Abstract
Objective
The purpose of this study was to test the hypothesis that local inhibition of nitric oxide and prostaglandin synthesis attenuates cutaneous vasodilator responses during post-menopausal hot flashes.
Methods
Four microdialysis membranes were inserted into forearm skin (dorsal surface) of 8 post-menopausal women (mean ± SD, 51±7 y). Ringers solution (control), 10mM Ketorolac (Keto) to inhibit prostaglandin synthesis, 10mM NG-L-arginine methyl ester (L-NAME) to inhibit nitric oxide synthase, and a combination of 10mM Keto + 10mM L-NAME were each infused at the separate sites. Skin blood flow at each site was indexed using laser-Doppler flowmetry. Cutaneous vascular conductance (CVC) was calculated as laser-Doppler flux/mean arterial blood pressure and was expressed as a percentage of the maximal calculated CVC (CVCmax) obtained following infusion of 50mM sodium nitropruside at all sites at the end of the study. Data from 13 hot flashes were analyzed.
Results
At the control site, the mean ± SD peak increase in CVC was 15.5±6% CVCmax units. This value was not different relative to the peak increase in CVC at the Keto site (13.0±5 % CVCmax units, P = 0.09). However, the peak increase in CVC during the flash was attenuated at the L-NAME and L-NAME + Keto sites (7.4±4 % CVCmax units and 8.7±7 % CVCmax units, respectively) relative to both the control and the Keto sites (P<0.05 for both comparisons). There were no significant differences in the peak increases in sweat rate between any of the sites (P = 0.24).
Conclusions
These data demonstrate that cutaneous vasodilation during a hot flash has a nitric oxide component. Increases in CVC despite the inhibition of prostaglandin synthesis suggest prostaglandins do not contribute to cutaneous vasodilation during a hot flash.
Keywords: Hot Flash, Nitric Oxide, Skin Blood Flow, Cutaneous Vasodilation
INTRODUCTION
Hot flashes are one of the most prominent symptoms of female menopause. They affect approximately 70% of women during the first 5 years following the onset of the menopause transition 1–3. A hot flash is defined as a sudden feeling of heat usually accompanied by flushed skin and perspiration. These sensations frequently begin in the chest and often include the face, head, and arms. Hot flashes can range in severity and can cause additional symptoms such as anxiety, embarrassment, depression, and nausea 4–7. Additionally, hot flashes can decrease quality of life by negatively affecting concentration, quality of sleep, mood, and sexual function 8, 9.
Previous studies have reported transient increases in blood flow in the finger, hand, forearm, and calf during a hot flash 10–13. Blood flow in these studies were measured using plethysmography, which is unable to distinguish between the vascular beds of skin and muscle; however, those authors speculated that the increases in blood flow were due to increases in skin blood flow (SkBF). A recent study in our laboratory confirmed that the hot flash is accompanied by increases in sternal and forearm SkBF 9.
The mechanism of cutaneous vasodilation occurring during a hot flash is unknown. Previous research has shown that ~85–95% of the elevation in skin blood flow during a whole-body heat stress occurs via a neurally-mediated active vasodilator system14. Moreover, both nitric oxide- (NO) and prostaglandin-dependent mechanisms contribute to this neurally-mediated vasodilatory response 15, 16. It may be that similar mechanisms are responsible for the increases in skin blood flow during a hot flash, but this remains unknown. Therefore, the first objective of this study was to test the hypothesis that NO and/or prostaglandins are responsible for, or contribute to, cutaneous vasodilator responses during post-menopausal hot flashes.
Increases in sweating also occur during hot flashes 4–7, 17. This response presumably occurs via similar mechanisms as sweating in hyperthermic humans in which acetylcholine is released from sympathetic cholinergic nerves to stimulate sweat production 18. Since NO has been shown to sensitize sweat glands 19–21, it is possible that sweating occurring during a hot flash may be modified by NO-related mechanisms. Therefore, a secondary purpose of this study was to test the hypothesis that sweating during a hot flash is modulated by NO mechanisms.
