Abstract
The effectiveness and safety of drug eluting stents (DES) compared to bare metal stents (BMS) in saphenous vein graft (SVG) disease remains unclear. In particular, there is a paucity of data on long term outcomes. We analyzed 395 patients enrolled in the National Heart, Lung, and Blood Institute Dynamic Registry who underwent stenting of a SVG lesion with a BMS (n=192) from 1999–2006 or a DES (n=203) from 2004–2006. Patients were followed prospectively for the occurrence of cardiovascular events and death at 3 years. Patients treated with DES were more likely to have diabetes mellitus and other comorbidities, and prior percutaneous coronary intervention (PCI). Treated lesions in DES patients were more complex than in BMS patients. At 3 years of follow-up, the adjusted risk of target vessel revascularization (TVR) (HR 1.03, 95% CI 0.65–1.62, p=0.91) and death or myocardial infarction (MI) (HR 0.72, 95% CI 0.49–1.04, p=0.08) was similar in DES and BMS treated patients. The combined outcome of death, MI, or TVR excluding peri-procedural MI was also similar (adjusted HR 0.82 95% CI 0.62–1.09, p=0.16). In conclusion, this multi-center non-randomized study of unselected patients showed no benefit of DES in SVG lesions including no reduction in TVR compared to BMS at 3 years. An adequately powered randomized controlled trial is needed to determine the optimal stent type for SVG PCI.
Keywords: Stents, Coronary bypass surgery, Registries
Saphenous vein graft (SVG) disease occurs frequently after coronary artery bypass grafting (CABG) and is a strong predictor of mortality.1 Percutaneous coronary intervention (PCI) with stent placement is the preferred treatment for SVG disease, but restenosis occurs in over 30% of patients treated with bare metal stents (BMS).2 Drug-eluting stents (DES) reduce the need for repeat revascularization compared to BMS in native coronary lesions, but studies of DES in SVG lesions have shown mixed results.3–11 Furthermore, the safety of DES in SVG lesions has been questioned, with a concern for late stent thrombosis and sudden cardiac death.12 Given the paucity of long term data on DES in SVG lesions we examined a subgroup of patients undergoing SVG PCI with either BMS or DES in a prospective observational multi-center registry with planned 5 year follow-up.
Methods
The National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry is a prospective observational study of consecutive patients undergoing PCI at selected centers in North America.13 Five enrollment waves of approximately 2,000 consecutive patients each have been collected since 1997. Waves 4 and 5 enrolled patients in the DES era. Stent selection (BMS vs. DES) during waves 4 and 5 was at the discretion of the operator. Each clinical center received approval from its Institutional Review Board, and data were compiled and analyzed at the University of Pittsburgh.
In each wave, baseline demographic, clinical, angiographic, and procedural data were collected at the time of the index PCI. In-hospital outcomes were obtained at the site of enrollment. All patients were followed for 1 year post-PCI, and patients enrolled in waves 2, 4, and 5 are followed yearly out to 5 years. Systematic collection of data on stent thrombosis began in wave 4. During follow-up, coronary angiography was obtained only if clinically indicated for symptoms or objective ischemia. For patients undergoing repeat PCI, lesion-specific data were collected to determine whether target vessel revascularization was performed. Patients were interviewed by telephone by trained data coordinators guided by standardized questionnaires.
This analysis includes patients enrolled in waves 2, 4, and 5 who underwent SVG PCI with at least 1 BMS or DES. Wave 1 and 3 patients were excluded because follow-up did not extend beyond 1 year. A total of 6394 patients were enrolled in waves 2, 4 and 5 and 457 had an SVG intervention. Patients with SVG lesions treated with balloon angioplasty only (n=50) or with a combination of BMS and DES (n=12) were excluded. The remaining 395 patients were included in this study. In wave 2, 119 patients underwent SVG stenting with BMS. In waves 4 and 5, 73 patients underwent SVG stenting with BMS, and 203 patients underwent SVG stenting with DES. Thus, 192 patients treated with BMS were compared to 203 patients treated with DES. Three -year follow-up rates by recruitment wave ranged from 97% to 100%.
