Abstract
Objective
Blau syndrome is an autoinflammatory disease resulting from mutations in NOD2 (nucleotide-binding oligomerization domain 2), wherein granulomatous arthritis, uveitis and dermatitis develop. The mechanisms by which aberrant NOD2 causes joint inflammation are poorly understood. Indeed very few studies have addressed NOD2 function in the joint. Here, we investigate NOD2 function in an experimental model of arthritis and explore the potential interplay between TLR2 and NOD2 in joint inflammation.
Methods
Mice deficient for TLR2, MyD88, or NOD2 and their wildtype controls were administered an intra-articular injection of muramyl dipeptide (MDP), peptidoglycan (PGN) (a metabolite of which is MDP), or Pam3CSK4, a synthetic TLR2 agonist. Joint inflammation was assessed using near-infrared fluorescence imaging and histology.
Results
Locally administered PGN results in joint inflammation, which was markedly reduced in mice deficient for either TLR2 or the TLR signaling mediator, MyD88. In addition to TLR2 signaling events, NOD2 mediated joint inflammation since mice deficient for NOD2 showed significantly reduced PGN-induced arthritis. TLR2 or MyD88 deficiency did not influence arthritis induced by the specific NOD2 agonist, MDP. In addition, NOD2 deficiency did not alter TLR2-dependent joint inflammation elicited by the synthetic TLR2 agonist, Pam3CSK4.
Conclusion
Whereas NOD2 and TLR2 are both critical for the development of PGN-arthritis, they appear to elicit inflammation independently of each other. Our studies support an inflammatory role for NOD2 in arthritis.
The NOD-like receptor family (NLR) plays a critical role in innate immunity. The members of the NLR family share many functional and structural characteristics and are thought to cooperate with Toll-like receptors (TLRs) in host defense. While much of the focus has been on the role of TLRs and their involvement in autoinflammatory diseases such as arthritis, the NLR family is emerging as an important participant in inflammation—perhaps even more so than TLRs in light of the association of the many NLR family members and inflammatory diseases (1, 2). One NLR family member in particular, NOD2 (also known as NLRC2 or CARD15), plays an important role in the health and function of diathrodial joints as evidenced by the fact that a single amino acid change in NOD2 causes Blau syndrome (3), which is characterized by inflammatory arthritis, uveitis and dermatitis (4, 5). In addition, most patients previously diagnosed with early onset sarcoidosis have been shown to have a mutation in the nucleotide oligomerization domain (NOD) of NOD2 (6). As such, understanding the function of NOD2 in the joints could clarify the pathogenesis of Blau syndrome and quite possibly other more common forms of arthritis.
NOD2 plays an important role in bacterial infections. It is understood that NOD2 functions as an intracellular sensor of muramyl dipeptide (MDP) (7-9), which is a breakdown product of peptidoglycan (PGN)—an ubiquitous component of bacterial cell walls. Once activated NOD2 plays a role in the induction of signal transduction pathways involving the kinase, RIP2, the transcription factor, NF-κB, and CARD9 and MAP kinases (10-13). Cross-talk between NOD2 and several different TLRs, including TLR2, has been observed, further linking TLR and NLR functioning and perhaps regulation. In some settings NOD2 amplifies the function of TLRs since suboptimal concentrations of specific TLR ligands and MDP delivered simultaneously can produce synergistic cytokine responses (14-17). Conversely, in models of colitis, activation of NOD2 by MDP has ability to suppress inflammation triggered by TLR activation (18). Interestingly, different polymorphisms in NOD2 increase the risk for developing Crohn's disease, a chronic inflammatory disorder of the intestinal tract (6). Mouse models of colitis support a negative regulatory role of NOD2 in intestinal inflammation and mice deficient in NOD2 have lost this negative regulatory function, making them prone to murine colitis when TLR2 is activated in the gut (18-20). Despite our understanding of the cellular function of NOD2, whether NOD2 exerts a similar regulatory capacity in the joint is far from understood.