METHODS
Subjects
Eight healthy, post-menopausal women participated in this study. Their mean ± standard deviation age, height, and weight were 51.2 ± 7.4 y, 164.8 ± 6.3 cm, and 68.1 ± 8.7 kg, respectively. All women were amenorrheic for at least 1 year and had a minimum of 4 hot flashes per day. Subjects completed a 7-day hot flash journal prior to participation in order to verify the average number of flashes per day. Subjects were healthy with no history of cardiovascular or metabolic disease and were not taking hormone replacement therapy or any other treatments for hot flashes. Institutionally-approved (University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas), written, informed consent was obtained from all subjects before they enrolled in the study.
Instrumentation
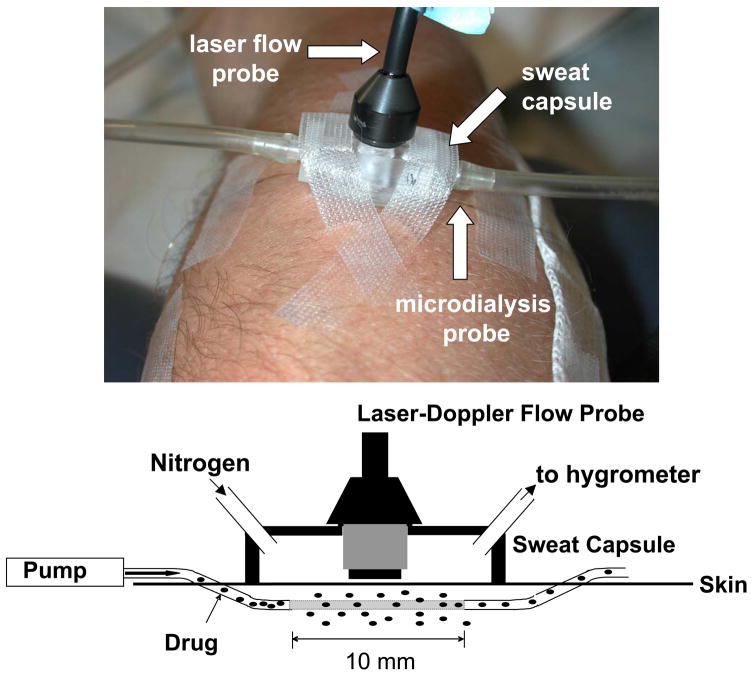
Upon arrival to the laboratory, each subject donned water-perfused tube-lined pants (Med-Eng, Ottawa, ON, Canada). Subjects rested quietly in a semirecumbant position while 4 microdialysis membranes (Bioanalytical Systems, West Lafayette, IN) were inserted into dorsal forearm skin. An integrating laser-Doppler probe (Model DP7a, Moor Instruments, Wilmington, DE), secured in an acrylic sweat rate capsule, was placed over each microdialysis membrane (see Figure 1). This setup allowed the simultaneous evaluation of sweating from a 0.78 cm2 area, with skin blood flow being indexed from the same location. Sweat rate was measured using the capacitance hygrometry ventilated-capsule method (Vaisala, Woburn, WA) with compressed nitrogen delivered at a rate of 150 ml/min. Two additional larger sweat rate capsules (2.83 cm2) were placed on the subjects, one on the chest and one on the forearm, neither of which were over a microdialysis membrane. Sweat rate in these capsules was measured using the same method, but with compressed nitrogen delivered at a rate of 300 ml/min. Continuous beat-by-beat arterial blood pressure was recorded from a finger (Finapres Medical Systems, Amsterdam, The Netherlands). Arterial blood pressure was also measured intermittently using electrosphygmomanometry of the brachial artery (Tango, SunTech Medical Instruments, Raleigh, NC).
Figure 1.