Death includes mortality from all causes. Myocardial infarction is defined by: (1) evolutionary ST-segment elevation, development of new Q-waves in 2 or more contiguous ECG leads, or new LBBB patterns on the ECG, or, (2) biochemical evidence of myocardial necrosis, manifested as (a) CK-MB ≥ 3 times the upper limit of normal, or, if CK-MB is not available, (b) total CK ≥ 3 times the upper limit of normal, or (c) troponin value above the upper limit of normal. Repeat PCI includes both target and non-target vessel interventions. Planned staged PCI procedures following the index procedure were not considered repeat PCI. Target vessel revascularization (TVR) is defined as a repeat revascularization involving the initially treated vein graft. Native vessel TVR was not included in the TVR endpoint. Stent thrombosis includes only angiographically-confirmed (definite) events.
Patient and lesion level characteristics pertaining to the index PCI were compared between stent types by Student t tests for continuous variables, and by chi-square test or Fisher’s exact test for categorical variables. Three-year event rates were calculated using the Kaplan-Meier approach, and unadjusted comparisons of survival curves were performed using the log-rank test. Multivariate Cox proportional hazards modeling was used to estimate 3-year hazard ratios (HRs) for adverse clinical events according to stent type. Covariate adjustment was performed such that clinical, demographic, and procedural variables were entered individually into outcome-specific models that included an indicator variable for stent type. Confounding variables were assessed in a forward stepwise manner to determine the final adjusted model. The screened variables with a p value of <0.20 were included initially, and those for which the p value was <0.10 remained in the model. Covariate adjustments for outcomes based on lesion and stent type were performed in a similar fashion. Proportional hazards assumptions were evaluated and met. Patients who did not experience the outcome of interest were censored at the last known date of contact or at 3 years if contact extended beyond that time. For all analyses, a 2-sided P value of ≤0.05 was considered to be statistically significant.
Results
Selected baseline clinical characteristics of the groups are shown in Table 1. Patients treated with DES were significantly more likely to present with diabetes mellitus, hypercholesterolemia, hypertension and a prior PCI. Other baseline characteristics were similar between the groups. At the index procedure, 287 lesions (248 SVG and 39 native) were stented in 192 BMS-treated patients, and 295 lesions (256 SVG and 39 native) were stented in 203 DES-treated patients. SVG lesion characteristics are shown in Table 2. DES-treated lesions were significantly more likely to have a prior stent, longer length, smaller reference diameters, and more complexity compared to BMS lesion. The results were similar when all lesions (SVG and native) were analyzed.
Table 1.
Baseline Demographics and Patient Characteristics According to Stent Type
| Variable | BMS (N = 192) | DES (N=203) | P Value |
|---|---|---|---|
| Mean age (years) | 70.4 | 69.7 | 0.42 |
| Women | 50 (26.0%) | 38 (18.7%) | 0.08 |
| Race/ethnicity | 0.59 | ||
| White | 171 (89.1%) | 173 (85.2%) | |
| Black | 16 (8.3%) | 20 (9.9%) | |
| Asian | 3 (1.6%) | 5 (2.5%) | |
| Hispanic | 2 (1.0%) | 5 (2.5%) | |
| Smoking status | 0.17 | ||
| Never smoked | 67 (37.0%) | 55 (30.4%) | |
| Current/former smoker | 114 (63.0%) | 126 (69.6%) | |
| Hypertension | 146 (76.4%) | 174 (87.0%) | 0.007 |
| Diabetes | 58 (30.4%) | 91 (44.8%) | 0.003 |
| Hypercholesterolemia* | 142 (75.9%) | 183 (91.0%) | <.0001 |
| Renal disease** | 20 (10.5%) | 33 (16.3%) | 0.09 |
| Peripheral vascular disease | 34 (17.8%) | 39 (19.3%) | 0.70 |
| Cerebrovascular disease | 24 (12.6%) | 25 (12.4%) | 0.95 |
| Previous percutaneous coronary intervention | 73 (38.0%) | 119 (58.6%) | <.0001 |
| Previous myocardial infarction | 87 (47.8%) | 83 (43.9%) | 0.45 |
| History of congestive heart failure | 40 (21.6%) | 45 (23.1%) | 0.73 |
| Mean left ventricular ejection fraction | 47.8 | 47.9 | 0.61 |
| Triple vessel coronary disease | 135 (70.3%) | 161 (79.3%) | 0.048 |
serum cholesterol >240 mg/dl or receiving medical treatment for high cholesterol,
renal failure or insufficiency diagnosed by a physician and treated with medication, low protein diet or dialysis.
