Abstract
Background
While the mortality burden of the devastating 1918 influenza pandemic has been carefully quantified in the US, Japan, and European countries, little is known about the pandemic experience elsewhere. Here, we compiled extensive archival records to quantify the pandemic mortality patterns in two Mexican cities, Mexico City and Toluca.
Methods
We applied seasonal excess mortality models to age-specific respiratory mortality rates for 1915–1920 and quantified the reproduction number from daily data.
Results
We identified 3 pandemic waves in Mexico City in spring 1918, fall 1918, and winter 1920, characterized by unusual excess mortality in 25–44 years old. Toluca experienced 2-fold higher excess mortality rates than Mexico City, but did not have a substantial 3rd wave. All age groups including those over 65 years experienced excess mortality during 1918–20. Reproduction number estimates were below 2.5 assuming a 3-day generation interval.
Conclusion
Mexico experienced a herald pandemic wave with elevated young adult mortality in spring 1918, similar to the US and Europe. In contrast to the US and Europe, there was no mortality sparing in Mexican seniors, highlighting potential geographical differences in pre-existing immunity to the 1918 virus. We discuss the relevance of our findings to the 2009 pandemic mortality patterns.
Keywords: 1918 influenza pandemic, Mexico, Toluca, Transmissibility, age-specific mortality rates
Introduction
The 1918 influenza pandemic is considered by some as the “mother of all pandemics” and may have caused upwards of 20–50 million deaths worldwide during 1918–1920 [1]. Historical influenza pandemics, in particular the 1918 A/H1N1 pandemic, have received increasing attention in the last few years in an effort to better understand the factors driving the emergence of novel influenza viruses and their impact on human populations. Archeo-epidemiology studies have shed light on the age, temporal, and transmissibility patterns of historical pandemics in several regions of the world [2–11]. Influenza pandemics are characterized by an “age shift” in the proportion of influenza-related mortality towards younger age groups, relative to seasonal epidemics; the occurrence of multiple waves over short time periods, sometimes outside of typical winter seasons; and increased transmission resulting from lack of population immunity [10]. In addition, substantial geographical variations in pandemic mortality impact can occur within and between countries [5], perhaps due to differences in prior immunity, economy, background mortality levels, and population density. In particular, 1918 studies from England and Wales [12] and New Zealand [8] suggest that influenza-related mortality rates were higher in cities than rural areas.
Quantitative studies of the 1918 pandemic are hampered by the amount of time and efforts required to access archival paper records and digitize data. Reports from the Americas are scarce, with only a few studies from the US [3, 4], Canada [13, 14], and Brazil [15]. With the recent emergence of the swine-origin A/H1N1-pdm virus in Mexico [16, 17], followed by global pandemic activity during 2009, it is pertinent to gain more knowledge about past pandemic experiences in the Americas. To start filling this gap, we collected archival data on age-specific respiratory mortality to characterize the epidemiology and transmissibility of the 1918 pandemic in two Mexican cities, Mexico City and Toluca.
Material And Methods
Data sources
For both cities, Mexico City and Toluca, we examined mortality archives for 2–3 years before the pandemic, in order to estimate baseline mortality in pre-pandemic years, and assess the impact of the pandemic in subsequent years 1918–1920.
Mexico City
Population data
Mexico City is located in a valley in the central part of Mexico at an elevation of 2,240 meters. The census of 1910 registered 720,753 inhabitants in the city, while the 1921 population size was 906,063, representing a mean annual increase of 2.3% [18]. We used age-specific population estimates from the 1910 decennial census to derive age-specific mortality rates.
Monthly pneumonia and influenza mortality statistics, 1916–1920
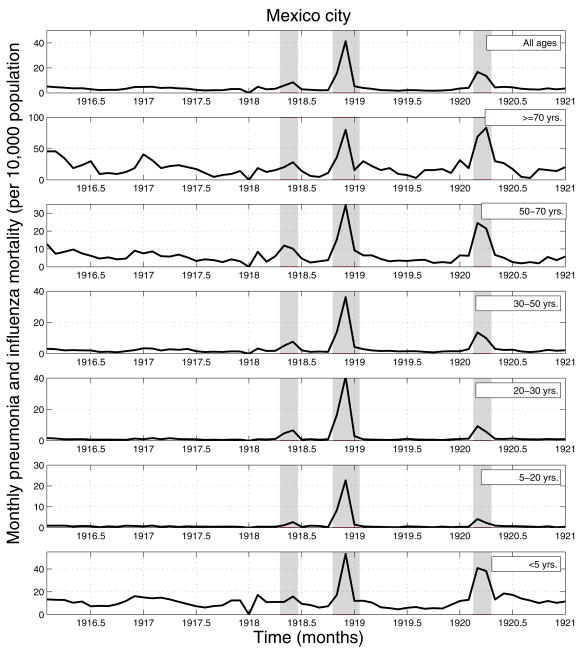
We obtained monthly numbers of pneumonia and influenza (P&I) deaths from the epidemiological Bulletins published by Mexico City’s Superior Council of Hygiene during the period 1916–1920 [19] and stratified by age group (<5, 5–19, 20–29, 30–49, 50–69, over 70 years of age; Figure 1). Monthly time series stratified into 8 smaller administrative regions comprising Mexico City were also available.
