Abstract
We describe herein an efficient new route to the trikentrins and their related structures using a tandem 6,7-indolyne cycloaddition/Negishi cross-coupling reaction starting from a 4,6,7-tribromoindole (obtained in good yield via the Bartoli indole synthesis). The key step of this second generation route to the trikentrins is based on our observation that the 7-bromo substituent appears to undergo selective metal–halogen exchange and elimination to give the 6,7-indolyne, which is trapped in the presence of excess cyclopentadiene. Subsequent Negishi cross-coupling at the 4-bromoindole position with Et2Zn gave directly the same intermediate obtained from our previous work. Application of this chemistry to the construction of trikentrin-related libraries using this general cycloaddition/cross-coupling tactic will also be described.
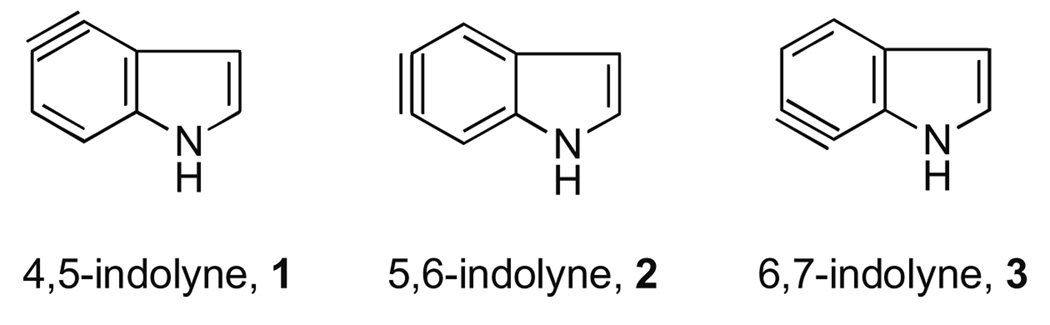
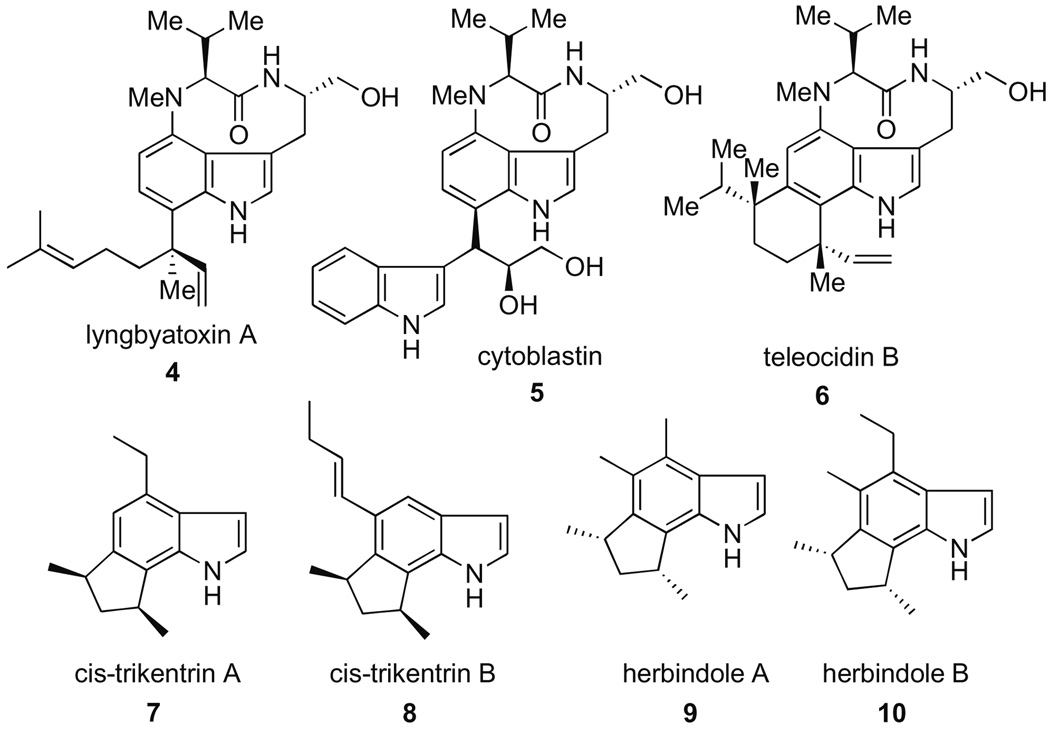
Arynes derived from all three benzenoid positions of the ubiquitous indole nucleus (indolynes 1–3, Fig. 1) represent potentially attractive and powerful new tools for the total synthesis of complex alkaloid natural products such as the trikentrins,1 herbindoles, 1a,c teleocidins,2 cytoblastin,3 and lyngbyatoxin4 (Fig. 2). Our group recently discovered the first successful and general route to these intermediates via metal–halogen exchange of o-dihalides5 and later, via fluoride-induced decomposition of o-silyltriflates.6 We subsequently examined the regioselectivity of indolynes in Diels–Alder reactions with 2-substituted furans.6a These studies quickly led to the first application of indolynes in the construction of natural products and culminated in a concise synthesis of (±)-cis-trikentrin A 7 and (±)-herbindole A, 9.7 This work clearly validated the utility of indolynes to gain quick access to architecturally challenging structures.8