Illustration of the intradermal microdialysis, laser-Doppler probe, and sweat capsule set-up (upper panel). A pump perfuses the drug solution through the microdialysis probe (lower panel). Some of the drug leaves the probe through the 10 mm semipermeable membrane in the middle of the probe. In the present experiment, the drugs L-NAME and ketorolac were infused at sufficient doses to inhibit nitric oxide synthase and prostaglandin synthesis, respectively, while the effects of those drugs on skin blood flow and sweat rate were simultaneous measured during hot flashes. Skin blood flow was indexed via laser-Doppler flowmetry and sweat rate was measured via capacitance hygrometry using compressed nitrogen as the perfusion gas.
Protocol
Immediately after microdialysis probe insertion, Ringers solution (control), 10mM Ketorolac (Keto), 10mM NG-L-arginine methyl ester (L-NAME), and a combination of 10mM Keto + 10mM L-NAME were each infused at the separate microdialysis sites at a rate of 2 μL/min using perfusion pumps (Harvard Apparatus, Holliston, MA). Ketorolac was perfused to inhibit prostaglandin synthesis, while L-NAME was perfused to inhibit NO synthase synthesis. These drugs and doses were selected given prior findings where successful blockade of NO and prostaglandin synthesis were achieved in humans skin 15, 22, 23. Subjects rested quietly and were monitored during a 4 to 5-hour evaluation period. Hot flashes are reported to be more frequent in higher ambient temperatures and during peripheral warming 1, 8. Therefore, if a subject did not experience a hot flash after a few hours of monitoring, attempts were made to induce hot flashes with a mild heat stress by perfusing the suit pants with moderately warm (43°C) water. At the end of the protocol, 1 M methacholine was infused through the 4 microdialysis sites to obtain maximal sweat responses followed by infusion of 50 mM sodium nitroprusside at all sites to obtain maximal skin blood flux.
Data Analysis
Data were sampled at 50 Hz with a data acquisition system (Biopac Systems, Santa Barbara, CA) and analyzed using a statistical software package (SigmaStat 3.11, Systat Software, Inc, San Jose, CA). Cutaneous vascular conductance (CVC) was calculated as laser-Doppler flux/mean arterial pressure. CVC was normalized relative to the percentage of the maximal CVC (CVCmax) obtained during sodium nitroprusside administration. Sweat rate was expressed as a percentage of maximal sweat rate (SRmax) normalized upon methacholine administration. The onset of a hot flash was defined as an increase in sternal sweating of at least 0.002 (mg sweat·cm−2·minute−1)/s, as has been previously reported 9. Because of the variability in the length of hot flashes, each hot flash was divided into 8 equal segments (each segment representing 12.5% of the hot flash duration). Five-second periods of data at the end of each segment and every 15 s over a period of 2 min before and after the hot flash were used in the statistical analysis. The highest CVC and sweat rate responses during each flash were identified. Differences in the peak change (e.g. peak – pre hot flash baseline) in CVC responses from pre hot flash baseline between sites were evaluated using a one-way repeated measures analysis of variance followed by a Student-Neuman Keuls test when a main effect was identified. Differences in the peak change in sweat rate responses between sites were analyzed via Friedman Repeated Measures analysis of variance on ranks given that these data failed a normality test. All values are reported as means ± standard deviation. P values less than 0.05 were considered statistically significant.
RESULTS
Data from 13 hot flashes were analyzed for skin blood flow responses. Subject characteristics and hot flash incidence for each subject are shown in Table 1. There were no differences in mean pre-flash baseline CVC at any of the sites (P = 0.34). At the control site, the average peak increase in CVC during the hot flash was 15.5 ± 6% CVCmax units (see Figure 2). This value was not different relative to the peak increase in CVC at the Keto site (13.0 ± 5% CVCmax units, P = 0.09). However, the peak increase in CVC during the flash was attenuated at the L-NAME and L-NAME + Keto sites (7.4 ± 4% and 8.7 ± 7% CVCmax units, respectively) relative to both the control site (P < 0.001 for both) and the Keto-only site (P < 0.004 for both; Figure 2).
Table 1.