Figure 1.
Age-specific monthly time series of pneumonia and influenza (P&I) mortality rates for Mexico City, 1916–1920. Areas shaded in red highlight 3 time periods of high mortality associated with three waves of the 1918–20 pandemic occurring in spring (Apr–May 1918), fall (Oct–Dec 1918), and winter (Feb–Mar 1920).
Daily historical death records, Civil Registry, 1918
To obtain more detailed information about the temporal dynamics of the pandemic waves during year 1918 in Mexico City, and allow estimation of transmission characteristics from daily time series, we explored Mexico City’s Civil Registry. We recorded all respiratory deaths during Apr–May and Sep–Dec 1918, which were periods of large increases in mortality based on the monthly statistics. For each death record from the Registry, we manually retrieved age, cause and exact date of death. Based on this information, we compiled daily and weekly respiratory mortality (influenza, pneumonia and bronchitis) time series. A total of 4,749 respiratory deaths were identified during the spring and autumn waves of 1918 through this system.
Toluca city, Mexico State
Population data
The city of Toluca is located in one of the valleys of central Mexico at an elevation of 2,667 meters. We selected this city because it experienced stable and slow population growth for at least eight years prior to the influenza pandemic of 1918. The census of 1910 registered 31,023 inhabitants in the city, while the population size in 1921 was 34,265, representing a mean annual increase of 0.9% [20]. We estimated the age-specific population size for Toluca from the 1910 decennial census data for the state of Mexico, where the city is located [21] (no city-specific census data were available).
Historical death records, General Cemetery, 1915–1920
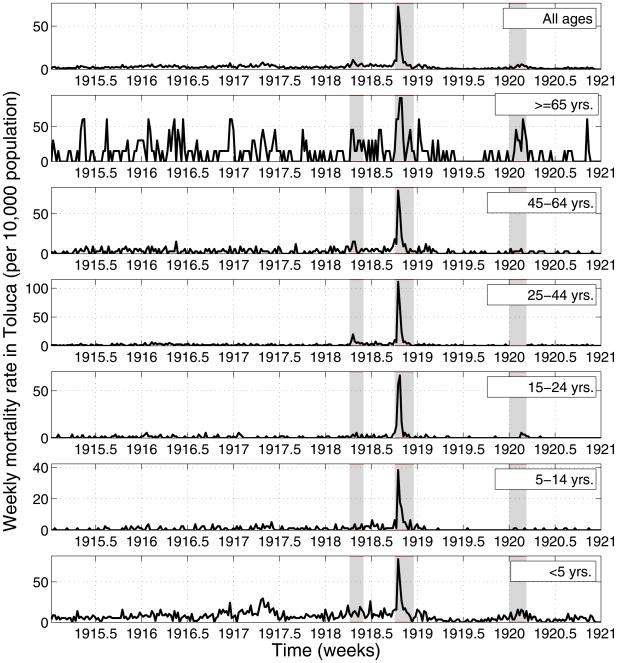
We manually retrieved a total of 2,998 mortality records for the period 1915–1920 from the Office of the General Cemetery in Toluca and recorded the exact age, cause, and date of death. Death certificates were completed by physicians and all burials were performed in a single cemetery (Panteon General); the complete set of records remains at the cemetery’s office. We compiled daily and weekly respiratory mortality (influenza, pneumonia and bronchitis) time series stratified into six age groups (0–4, 5–14, 15–24, 25–44, 45–64, and >=65; Figure 2). These age groups were chosen for comparison with a detailed quantitative study of the 1918 pandemic in New York City [4].
Figure 2.
Age-specific weekly time series of respiratory mortality per 10,000 people in the city of Toluca, Mexico State, 1915–1920. Areas shaded in red highlight 3 time periods of high mortality associated with three waves of the 1918–20 pandemic occurring in spring (Apr-2 to Jun-3, 1918), fall (Oct-1 to Dec-23, 1918), and winter (Jan-1 to Mar-11, 1920).