Figure 1.
Benzenoid indole aryne (indolyne) systems.
Figure 2.
Representative indole natural products.
We now describe an efficient and flexible new route to the trikentrins using a novel tandem intermolecular 6,7-indolyne cycloaddition/Negishi cross-coupling reaction starting from a versatile 4,6,7-tribromoindole, which itself was obtained via the Bartoli indole synthesis.9
Application of this general cycloaddition/cross-coupling tactic to the construction of novel small-molecule libraries will also be introduced.
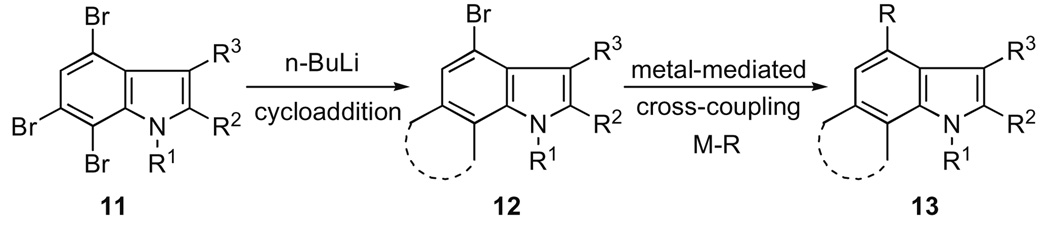
We were intrigued by the idea of using a potentially new scaffold, namely, a 4,6,7-tribromoindole 11 for the synthesis of the antibacterial cis-trikentrin A as well as libraries derived from it (Scheme 1).
Scheme 1.
Versatile 4,6,7-tribromoindole scaffold.
Based on our earlier work with the various reaction manifolds of 6,7-indolynes,6a it occurred to us that it might be possible to generate selectively the 6,7-indolyne as described previously5,6a without simultaneously inducing metal–halogen exchange at the 4-bromo position. In this manner indolyne cycloaddition would be followed by further elaboration of the scaffold at the 4-bromo position of 12 by any of the several metal-mediated coupling strategies (e.g., Buchwald–Hartwig, Heck, Negishi, Sonogashira, Stille, and Suzuki). We are now pleased to report that this is indeed the case.