We have previously demonstrated that NOD2 deficiency did not alter a T-cell dependent model of chronic and sterile arthritis induced by immunization with the cartilage component proteoglycan (21). However, our finding that MDP activation of NOD2 exacerbated proteoglycan-induced disease prompted us to explore the role of NOD2 in an acute model of inflammatory arthritis triggered by innate immunity. Evidence demonstrating expression of NOD2 within joint tissue (22, 23) along with the presence of bacterial cell wall components such as PGN and MDP within the joints of patients with rheumatoid arthritis (RA) (23, 24) would support the notion that bacterial components could directly activate NOD2 within the joints themselves to trigger local inflammation. In order to gain insight into this question, we have studied arthritis induced following intra-articular injection of two TLR2 ligands, peptidoglycan (PGN) and synthetic lipopeptide, N, palmityol(S)-[2,3-bis(palmitoyloxy)-(2Rs)-propyl]Cys-Ser-Lys4 (Pam3CSK4), along with muramyl dipeptide (MDP), a breakdown product of PGN that is sensed by NOD2. Using mice deficient in TLR2 and NOD2, we have explored the functioning of these proteins in acute inflammatory arthritis.
MATERIALS AND METHODS
Reagents
Synthetic MDP (Bachem; Torrance, CA) and Pam3CSK4 (Invivogen; San Diego, CA) or PGN from S. Aureus (Invivogen) were dissolved in pyrogen free, sterile saline for injections. All three reagents tested below the lower limit of detection of endotoxin activity.
Mice
Age-matched (10-12 week-old) female TLR2 deficient and NOD2 deficient mice and their wild-type C57Bl/6 controls were purchased from Jackson Laboratories (Bar Harbor, ME). MyD88 deficient mice on a BALB/c background were generously provided by Drs. Daniel Goldstein and Shizuo Akira (Yale University School of Medicine and Osaka University, respectively); BALB/c controls were purchased from Jackson Laboratories. Mice were housed in a facility approved by the Association of Assessment and Accreditation of Laboratory Animal Care International. Procedures were carried out in accordance with National Institutes of Health and guidelines designated by Oregon Health & Science University Institutional Animal Care and Use policies.
Arthritis
Mice were administered an intraarticular (i.a.) injection (5-10 μl volume) via a Hamilton syringe with a 30-gauge, half-inch needle. The contralateral knee was administered an i.a. injection of an equal volume of saline.
Neutrophil-depletion
Mice were administered an intravenous (i.v.) injection of 200 μg anti-Ly6G/Gr-1 rat mAb (clone RB6-8C5 from eBioscience) 18 hours prior to the i.a. injection of PGN. This antibody has been demonstrated to deplete neutrophils for 3 days (25). Our own data indicate that this antibody treatment regimen results in >90% reduction in circulating neutrophils over a 3 day period, as determined by flow cytometry. Control mice received an i.v. injection of 200 μg isotype control antibody rat IgG2bκ (eBioscience).
Near-Infrared (NIR) fluorescence imaging
The early events of inflammation within the joints involving protease activity were quantified according to a previously established method using Near-Infrared (NIR)-fluorescence imaging (21). Briefly, 24 hours prior to the time of imaging, animals were administered an i.v. injection of the protease substrate, Prosense (2 nmol/150 μl volume; ViseEn Medical, Woburn, MA). Prosense is a NIR fluorescent protease substrate that is incorporated into bone tissue and allows us to specifically visualize ongoing in vivo protease activity of cathepsins B, C, D, G, K, L and S, plasmin, and plasma kallikrein. NIR-fluorescence scanning of dissected knees was performed at the time of sacrifice using the Licor-Odessey infrared imaging system with a MousePod attachment. Images were then analyzed using commercially available software (Licore-Odessey, Lincoln, NE) for regions of interest within each knee and the mean differences in fluorescent intensity were quantified. Data are represented as mean fold induction relative the saline controls, which were scanned and analyzed at the same time.
Histology
Mouse joints were prepared for histological assessment as previously described (21). Briefly, joints were dissected, fixed in 10% neutral-buffered formalin, de-calcified and embedded in paraffin for sectioning. Seven-micrometer tissue sections were stained with hematoxylin and eosin and assessed by an observer masked to treatments. Slides were photographed at 200X using a microscope (DM500B; Leica, Wetzlar, Germany) and a digital camera (CD500; Leica).
Statistical Analysis
Data are represented as mean ± SEM. Mean differences between treatment and genotype controls were analyzed using a two-way and one-way analysis of variance with Bonferroni test or t-test post hoc analyses. Differences were considered statistically significant when p < 0.05.
