Abstract
The genus Cladosporium is one of the largest genera of dematiaceous hyphomycetes, and is characterised by a coronate scar structure, conidia in acropetal chains and Davidiella teleomorphs. Based on morphology and DNA phylogeny, the species complexes of C. herbarum and C. sphaerospermum have been resolved, resulting in the elucidation of numerous new taxa. In the present study, more than 200 isolates belonging to the C. cladosporioides complex were examined and phylogenetically analysed on the basis of DNA sequences of the nuclear ribosomal RNA gene operon, including the internal transcribed spacer regions ITS1 and ITS2, the 5.8S nrDNA, as well as partial actin and translation elongation factor 1-α gene sequences. For the saprobic, widely distributed species Cladosporium cladosporioides, both a neotype and epitype are designated in order to specify a well established circumscription and concept of this species. Cladosporium tenuissimum and C. oxysporum, two saprobes abundant in the tropics, are epitypified and shown to be allied to, but distinct from C. cladosporioides. Twenty-two species are newly described on the basis of phylogenetic characters and cryptic morphological differences. The most important phenotypic characters for distinguishing species within the C. cladosporioides complex, which represents a monophyletic subclade within the genus, are shape, width, length, septation and surface ornamentation of conidia and conidiophores; length and branching patterns of conidial chains and hyphal shape, width and arrangement. Many of the treated species, e.g., C. acalyphae, C. angustisporum, C. australiense, C. basiinflatum, C. chalastosporoides, C. colocasiae, C. cucumerinum, C. exasperatum, C. exile, C. flabelliforme, C. gamsianum, and C. globisporum are currently known only from specific hosts, or have a restricted geographical distribution. A key to all species recognised within the C. cladosporioides complex is provided.
Keywords: Cladosporium oxysporum, Cladosporium tenuissimum, epitypification, new species, phylogeny, taxonomy
INTRODUCTION
The genus Cladosporium, which comprises more than 772 names (Dugan et al. 2004), has been studied extensively in recent years. Based on morphological examinations (Schubert & Braun 2004, Heuchert et al. 2005, Schubert 2005a, b, 2005a, b, 2007, Braun et al. 2006, 2008a, b, Crous et al. 2006a, b, Schubert et al. 2006, Braun & Schubert 2007) and molecular studies (Crous et al. 2006a, 2007a, b, c, Arzanlou et al. 2007, Schubert et al. 2007a, b), a modern generic concept of Cladosporium was established, including clear delimitations from morphologically similar genera (Crous et al. 2007b, de Hoog et al. 2007, Seifert et al. 2007). Species belonging to Cladosporium are characterised by having a unique coronate scar structure (David 1997) and by being linked to Davidiella teleomorphs (Braun et al. 2003, Schubert et al. 2007b, Crous et al. 2009c). They cluster apart from species of Mycosphaerella and represent a separate family, Davidiellaceae (Schoch et al. 2006, 2009a, b, Crous et al. 2009c). Taxonomic studies using polyphasic approaches were undertaken to define distinct phylogenetic and morphological entities within the genus (Schubert et al. 2009), especially in the species complexes of C. herbarum (Schubert et al. 2007b) and C. sphaerospermum (Zalar et al. 2007, Dugan et al. 2008).
The present study is a contribution in this series, dealing with the C. cladosporioides complex. Cladosporium cladosporioides is a very common, cosmopolitan, saprobic species. It often occurs as a secondary invader on necrotic parts of many different host plants, has been isolated from air, soil, textiles and several other substrates (Ellis 1971), and is a common endophytic or quiescent fungus (Riesen & Sieber 1985, El-Morsy 2000, Kumaresan & Suryanarayanan 2002). In the past C. cladosporioides has also been reported to be involved in several pulmonary and cutaneous infections and other human health problems (de Hoog et al. 2000). Races associated with leaf-spotting have also been reported (Anilkumar & Seshadri 1975, Arya & Arya 2003), though this could not be confirmed during the course of the present study.