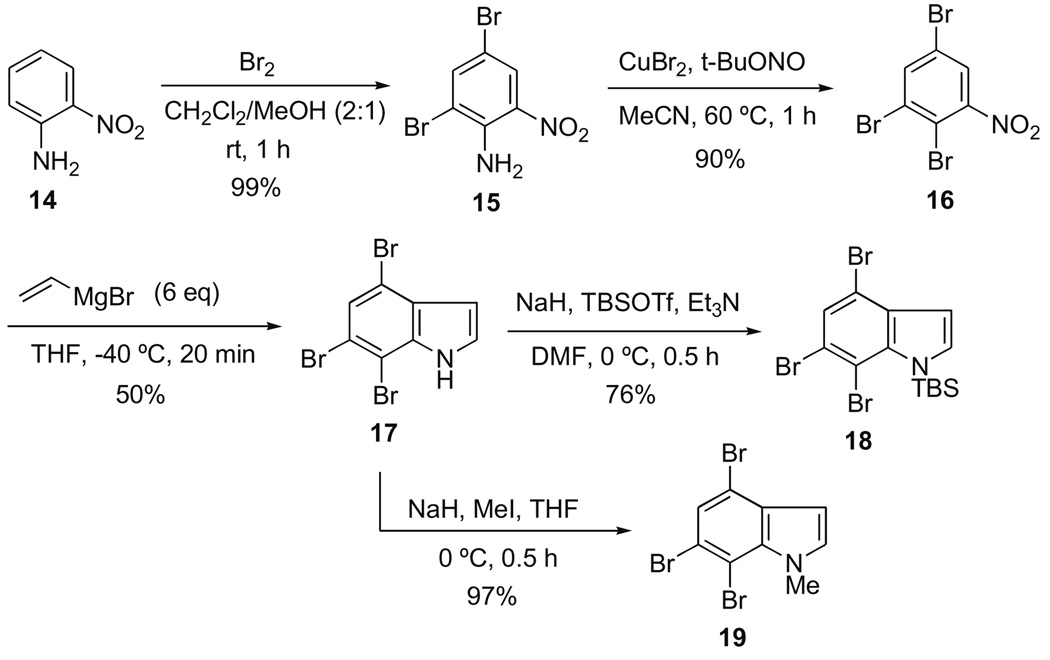
Whereas our first-generation synthesis of cis-trikentrin A relied on 4-ethylaniline,7 a potentially far more flexible approach required the preparation of the versatile silyl protected 4,6,7-tribromoindole 18 (Scheme 2). Thus inexpensive o-nitroaniline 14 was brominated [Br2 (4 equiv), CH2Cl2/MeOH (2:1), rt, 1 h] in nearly quantitative yield to give 4,6-dibromo-2-nitroaniline 15. Subsequent diazotization of this aniline with a stoichiometric amount of cupric bromide [CuBr2 (1.3 equiv), t-BuONO (1.0 equiv), MeCN, 60 °C, 1 h] afforded the heretofore unknown 2,3,5-tribromonitrobenzene 16 in 90% yield on a multi-gram scale.
Scheme 2.
Synthesis of the 4,6,7-tribromoindole scaffold.
Application of the Bartoli indole synthesis9 to 16 (vinyl Grignard, 6 equiv; THF, −40 °C, 20 min) on a gram-scale gave cleanly a very respectable 50% yield of the desired 4,6,7-tribromoindole 17.10 Finally, protection of the indole N–H group as its TBS ether 18 was accomplished in 78% yield with NaH (4.0 equiv), TBSOTf (2.0 equiv), and Et3N (2.0 equiv) in DMF at 0 °C for 0.5 h. Alternatively, N-methylation could be achieved in 97% yield (NaH (2.0 equiv; MeI, 1.5 equiv, THF, 0 °C, 0.5 h) to give 19.