RESULTS
TLR2 signaling is required for PGN-induced arthritis
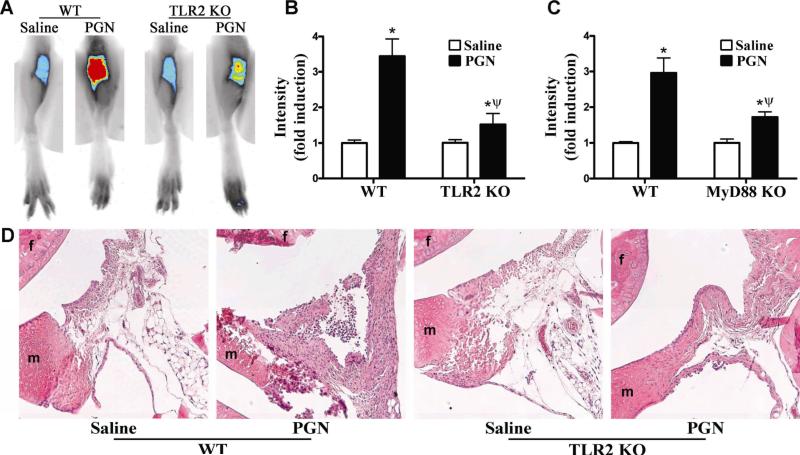
In order to examine the potential effect of NOD2 on arthritis triggered by the TLR2 pathway we chose to use known agonists of TLR2 as opposed to the previously established streptococcal cell wall (SCW) induced arthritis model, wherein a mixture of bacterial cell wall components and lipoproteins are used to induce disease locally (26). Our preliminary studies optimized the dose and timing of PGN-induced arthritis in mice. As shown in Figure 1A and B, locally administered PGN elicts joint inflammation in wild-type (WT) mice as assessed by Near-Infrared (NIR) fluorescence. NIR fluorescence imaging is a very sensitive and reproducible means of quantifying early inflammatory events that involve protease activity (as previously described (21)). This imaging technique has recently been applied to imaging various processes including inflammation in a different mouse model of inflammatory joint disease (27). Histological examination of WT mice supported the inflammatory effects of PGN on the joint as assessed by NIR imaging (Figure 1D, left panels). By day 3 the synovium has lost its normal fat content and has been infiltrated with mononuclear cells. The synovial membrane at the cartilage-synovial interface has become thickened and is starting to cover the cartilage surface.
Figure 1. TLR2 signaling events are involved in PGN-induced arthritis.
Mice were administered an intra-articular injection of 75 μg PGN in one knee and saline was administered to the contralateral knee. Inflammation within the knee joint was assessed 3 days later. A) Representative NIR-fluorescent images of knee joints of wild-type (WT) controls or TLR2 knockout (KO) mice, B) Quantification of mean NIR-intensity in TLR2 KOs versus WT controls, C) Quantification of mean NIR-intensity in MyD88 KO versus WT controls; D) Histological assessment of knee joints of TLR2 KOs versus WT controls (f: femur, m: meniscus). Data are the mean ± SEM; * p<0.05, comparison between treatments within a genotype; ψ p<0.05 comparison between KO and WT mice treated with PGN (n = 6 mice/treatment/genotype).
We assessed the role of TLR2 in PGN-induced arthritis using TLR2 KO mice. Mice deficient in TLR2 expression showed an almost complete abolishment in inflammation as assessed by NIR-intensity (Figure 1A-B); albeit the response remaining was still statistically different from saline-injected control knees. Histological assessment of TLR2 KO mice demonstrates the correlation between the reduction in NIR-fluorescence and degree of cellular infiltration within the joint, further supporting the role for TLR2 in PGN-induced arthritis (Figure 1D, right panels). We then further confirmed the contribution of TLR2 signaling events in PGN-induced arthritis using MyD88 KO mice. Deficiency in the TLR signaling mediator MyD88 significantly diminished joint inflammation (although not completely abolished) in response to PGN (Figure 1C). Taken together these data support a critical role for TLR2 signaling events in the development of joint inflammation induced by PGN.