Table 2.
Saphenous Vein Graft Lesion Characteristics According to Stent Type
| Variable | Bare-Metal Stent (N=248) | Drug-Eluting Stent (N=256) | P value |
|---|---|---|---|
| Lesion previously treated with stent | 16 (6.5%) | 44 (17.2%) | 0.0002 |
| Reference vessel size (mm) | 3.6 | 3.3 | <.0001 |
| Mean lesion length (mm) | 13.2 | 18.5 | <.0001 |
| Lesion characteristics | |||
| Evidence of thrombus | 51 (21.7%) | 46 (18.1%) | 0.32 |
| Calcified | 27 (11.3%) | 26 (10.3%) | 0.73 |
| Ulcerated | 31 (13.1%) | 37 (14.7%) | 0.60 |
| Ostial lesion | 28 (11.3%) | 35 (13.7%) | 0.43 |
| Total occlusion | 7 (2.8%) | 14 (5.5%) | 0.14 |
| Lesion classification* | 0.001 | ||
| A | 18 (7.7%) | 11 (4.3%) | |
| B1 | 73 (31.3%) | 69 (27.0%) | |
| B2 | 90 (38.6%) | 78 (30.5%) | |
| C | 52 (22.3%) | 98 (38.3%) | |
According to the American College of Cardiology and American Heart Association Classification
Table 3 shows the characteristics and in-hospital outcomes of the PCI procedures. Procedural indications were similar in BMS and DES-treated patients. Distal protection devices, which came into widespread use after wave 2, were used in 18.3% of BMS and 32.5% of DES patients (P=0.001). There was a higher incidence of in-hospital death and myocardial infarction in the BMS-treated group compared to the DES-treated group (7.3% vs. 2.5%, p=0.03). There was a trend towards a higher rate of MI in BMS patients, but no differences were observed between BMS- and DES-treated patients in the rates of in-hospital Q-wave MI, death, CABG, or stroke.
Table 3.
Procedural Characteristics and Outcomes
| Variable | Bare-Metal Stent (N=192) | Drug-Eluting Stent (N=203) | P Value |
|---|---|---|---|
| Primary indication for revascularization | |||
| Asymptomatic coronary artery disease | 11 (5.7%) | 13 (6.4%) | 0.78 |
| Stable angina pectoris | 40 (20.8%) | 45 (22.2%) | 0.75 |
| Unstable angina pectoris | 100 (52.1%) | 93 (45.8%) | 0.21 |
| Acute myocardial infarction | 37 (19.3%) | 43 (21.2%) | 0.64 |
| Cardiogenic shock on presentation | 1 (0.5%) | 2 (1.0%) | 0.60 |
| Glycoprotein IIb/IIIa inhibitors used | 78 (40.6%) | 57 (28.1%) | 0.009 |
| Distal protection device used | 35 (18.3%) | 66 (32.5%) | 0.001 |
| Number of lesions attempted | 0.27 | ||
| 1 | 120 (62.5%) | 138 (68.0%) | |
| 2 | 57 (29.7%) | 46 (22.7%) | |
| ≥3 | 15 (7.8%) | 19 (9.4%) | |
| Attempted stent location | 0.72 | ||
| Graft only | 163 (84.9%) | 175 (86.2%) | |
| Graft + 1 native vessel | 24 (12.5%) | 21 (10.3%) | |
| Graft + 2 native vessels | 5 (2.6%) | 7 (3.4%) | |
| In-hospital outcomes | |||
| Myocardial infarction | 11 (5.7%) | 4 (2.0%) | 0.05 |
| Coronary bypass surgery | 0 (0%) | 0 (0%) | 1.00 |
| Stroke | 0 (0%) | 1 (0.5%) | 0.33 |
| Death | 3 (1.6%) | 1 (0.5%) | 0.29 |
| Death or Q-wave MI | 3 (1.6%) | 2 (1.0%) | 0.61 |
| Death or MI | 14 (7.3%) | 5 (2.5%) | 0.03 |
| Procedural Success* | 189 (98.4%) | 200 (98.5%) | 0.95 |
Procedural Success is defined as partial or total angiographic success, without Q-wave myocardial infarction, emergency CABG, or death.