Subject and Hot Flash Characteristics
| Subject | Age | Body Mass (kg) | # Flashes | Ave Duration | Occurrence |
|---|---|---|---|---|---|
| A | 56 | 75 | 2 | 135.5 | N, H |
| B | 51 | 52 | 2 | 306.5 | N, H |
| C | 48 | 72 | 1 | 91 | N |
| D | 53 | 66 | 3 | 84.3 | N, N, N |
| E | 66 | 64 | 2 | 204.5 | H, H |
| F | 42 | 77 | 1 | 207 | N |
| G | 49 | 64 | 1 | 152 | H |
| H | 45 | 80 | 1 | 719 | H |
Table summarizes subject characteristics as well as the number and average duration (in seconds) of hot flashes for the indicated subject. Occurrence indicates if the flash occurred naturally (N) or was induced by mild heat stress (H).
Figure 2. Skin Blood Flow Results.
Mean (± standard deviation) peak increases in cutaneous vascular conductance from baseline during 13 hot flashes. While nitric oxide synthase inhibition (L-NAME) attenuated the magnitude of cutaneous vasodilation, prostaglandin synthesis inhibition did not alter cutaneous vasodilation during the flashes. * indicates significant difference from control (P < 0.001). ¥ indicates significant difference from Ketorolac (P < 0.004).
Data from 9 flashes were analyzed for sweating responses. Four fewer flashes were analyzed for this variable relative to skin blood flow due to the fact that some flashes were not strong enough to elicit a forearm sweating response at the control site, despite increases in skin blood flow and sternal sweat rate during those flashes. There were no significant differences in the peak increases in sweat rate between any of the 4 sites (P = 0.24; Figure 3). The mean peak increase in sweat rate at the control site was 12.6 ± 3.3% SRmax, 12 ± 3.8% SRmax at the Keto site, 12.2 ± 6.9% SRmax at the L-NAME site and 9.8 ± 4.4% SRmax at the L-NAME + Keto site.
Figure 3. Sweat Rate Results.
Mean (± standard deviation) peak increase in sweat rate from baseline during 9 hot flashes. The increase in sweat rate during the hot flashes was not affected by inhibition of nitric oxide or prostaglandin synthesis (P = 0.24).
DISCUSSION
The primary finding of this study is that cutaneous vasodilation during a hot flash is mediated in part by NO mechanisms given that inhibition of NO synthase via L-NAME attenuated increases in cutaneous vascular conductance during the hot flashes. In contrast, normal increases in cutaneous vascular conductance at the Ketorolac site suggest that prostaglandins do not contribute to cutaneous vasodilation during hot flashes. The absence of differences in sweat rate during hot flashes at the sites where NO and prostaglandin synthesis were inhibited suggest these mechanisms do not contribute to the sweating response during hot flashes.
Previously, a number of investigators hypothesized that skin blood flow increases during a hot flash 10–13. This hypothesis was recently confirmed upon assessment of cutaneous vascular responses via laser-Doppler flowmetry during hot flashes 9. Both data sets demonstrate that increases in skin blood flow during a flash occur quite rapidly, even before measurable increases in sweat rate or indices of sweat rate (e.g., galvanic skin response) are identifiable.
The primary mechanism by which skin blood flow increases during a hot flash is unknown. Freedman and colleagues 11 conducted a study in which the nerves of one hand were blocked with a local injection of lidocaine in menopausal women. Significant increases in finger temperature and blood flow occurred during hot flashes in both the nerve-blocked and non-nerve-blocked fingers. Based upon these findings, it was proposed that a circulating vasodilating substance may cause vasodilation during a hot flash. Counter to Freedman’s hypothesis, the rapid rise in skin blood flow observed in this study and others 9 strongly suggests this response may be neurally-mediated.