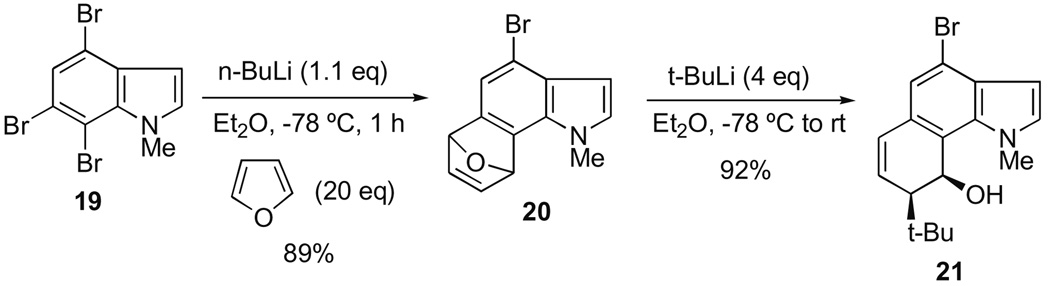
To test the idea of reaction orthogonality in these systems, we first subjected 19 to our indolyne-forming conditions in the presence of an excess of furan (Scheme 3).
Scheme 3.
Test of reaction orthogonality in the 4,6,7-tribromoindole system.
Gratifyingly, the corresponding cycloadduct 20 was cleanly formed in 89% yield with no evidence of additional metal–halogen exchange occurring at C-4. Indeed, efforts to force metal–halogen exchange at the 4-bromoindole position with an excess of t-BuLi gave only the ring-opened product 21 in excellent yield via regioand exoselective attack by the alkyllithium. This observation is consistent with what we found in similar systems and which we documented in an earlier Letter.5,6a
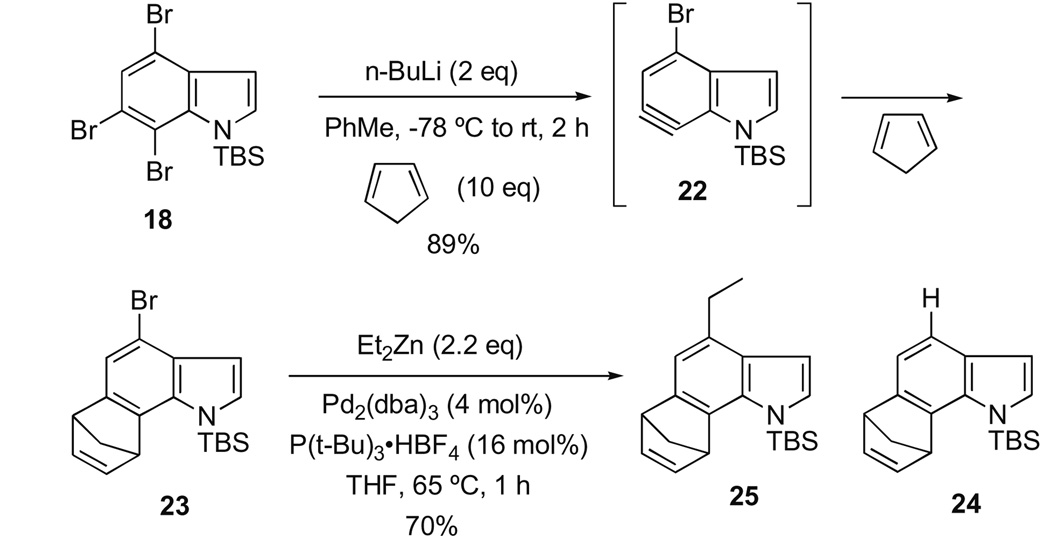
Encouraged by these results, we proceeded with our objective of executing a tandem intermolecular 6,7-indolyne cycloaddition/Negishi cross-coupling reaction (Scheme 4) enroute to cis-trikentrin A.
Scheme 4.
Tandem 6,7-indolyne cycloaddition/Negishi cross-coupling.
Reaction of 18 with n-BuLi in PhMe11 at −78 °C presumably resulted in selective metal–halogen exchange at C-7 and elimination to give the 4-bromo-6,7-indolyne 22, which was trapped with cyclopentadiene to afford the desired 4-bromoindole 23 in 89% yield. Although the possibility of some metal–halogen exchange occurring at the 4-bromo position cannot be rigorously excluded at this time, it is important to note that no evidence for the formation of compound 24 has been found thus far. Application of the Negishi cross-coupling [Et2Zn, 2.2 equiv; Pd2(dba)3 (4 mol %); P(t-Bu)3·HBF4 (16 mol %); THF, 60 °C, 1 h] afforded the desired product 25 in 70% yield.12 The use of the somewhat more exotic Fu catalyst/ligand combination13 was found to be the best conditions in this case to achieve the optimal yield of the 4-ethylindole. This intermediate is identical in all respects to that synthesized by our first-generation method beginning with 4-ethylaniline,7 and thus constitutes a formal total synthesis of (±)-cis-trikentrin A, as well as the shortest route to this target reported to date.
