiac cells (EFCT) (Fig. 6). MDSC-3DGB as well as culture day 7 EFCT displayed a positive Frank-Starling response to increased construct length, whereas 3D-MDSC did not (p < 0.05; Fig. 6B, C). MDSC-3DGB generated a greater maximum active force (0.41 ± 0.06 mN [n = 7, p < 0.05]) compared with 3D-MDSC (0.22 ± 0.04 mN [n = 5]), which was approximately one-third of EFCT (1.45 ± 0.39 mN [n = 7, p < 0.05]) (Fig. 6C).
FIG. 6.
Biomechanical testing of MDSC-3DGB. (A) MDSC-3DGB mounted on a mechanical testing station (white arrow). Scribed x-axis minor scale divisions represent 1 mm. (B) Representative contractile force tracing of MDSC-3DGB at increasing resting lengths. (C) Active force–strain relations of culture day 7 MDSC-3DGB, culture day 7 3D-MDSC, and EFCT at strain deviations of 0 to 0.15. †p < 0.05 versus MDSC-3DGB. Active force increased in response to increased strain (positive Frank-Starling response, *p < 0.05, analysis of variance). Color images available online at www.liebertonline.com/ten.
[Ca2+]i transients in MDSC-3DGB
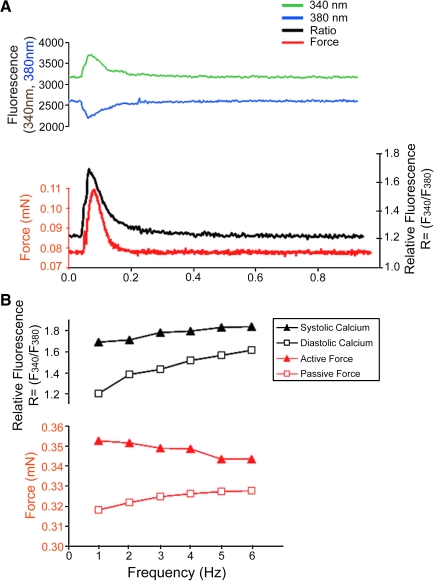
We further investigated calcium handling by simultaneously recording contractile force and [Ca2+]i transients from culture day 7 MDSC-3DGB in which we observed spontaneous tissue contraction. A rise in [Ca2+]i preceded force generation (Fig. 7A) and each [Ca2+]i transient was associated with a concurrent contraction. MDSC-3DGB displayed a negative force–frequency relationship between 1 and 6 Hz based on reduced [Ca2+]i transients and active force at increasing pacing rates and was associated with increased diastolic ratio (suggesting increased diastolic [Ca2+]i and passive force) (Fig. 7B), similar to immature myocardium.33,34 MDSC-3DGB force as well as [Ca2+]i transient developed calcium (ratio = F340/F380) (0.48 ± 0.16 [n = 3]) was similar to ENCT (engineered cardiac tissue from cardiac cells isolated from day 1 neonatal rats) (0.56 ± 0.11 [n = 5]). These data indicate that MDSC-3DGB display the [Ca2+]i transient features of immature CM with incomplete maturation of excitation–contraction coupling.
FIG. 7.
Simultaneous contractile force and [Ca2+]i transient measurement of MDSC-3DGB. (A) Representative single beat force (red) fluorescence at 340 nm (green) and at 380 nm (blue), and the relative fluorescence (ratio [R = F340/F380]) (black) tracing of culture day 7 MDSC-3DGB electrically stimulated at 1 Hz at 50 V and 4 ms duration. A rise in [Ca2+]i preceded force generation and each [Ca2+]i transient was associated with a concurrent contraction. (B) Culture day 7 MDSC-3DGB electrically stimulated at rates of 1 to 6 Hz at 50 V and 4 ms duration. MDSC-3DGB showed increased diastolic [Ca2+]i and reduced systolic [Ca2+]i transient ratios (black) associated with increased diastolic and decreased active force (red) at increasing pacing rates, similar to immature myocardium. [Ca2+]i, intracellular free calcium ion. Color images available online at www.liebertonline.com/ten.