NO and prostaglandins contribute to cutaneous vasodilation during a number of perturbations. For example, both have been reported to contribute to cutaneous vasodilation during exogenous administration of acetylcholine 22–24. Likewise, studies have reported clear NO and prostaglandin contributions to heat stress induced cutaneous vasodilation 15, 16, 25. Sympathetic cholinergic nerves recognized to cause vasodilation during heat stress release acetylcholine as well as other co-transmitters 18. Given these observations, if the cutaneous vasodilation during a hot flash occurs through engagement of the sympathetic cholinergic system, then one would likewise expect a NO and prostaglandin component in mediating vasodilation during a hot flash. However, the absence of an effect of prostaglandin inhibition on vasodilation during a hot flash raises the possibility of differing mechanisms of cutaneous vasodilation relative to that which occurs during heat stress.
With the present data, we are unable to identify the source of the NO contributing to the cutaneous vasodilation. One possibility could be secondary from acetylcholine binding to muscarinic receptors on the endothelium thereby stimulating the release of NO. Alternatively, hot flash-induced cutaneous vasodilation would be expected to cause an increase in shear stress within the cutaneous vasculature resulting in an increase in NO release and facilitated vasodilation. Finally, it has been reported that neuronally-released NO contributes to vasodilation during heat stress 26, and thus neuronally-released NO may directly cause vasodilation during a hot flash.
It is intriguing to note that neither NO nor prostaglandin inhibition altered the sweating response during the hot flash. Recent findings have demonstrated that NO is capable of modulating sweat rate to exogenous acetylcholine administration as well as during pronounced heat stress 19, 27. However, other data suggest that sweat rate during more moderate heat stress is not affected by inhibition of NO synthesis 16, 28. Taken together, it may be that NO affects sweating only after sweat rate has reached a sufficiently high level. Consequently, the modest sweat rates observed in the present study were apparently insufficient to be alterable by inhibition of NO synthase. One may speculate that NO synthase inhibition may attenuate the sweat response during a hot flash in which the individual sweats profusely.
A limitation to the interpretation of these results is the low number of hot flashes experienced by the subjects from which the data could be analyzed. Despite the application of a mild heat stress, the majority of subjects exhibited only one or two flashes during the observation period. For the skin blood flow assessment, this resulted in 13 hot flashes analyzed, which was clearly sufficient to identify an effect of NO in mediating a component of the cutaneous vasodilator response (see Figure 2). However, four of these flashes were not of significant magnitude to evoke a forearm sweating response, despite clearly cutaneous vasodilation and sternal sweating, resulting in only 9 hot flashes being analyzed for sweat rate. This low number of flashes, coupled with the absence of a significant difference between drugs sites for sweat rate (see Figure 3), raises the issue of whether the analysis was sufficiently powered to test the proposed hypothesis. For this reason a power analysis was performed, which confirmed that the statistical analysis for sweat rate was underpowered. However, to achieve sufficient power (0.80) to confirm the absence of a statistical difference between the control site and the LNAME + Ketorolac sites using the obtained data (see Figure 3), over 400 hot flashes would be necessary. Thus, given the recognized under-powered analysis for sweat rate, these data must be viewed with the understanding of a remote possibility of a type II error.
CONCLUSION
In conclusion, the main finding of this study was that NO contributes to cutaneous vasodilation occurring during a hot flash, while inhibition of prostaglandin synthesis did not attenuate cutaneous vasodilation during flashes. In addition, inhibition of NO and prostaglandin synthesis did not significantly alter sweating during hot flashes. These data provide valuable insight into the mechanisms responsible for cutaneous vasodilation and sweating during hot flashes.
Acknowledgments
This project was supported by the NIH, National Institute on Aging (AG030189).
We would like to thank the subjects for their willing participation in this study. We also express appreciation to Jena Porterfield, RN, BSN for her assistance with this project.