David (1997) introduced section Hormodendropsis of the subgenus Cladosporium, typified by C. cladosporioides, characterised by determinate, non-proliferating conidiophores. However, type material of C. cladosporioides, cited by Fresenius (1850) for Penicillium cladosporioides, could not be traced in the Fresenius herbarium at the Senckenberg-Museum in Frankfurt and is undoubtedly not preserved. De Vries (1952) discussed the fact that C. cladosporioides has often been considered a form of C. herbarum, compared these two species and found sufficient morphological differences to justify the recognition of C. cladosporioides as a distinct taxon. As “lectotype” of this species he invalidly and erroneously proposed to choose Bisby's dried “standard culture” [isol. fr. Arundo leaves, Bamboo Garden, Kew, 1943 (IMI 25324, 60507, 60509)] which, however, proved to belong to the C. herbarum complex. Hence, it is necessary to designate a neotype and, above all, an epitype with ex-type culture close to the current concept of C. cladosporioides, which is mainly based on Ellis (1971).
In recent decades the name C. cladosporioides has been applied to several taxa now demonstrated as distinct, but all united by a superficial resemblence to the taxon described and illustrated in Ellis (1971). To establish the identity and clarify the taxonomic status of fungi previously lumped under this name, it was necessary to re-examine numerous cultures deposited as “C. cladosporioides” as well as undetermined isolates from diverse substrates and geographical origins. Therefore, a multilocus DNA sequence typing approach employing three gene regions (ITS, actin, translation elongation factor 1-α) supplemented with morphological and cultural examinations (following protocols outlined in Schubert et al. 2007b), was used to elucidate species diversity within the C. cladosporioides complex.
MATERIAL AND METHODS
Isolates
Isolates included in this study were obtained from the culture collection of the Centraalbureau voor Schimmelcultures (CBS-KNAW Fungal Biodiversity Centre), Utrecht, Netherlands, or were freshly isolated from a range of different substrates and placed in the working collection of Pedro Crous (CPC), housed at CBS. Single-conidial and ascospore isolates were obtained using techniques in Crous et al. (1991) and Crous (1998). Isolates were inoculated onto 2 % potato-dextrose agar (PDA), synthetic nutrient-poor agar (SNA), 2 % malt extract agar (MEA) and oatmeal agar (OA) (Crous et al. 2009f, Crous et al. 2009f), and incubated under continuous near-ultraviolet light at 25 °C to promote sporulation. All cultures in this study are maintained at the CBS (Table 1). Nomenclatural novelties and descriptions were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004a).
Table 1.
Cladosporium isolates included for sequence and morphological analyses.
| Species | Accession number1 | Substrate | Country | Collector | GenBank numbers2(ITS, TEF, ACT) |
|---|---|---|---|---|---|