Rates of complications for SVG lesions, including embolization (1.2% BMS vs. 1.6% DES, p=0.74), major dissection (0.8% BMS vs. 1.6% DES, p=0.44), and perforation (1.6% vs. 0.4%, p=0.16), were low and similar between BMS-treated and DES-treated patients. Thrombosis in myocardial infarction flow grade was also similar. Post-procedure TIMI-3 flow was seen in 98.0% of BMS-treated SVG lesions and 96.9% of DES-treated SVG lesions (p=0.57). Angiographic success was high and was achieved in 99.6% of BMS-treated SVG lesions and 98.4% of DES-treated SVG lesions (p=0.19). Of the patients treated with DES, 29.6% received paclitaxel-eluting stents, 63.1% received sirolimus-eluting stents and 7.4% of the patients received both types of drug-eluting stent. In hospital event rates including death and MI did not differ according to DES type.
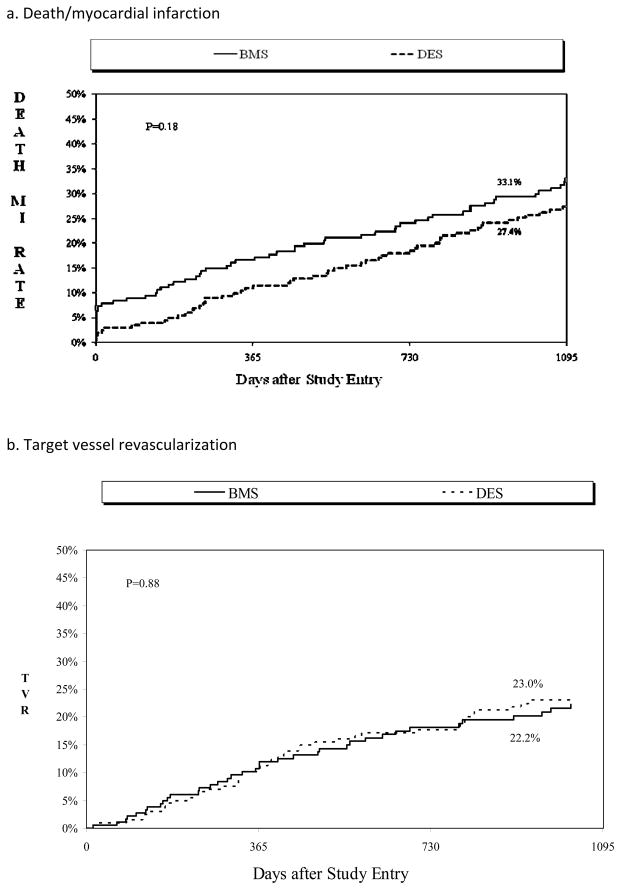
Cumulative event rates are shown in Table 4. By 3 years, there were no statistically significant differences in the individual endpoints of death, MI, repeat PCI, or TVR in DES compared to BMS -treated patients. There was a trend towards more repeat CABG in BMS-treated patients. The combined safety endpoint of death and MI occurred in 33.1% of patients treated with BMS versus 27.4% of patients treated with DES (Figure 1a). Among BMS patients, those enrolled in wave 2 (n=119) compared to waves 4–5 (n=73) had non-significantly higher 3 year rates of MI (20.8% vs. 15.1%, p=0.33) and peri-procedural MI (9.1% vs. 7.2%, 0.67). In terms of efficacy, target vessel revascularization occurred in 22.2% of patients treated with BMS versus 23.0% of patients treated with DES (Figure 1b).