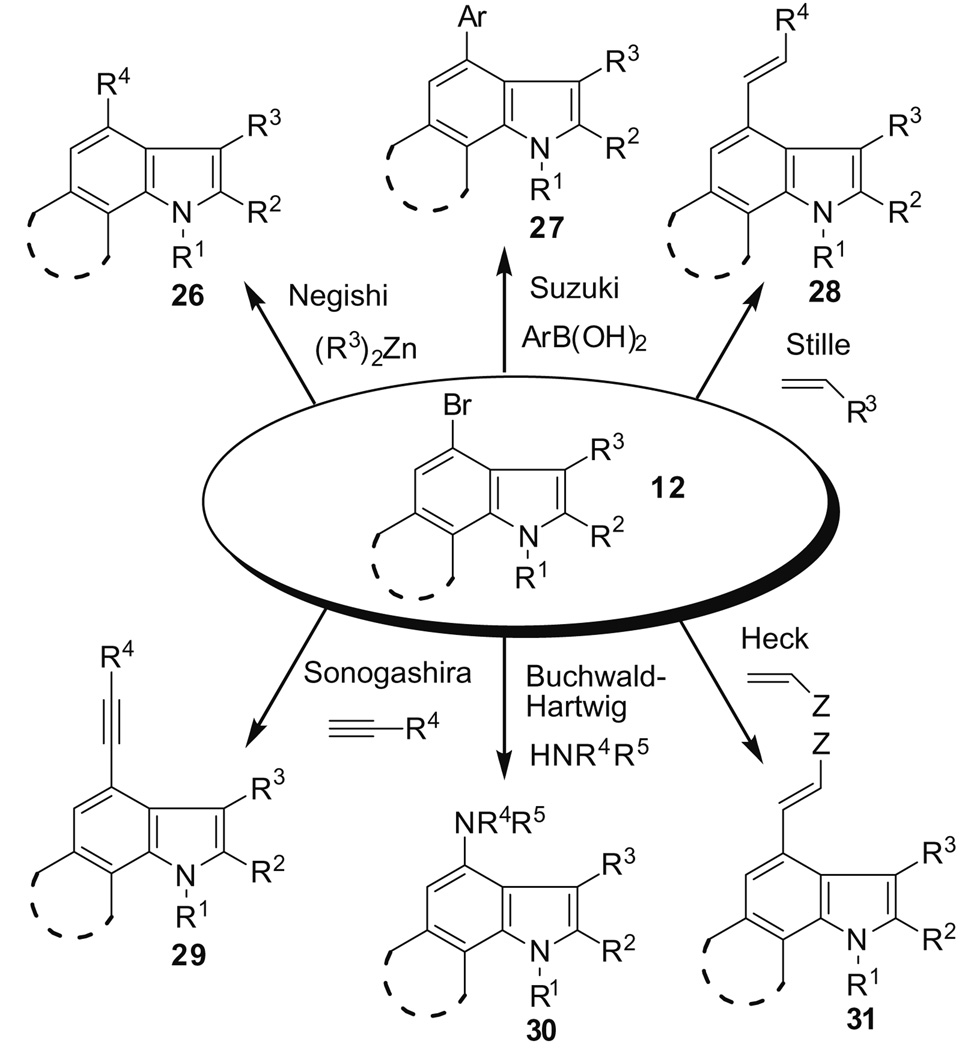
The ability to exploit reaction orthogonality in 4,6,7-tribromoindole makes it a potentially valuable scaffold for the construction of libraries using a general tandem 6,7-indolyne cycloaddition/cross-coupling strategy as depicted in Figure 3.