Estimation of excess mortality attributable to influenza
To estimate the mortality attributable to the influenza pandemic, we calculated excess mortality for each wave during 1918–1920 over a traditional Serfling model baseline [7, 22, 23]. We established the baseline by applying a cyclical Serfling linear regression model to weekly or monthly respiratory mortality time series, after excluding data from year 1918 and winter months (December–March) in other years. Influenza periods were defined as months or weeks when mortality exceeded the upper limit of the 95% confidence interval on this baseline. Weekly or monthly excess mortality was defined as mortality in excess of the baseline during influenza periods. We summed the excess deaths above the model baseline during each influenza period identified during 1918–20 to estimate the mortality burden of each pandemic wave. Separate models were fitted to each age group and city; all model fits were good (0.65 ≤ R2 ≤ 0.73).
As a sensitivity analysis, we also estimated excess mortality associated with each pandemic wave using a “model-free” approach, in which reference months in pre-pandemic years are used to estimate baseline mortality (adapted from [5]). Finally, we also calculated the relative risk of pandemic death, defined as the ratio of excess mortality during pandemic periods to the expected mortality in the absence of influenza virus activity from the model baseline. The relative risk facilitates comparison between age groups and locations which have different baseline risks of deaths [4, 23].
Estimation of transmission characteristics (Reproduction number)
We also characterized the intrinsic transmission parameter for each pandemic wave. The basic reproduction number (R0) is defined as the average number of secondary cases generated by a primary case during the initial epidemic period in an entirely susceptible population [24, 25] while the reproduction number, R, measures transmission potential at the beginning of an epidemic in a partially immune population [12]. During the initial wave of a pandemic, there is little or no background population immunity, and hence we can expect R to approximate R0. Nevertheless, the reproduction number could vary spatially and temporally depending on the season in which the novel influenza virus is introduced into local populations.
We estimated the reproduction number, R, using the intrinsic growth rate method, as in [12, 26]. The growth rate was estimated by fitting an exponential function to the initial increase in the daily number of respiratory deaths [27], assuming exponentially distributed latent and infectious periods [26, 28] or a fixed generation interval [26]. We also tested the robustness of R to the choice of mortality outcomes and compared estimates derived from crude respiratory deaths and excess respiratory deaths.
To account for the uncertainty associated with the generation interval for influenza, we considered two extreme values used in past research: a short interval of 3 days [26, 29, 30] and a longer interval of 6 days [3, 31]. The same approach was used by Andreasen et al. [7] to quantify R for the summer and autumn 1918 pandemic waves in Copenhagen, Denmark, so that the Copenhagen and Mexico estimates are directly comparable.
Results
Timing of pandemic waves and age mortality patterns
The age-stratified time series of P&I mortality in Mexico City (Figure 1) reveals a pattern of three successive waves of increased mortality occurring in spring (Apr–May 1918), fall (Sep–Dec 1918) and winter (Jan–Apr 1920). These mortality waves were synchronized across the 8 administrative regions of Mexico City (not shown). In the spring wave (Apr–May 1918), P&I mortality rates increased by 10% to 150% above baseline levels, depending on the administrative region. In the main autumn pandemic wave, P&I mortality rates increased by 400% to 1,100% over baseline. By contrast, in the 3rd wave occurring during winter 1919–1920, the increase in P&I mortality rates was more moderate, ranging from 23% to 76% across all administrative regions of Mexico City.
In contrast to Mexico City, the smaller city of Toluca (Figure 2) experienced a small increase in respiratory mortality rates during spring (April 2nd to June 3rd, 1918), a large increase during fall (October 1 to December 23, 1918), and little excess mortality in the winter of 1919–1920. Of note in Toluca, respiratory mortality remained elevated throughout summer 1918, persisting at levels 2–3 fold above that of baseline pre-pandemics summers.
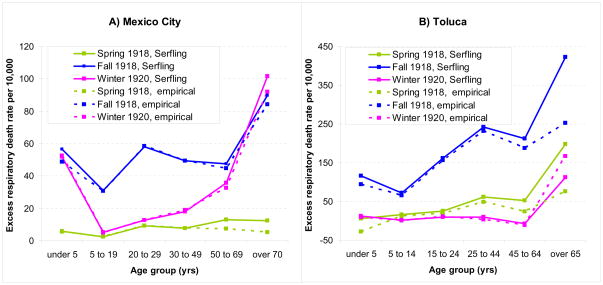
Figure 3 illustrates the comparison between seasonal regression and model-free approaches to estimate age-specific excess respiratory mortality rates. Age-specific estimates were consistent with the two approaches, with correlation coefficients above 0.90 (P<0.01), although the Serfling approach tended to produce somewhat higher estimates (by ~8% on average for Mexico City and 14% for Toluca). Overall, the age-specific excess mortality rates were consistent across cities and were reminiscent of a W-shaped pattern, with lowest excess mortality rates in children and teenagers 5–19 yrs and adults 45–64 yrs, and high excess mortality in all other age groups including seniors (Table 1 and 2). Although few deaths occurred during the spring 1918 wave, young adults aged 20–50 yrs experienced unusually high excess death rates. The unusual mortality elevation in young adults persisted in autumn 1918 and almost disappeared by the following winter in 1920.