Footnotes
Conflicts of Interest: None
Cited References
- 1.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–133. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F. Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol. 1994 May–Aug;29:319–336. doi: 10.1016/0531-5565(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann GA. Vasomotor flushes in menopausal women. Am J Obstet Gynecol. 1999 Mar;180:S312–316. doi: 10.1016/s0002-9378(99)70725-8. [DOI] [PubMed] [Google Scholar]
- 4.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998 Aug;70:332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 5.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil Steril. 1996 Jun;65:1141–1144. [PubMed] [Google Scholar]
- 6.Molnar GW. Body temperatures during menopausal hot flashes. J Appl Physiol. 1975 Mar;38:499–503. doi: 10.1152/jappl.1975.38.3.499. [DOI] [PubMed] [Google Scholar]
- 7.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995 Aug;80:2354–2358. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 8.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989 Sep;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 9.Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008 Mar–Apr;15:290–295. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cignarelli M, Cicinelli E, Corso M, et al. Biophysical and endocrine-metabolic changes during menopausal hot flashes: increase in plasma free fatty acid and norepinephrine levels. Gynecol Obstet Invest. 1989;27:34–37. doi: 10.1159/000293612. [DOI] [PubMed] [Google Scholar]
- 11.Freedman RR, Woodward S, Mayes MM. Nonneural mediation of digital vasodilation during menopausal hot flushes. Gynecol Obstet Invest. 1994;38:206–209. doi: 10.1159/000292480. [DOI] [PubMed] [Google Scholar]
- 12.Ginsburg J, Swinhoe J, O’Reilly B. Cardiovascular responses during the menopausal hot flush. Br J Obstet Gynaecol. 1981 Sep;88:925–930. doi: 10.1111/j.1471-0528.1981.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg F, Carraway RE. Changes in neurotensin-like immunoreactivity during menopausal hot flashes. J Clin Endocrinol Metab. 1985 Jun;60:1081–1086. doi: 10.1210/jcem-60-6-1081. [DOI] [PubMed] [Google Scholar]
- 14.Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006 May;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 15.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol. 2006 Sep;291:R596–602. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- 16.Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998 Sep;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 17.Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002 Mar;77:487–490. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg DL, Jr, Pergola PE, Piest KL, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995 Dec;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 19.Lee K, Mack GW. Role of nitric oxide in methacholine-induced sweating and vasodilation in human skin. J Appl Physiol. 2006 Apr;100:1355–1360. doi: 10.1152/japplphysiol.00122.2005. [DOI] [PubMed] [Google Scholar]
- 20.Mills PC, Marlin DJ, Scott CM, Smith NC. Nitric oxide and thermoregulation during exercise in the horse. J Appl Physiol. 1997 Apr;82:1035–1039. doi: 10.1152/jappl.1997.82.4.1035. [DOI] [PubMed] [Google Scholar]
- 21.Mills PC, Scott CM, Marlin DJ. Effects of nitric oxide inhibition on thermoregulation during exercise in the horse. Ann N Y Acad Sci. 1997 Mar 15;813:591–599. doi: 10.1111/j.1749-6632.1997.tb51750.x. [DOI] [PubMed] [Google Scholar]
- 22.Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005 Feb;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 23.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005 Mar 15;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation. 2008 Aug;15:569–579. doi: 10.1080/10739680802091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holowatz LA, Jennings JD, Lang JA, Kenney WL. Ketorolac alters blood flow during normothermia but not during hyperthermia in middle aged human skin. J Appl Physiol. 2009 Aug 6; doi: 10.1152/japplphysiol.00750.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellogg DL, Jr, Zhao JL, Wu Y. Roles of Nitric Oxide Synthase Isoforms in Cutaneous Vasodilation Induced by Local Warming of the Skin and Whole Body Heat Stress in Humans. J Appl Physiol. 2009 Sep 10; doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch G, Foote KM, Hansen C, Mack GW. Nonselective NOS inhibition blunts the sweat response to exercise in a warm environment. J Appl Physiol. 2009 Mar;106:796–803. doi: 10.1152/japplphysiol.90809.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietz NM, Rivera JM, Warner DO, Joyner MJ. Is nitric oxide involved in cutaneous vasodilation during body heating in humans? J Appl Physiol. 1994 May;76:2047–2053. doi: 10.1152/jappl.1994.76.5.2047. [DOI] [PubMed] [Google Scholar]