NOD2 is required for PGN-induced arthritis
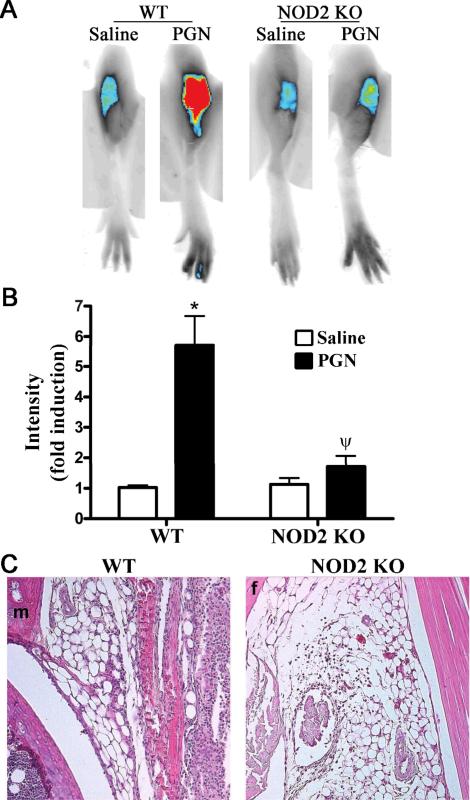
We went on to test whether the absence of NOD2 alters arthritis triggered by PGN. In comparison to WT mice administered PGN, NIR-fluorescence intensity was almost completely abolished in NOD2 KO mice (Figure 2A-B). The protective effect of NOD2 deficiency is also evident at the histopathological level (Figure 2C), wherein minimal cellular infiltration is observed in the NOD2 KO mice compared to the WT controls challenged with PGN. We have determined that maximal inflammation occurs at 3 days following PGN injection, as assessed here. However, we have also noted that NOD2 deficiency results in diminished arthritis at 24 hours following PGN treatment as well (data not shown), indicating its role in the initiation of joint inflammation. Taken together, these data support a role for NOD2 in mediating PGN-joint inflammation. This finding would be consistent with our data (Figure 1) indicating that deficiency in TLR2 or MyD88 does not completely abolish PGN-induced arthritis.
Figure 2. NOD2 is an important contributor in PGN-induced arthritis.
Mice were administered an intra-articular injection of 75 μg PGN in one knee and saline was administered to the contralateral knee. Inflammation within the knee joint was assessed 3 days later. A) Representative NIR-fluorescent images of knee joints of wild-type (WT) controls or NOD2 knockout (KO) mice, B) Quantification of mean NIR-intensity in NOD2 KOs versus WT controls, C) Histological assessment of PGN-treated WT or NOD2 KO mice (f: femur, m: meniscus). Data are the mean ± SEM; * p<0.05, comparison between treatments within a genotype; ψ p<0.05 comparison between KO and WT mice treated with PGN (n = 9 mice/treatment/genotype).
MDP-triggered NOD2 pathway operates independently of TLR2 to promote joint inflammation
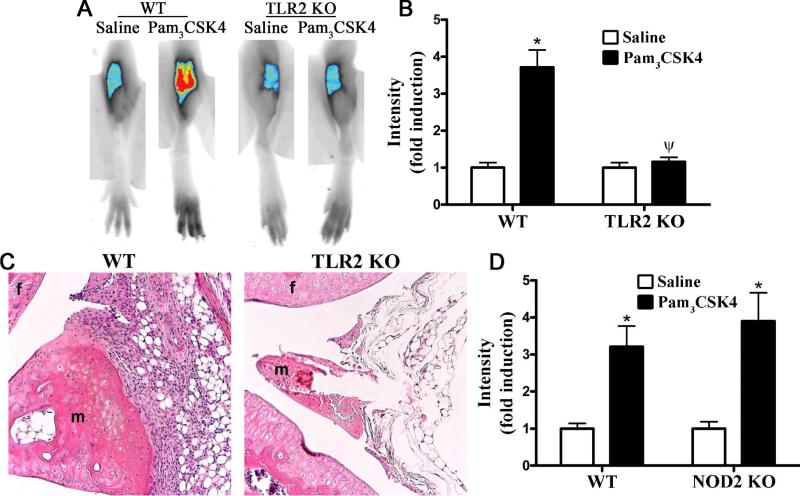
Our data presented above suggests that participation of both the TLR2/MyD88 pathway and NOD2 pathway is necessary for maximum joint inflammation caused by locally administered PGN. Prior work in experimental models of colitis indicates that TLR2 and NOD2 signaling are linked such that a function of NOD2 is to negatively regulate TLR2 signaling. However, since MDP is derived from PGN itself another possibility is that PGN and PGN-derived MDP function as agonists for TLR2 and NOD2, respectively. In this scenario, NOD2 would be directly activated rather than functioning in an indirect fashion to regulate TLR2-inflammation. We therefore further explored the potential interplay between NOD2 and TLR2-dependent inflammatory responses in the joint. To test whether NOD2 regulates TLR2 responses we used a synthetic TLR2 agonist (Pam3CSK4), which does not contain MDP as part of its structure, in order to induce arthritis. As the induction of arthritis by Pam3CSK4 has not been previously demonstrated, our preliminary work established an effective dose of Pam3CSK4 and time frame of arthritis (data not shown). We show here that locally administered Pam3CSK4 induces arthritis within 3 days as assessed by NIR-imaging (Figure 3A-B). Histologically, Pam3CSK4-induced arthritis was characterized by edema and cellular infiltration within the synovial membrane, subsynovial tissue, synovial space and articular cartilaginous tissues (Figure 3C). As would be expected, TLR2 deficiency completely abolishes joint inflammation (Figure 3A-C), indicating the specificity of Pam3CSK4 for TLR2 in vivo in the joint. We then tested whether the absence of NOD2 alters Pam3CSK4-induced joint inflammation. NOD2 KO mice develop arthritis to the same extent as WT controls (Figure 3D, solid bars, P value insignificant), suggesting that NOD2 is not directly involved in regulating TLR2 signaling events in the murine joint.