Figure 3.
Age-specific estimates of excess respiratory mortality rates during the spring 1918, fall 1918 and winter 1919–20 influenza pandemic waves in Mexico City and Toluca. Estimates are based on 2 independent methods: 1) a Serfling approach using seasonal linear regression to estimate baseline non-influenza mortality [7, 22, 23] and 2) an empirical method using mortality in pre-pandemic years 1915–17 as baseline [5].
Table 1.
Age-specific mortality impact associated with the spring, fall, and winter waves of the 1918–20 influenza pandemic in Mexico City, Mexico. Excess mortality estimates are based on a seasonal regression approach applied to monthly respiratory mortality and presented as rates per 10,000. A relative risk of death is also presented, based on the ratio of excess mortality to baseline mortality, facilitating comparisons across age groups which have different background risk of death.
| Spring 1918 wave Apr–May, 1918 | Fall 1918 wave Oct–Dec, 1918 | Winter 1920 wave Feb–Mar, 1920 | ||||
|---|---|---|---|---|---|---|
| Age Group | Excess Mortality rate per 10,000 | Relative risk over baseline mortality | Excess Mortality rate per 10,000 | Relative risk over baseline mortality | Excess Mortality rate per 10,000 | Relative risk over baseline mortality |
| all ages | 6.6 | 1.2 | 47.0 | 7.0 | 19.3 | 2.6 |
| 0–4 yrs | 5.9 | 0.3 | 56.6 | 2.2 | 52.0 | 1.8 |
| 5–19 yrs | 2.6 | 2.3 | 31.2 | 24.5 | 5.4 | 4.5 |
| 20–29 yrs | 9.2 | 4.7 | 58.3 | 26.3 | 12.7 | 5.0 |
| 30–49 yrs | 7.9 | 7.7 | 49.5 | 9.9 | 18.0 | 18.9 |
| 50–69 yrs | 13.0 | 1.4 | 47.5 | 4.2 | 35.8 | 3.3 |
| >=70 yrs | 12.5 | 0.3 | 89.8 | 2.1 | 101.8 | 2.0 |
Table 2.
Age-specific mortality impact associated with the spring, fall, and winter waves of the 1918–20 influenza pandemic in the city of Toluca, Mexico. Excess mortality estimates are based on a seasonal regression approach applied to weekly respiratory mortality and presented as rates per 10,000. A relative risk of death is also presented, based on the ratio of excess mortality to baseline mortality, facilitating comparisons across age groups which have different background risk of death.
| Spring 1918 wave Apr-5 to Jun-7, 1918 | Autumn 1918 wave Sep-27 to Dec-20, 1918 | Winter 1920 wave Feb-27 to Mar-26, 1920 | ||||
|---|---|---|---|---|---|---|
| Age Group | Excess Mortality rate per 10,000 | Relative risk over baseline mortality* | Excess Mortality rate per 10,000 | Relative risk over baseline mortality* | Excess Mortality rate per 10,000 | Relative risk over baseline mortality* |
| all ages | 18.6 | 1.0 | 162.3 | 14.4 | 9.9 | 1.3 |
| 0–4 yrs | 1.3 | 0.1 | 118.2 | 3.8 | 15.0 | 0.7 |
| 5–14 yrs | 4.6 | 0.8 | 72.6 | 27.1 | 0.6 | 1.0 |
| 15–24 yrs | 12.5 | 2.1 | 161.6 | 39.6 | 10.8 | 5.0 |
| 25–44 yrs | 47.5 | 4.8 | 245.1 | 53.1 | 12.1 | 4.2 |
| 45–64 yrs | 25.8 | 1.1 | 208.6 | 13.1 | 0 | 0 |
| >=65 yrs | 0 | 0 | 381.3 | 6.5 | 106.8 | 1.4 |
calculated as excess mortality divided baseline mortality during influenza epidemic months
Estimates of the relative risk of death associated with each pandemic wave, age group, and city, are provided in Tables 1 and 2, facilitating comparison between population groups experiencing different baseline risks of deaths. In both cities, a substantial increase in mortality rates was observed among seniors >=65 years of age during the autumn 1918 and winter 1920 wave, with a 2–6 fold elevation over baseline. Despite the high absolute excess mortality rates in seniors, however, the highest relative pandemic risk increase was experienced by young adults with a 25–50 fold increase above baseline during autumn 1918. In Mexico City where a more detailed age break-down is available, the peak relative risk of death was observed at age 20–29 yrs during the autumn wave. During the same period in Toluca, the peak relative risk of death was found in the broader age group 25–44 yrs.