| Cladosporium acalyphae | CBS 125982*; CPC 11625 | Acalypha australis | South Korea | H.D. Shin | HM147994, HM148235, HM148481 |
| Cladosporium angustisporum | CBS 125983*; CPC 12437 | Alloxylon wickhamii | Australia | B.A. Summerell | HM147995, HM148236, HM148482 |
| Cladosporium asperulatum | CBS 113744 | Grape bud | U.S.A.: Washington | F.M. Dugan | HM147996, HM148237, HM148483 |
| CBS 126339; CPC 11158 | Eucalyptus leaf litter | India | W. Gams | HM147997, HM148238, HM148484 | |
| CBS 126340*; CPC 14040 | Protea susannae | Portugal | — | HM147998, HM148239, HM148485 | |
| Cladosporium australiense | CBS 125984*; CPC 13226 | Eucalyptus moluccana | Australia | B.A. Summerell | HM147999, HM148240, HM148486 |
| Cladosporium basiinflatum | CBS 822.84* | Hordeum vulgare | Germany | — | HM148000, HM148241, HM148487 |
| Cladosporium chalastosporoides | CBS 125985*; CPC 13864 | Fruiting bodies of Teratosphaeria proteae-arboreae on leaves of Protea nitida | South Africa | P.W. Crous | HM148001, HM148242, HM148488 |
| Cladosporium chubutense | CBS 124457*; CPC 13979; CIEFAP 321 | Pinus ponderosa | Argentina | A. Greslebin | FJ936158, FJ936161, FJ936165 |
| Cladosporium cladosporioides | CBS 101367 | Soil | Brazil | — | HM148002, HM148243, HM148489 |
| CBS 112388* | Indoor air | Germany | Ch. Trautmann | HM148003, HM148244, HM148490 | |
| CBS 113738 | Grape bud | U.S.A.: Washington | F.M. Dugan | HM148004, HM148245, HM148491 | |
| CBS 113739 | Culm node of crested wheat grass | U.S.A.: Washington | F.M. Dugan | HM148005, HM148246, HM148492 | |
| CBS 113740 | Grape berry | U.S.A.: Washington | F.M. Dugan | HM148006, HM148247, HM148493 | |
| CBS 117483; CPC 11684 | — | U.S.A. | M. Blackwell | HM148007, HM148248, HM148494 | |
| CBS 122130; ATCC 38012; IFO 6539; JCM 10684; NBRC 6539 | Bamboo slats | Japan | — | HM148008, HM148249, HM148495 | |
| CBS 126341; CPC 12763 | Spinach seed, Spinacia oleracea | U.S.A.: Washington | L. du Toit | HM148009, HM148250, HM148496 | |
| CBS 132.29 | — | — | C.L. Shear | HM148010, HM148251, HM148497 | |
| CBS 143.35; MUCL 10090 | Pisum sativum | South Africa | B.J. Dippenaar | HM148011, HM148252, HM148498 | |
| CBS 144.35; ATCC 11284; IFO 6371; IMI 049627 | Pisum sativum | U.S.A.: California | — | HM148012, HM148253, HM148499 | |
| CBS 145.35; MUCL 926 | Pisum sativum | Germany | — | HM148013, HM148254, HM148500 | |
| CBS 674.82; CBS 320.87; ATCC 38026; ATCC 200936; IMI 126640 | Gossypium seeds | Israel | M. Gonen | HM148014, HM148255, HM148501 | |
| CPC 10142 | Chenopodium ficifolium | South Korea | H.D. Shin | HM148015, HM148256, HM148502 | |
| CPC 11119 | Ricinus communis | South Korea | H.D. Shin | HM148016, HM148257, HM148503 | |
| CPC 11120 | Viola mandshurica | South Korea | H.D. Shin | HM148017, HM148258, HM148504 | |
| CPC 11121 | Celosia cristata | South Korea | H.D. Shin | HM148018, HM148259, HM148505 | |
| CPC 11122 | Phytolacca americana | South Korea | H.D. Shin | HM148019, HM148260, HM148506 | |
| CPC 11123 | Vigna unguiculata | South Korea | H.D. Shin | HM148020, HM148261, HM148507 | |