Figure 3.
Cross-coupling manifolds in the 4-bromoindole scaffold.
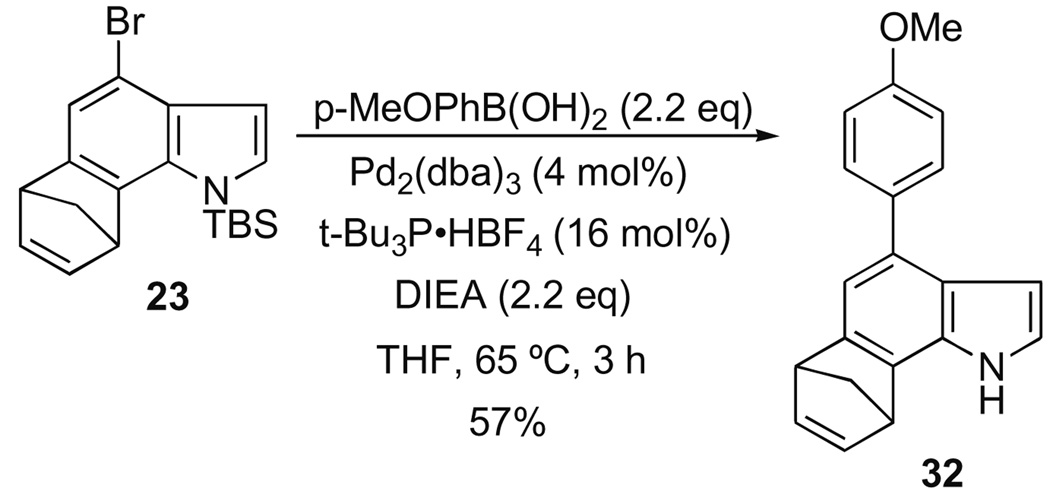
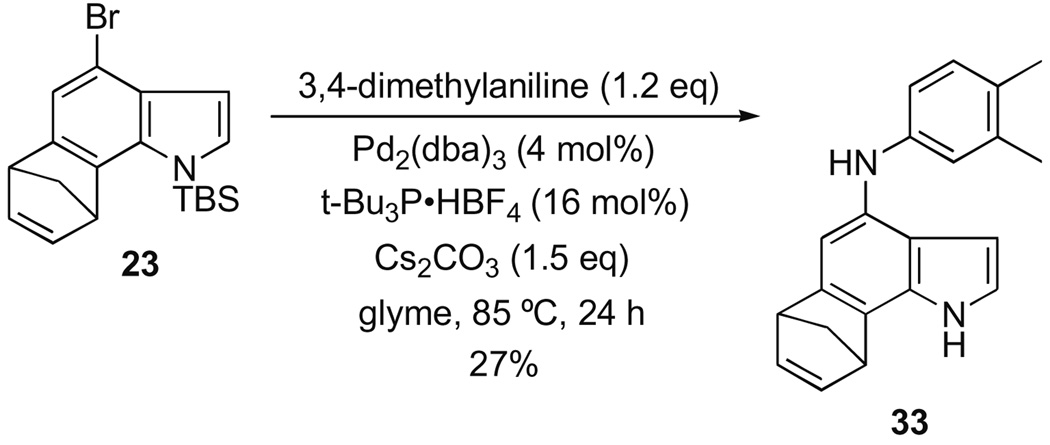
We have provided a proof-of-concept for two other manifolds in addition to the Negishi reaction. For example, coupling a boronic acid to 23 in the Suzuki–Miyaura reaction gave a good yield of the desired product 32 (Scheme 5), while the use of an aniline afforded the corresponding Buchwald–Hartwig coupled product 33, albeit in modest yield (Scheme 6).
Scheme 5.
Suzuki–Miyaura coupling with 4-bromoindoles.
Scheme 6.