Transmissibility estimates
Estimates of the reproduction number and confidence intervals for the spring and autumn waves of the 1918 influenza pandemic in Toluca and Mexico City are provided in Tables 3 and 4. For Mexico City, the mean reproduction number ranged from 1.3–1.8 for the spring wave and 1.3–1.7 for the fall wave assuming a serial interval of 3 or 6 days. Estimates of the reproduction number were higher for the city of Toluca, ranging from 1.6–3.1 for the spring wave and 2.1–6.1 for the autumn wave. These estimates did not change substantially when basing the estimation on excess respiratory deaths instead of all respiratory deaths.
Table 3.
Mean estimates of transmissibility for the summer 1918 and fall 1918 waves of the pandemic in Mexico City, assuming a serial interval of 3 or 6 days that is either exponentially distributed or fixed (delta distribution).
| 3-day serial interval | 6-day serial interval | |||
|---|---|---|---|---|
| Exp dist. | Delta dist. | Exp. Dist. | Delta dist. | |
| Spring wave | 1.3 (1.3, 1.3) | 1.3 (1.3, 1.3) | 1.7 (1.6, 1.7) | 1.8 (1.8, 1.8) |
| Autumn wave | 1.3 (1.3, 1.3) | 1.3 (1.3, 1.3) | 1.6 (1.6, 1.6) | 1.7 (1.7, 1.8) |
Table 4.
Mean estimates of transmissibility for the spring 1918 and autumn 1918 waves of the in the city of Toluca, Mexico assuming a serial interval of 3 or 6 days that is either exponentially distributed or fixed (delta distribution).
| 3-day serial interval | 6-day serial interval | |||
|---|---|---|---|---|
| Exp dist. | Delta dist. | Exp. Dist. | Delta dist. | |
| Estimates based on daily number of respiratory deaths | ||||
| Spring wave | 1.6 (1.5, 1.7) | 1.8 (1.6, 1.9) | 2.4 (2.2, 2.6) | 3.1 (2.6, 3.6) |
| Autumn wave | 2.1 (2.1, 2.1) | 2.5 (2.4, 2.5) | 3.5 (3.4, 3.6) | 6.1 (5.9, 6.3) |
| Estimates based on daily number of excess respiratory deaths | ||||
| Spring wave | 1.7 (1.6, 1.7) | 1.8 (1.7, 1.8) | 2.4 (2.4, 2.5) | 3.1 (3.0, 3.3) |
| Autumn wave | 2.0 (2.0, 2.1) | 2.3 (2.2, 2.3) | 3.2 (3.2, 3.2) | 5.1 (5.0, 5.2) |
Discussion
To the best of our knowledge, this is the first study to quantify the age-specific excess mortality impact of the devastating 1918 influenza pandemic in Mexico, a country that remained neutral during World War I. This work involved intense primary data collection efforts to compile archival age-stratified respiratory mortality rates for years before and during the pandemic in two cities, Mexico City and Toluca. We document a pattern of 3 successive pandemic waves in Mexico City and Toluca in Spring 1918, Fall 1918 and Winter 1920, although the third pandemic wave was very minor in Toluca. In line with reports from the US and Europe [4, 7, 32], young Mexican adults aged 25–44 years old experienced an unusually elevated risk of respiratory mortality, especially during the first 2 pandemics waves. However, in contrast to previous studies, the mortality data available from 2 Mexican cities suggest that individuals aged 65 and over were not spared by this pandemic and experienced substantial influenza-related excess mortality.
The early wave of respiratory mortality reported in Mexico City and Toluca in spring 1918 was associated with increased death rates in young adults as compared to baseline mortality in prior years, consistent with the signature age mortality patterns of 1918 A/H1N1 pandemic virus [4, 7]. Similar herald waves of excess respiratory mortality in young adults have been reported in spring and summer 1918 in other regions of the world, including New York City [4], Geneva [2, 33], Copenhagen [7], the US military [7] the UK [12], and Singapore [34]. Hence our study and others are suggestive of the early emergence and circulation of a mild form of the pandemic A/H1N1 virus in February–May 1918 in North America. We note that while the pattern of increased mortality in young adults was particularly marked in the fall 1918 wave in Mexico City, it had almost disappeared by the 1920 winter, suggesting high immunity levels achieved in the young adult population from infection in prior waves, possibly combined with decreasing severity of the A/H1N1 infection.