Figure 3. TLR2 activation induces arthritis independently of NOD2.
Mice were administered an intra-articular injection of 75 μg Pam3CSK4 in one knee and saline was administered to the contralateral knee. Inflammation within the knee joint was assessed 3 days later. A) Representative NIR-fluorescent images of knee joints of wild-type (WT) controls or TLR2 KO knockout (KO) mice, B) Quantification of mean NIR-intensity in WT controls versus TLR2 KO mice, C) Histological assessment of Pam3CSK4-treated WT or TLR2 KO mice (f: femur, m: meniscus); D) Quantification of mean NIR-fluorescence intensity in WT controls versus NOD2 KO mice. Data are the mean ± SEM; * p<0.05, comparison between treatments within a genotype; ψ p<0.05 comparison between KO and WT mice treated with PGN (n = 6 mice/treatment/genotype). There was no statistical effect of genotype on Pam3CSK4-induced joint inflammation.
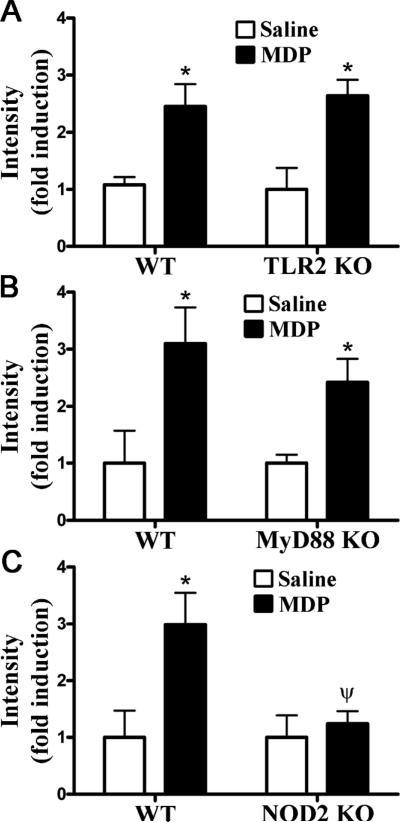
To further explore the putative regulation between TLR2 and NOD2-elicited inflammation in the joint, we examined the converse question and tested the functional role of TLR2 and its signaling mediator MyD88 in responsiveness to the NOD2 agonist MDP. As previously demonstrated (21), mice that were administered MDP developed arthritis within 24 hours following treatment based on NIR imaging (Figure 4). We demonstrate that deficiency in TLR2 expression did not alter MDP-induced arthritis, as assessed by NIR-imaging (Figure 4A) and further confirmed by histology (data not shown). Consistent with this finding, the absence of MyD88 did not significantly alter MDP-induced arthritis (Figure 4B). As would be expected however, MDP did elicit joint inflammation in a NOD2-dependent manner (Figure 4C), since arthritis was abolished in NOD2 deficient mice.
Figure 4. NOD2 activation induces arthritis independently of TLR2.
Mice were administered an intra-articular injection of 100 μg MDP in one knee and saline was administered to the contralateral knee. Inflammation within the knee joint was assessed 3 days later. Quantification of mean NIR-intensity in WT controls versus TLR2 KO mice (panel A), MyD88 KO mice (panel B), or NOD2 KO mice (panel C). Data are the mean ± SEM; * p<0.05, comparison between treatments within a genotype; ψ p<0.05 comparison between KO and WT mice treated with PGN (n = 6 mice/treatment/genotype).