| CPC 11131 | Dalbergia sp. | India | W. Gams | HM148021, HM148262, HM148508 | |
| CPC 11161 | Eucalyptus sp. | India | W. Gams | HM148022, HM148263, HM148509 | |
| CPC 11393 | Valeriana fauriei | South Korea | H.D. Shin | HM148023, HM148264, HM148510 | |
| CPC 11398 | Phragmidium griseum on Rubus crataegifolius | South Korea | H.D. Shin | HM148024, HM148265, HM148511 | |
| CPC 11404 | Rubus coreanus | South Korea | H.D. Shin | HM148025, HM148266, HM148512 | |
| CPC 11406 | Plectranthus sp. | South Korea | H.D. Shin | HM148026, HM148267, HM148513 | |
| CPC 12187 | Leaves of Stellaria aquatica | South Korea | H.D. Shin | HM148027, HM148268, HM148514 | |
| CPC 12214 | Leaves of Morus rubra | Germany | N. Ale-Agha | HM148028, HM148269, HM148515 | |
| CPC 12760 | Spinach seed, Spinacia oleracea | U.S.A.: Washington | L. du Toit | HM148029, HM148270, HM148516 | |
| CPC 12762 | Spinach seed, Spinacia oleracea | U.S.A.: Washington | L. du Toit | HM148030, HM148271, HM148517 | |
| CPC 12764 | Spinach seed, Spinacia oleracea | U.S.A.: Washington | L. du Toit | HM148031, HM148272, HM148518 | |
| CPC 12852 | Pruned wood | U.S.A.: Louisiana | K. Seifert | HM148032, HM148273, HM148519 | |
| CPC 13235 | Eucalyptus sp. | Australia | P.W. Crous | HM148033, HM148274, HM148520 | |
| CPC 13667 | Eucalyptus robertsonii ssp. hemisphaerica | Australia | B.A. Summerell | HM148034, HM148275, HM148521 | |
| CPC 13669 | Eucalyptus robertsonii ssp. hemisphaerica | Australia | B.A. Summerell | HM148035, HM148276, HM148522 | |
| CPC 13734 | Areca sp. | Thailand | I. Hidayat | HM148036, HM148277, HM148523 | |
| CPC 14009; MRC 10150 | Wheat | South Africa | — | HM148037, HM148278, HM148524 | |
| CPC 14015; MRC 10260 | Wheat | South Africa | — | HM148038, HM148279, HM148525 | |
| CPC 14017; MRC 10809 | Wheat | South Africa | — | HM148039, HM148280, HM148526 | |
| CPC 14018; MRC 10810 | Wheat | South Africa | — | HM148040, HM148281, HM148527 | |
| CPC 14019; MRC 10813 | Wheat | South Africa | — | HM148041, HM148282, HM148528 | |
| CPC 14021; MRC 10827 | Wheat | South Africa | — | HM148042, HM148283, HM148529 | |
| CPC 14024; MRC 11280 | Pawpaw | South Africa | — | HM148043, HM148284, HM148530 | |
| CPC 14244 | Magnolia sp. | U.S.A.: Louisiana | P.W. Crous | HM148044, HM148285, HM148531 | |
| CPC 14271 | Twigs of an unidentified tree | France | P.W. Crous | HM148045, HM148286, HM148532 | |
| CPC 14292; BA1691 | Soil, pea field | Denmark | B. Andersen | HM148046, HM148287, HM148533 | |
| CPC 14293; BA1692 | Cellulose powder, paint manufacturer | Denmark | B. Andersen | HM148047, HM148288, HM148534 | |
| CPC 14355; BA1676 | Food, mouldy pea | U.S.A.: Laramie | B. Andersen | HM148048, HM148289, HM148535 | |
| CPC 14356; BA1677 | Food, coffee leaf | Uganda | B. Andersen | HM148049, HM148290, HM148536 | |
| CPC 14705 | Chasmothecia of Phyllactinia sp. on Fraxinus rhynchophylla | South Korea | H.D. Shin | HM148050, HM148291, HM148537 | |
| CPC 15038 | Eucalyptus sp., endophyte | Indonesia | M.J. Wingfield | HM148051, HM148292, HM148538 | |