Discussion
Previous studies have shown that stem cells isolated from skeletal muscle specimens have the ability to differentiate into CMs;8,14,16–19 however, the efficiency of CM differentiation remains unclear and with limited functional characterization. In the current study we found that (1) low-serum growth medium–treated rat MDSCs expressed cardiac-specific genes similar to native adult myocardium, consistent with the findings of others in both human and mouse skeletal muscle–derived cells, and (2) the combination of MDSC-aggregate formation and 3DGB culture significantly increased cardiac-specific protein expression, spontaneous beating cell activity, and contractile properties. Our results suggest that 3D microenvironmental cues provided by MDSC-aggregate and 3DGB culture play an important role in differentiation of cells with a functional CM phenotype from MDSCs and the 3DGB system provides the necessary environment to evaluate the contractile properties of these differentiated CMs.
Various experiments have highlighted the importance of the microenvironment on stem cell–derived CM induction, differentiation, and survival.35,36 Tamaki et al. have recently shown that skeletal muscle–derived multipotent Sk-34 cells can give rise to CMs and that cell-to-cell relationships and cellular milieu were important for this differentiation.18 However, these results were achieved with coculture with embryonic CMs, and there was no functional characterization of the SK-34–derived CMs in vitro. Thus, it is unclear how these cells function in comparison to native CMs in either a healthy or diseased heart. Rota et al. have shown that bone marrow stem cells can engraft into the injured myocardium and differentiate into functionally competent CMs and vascular structures by establishing a microenvironment necessary to adopt the cardiac phenotype.5 However, despite these positive results there is still limited information on the use of physical stimuli and unique microenvironments to control CM induction from progenitor/stem cells, CM maturation, and contractile function.
Three-dimensional growth of cells in aggregate spheres has been shown to direct and facilitate cell–cell interactions as well as to modify the differential expression of both morphogenic and angiogenic pathways in CMs22 and hepatocytes.37 Cell aggregate culture has been shown to enhance CM gene expression patterns,22 increase the synthesis and release of ECM components,26 and accelerate CM differentiation efficiency of embryonic stem cells25 and liver stem cells.23 Similarly, Albrecht et al. found that chondrocyte matrix biosynthesis was dependent on cell cluster size, rather than overall cell density.38 Aggregate culture has also been used to enhance survival and differentiation of various stem cell types,25,39,40 versus static culture. These studies suggest that aggregation imparts many of the necessary structural cues required for maintaining differentiated phenotype, including proper dimensionality, shape, cell–ECM, and cell–cell interactions.
Cardiac cells within 3D cultured tissue display distinct features that are more representative of native myocardium than do cells within 2D culture.32,41 Bursac et al. investigated the effect of 3D versus 2D culture on CM properties and found that the 3D microenvironment plays a critical role in maintenance of CM metabolism, sarcomere formation, cell-to-cell connections, and electrophysiological properties.42 Three-dimensional growth of fibroblasts,1,4 endothelial cells,6 and mammary gland cells7 also exhibits cell morphology and function similar to native tissue over 2D culture.43 In the current study we showed that the 3D culture condition increased differentiation of cells with a functional CM phenotype at both the gene and protein levels. Further, the combination of MDSC-aggregate formation and 3DGB culture significantly increased expression of the cardiac-specific proteins cTn-I and Cx-43 in comparison to the MDSC-aggregate group, indicating that MDSC-aggregate formation followed by 3DGB culture synergistically promoted differentiation and maturation of cells with a functional CM phenotype in vitro. Chronotropic effects of ISP and cadmium chloride treatment clearly showed that MDSC-3DGB contractile properties are mimicking cardiac tissue, not twitching mature skeletal muscle or tissue derived from myoblasts. MDSC-3DGB exhibited synchronous contraction and cardiac [Ca2+]i transients in response to electric field stimulation similar to engineered cardiac tissue from native CMs, suggesting that cells within MDSC-3DGB are a functional syncytium. MDSC-3DGB exhibited a positive force–length (Frank-Starling) and a negative force–frequency relationship consistent with incomplete maturation of CM calcium cycling32,44 that was similar to engineered cardiac tissue from native fetal CMs. These results suggested that the combination of MDSC-aggregate formation and 3DGB tissue culture was required for differentiation of cells with a functioning CM phenotype from MDSCs.