Buchwald–Hartwig coupling of anilines with 4-bromoindoles.
In both cases, the cross-coupling event proceeded with concomitant loss the N-silyl protecting group. This unexpected outcome is advantageous in that it saves a deprotection step, and allows for the subsequent introduction of further diversity elements, for example, by way of N-alkylation or N-acylation,. The yields of each reaction warrant comment. While the yield of product for the Negishi reaction is certainly respectable, the first attempts at the Suzuki– Miyaura and Buchwald–Hartwig cases gave disappointing results. Both are much lower than would be expected for simple cross-coupling reactions with an aryl bromide. This observation required us, after much experimentation, to finally adopt the less conventional catalyst and ligand combinations to achieve even the stated yields. Although cross-coupling reactions with haloindoles are certainly precedented,14 their yields, along with other heteroarenes, tend to be highly variable, and usually low.15 In our systems, annulation leads to a more electron-rich bromoarene, and this factor is also known to suppress yields in many cases. We are continuing to pursue improved conditions in support of our library objectives.
In conclusion, we have devised a shorter, improved route to (±)-cis-trikentrin A that utilizes an efficient tandem 6,7-indolyne cycloaddition/Negishi coupling sequence. We have shown that the 4,6,7-tribromoindole system appears to undergo selective metal–halogen exchange with n-BuLi at the 7-bromo position and subsequent elimination to give the 6,7-indolyne, followed by cycloaddition with dienes. The remaining 4-bromo position is then available for further elaboration using well-known cross-coupling protocols. This heretofore unrecognized reaction orthogonality renders the 4,6,7-tribromoindole a versatile platform for the total synthesis of natural products, and holds enormous potential for the construction of diverse small-molecule libraries. We are presently exploiting this latter application for use in library development. The results of these efforts will be reported in due course.
Acknowledgments
We acknowledge support of this work by the National Institutes of Health, NIGMS, Grant R01 GM069711 (K.R.B.). Additional support of this work was provided by the NIH, NIGMS, Grant P50 GM069663 via the University of Kansas Chemical Methodologies and Library Development Center of Excellence (KU-CMLD).
References and notes
- 1.For recent total syntheses, see: Jackson SK, Kerr MA. J. Org. Chem. 2007;72:1405–1411. doi: 10.1021/jo062350v. Huntley RJ, Funk RL. Org. Lett. 2006;8:3403–3406. doi: 10.1021/ol061259a. Jackson SK, Banfield SC, Kerr MA. Org. Lett. 2005;7:1215–1218. doi: 10.1021/ol047498k. MacLeod JK, Monahan LC. Tetrahedron Lett. 1988;29:391–392.
- 2.(a) Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1987;38:3671–3674. [Google Scholar]; (b) Nakatsuka S, Matsuda T, Goto T. Tetrahedron Lett. 1986;27:6245–6248. [Google Scholar]; (c) Nakatsuka S, Masuda T, Asano O, Terame T, Goto T. Tetrahedron Lett. 1986;27:4327–4330. [Google Scholar]
- 3.Moreno OA, Kishi Y. J. Am. Chem. Soc. 1996;118:8180–8181. [Google Scholar]
- 4.Muratake H, Natsume M. Tetrahedron Lett. 1987;28:2265–2268. [Google Scholar]
- 5.Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org. Lett. 2007;9:4135–4137. doi: 10.1021/ol701595n. [DOI] [PubMed] [Google Scholar]
- 6. Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65. doi: 10.1016/j.tetlet.2008.10.086. Another o-silyltriflate method for indolyne formation and further cycloaddition reactions has subsequently been reported: Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007–1010. doi: 10.1021/ol802958a.
- 7.Buszek KR, Brown N, Luo D. Org. Lett. 2009;11:201–204. doi: 10.1021/ol802425m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A synthesis of the bicyclic scaffold of N-methylwelwit-indolinone C isothiocyanate via indolyne cyclization has been reported: Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. doi: 10.1021/ol9007684.