| CPC 15167; HJS1069 | Living mite inhabiting a strawberry leaf | Slovenia | H.J. Schroers | HM148052, HM148293, HM148539 | |
| Cladosporium cladosporioides s. lat. Lineage 1 | CBS 116744 | Leaves of Acer pseudoplatanus | Germany | L. Pehl | HM148053, HM148294, HM148540 |
| CBS 125.80 | Seadcoat of Cirsium vulgare | Netherlands | — | DQ780941, HM148295, EF101351 | |
| CPC 13220 | Lichens on leaves of Acer platanoides | Germany | B. Heuchert | HM148054, HM148296, HM148541 | |
| CPC 14238 | Fruits of Sambucus nigra | Netherlands | P.W. Crous | HM148055, HM148297, HM148542 | |
| CPC 14296; BA1695 | Indoor building material, school | Denmark | B. Andersen | HM148056, HM148298, HM148543 | |
| Cladosporium cladosporioides s. lat. Lineage 2 | CBS 306.84 | Urediniospores of Puccinia allii | U.K. | G.S. Taylor | HM148057, HM148299, HM148544 |
| CPC 11664; Hill 1076-2 | Oncoba spinosa | New Zealand | C.F. Hill | HM148058, HM148300, HM148545 | |
| CPC 13867 | Leptosphaeria sp. | South Africa | P.W. Crous | HM148059, HM148301, HM148546 | |
| CPC 15457 | Imported buds of Prunus avium | New Zealand | J. Rennie | HM148060, HM148302, HM148547 | |
| Cladosporium cladosporioides s. lat. Lineage 3 | CBS 109082 | Silene maritima | U.K. | A. Aptroot | EF679354, EF679429, EF679506 |
| Cladosporium cladosporioides s. lat. Lineage 4 | CBS 113746 | Bing cherry fruits | U.S.A.: Washington | R.G. Roberts | HM148061, HM148303, HM148548 |
| CPC 10150 | Fatoua villosa | South Korea | H.D. Shin | HM148062, HM148304, HM148549 | |
| CPC 13362 | Paeonia obovata | Germany | P.W. Crous | HM148063, HM148305, HM148550 | |
| CPC 13978 | Needles of Pinus ponderosa | Argentina | A. Greslebin | HM148064, HM148306, HM148551 | |
| CPC 14284; BA1674 | Wheat grain, Triticum sp. | Germany | B. Andersen | HM148065, HM148307, HM148552 | |
| Cladosporium colocasiae | CBS 115191; CPC 4323 | Colocasia esculenta | Fiji | C.F. Hill | AY251075, HM148308, HM148553 |
| CBS 119542; CPC 12726; ICM 13264 | Colocasia esculenta | Japan | — | HM148066, HM148309, HM148554 | |
| CBS 386.64*; ATCC 200944; MUCL 10084 | Colocasia esculenta | Taiwan | K. Sawada | HM148067, HM148310, HM148555 | |
| CPC 5124 | Apium graveolens | New Zealand | C.F. Hill | AY251076, HM148311, HM148556 | |
| Cladosporium colombiae | CBS 274.80B* | Cortaderia sp. | Colombia | W. Gams | FJ936159, FJ936163, FJ936166 |
| Cladosporium cucumerinum | CBS 108.23 | Cucumis sativus | — | W.W. Gilbert | HM148068, HM148312, HM148557 |
| CBS 109.08 | Cucumis sativus | — | — | HM148069, HM148313, HM148558 | |
| CBS 123.44 | Cucumis sativus | Netherlands | — | HM148070, HM148314, HM148559 | |
| CBS 158.51; ATCC 11279; IFO 6370; IMI 049628; VKM F-817 | Cucumis sativus | Netherlands | — | HM148071, HM148315, HM148560 | |
| CBS 171.52*; MUCL 10092 | Cucumis sativus | Netherlands | — | HM148072, HM148316, HM148561 | |
| CBS 172.54 | Cucumis sativus | Netherlands | G.W. van der Helm | HM148073, HM148317, HM148562 | |
| CBS 173.54 | Cucumis sativus | Netherlands | G.W. van der Helm | HM148074, HM148318, HM148563 | |