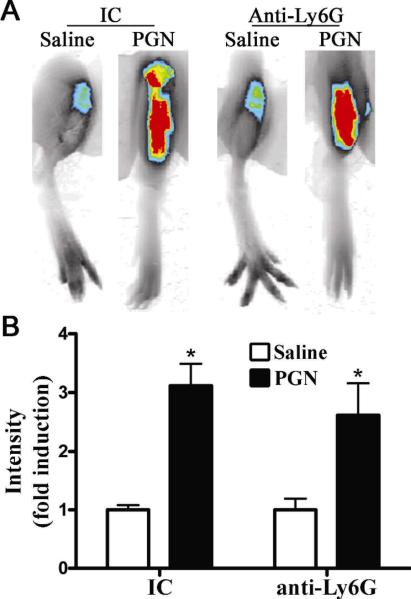
Neutrophils are not essential cellular mediators of locally triggered PGN-arthritis
We sought to clarify the contribution of neutrophils in our model of PGN-induced arthritis, wherein both TLR2 and NOD2 are involved. As such, we tested the functional contribution of neutrophils in locally administered PGN-induced arthritis. As previously reported (25) intravenous administration of anti-Ly6G neutrophil antibody resulted in depletion of circulating neutrophils. We found that by flow cytometry administration of anti-Ly6G neutrophil antibody depleted circulating levels of neutrophils >90% within 18 h of its administration and that depletion was sustained out to 3 days, at which point the experiment was terminated. We find that neutrophil depletion did not alter PGN-induced arthritis as assessed by NIR imaging (Figure 5). Consistent with the NIR-imaging results, histopathological assessment revealed no difference in infiltrating mononuclear cells and cellular proliferation within the articular cartilage (data not shown).
Figure 5. Neutrophils are not essential cellular mediators of arthritis induced by locally administered PGN.
For neutrophil depletion, mice were pretreated with anti-Ly6G neutralizing antibody or isotype control (IC) 18 hours prior to the administration PGN. Mice received an intra-articular injection of 75 μg PGN in one knee and saline was administered to the contralateral knee. Inflammation within the knee joint was assessed 3 days later by NIR fluorescent scanning and histology. A) Representative NIR-fluorescent images of knee joints of isotype control treated (IC) or neutrophil-depleted mice (Anti-Ly6G); B) Quantification of mean NIR-intensity in IC versus anti-Ly6G-treated mice. Data are the mean ± SEM; * p<0.05, comparison between treatments within a genotype; ψ p<0.05 comparison between KO and WT mice treated with PGN (n = 6 mice/treatment/genotype).
DISCUSSION
Despite the link between NOD2 and arthritis very few studies have examined NOD2 function within the joint. In murine experimental models of colitis, NOD2 functions to negatively regulate TLR2 signaling events in the setting of a significant bacterial load. Given that the joint is a sterile environment, we investigated TLR2-NOD2 interactions within the joint to determine if NOD2 negatively regulates inflammation in this organ as well.
We found that mice deficient in NOD2 developed significantly less PGN-induced arthritis than their WT controls, indicating that NOD2 promotes inflammation in the joint rather than suppressing inflammation. We show that PGN-induced arthritis does indeed involve TLR2 signaling events as mice deficient in either TLR2 or its signaling mediator, MyD88, demonstrated impaired PGN-induced arthritis. Taken together, these findings indicate that both NOD2 and TLR2 when activated within the joint promote inflammation. The possibility of NOD2 exerting a regulatory role on TLR2-induced inflammation was further explored in our experiments using the synthetic TLR2 agonist, Pam3CSK4, or the NOD2 agonist, MDP, to dissect the possible cooperation between NOD2 and TLR2 pathways. These data support a role for both NOD2 and TLR2 in promoting joint inflammation, albeit completely independently. It seems reasonable to postulate that joint inflammation triggered by PGN involves cellular recognition of PGN at the cell surface by TLR2 and then subsequent activation of NOD2 within the cell by PGN-derived MDP. In support of this theory, we have observed that co-treatment with PGN and MDP did not have an additive effect with regard to joint inflammation 3 days later (data not shown), suggesting both receptor systems had reached saturation and addition of more MDP could not be sensed.
The exact nature of how NOD2 influences the function of TLRs has yet to be completely resolved. The synergistic cooperation between NOD2 and TLRs that has been observed could be protective to the host in the elimination of pathogens. However, overproduction of cytokines can be harmful leading to tissue injury as in the case of colitis or inflammatory arthritis. While global suppression of TLR responses by NOD2 has been demonstrated (18, 19), our studies in an arthritis model would demonstrate the opposite that in fact, NOD2 does not participate in TLR2 suppression in the joint. This is not necessarily in opposition to other reports of NOD2 demonstrating its ability to regulate TLR-triggered inflammatory responses. Rather it indicates that the tissue type, the context of inflammation, or doses and timing, may influence the inflammatory effects of NOD2.