Studies in cellular cardiomyoplasty showed that the microenvironment of the injured myocardium includes the release of matrix factors and cytokines that are clearly not conducive to supporting CM induction and/or survival from implanted stem cells.45,46 This may inhibit proper CM regeneration and/or lead to the potential for inappropriate differentiation in the absence of the proper cell-specific milieu. Therefore, our MDSC-3DGB provides a novel method for studying and optimizing in vitro conditioning of the micromechanical environment with direct assessment of contractile properties, and its role in stem cell commitment to functioning CMs from MDSCs in vitro. Although the results of our current study clearly indicate that combined MDSC-aggregate formation and 3DGB culture drives induction of cells with a functioning cardiac phenotype from MDSCs and that the contractile properties mimic those of engineered tissue from native fetal cells, the underlying mechanisms as to how the 3D microenvironment regulates this induction from MDSCs remains unclear and necessitates further studies.
There are several limitations that need to be mentioned. A major limitation to the current study is that we cannot definitively determine the state of the CM-like cells within MDSC-3DGB. This is most likely due to the coexistance of many cardiac and skeletal muscle–specific proteins (MHCs, troponins, etc.) as well as excitation–contraction coupling mechanisms that occur not only in cultured cells, especially those that are considered to be immature, but also within the developing tissue. Cognard et al. have shown that cardiac and skeletal excitation–contraction coupling mechanisms coexist in the developing skeletal muscle with the cardiac type dominant in the early phases of myogenesis and the skeletal dominating in more mature muscle.47 Rose et al. have reported bone marrow–derived mesenchymal stromal cells that acquire expression of CM genes in in vitro coculture with native CMs but that did not generate action potentials or display ionic currents typical of CMs, and thus remain functionally non-CM.48 Therefore, further studies are necessary to determine whether CM phenotypic cells within MDSC-3DGB develop further and mature similarly to the native developing immature myocardium, and whether the 3DGB culture in its present form is able to do this. In the present study, we showed that cTn-T–positive cells consist of approximately 20% of the entire number of MDSCs within the MDSC-3DGB, a population which may contain both undifferentiated MDSCs and MDSCs differentiated into other types of cells. Thus, it is necessary to develop a method to enrich CM phenotype cells from the MDSC-3DGB. It is likely that more contractile apparatuses within each differentiated CM as well as more CMs within MDSC-3DGB (∼20% compared to ∼60–70% within EFCT) and a better organization and communication within the tissue would serve to increase the contractile properties to be similar to the EFCT or native tissue; however, further studies are necessary. Similarly, it is also necessary to observe the CM phenotypic cells within MDSC-3DGB over longer culture periods. It is possible that the CM phenotypic cells will (1) acquire a more mature CM phenotype, (2) remain an immature CM population, or (3) return to an undifferentiated state. It remains unknown whether CM differentiation and maturation from MDSCs require other types of cells in vitro. Previous studies have shown that preconditioned MDSCs transplanted into injured myocardium survive and differentiate into a more matured CM phenotype based on histological assessment,16–18 while in the current study, differentiated cells exhibited a more immature CM phenotype. The factors that drive differentiated CM phenotypic cells toward a more mature CM phenotype remain unknown.
Conclusion
In summary, our results suggest that 3D environmental cues provided by MDSC-aggregate formation and 3DGB culture are complementary and sufficient to trigger differentiation of cells with an immature functioning CM phenotype from rat skeletal MDSCs in vitro and that this 3DGB culture can be used as a method to directly assess the contractile properties of differentiated CMs from MDSCs in vitro. This novel induction approach may be useful in generating scalable, functioning, donor CMs for cardiac repair and regeneration.