- 9.(a) Bartoli G, Palmieri G. Tetrahedron Lett. 1989;30:2129–2132. [Google Scholar]; (b) Bartoli G, Bosco M, Dalpozzo R, Palmieri G, Marcantoni E. J. Chem. Soc. PT1. 1991:2757–2761. [Google Scholar]; (c) Bosco M, Dalpozzo R, Bartoli G, Palmieri G, Petrini M. J. Chem. Soc., Perkin Trans. 2. 1991:657–663. [Google Scholar]; (d) Dalpozzo R, Bartoli G. Curr. Org. Chem. 2005;9:163–178. [Google Scholar]
- 10.All new compounds reported herein exhibited satisfactory 1H NMR, 13C NMR, high resolution mass spectral, or elemental analysis data consistent with their structures. 15: 1H NMR: δ 8.28 (d, 1H, J = 6.2 Hz); δ 7.80 (d, 1H, J = 6.4 Hz); δ 6.60 (br s, 2H, NH2). 13C NMR: δ 141.2, 140.8, 128.2 (2 C), 112.7, 106.8. Mp = 127.8–129.3 °C; 16: 1H NMR: δ 7.98 (d, 1H, J = 2.2 Hz); δ 7.76 (d, 1H, J = 2.2 Hz). 13C NMR: 138.8, 128.8, 126.5, 121.6, 116.3. Mp = 83.5–84.8 °C; 17: 1H NMR: δ 8.46 (br s, 1H, NH); δ 7.53 (s, 1H); δ 7.28 (m, 1H); δ 6.65 (m, 1H). 13C NMR: δ 153.3, 128.2, 126.7, 125.7, 117.2, 114.1, 106.5, 104.7. Mp = 97.0–99.0 °C; 18: 1H NMR: δ 7.62 (s, 1H); δ 7.43 (d, 1H, J = 3.6 Hz); δ 6.71 (d, 1H, J = 3.3 Hz); δ 0.99 (s, 9H); δ 0.73 (s, 6H). 13C NMR: δ 140.7, 135.4, 133.4, 127.6, 119.4, 113.7, 108.0, 106.2, 27.4, 19.9, 2.2. Mp = 90.5–91.6 °C; 23: 1H NMR: δ 7.34 (s, 1H); 7.22 (d, 1H, J = 3.3 Hz); δ 6.92 (m, 1H); δ 6.84 (m, 1H); δ 6.64 (d, 1H, J = 3.5 Hz); δ 4.48 (s, 1H); δ 3.98 (s, 1H); δ 2.36–2.26 (m, 2H); δ 1.04 (s, 9H); δ 0.69 (s, 3H); δ 0.61 (s, 3H). 13C NMR: δ 148.6, 145.0, 142.0, 136.9, 134.2, 132.4, 130.8, 118.6, 109.2, 105.4, 70.4, 51.0, 50.6, 26.6, 19.4, −1.5, −1.8. Mp = 114.1–115.2 °C.
- 11.Coe JW, Wirtz MC, Bashore CG, Candler J. Org. Lett. 2004;6:1589–1592. doi: 10.1021/ol049655l. [DOI] [PubMed] [Google Scholar]
- 12.This product is identical in all respects to that previously reported by us (see Ref. 7).
- 13.(a) González-Bobes F, Fu GC. J. Am. Chem. Soc. 2006;128:5360–5361. doi: 10.1021/ja0613761. [DOI] [PubMed] [Google Scholar]; (b) Zhou J, Fu GC. J. Am. Chem. Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]; (c) Netherton MR, Fu GC. Angew. Chem., Int. Ed. 2002;41:3910–3912. doi: 10.1002/1521-3773(20021018)41:20<3910::AID-ANIE3910>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Billingsley K, Buchwald SL. J. Am. Chem. Soc. 2007;129:3358–3366. doi: 10.1021/ja068577p. [DOI] [PubMed] [Google Scholar]
- 15.Tyrell E, Brookes P. Synthesis. 2004;4:469–483. [Google Scholar]