Our finding that NOD2 promotes PGN-induced arthritis would be consistent with a recent report by Saha et al wherein mice that were administered PGN systemically developed joint inflammation (25). However, several distinctions are also noted. Consistent with SCW-induced arthritis (23, 26) our data support an essential role for TLR2 and MyD88 in arthritis induced by locally administered PGN whereas Saha et al report a TLR2-independent mechanism (25). The importance of either TLR2 or NOD2 in PGN-induced arthritis is inferred from studies with KO mice. However, KO mice may have compensatory mechanisms that complicate our interpretation of experimental data. For example, TLR4 deficient mice have a defect in neutrophil development (28). In our own data, analysis of TLR2 KO mice would support the interpretation that TLR2 is the dominant receptor for PGN-induced arthritis. However, the use of NOD2 KO mice would argue that TLR2 plays a minimal role and NOD2 is the key determinant of PGN-induced arthritis, presumably after it has been degraded to MDP. While our study does not allow identification of whether or not TLR2 or NOD2 is the dominant receptor, it is clear that NOD2 is contributing to arthritis in a positive, enhancing manner.
In contrast to systemically administered PGN-induced arthritis, wherein neutrophils played an essential role in joint inflammation (25), our data indicate that neutrophils are not the major cellular mediator of arthritis induced by intra-articular PGN as neutrophil depletion had no marked effect on arthritis. The non-essential role of neutrophils would be consistent with a report of SCW-induced arthritis, which likely involves macrophages and other tissue resident cells (23, 26). Indeed by immunohistochemistry we detect minimal neutrophils present within the joint after i.a. injection of PGN and mice deficient in CD11b, an adhesion molecule considered necessary for neutrophil extravasation and infiltration into inflamed tissues, are still capable of developing PGN-induced arthritis (data not shown). NOD2 is thought to be predominantly expressed in myeloid cells. However, NOD2 is expressed in several other cell types within tissues including vascular endothelial cells, epithelial cells, osteoclasts and macrophages and fibroblasts in the synovium (22, 23, 29-31). Together with our finding, this would underscore the importance of NOD2 on cellular functions within the joint itself in the initiation and perpetuation of the inflammation and joint destruction within the joint. In contrast, i.v. administered PGN may upregulate NOD2 in or around joints spaces thereby acting on different cell types. This process has been shown to involve PGLYRP-2 expression and the induction of cytokine and chemokine expression, neutrophil recruitment and subsequent arthritis (25). Neutrophils may be involved in this model at other points in the pathway, such as upstream at the point of degrading PGN into MDP.
In conclusion, our studies here used different mouse models of arthritis to explore the interplay between NOD2 and TLR2 in inflammation within the joint. We provide evidence that in addition to TLR2, NOD2 functions within the joint promote arthritis. Each receptor system functions independently and NOD2 did not exert a negative regulatory effect on TLR2 signaling.
ACKNOWLEDGEMENTS
The authors are grateful for the technical assistant of Jeff Jensen and Marc Blackledge in obtaining the mouse knee NIR-images. We thank Drs. Shizuo Akira and Daniel Goldstein for the provision of the MyD88 KO mice.
Grant support: This work was supported by the US Department of Veterans Affairs Merit Review grant (MPD), National Eye Institute grants F32-EY017254 (HLR) and EY-013093 (JTR) along with a Gerlinger Award (through an award to OHSU to MDP/HLR), the Stan and Madelle Rosenfeld Family Trust, and the Fund for Arthritis and Infectious Disease Research. Funding for this project was provided by the American College of Rheumatology Research and Education Foundation New Investigator Award in addition the Research to Prevent Blindness Foundation (HLR).