Table 4.
Cumulative Event Rates at 3 years
| Outcome | Bare-Metal Stent (N=192) | Drug-Eluting Stent (N=203) | P Value |
|---|---|---|---|
| Death | 32 (18.1%) | 33 (16.8%) | 0.73 |
| Myocardial infarction | 32 (18.6%) | 30 (16.1%) | 0.39 |
| Death/myocardial infarction | 59 (33.1%) | 54 (27.4%) | 0.18 |
| Target vessel revascularization | 37 (22.2%) | 43 (23.0%) | 0.88 |
| Repeat percutaneous coronary intervention | 56 (33.0%) | 60 (32.2%) | 0.75 |
| Coronary bypass surgery | 12 (7.2%) | 6 (3.5%) | 0.09 |
| Death/myocardial infarction/Target vessel revascularization | 83 (46.6%) | 81 (40.9%) | 0.16 |
| Stent thrombosis* | 1 (1.6%) | 3 (1.6%) | 0.97 |
Stent thrombosis data was collected in waves 4 and 5 only (BMS N=73, DES N=203)
Figure 1.
Three-year Cumulative incidence of (a) Death or myocardial infarction, and (b) target vessel revascularization by stent type
Following adjustment for important covariates, the risk for TVR and the combined endpoint of death or MI was similar in both stent groups (Table 5). There was significantly lower risk of combined death, MI or TVR in DES treated patients, however, after excluding peri-procedural MI there was no difference between BMS and DES.
Table 5.
Relative Risk Models for 3 Year Events in Drug Eluting Versus Bare Metal Stent Patients
| Event | Unadjusted Hazard Ratio | N (AHR model) | Adjusted Hazard Ratio(AHR) | 95% C.I. | P- value |
|---|---|---|---|---|---|
| Death/myocardial infarction | 0.78 | 394 | 0.72 | 0.49, 1.04 | 0.08 |
| Target vessel revascularization | 1.04 | 395 | 1.03 | 0.65, 1.62 | 0.91 |
| Death/myocardial infarction/Target vessel revascularization | 0.81 | 395 | 0.70 | 0.51, 0.95 | 0.02 |
| Death/myocardial infarction/Target vessel revascularization excluding peri-procedural MI | 0.97 | 395 | 0.82 | 0.62, 1.09 | 0.16 |
Among patients recruited in waves 4 and 5, definite stent thrombosis occurred in 1.6% (3 patients) of DES patients and 1.6% (1 patient) of BMS patients. One stent thrombosis occurred in a DES stent from the initial procedure and the remainder originated in stents that were placed either before or after initial enrollment into the Dynamic Registry emphasizing that repeat procedures are common in this patient population. There were 2 cases of sub-acute stent thrombosis both in DES-treated SVG lesions (on day 1 and day 10) although 1 of these patients was classified in the BMS group because this patient initially received BMS stents. A third patient experienced 2 concurrent stent thromboses 139 and 348 days after SVG lesions were treated with DES.
Discussion
We observed no benefit in treating SVG disease with DES compared with BMS after 3 years of follow-up including no difference in the rates of death or MI, TVR, and stent thrombosis. Previous studies have shown mixed results regarding the effectiveness of DES versus BMS for SVG disease. 3–12 Two recent meta-analyses reported a modest, statistically significant benefit with the use of DES compared to BMS in SVG intervention. Joyal, et al, found a reduction in MACE, death, TVR, and target lesion revascularization (TLR) with use of DES.14 Lee, et al, found a lower rate of TVR and MI with DES, and no difference in death.15 The explanation for the observed lower risk of death and MI in DES patients in these analyses is unclear and raises concern regarding the ability of the meta-analysis to appropriately account for baseline and procedural differences in the BMS and DES patients.