Funding Sources
This research was supported by the NIH-NHLBI training Grant T32-HL76124 (K.C.C.), NIH R21HL79998, NIH BRP HL069368, NIH RO1 HL085777-01-A2, the Pennsylvania Department of Health, Children's Hospital of Pittsburgh Foundation, and McGinnis Endowed Chair.
Supplementary Material
Supplementary Material
Disclosure Statement
No competing financial interests exist.
References
- 1.Dimmeler S. Zeiher A.M. Schneider M.D. Unchain my heart: the scientific foundations of cardiac repair. J Clin Investig. 2005;115:572. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowell J.D. Rubart M. Pasumarthi K.B. Soonpaa M.H. Field L.J. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 3.Roell W. Lu Z.J. Bloch W. Siedner S. Tiemann K. Xia Y. Stoecker E. Fleischmann M. Bohlen H. Stehle R. Kolossov E. Brem G. Addicks K. Pfitzer G. Welz A. Hescheler J. Fleischmann B.K. Cellular cardiomyoplasty improves survival after myocardial injury. Circulation. 2002;105:2435. doi: 10.1161/01.cir.0000016063.66513.bb. [DOI] [PubMed] [Google Scholar]
- 4.Christoforou N. Gearhart J.D. Stem cells and their potential in cell-based cardiac therapies. Prog Cardiovasc Dis. 2007;49:396. doi: 10.1016/j.pcad.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Rota M. Kajstura J. Hosoda T. Bearzi C. Vitale S. Esposito G. Iaffaldano G. Padin-Iruegas M.E. Gonzalex A. Rizzi R. Small N. Muraski J. Alvarez R. Chen X. Urbanek K. Bolli R. Houser S.R. Leri A. Sussman M.A. Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. PNAS. 2007;104:17783. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ott H.C. Davis B.H. Taylor D.A. Cell therapy for heart failure—muscle, bone marrow, blood, and cardiac-derived stem cells. Semin Thorac Cardiovasc Surg. 2005;17:348. doi: 10.1053/j.semtcvs.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Tossios P. Muller-Ehmsen J. Schmidt M. Scheid C. Unal N. Moka D. Schwinger R.H. Mehlhorn U. No evidence of myocardial restoration following transplantation of mononuclear bone marrow cells in coronary bypass grafting surgery patients based upon cardiac SPECT and 18F-PET. BMC Med Imaging. 2006;6:7. doi: 10.1186/1471-2342-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okada M. Payne T.R. Bo Z. Oshima H. Momoi N. Tobita K. Keller B.B. Peault B. Huard J. Identification of a novel progenitor cell population in human skeletal muscle with a superior ability for myocardial repair in comparison to commited skeletal myoblast. JACC. 2008;52:1869. doi: 10.1016/j.jacc.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolossov E. Bostani T. Roell W. Breitbach M. Pillekamp F. Nygren J.M. Sasse P. Rubenchik O. Fries J.W. Wenzel D. Geisen C. Xia Y. Lu Z. Duan Y. Kettenhofen R. Jovinge S. Bloch W. Bohlen H. Welz A. Hescheler J. Jacobsen S.E. Fleischmann B.K. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng H. Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transplant Immunol. 2004;12:311. doi: 10.1016/j.trim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.Y. Qu-Petersen Z. Cao B. Kimura S. Jankowski R. Cummins J. Usas A. Gates C. Robbins P. Wernig A. Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankowski R.J. Haluszczak C. Trucco M. Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 13.Zheng B. Cao B. Crisan M. Sun B. Li G. Logar A. Yap S. Pollett J.B. Drowley L. Cassino T. Gharaibeh B. Deasy B.M. Huard J. Péault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 14.Oshima H. Payne T.R. Urish K.L. Sakai T. Ling Y. Gharaibeh B. Tobita K. Keller B.B. Cummins J.H. Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 15.Zuba-Surma E.K. Abdel-Latif A. Case J. Tiwari S. Hunt G. Kucia M. Vincent R.J. Ranjan S. Ratajszak M.Z. Srour E.F. Bolli R. Dawn B. Sac-1 expression is associated with decreased cardiomyogenic differentiation potential of skeletal muscle-derived adult primitive cells. JMCC. 2006;41:650. doi: 10.1016/j.yjmcc.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Winitsky S.O. Gopal T.V. Hassanzadeh S. Takahashi H. Gryder D. Rogawski M.A. Takeda K. Yu Z.X. Xu Y.H. Epstein N.D. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 2005;3:e87. doi: 10.1371/journal.pbio.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Invernici G. Cristini S. Madeddu P. Brock S. Spillmann F. Bernasconi P. Cappelletti C. Calatozzolo C. Fascio U. Bisleri G. Muneretto C. Alessandri G. Parati E.A. Human adult skeletal muscle stem cells differentiate into cardiomyocyte phenotype in vitro. Exp Cell Res. 2008;314:366. doi: 10.1016/j.yexcr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Tamaki T. Akatsuka A. Okada Y. Uchiyama Y. Tono K. Wada M. Hoshi A. Iwaguro H. Iwasaki H. Oyamada A. Asahara T. Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium. PLoS ONE. 2008;3:e1789. doi: 10.1371/journal.pone.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arsic N. Mamaeva D. Lamb N.J. Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Payne T.R. Oshima H. Okada M. Momoi N. Tobita K. Keller B.B. Peng H. Huard J. A relationship between VEGF, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. JACC. 2007;23:1677. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg M.S. Reconstruction of tissues by dissociated cells. Science. 1963;141:401. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 22.Akins R.E. Gratton K. Quezada E. Rutter H. Tsuda T. Soteropoulos P. Gene expression profile of bioreactor-cultured cardiac cells: activation of morphogenetic pathways for tissue engineering. DNA Cell Biol. 2007;26:425. doi: 10.1089/dna.2006.0543. [DOI] [PubMed] [Google Scholar]
- 23.Anderson P.A. Muller-Borer B.J. Esch G.L. Coleman W.B. Grisham J.W. Malouf N.N. Calcium signals induce liver stem cells to acquire a cardiac phenotype. Cell Cycle. 2007;6:1565. doi: 10.4161/cc.6.13.4454. [DOI] [PubMed] [Google Scholar]
- 24.Dertinger H. Hulser D.F. Durand R. Intercellular communication in spheroids. In: Acker H., editor; Carlsson J., editor; Sutherland R.M., editor. Spheroids in Cancer Research: Methods and Perspectives. Berlin: Springer-Verlag; 1984. pp. 67–83. [DOI] [PubMed] [Google Scholar]
- 25.Carpenedo R.L. Sargent C.Y. McDevitt T.C. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 26.Watzka S.B. Lucien J. Shimada M. Edwards V. Yeger H. Hannigan G. Coles J.G. Selection of viable cardiomyocytes for cell transplantation using three-dimensional tissue culture. Transplantation. 2000;70:1310. doi: 10.1097/00007890-200011150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann W.H. Didie M. Doker S. Melnychenko I. Naito H. Rogge C. Tiburcy M. Eschenhagen T. Heart muscle engineering: an update on cardiac muscle replacement therapy. Cardiovasc Res. 2006;71:419. doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann W.H. Melnychenko I. Wasmeier G. Didié M. Naito H. Nixdorff U. Hess A. Budinsky L. Brune K. Michaelis B. Dhein S. Schwoerer A. Ehmke H. Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 29.Jankowski R.J. Deasy B.M. Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 30.Deasy B.M. Gharaibeh B. Pollett J.B. Jones M.M. Lucas M.A. Kanda Y. Huard J. Long-term self-renewal of postnatal muscle-derived Stem cells. Mol Bio Cell. 2005;16:3323. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gharaibeh B. Lu A. Tebbets J. Zheng B. Feduska J. Crisan M. Peault B. Cummins J. Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Prot. 2008;3:1501. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 32.Tobita K. Liu L.J. Janczewski A.M. Tinney J.P. Nonemaker J.M. Augustine S. Stolz D.B. Shroff S.G. Keller B.B. Engineered early embryonic cardiac tissue (EEECT) retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1829. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- 33.Penefsky Z.J. Wolfe J. Greengard O. Bernstein J. Mechanical responses of developing Fisher rat heart. Effects of steroid hormone. J Dev Physiol. 1986;8:333. [PubMed] [Google Scholar]
- 34.Liu J. Fu J.D. Siu C.W. Li R.A. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 35.Iijima Y. Nagai T. Mizukami M. Matsuura K. Ogura T. Wada H. Toko H. Akazawa H. Takano H. Nakaya H. Komuro I. Beating is necessary for transdifferentiation of skeletal muscle-derived cells into cardiomyocytes. FASEB J. 2003;17:1361. doi: 10.1096/fj.02-1048fje. [DOI] [PubMed] [Google Scholar]
- 36.Rudy-Reil D. Lough J. Avian precardiac endoderm/mesoderm induces cardiac myocyte differentiation in murine embryonic stem cells. Circ Res. 2004;94:e107. doi: 10.1161/01.RES.0000134852.12783.6e. [DOI] [PubMed] [Google Scholar]
- 37.Hansen L.K. Hsiao C. Friend J.R. Wu F.J. Bridge G.A. Remmel R.P. Cerra F.B. Hu W. Enhanced morphology and function in hepatocyte spheroids: a model of tissue self-assembly. Tissue Eng. 1998;4:65. [Google Scholar]
- 38.Albrecht D.R. Underhill G.H. Wassermann T.B. Sah R.L. Bhatia S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 39.Suenaga H. Furukawa K.S. Ushida T. Takato T. Tateishi T. Aggregate formation of bone marrow stromal cells by rotation culture. Mat Sci Eng. 2004;C24:421. [Google Scholar]
- 40.Kehat I. Kenyagin-Karsenti D. Snir M. Segev H. Amit M. Gepstein A. Livne E. Binah O. Itskovitz-Eldor J. Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmermann W.H. Schneiderbanger K. Schubert P. Didie M. Munzel F. Heubach J.F. Kostin S. Neuhuber W.L. Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;8:223. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 42.Bursac N. Papadaki M. White J.A. Eisenberg S.R. Vunjak-Novakovic G. Freed L.E. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 43.Panorchan P. Lee J.S.H. Kole T.P. Tseng Y. Wirtz D. Microrheology and ROCK signaling of human endothelial cells embedded in a 3D matrix. Biophys J. 2006;91:3499. doi: 10.1529/biophysj.106.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolnikov K. Shilkrut M. Zeevi-Levin N. Gerecht-Nir S. Amit M. Danon A. Itskovitz-Eldor J. Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 45.Frangogiannis N.G. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 46.Kastrup J. Ripa R.S. Wang Y. Jorgensen E. Myocardial regeneration induced by granulocyte-colony-stimulating factor mobilization of stem cells in patients with acute or chronic ischaemic heart disease: a non-invasive alternative for clinical stem cell therapy? Eur Heart J. 2006;27:2748. doi: 10.1093/eurheartj/ehl339. [DOI] [PubMed] [Google Scholar]
- 47.Cognard C. Rivet-Bastide M. Constantin B. Raymond G. Progressive predominance of “skeletal” versus “cardiac” types of excitation-contraction coupling during in vitro skeletal myogenesis. Eur J Phys. 1992;422:207. doi: 10.1007/BF00370424. [DOI] [PubMed] [Google Scholar]
- 48.Rose R.A. Juang H. Wang X. Helke S. Tsoporis J.N. Gong N. Keating S.C.J. Parker T.G. Backx P.H. Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26:2884. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.