Footnotes
AUTHOR CONTRIBUTIONS:
Dr. Rosenzweig had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design: Rosenzweig, Davey
Acquisition of data: Rosenzweig, Jann, Vance
Analysis and Interpretation of data: Rosenzweig, Jann, Davey, Rosenbaum, Planck
Manuscript preparation: Rosenzweig, Davey, Rosenbaum
REFERENCES
- 1.Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8(6):465–79. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Shaw MH, Kim YG, Nunez G. Nod-like Receptors: Role in Innate Immunity and Inflammatory Disease. Annu Rev Pathol. 2008 doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 3.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. National Genetics. 2001;29(1):19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 4.Blau EB. Familial granulomatous arthritis, iritis, and rash. The Journal of Pediatrics. 1985;107:689–693. doi: 10.1016/s0022-3476(85)80394-2. [DOI] [PubMed] [Google Scholar]
- 5.Jabs DA, Houk JL, Bias WB, Arnett FC. Familial granulomatous synovitis, uveitis, and cranial neuropathies. American Journal of Medicine. 1985;78:801–804. doi: 10.1016/0002-9343(85)90286-4. [DOI] [PubMed] [Google Scholar]
- 6.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. Embo J. 2004;23(7):1587–97. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 9.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, et al. Peptidoglycan Molecular Requirements Allowing Detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178(4):2380–6. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 11.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, et al. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2(8):736–42. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416(6877):194–9. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 13.Pauleau A-L, Murray PJ. Role of Nod2 in the Response of Macrophages to Toll-Like Receptor Agonists. Molec. Cell. Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol. 2005;35(8):2459–70. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 15.Tada H, Aiba S, Shibata K, Ohteki T, Takada H. Synergistic effect of Nod1 and Nod2 agonists with toll-like receptor agonists on human dendritic cells to generate interluekin-12 and T helper type 1 cells. Infect. Immun. 2005;73(12):7967–76. doi: 10.1128/IAI.73.12.7967-7976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uehara A, Yang S, Fujimoto Y, Fukase K, Kusomoto S, Shibata K, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with a chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocyte cells in culture. Cell Microbiol. 2005;7(1):53–61. doi: 10.1111/j.1462-5822.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.van Heel DA, Ghosh S, Butler M, Hunt K, Foxwell BM, Mengin-Lecreulx D, et al. Synergistic enhancement of Toll-like receptor responses by NOD1 activation. Eur J Immunol. 2005;35(8):2471–6. doi: 10.1002/eji.200526296. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Asano N, Murray PJ, Ozato K, Tailor P, Fuss IJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118(2):545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5(8):800–8. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25(3):473–85. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Rosenzweig HL, Jann MM, Glant T, Martin TM, Planck SR, Van Eden W, et al. Activation of nucleotide oligomerization domain 2 exacerbates a murine model of proteoglycan-induced arthritis. J Leukoc Biol. 2009;85(4):711–8. doi: 10.1189/jlb.0808478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ospelt C, Brentano F, Jungel A, Rengel Y, Kolling C, Michel BA, et al. Expression, regulation, and signaling of the pattern-recognition receptor nucleotide-binding oligomerization domain 2 in rheumatoid arthritis synovial fibroblasts. Arth Rheum. 2009;60(2):355–63. doi: 10.1002/art.24226. [DOI] [PubMed] [Google Scholar]
- 23.Joosten LA, Heinhuis B, Abdollahi-Roodsaz S, Ferwerda G, Lebourhis L, Philpott DJ, et al. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci U S A. 2008;105(26):9017–22. doi: 10.1073/pnas.0710445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arth Rheum. 2000;43(3):593–8. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Saha S, Qi J, Wang S, Wang M, Li X, Kim YG, et al. PGLYRP-2 and Nod2 are both required for peptidoglycan-induced arthritis and local inflammation. Cell Host Microbe. 2009;5(2):137–50. doi: 10.1016/j.chom.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joosten LA, Koenders MI, Smeets RL, Heuvelmans-Jacobs M, Helsen MM, Takeda K, et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171(11):6145–53. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 27.Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004;50(8):2459–65. doi: 10.1002/art.20379. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Gao XP, J. F, Liu Q, Anwar KN, Frey RS, et al. LPS activation of Toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L655–62. doi: 10.1152/ajplung.00327.2004. [DOI] [PubMed] [Google Scholar]
- 29.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc Res. 2006;71(2):103–7. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara Y, Uehara A, Fujimoto Y, Kusumoto S, Fukase K, Shibata K, et al. Toll-like receptors, NOD1 and NOD2 in oral epithelial cells. J Dent Res. 2006;85(6):524–9. doi: 10.1177/154405910608500609. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Takahashi N, Yamashita T, Sato N, Takahashi M, Mogi M, et al. Muramyl dipeptide enhances osteoclast formation induced by lipopolysaccharide, IL-1 alpha, and TNF-alpha through nucleotide-binding oligomerization domain 2-mediated signaling in osteoblasts. J Immunol. 2005;175(3):1956–64. doi: 10.4049/jimmunol.175.3.1956. [DOI] [PubMed] [Google Scholar]