The 2 randomized controlled trials of DES versus BMS in SVG disease were small and the results were not conclusive. The RRISC randomized 75 patients to SES versus BMS and at 6 months there was a reduction in TVR in the SES-treated patients, but no difference in death and MI. At 32 months, however, there was no difference in TVR, and an increased risk of death and MI in the SES-treated patients.4, 12 In the SOS trial, 80 patients undergoing SVG intervention were randomized to PES versus BMS. At 18 months, the PES-treated patients had a lower rate of TLR, and no significant difference in TVR, MI, or death.3
The largest published registry data, from the STENT (Strategic Transcatheter Evaluation of New Therapies) Group, assessed 9-month and 2 year outcomes in 785 patients treated with DES and 343 patients treated with BMS. The authors found a lower rate of TVR in the DES-treated patients after 9 months, with most of the advantage lost after 2 years. There was a trend toward a lower rate of death and MI in the DES-treated patients, which did not reach statistical significance. 16 In our study, there was no advantage with DES compared to BMS in SVG disease at 3 years. There was a lower risk of the composite of death, MI, or TVR in DES-treated patients in our study, but this advantage was lost after the exclusion of peri-procedural MI which tended to be lower in later recruitment waves and is likely the result of improved procedural techniques including distal protection devices, adjunctive pharmacology such as thienopyridines, and improved stent designs over time.
Similar to our study, it is not certain to what extent temporal changes in clinical practice may have influenced outcomes in previously published observational trials. For the reasons described above, previous trials may have overestimated the benefit of DES compared to BMS in SVG lesions. Increased use of dual antiplatelet therapy in patients treated with DES may have reduced the risk of death and MI in high-risk patients, independently of outcomes in the treated vessel.
DES have a strong benefit in native coronary artery lesions, but have shown less impressive or no benefit in SVG lesions. One explanation for the lack of clinical advantage of DES compared to BMS is that SVGs are usually large diameter vessels. In native coronary artery lesions, the greatest benefit of DES occurs in small diameter vessels, which are most prone to in-stent restenosis. 17 Brodie, et al, noted that improvement in TVR with DES compared to BMS was observed in SVGs of diameter < 3.5 mm, but not in SVGs of diameter ≥ 3.5 mm.16 In our study, the mean vessel diameter was 3.4 mm. We observed no difference in TVR at 3 years according to a reference vessel diameter <3.5 mm or ≥ 3.5 mm (data not shown). Until recently, DES were not available in sizes > 3.5 mm, which may have introduced another source of bias in previous observational trials.
Another important reason for the lack of benefit of DES in SVG lesions is that the pathophysiology of atherosclerosis is different in veins and arteries. Eluted drugs that inhibit restenosis may be less effective in veins. Vein grafts are subject to unique mechanisms of graft failure, such as anastomotic lesions, kinking, and entrapment, in which DES may offer no benefit over BMS. The lack of long-term benefit of DES in SVG lesions is also likely related to the rapid and inexorable progression of SVG disease. Once SVGs begin to degenerate, there is a high rate of graft failure.18 This is reflected in our study by the high rate of death, MI, and TVR at 3 years, which was greater than 40% in each group studied. Even after successful treatment of an SVG lesion, graft failure is likely to occur elsewhere within the graft. The recent VELETI pilot trial reported a possible benefit for prophylactic treatment of moderate SVG lesions with PES, using a strategy of “plaque sealing”.19
Similar to previous publications on DES for SVG disease, our study has several limitations but offers longer term follow-up. The data is from a relatively small number of patients in a non-randomized, observational registry. As described above, patients were enrolled during different time periods, which introduced significant differences between BMS-treated patients and DES-treated patients. Statistical models were used to adjust for these differences but are imperfect. Patients were included in the analysis if they had concomitant native vessel PCI, but the number was similar in each group and TVR was reported specifically for SVG lesions. Only sirolimus- and paclitaxel-eluting stents were used during the time period of data collection, so data regarding other DES types are not available.