The exact mortality patterns associated with the 1918 pandemic virus have long been debated [4, 7, 35, 36]. Visual inspection of age-specific respiratory mortality rates for year 1918 suggests a W-shape pattern of death in many locations, characterized by high mortality in infants, young adults, and seniors over 65 years [35]. However, annual respiratory mortality is a crude and biased indicator of the actual burden of pandemic influenza, because it includes background death rates from other pathogens, which are particularly high in infants and elderly. Careful studies quantifying monthly mortality occurring in excess of background have shown that seniors over 65 years in New York City and Copenhagen experienced little to no excess mortality attributable to influenza during the pandemic [4, 7]. A recent study exploring influenza-specific mortality in Madrid and Paris reported that the proportion of deaths in seniors over 65 was only 5–6% in fall 1918, much lower than in previous inter-pandemic seasons (36–42%), suggesting that seniors were at least partially spared during the 1918 pandemic in these European cities [11]. In contrast to these studies, our Mexican data suggest that seniors over the age of 65 years experienced 1.5–2.4 fold higher excess mortality rates than young adults during fall 1918, a greater than 2-fold elevation over their baseline mortality rate. This is the first quantitative study to document high excess mortality among seniors during the 1918 pandemic and to produce a true W-like pattern of excess mortality risk by age, even after carefully accounting for the high background risk of deaths in seniors. This finding of elevated mortality in seniors is in agreement with anecdotal evidence from aboriginal populations in Alaska in 1918 [37], although with such remote populations it is difficult to integrate data on background risk of death, and obtain reliable population size estimates. Overall, the Mexican experience suggests that the pronounced mortality sparing of seniors documented in the historical US and European studies may not have been a global phenomenon.
The biological and immunological reasons behind the complicated age pattern of mortality associated with the 1918 pandemic remain debated. Previous studies have put forward the hypothesis that childhood exposure to antigenically-related A/H1N1 viruses before 1870 might account for mortality sparing in seniors, consistent with the pandemic age mortality profiles described in the US and Europe [4, 7]. Our Mexican data provide evidence that some urban North American senior populations lacked protection against pandemic mortality, suggesting differences in prior immunity to the 1918 H1N1 virus between countries. Such differences may result from heterogeneous circulation of influenza viruses in the 19th century, at a time when long-distance population travels were much less developed than today.
A theoretical scenario has been proposed for populations lacking prior immunity to the 1918 pandemic virus, in which the age-specific mortality curve follows a V-shape bottoming in early teenage and increasing rapidly and monotonously in older age groups [38]. Our Mexican mortality data does not support this pessimistic scenario but suggests that two factors may drive the age distribution of pandemic-related mortality in 1918: an unidentified factor increasing the risk of deaths in young adults and likely present globally, and a partially protective factor in people ~65 and over and present in Europe [7], the US [4] and Japan [32] but absent in Mexico and remote populations [37]. It has been hypothesized that the mortality risk factor in young adult may be mediated by an increased probability of cytokine storm upon influenza infection, although this remains a subject of debate [39].
Overall, we estimated that 0.7% of the population of Mexico City died of influenza during 1918–20. This estimate falls in the low range of reported excess pandemic death rates in countries in Europe [40] and elsewhere [5], but it is about twice as high as that experienced in New York City [4] or Copenhagen [7]. By contrast, pandemic-related excess death rate in Toluca is in the mid-range of the available global estimates at 1.9% [5]. Substantial variability in pandemic excess mortality rate within and between countries has been linked with variation in socio-economic conditions [5] and latitude [40], but remains poorly understood. It is possible that poorer socio-economic conditions, issues with access to health care, or environmental conditions may explain the higher death rate in Toluca than Mexico City.
In past research, transmissibility estimates derived from 1918–20 pandemic morbidity and mortality data were in the range 1.5 to 5.4 for community-based settings in several regions of the world [3, 7, 41–43] and 2.1–7.5 for some confined settings [41]. In this study, the reproduction number was significantly higher for Toluca than Mexico City. Of note, Toluca is located at a higher elevation than Mexico City (2667m vs. 2240m), and hence absolute humidity is generally lower in Toluca than in Mexico City. Therefore, it is possible that aerosol spread is more efficient in Toluca than in Mexico City, perhaps due to increased survival of the virus within aerosolized droplets as recently suggested by experimental and epidemiological studies [44, 45], and potentially explaining higher influenza transmissibility.
It is interesting to compare reproduction number estimates across successive pandemic waves to gauge potential changes in virus characteristics and population immunity. While the reproduction number estimate was lower in spring 1918 than autumn 1918 in Toluca, it was similar for both waves in Mexico City. Low estimates for the spring wave in both cities are in line with a previous study in Geneva (R~1.5) [33] and in the lower range of previous estimates for Copenhagen [7]. The apparent increase in reproduction number from Spring to Autumn in Toluca is in agreement with the Geneva study [33] and perhaps partially explained by increased fitness of the influenza virus during more propitious weather conditions in the fall, even after accounting for decreased population susceptibility following spring and summer outbreaks. In contrast, reproduction number estimates substantially decreased from spring to autumn in Copenhagen [7]. Differences in reproduction number estimates across locations and pandemic waves may reflect true differences attributable to spatiotemporal variation in attack rates of successive waves or local factors affecting transmission, or may simply illustrate difficulties measuring this important parameter with precision [27].
Although mortality data for the 2009 A/H1N1 pandemic are still preliminary, seniors appear to be partially spared, with only about 12% of influenza-related deaths occurring in people over 60 yrs, as compared with more than 90% in typical inter-pandemic seasons [17, 46–49]. This pattern is reminiscent of the 1918 pandemic in Europe and the US where only 0–6% of excess deaths occurred in people over 65 yrs, as compared with 33–42% in pre-pandemic influenza seasons [4, 7]. The multiple pandemic wave pattern of the H1N1-pdm virus in most of the Northern Hemisphere is also reminiscent of the 1918 pandemic, with a first wave in spring 2009 followed by a fall wave associated with high attack rates in most places. Although the impact of the novel H1N1pdm influenza virus has been significantly lower than that of the 1918 pandemic, and the fear of a returning lethal fall wave has not materialized so far, planning for the worse and monitoring pandemic mortality burden across age groups and countries is a prudent course of action until this virus has circulated in the population for several years. In parallel, our study highlights the importance of collecting historical mortality data from multiple locations around the world to quantify the impact of past influenza pandemics on populations, especially in lesser studied areas of the Americas, Asia and Africa.
Acknowledgments
Funding information
This work was supported in part (LS) by the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and Fogarty International Center, National Institutes of Health. This research was conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), an on-going international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php). Funding for this project comes from the Office of Global Health Affairs’ International Influenza Unit in the Office of the Secretary of the Department of Health and Human Services.
Footnotes
Competing interests
The authors have declared that no competing interests exist.
References
- 1.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–15. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 2.Ammon CE. Spanish flu epidemic in 1918 in Geneva, Switzerland. Euro Surveill. 2002;7:190–2. doi: 10.2807/esm.07.12.00391-en. [DOI] [PubMed] [Google Scholar]
- 3.Mills CE, Robins JM, Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432:904–6. doi: 10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci U S A. 2005;102:11059–63. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368:2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 6.Gottfredsson M, Halldorsson BV, Jonsson S, et al. Lessons from the past: familial aggregation analysis of fatal pandemic influenza (Spanish flu) in Iceland in 1918. Proc Natl Acad Sci U S A. 2008;105:1303–8. doi: 10.1073/pnas.0707659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–8. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McSweeny K, Colman A, Fancourt N, et al. Was rurality protective in the 1918 influenza pandemic in New Zealand? N Z Med J. 2007;120:U2579. [PubMed] [Google Scholar]
- 9.Nishiura H, Chowell G. Rurality and pandemic influenza: geographic heterogeneity in the risks of infection and death in Kanagawa, Japan (1918–1919) N Z Med J. 2008;121:18–27. [PubMed] [Google Scholar]
- 10.Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics--implications for policy. N Engl J Med. 2009;360:2595–8. doi: 10.1056/NEJMp0903906. [DOI] [PubMed] [Google Scholar]
- 11.Eroreka A. The Spanish influenza pandemic in occidental Europe (1918–1920) and victim age. Influenza and Other Respiratory Viruses. 2010;4:81–89. doi: 10.1111/j.1750-2659.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowell G, Bettencourt LM, Johnson N, Alonso WJ, Viboud C. The 1918–1919 influenza pandemic in England and Wales: spatial patterns in transmissibility and mortality impact. Proc Biol Sci. 2008;275:501–9. doi: 10.1098/rspb.2007.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowell G, Brauer F. The basic reproduction number of infectious diseases: Computation and estimation using compartmental epidemic models. In: Chowell G, Hyman JM, Bettencourt LM, Castillo-Chavez C, editors. Mathematical and Statistical Estimation Approaches in Epidemiology. Springer; 2009. [Google Scholar]