Acknowledgments
Funding: This study was supported by grant HL033292 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
References
- 1.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 2.Savage MP, Douglas JS, Jr, Fischman DL, Pepine CJ, King SB, 3rd, Werner JA, Bailey SR, Overlie PA, Fenton SH, Brinker JA, Leon MB, Goldberg S. Stent placement compared with balloon angioplasty for obstructed coronary bypass grafts. Saphenous Vein De Novo Trial Investigators. N Engl J Med. 1997;337:740–747. doi: 10.1056/NEJM199709113371103. [DOI] [PubMed] [Google Scholar]
- 3.Brilakis ES, Lichtenwalter C, de Lemos JA, Roesle M, Obel O, Haagen D, Saeed B, Gadiparthi C, Bissett JK, Sachdeva R, Voudris VV, Karyofillis P, Kar B, Rossen J, Fasseas P, Berger P, Banerjee S. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (Stenting of Saphenous Vein Grafts) trial. J Am Coll Cardiol. 2009;53:919–928. doi: 10.1016/j.jacc.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Vermeersch P, Agostoni P, Verheye S, Van den Heuvel P, Convens C, Bruining N, Van den Branden F, Van Langenhove G. Randomized Double-Blind Comparison of Sirolimus-Eluting Stent Versus Bare-Metal Stent Implantation in Diseased Saphenous Vein Grafts: Six-Month Angiographic, Intravascular Ultrasound, and Clinical Follow-Up of the RRISC Trial. J Am Coll Cardiol. 2006;48:2423–2431. doi: 10.1016/j.jacc.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Okabe T, Lindsay J, Buch AN, Steinberg DH, Roy P, Slottow TLP, Smith K, Torguson R, Xue Z, Satler LF, Kent KM, Pichard AD, Weissman NJ, Waksman R. Drug-eluting stents versus bare metal stents for narrowing in saphenous vein grafts. Am J Cardiol. 2008;102:530–534. doi: 10.1016/j.amjcard.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Assali A, Raz Y, Vaknin-Assa H, Ben-Dor I, Brosh D, Teplitsky I, Fuchs S, Kornowski R. Beneficial 2-years results of drug-eluting stents in saphenous vein graft lesions. Eurointervention. 2008;4:108–114. doi: 10.4244/eijv4i1a18. [DOI] [PubMed] [Google Scholar]
- 7.Gioia G, Benassi A, Mohendra R, Chowdhury K, Masood I, Matthai W. Lack of clinical long-term benefit with the use of a drug eluting stent compared to use of a bare metal stent in saphenous vein grafts. Catheter Cardiovasc Interv. 2008;72:13–20. doi: 10.1002/ccd.21599. [DOI] [PubMed] [Google Scholar]
- 8.Jeger RV, Schneiter S, Kaiser C, Bonetti PO, Brunner-La Rocca H, Handke M, Osswald S, Buser PT, Pfisterer ME, Investigators B. Drug-eluting stents compared with bare metal stents improve late outcome after saphenous vein graft but not after large native vessel interventions. Cardiology. 2009;112:49–55. doi: 10.1159/000137699. [DOI] [PubMed] [Google Scholar]
- 9.Pucelikova T, Mehran R, Kirtane AJ, Kim Y-H, Fahy M, Weisz G, Lansky AJ, Moussa I, Gray WA, Collins MB, Kodali SK, Stone GW, Moses JW, Leon MB, Dangas G. Short- and long-term outcomes after stent-assisted percutaneous treatment of saphenous vein grafts in the drug-eluting stent era. Am J Cardiol. 2008;101:63–68. doi: 10.1016/j.amjcard.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 10.Ramana RK, Ronan A, Cohoon K, Homan D, Sutherland J, Steen L, Liu J, Loeb H, Lewis BE. Long-term clinical outcomes of real-world experience using sirolimus-eluting stents in saphenous vein graft disease. Catheter Cardiovasc Interv. 2008;71:886–893. doi: 10.1002/ccd.21552. [DOI] [PubMed] [Google Scholar]