- 14.Palmer C, Sattenspiel L, Cassidy C. The Spread of the Spanish Flu on the Island of Newfoundland. Newfoundland and Labrador Studies. 2007;22:1719–1726. [Google Scholar]
- 15.Massad E, Burattini MN, Coutinho FA, Lopez LF. The 1918 influenza A epidemic in the city of Sao Paulo, Brazil. Med Hypotheses. 2007;68:442–5. doi: 10.1016/j.mehy.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 18.Cuadernos Estatales. Secretaria de Salud. 1996. Mexico City: 1996. [Google Scholar]
- 19.Monthly Epidemiological Bulletin. Mexico’s Superior Council of Hygiene. 1916–1920. [Google Scholar]
- 20.Instituto Nacional de Geografia Estadistica e Informatica. Estadisticas Historicas de Mexico. Aguascalientes, Ags; 1999. p. 27. [Google Scholar]
- 21.Instituto Nacional de Geografia Estadistica e Informatica. Estadisticas Historicas de Mexico. Aguascalientes, Ags; 1999. p. 33. [Google Scholar]
- 22.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506. [PMC free article] [PubMed] [Google Scholar]
- 23.Viboud C, Grais RF, Lafont BA, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192:233–48. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RM, May RM. Infectious diseases of humans. Oxford: Oxford University Press; 1991. [Google Scholar]
- 25.Diekmann O, Heesterbeek J. Mathematical epidemiology of infectious diseases: model building, analysis and interpretation. Wiley; 2000. [Google Scholar]
- 26.Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci. 2007;274:599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowell G, Nishiura H, Bettencourt LM. Comparative estimation of the reproduction number for pandemic influenza from daily case notification data. J R Soc Interface. 2007;4:155–66. doi: 10.1098/rsif.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipsitch M, Cohen T, Cooper B, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–70. doi: 10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boelle PY. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23:3469–87. doi: 10.1002/sim.1912. [DOI] [PubMed] [Google Scholar]
- 31.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159:623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 32.Richard SA, Sugaya N, Simonsen L, Miller MA, Viboud C. A comparative study of the 1918–1920 influenza pandemic in Japan, USA and UK: mortality impact and implications for pandemic planning. Epidemiol Infect. 2009;137:1062–72. doi: 10.1017/S0950268809002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowell G, Ammon CE, Hengartner NW, Hyman JM. Estimation of the reproductive number of the Spanish flu epidemic in Geneva, Switzerland. Vaccine. 2006;24:6747–50. doi: 10.1016/j.vaccine.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 34.Lee VJ, Chen MI, Chan SP, et al. Influenza pandemics in Singapore, a tropical, globally connected city. Emerg Infect Dis. 2007;13:1052–7. doi: 10.3201/eid1307.061313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195:1018–28. doi: 10.1086/511989. [DOI] [PubMed] [Google Scholar]
- 36.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 37.Crosby AW. Epidemic and Peace, 1918. Westport, CT: Greenwood Press; 1976. [Google Scholar]
- 38.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 39.Fedson DS. Was bacterial pneumonia the predominant cause of death in the 1918–1919 influenza pandemic? J Infect Dis. 2009;199:1408–9. doi: 10.1086/597621. author reply 1409–10. [DOI] [PubMed] [Google Scholar]
- 40.Ansart S, Pelat C, Boelle PY, Carrat F, Flahault A, Valleron AJ. Mortality burden of the 1918–1919 influenza pandemic in Europe. Influenza Other Respi Viruses. 2009;3:99–106. doi: 10.1111/j.1750-2659.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vynnycky E, Trindall A, Mangtani P. Estimates of the reproduction numbers of Spanish influenza using morbidity data. Int J Epidemiol. 2007;36:881–9. doi: 10.1093/ije/dym071. [DOI] [PubMed] [Google Scholar]
- 42.Nishiura H. Time variations in the transmissibility of pandemic influenza in Prussia, Germany, from 1918–19. Theor Biol Med Model. 2007;4:20. doi: 10.1186/1742-4682-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White LF, Pagano M. Transmissibility of the influenza virus in the 1918 pandemic. PLoS ONE. 2008;3:e1498. doi: 10.1371/journal.pone.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009;106:3243–8. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaman J, Pitzer V, Viboud C, Lipsitch M, Grenfell BT. Absolute Humidity and the Seasonal Onset of Influenza in the Continental US. PLoS Biology. 2010 doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized Patients with 2009 H1N1 Influenza in the United States, April–June 2009. N Engl J Med. 2009 doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 47.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009:14. doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 48.Critical Care Services and 2009 H1N1 Influenza in Australia and New Zealand. N Engl J Med. 2009 doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 49.Mexico Ministry of Health. Update on the influenza H1N1-pdm virus in Mexico, 12 October 2009. 2009 Available online: http://portal.salud.gob.mx/sites/salud/descargas/pdf/influenza/situacion_actual_epidemia_121009.pdf.