- 11.van Twisk P-H, Daemen J, Kukreja N, van Domburg RT, Serruys PW. Four-year safety and efficacy of the unrestricted use of sirolimus- and paclitaxel-eluting stents in coronary artery bypass grafts. Eurointervention. 2008;4:311–317. doi: 10.4244/eijv4i3a57. [DOI] [PubMed] [Google Scholar]
- 12.Vermeersch P, Agostoni P, Verheye S, Van den Heuvel P, Convens C, Van den Branden F, Van Langenhove G, Investigators DR. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J Am Coll Cardiol. 2007;50:261–267. doi: 10.1016/j.jacc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Williams DO, Holubkov R, Yeh W, Bourassa MG, Al-Bassam M, Block PC, Coady P, Cohen H, Cowley M, Dorros G, Faxon D, Holmes DR, Jacobs A, Kelsey SF, King SB, 3rd, Myler R, Slater J, Stanek V, Vlachos HA, Detre KM. Percutaneous coronary intervention in the current era compared with 1985–1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000;102:2945–2951. doi: 10.1161/01.cir.102.24.2945. [DOI] [PubMed] [Google Scholar]
- 14.Joyal D, Filion KB, Eisenberg MJ. Effectiveness and safety of drug-eluting stents in vein grafts: a meta-analysis. Am Heart J. 2010;159:159–169.e154. doi: 10.1016/j.ahj.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS, Yang T, Kandzari DE, Tobis JM, Liao H, Mahmud E. Comparison by Meta-Analysis of Drug-Eluting Stents and Bare Metal Stents for Saphenous Vein Graft Intervention. Am J Cardiol. 2010;105:1076–1082. doi: 10.1016/j.amjcard.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Brodie BR, Wilson H, Stuckey T, Nussbaum M, Laurent S, Bradshaw B, Humphrey A, Metzger C, Hermiller J, Krainin F, Juk S, Cheek B, Duffy P, Simonton CA, Group ftS. Outcomes With Drug-Eluting Versus Bare-Metal Stents in Saphenous Vein Graft Intervention Results From the STENT (Strategic Transcatheter Evaluation of New Therapies) Group. J Am Coll Cardiol Intv. 2009;2:1105–1112. doi: 10.1016/j.jcin.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Lemos PA, Hoye A, Goedhart D, Arampatzis CA, Saia F, van der Giessen WJ, McFadden E, Sianos G, Smits PC, Hofma SH, de Feyter PJ, van Domburg RT, Serruys PW. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation. 2004;109:1366–1370. doi: 10.1161/01.CIR.0000121358.26097.06. [DOI] [PubMed] [Google Scholar]
- 18.Ellis SG, Brener SJ, DeLuca S, Tuzcu EM, Raymond RE, Whitlow PL, Topol EJ. Late myocardial ischemic events after saphenous vein graft intervention--importance of initially “nonsignificant” vein graft lesions. Am J Cardiol. 1997;79:1460–1464. doi: 10.1016/s0002-9149(97)00171-9. [DOI] [PubMed] [Google Scholar]
- 19.Rodes-Cabau J, Bertrand OF, Larose E, Dery J-P, Rinfret S, Bagur R, Proulx G, Nguyen CM, Cote M, Landcop M-C, Boudreault J-R, Rouleau J, Roy L, Gleeton O, Barbeau G, Noel B, Courtis J, Dagenais GR, Despres J-P, DeLarochelliere R. Comparison of plaque sealing with paclitaxel-eluting stents versus medical therapy for the treatment of moderate nonsignificant saphenous vein graft lesions: the moderate vein graft lesion stenting with the taxus stent and intravascular ultrasound (VELETI) pilot trial. Circulation. 2009;120:1978–1986. doi: 10.1161/CIRCULATIONAHA.109.874057. [DOI] [PubMed] [Google Scholar]