| CBS 174.54 | Cucumis sativus | Netherlands | G.W. van der Helm | HM148075, HM148319, HM148564 | |
| CBS 174.62; ATCC 16022; ATHUM 2861; CECT 2110; IFO 31006; IMI 045534; MUCL 19019; VTT D-92188 | Painted floor | U.S.A. | M.H. Downing | HM148076, HM148320, HM148565 | |
| CBS 175.54 | Cucumis sativus | Netherlands | G.W. van der Helm | HM148077, HM148321, HM148566 | |
| CBS 176.54 | Cucumis sativus | Netherlands | G.W. van der Helm | HM148078, HM148322, HM148567 | |
| Cladosporium delicatulum | CBS 126342; CPC 14287; BA 1681 | Indoor air | Denmark | B. Andersen | HM148079, HM148323, HM148568 |
| CBS 126343; CPC 14299; BA 1698 | Building material | Denmark | B. Andersen | HM148080, HM148324, HM148569 | |
| CBS 126344; CPC 11389 | Tilia cordata | Germany | K. Schubert | HM148081, HM148325, HM148570 | |
| CPC 13148 | Puccinia bromina ssp. symphyti-bromarum | Germany | K. Schubert | HM148082, HM148326, HM148571 | |
| CPC 14285; BA 1679 | Indoor air | Denmark | B. Andersen | HM148083, HM148327, HM148572 | |
| CPC 14286; BA 1680 | Indoor air | Denmark | B. Andersen | HM148084, HM148328, HM148573 | |
| CPC 14289; BA 1683 | Door frame | Denmark | B. Andersen | HM148085, HM148329, HM148574 | |
| CPC 14307; BA 1706 | Sea weed | Denmark | B. Andersen | HM148086, HM148330, HM148575 | |
| CPC 14360; BA 1718 | Indoor air | Denmark | B. Andersen | HM148087, HM148331, HM148576 | |
| CPC 14363; BA 1724 | Indoor air | Denmark | B. Andersen | HM148088, HM148332, HM148577 | |
| CPC 14372; BA 1740 | Dust, school | Denmark | B. Andersen | HM148089, HM148333, HM148578 | |
| Cladosporium exasperatum | CBS 125986*; CPC 14638 | Eucalyptus tintinnans | Australia | B.A. Summerell | HM148090, HM148334, HM148579 |
| Cladosporium exile | CBS 125987*; CPC 11828 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148091, HM148335, HM148580 |
| Cladosporium flabelliforme | CBS 126345*; CPC 14523 | Melaleuca cajuputi | Australia | B.A. Summerell | HM148092, HM148336, HM148581 |
| Cladosporium funiculosum | CBS 122128; ATCC 16160; IFO 6536; JCM 10682 | Ficus carica | Japan | — | HM148093, HM148337, HM148582 |
| CBS 122129*; ATCC 38010; IFO 6537; JCM 10683 | Vigna umbellata | Japan | — | HM148094, HM148338, HM148583 | |
| Cladosporium gamsianum | CBS 125989*; CPC 11807 | Strelitzia sp. | South Africa | W. Gams | HM148095, HM148339, HM148584 |
| Cladosporium globisporum | CBS 812.96* | Meat stamp | Sweden | M. Olsen | HM148096, HM148340, HM148585 |
| Cladosporium hillianum | CBS 125988*; CPC 15459; C92 | Leaf mold of Typha orientalis | New Zealand | R. Beever | HM148097, HM148341, HM148586 |
| CPC 15458 | Leaf mold of Typha orientalis | New Zealand | R. Beever | HM148098, HM148342, HM148587 | |
| Cladosporium inversicolor | CBS 131.29; ATCC 200942, ATCC 11275; IMI 049623; LCP 52.404 | Triticum aestivum | — | F.T. Bennett | HM148099, HM148343, HM148588 |
| CBS 143.65 | Leaf of Tilia sp. | Netherlands | — | HM148100, HM148344, HM148589 | |
| CBS 401.80*; ATCC 200941 | Leaf of Triticum aestivum | Netherlands | — | HM148101, HM148345, HM148590 | |
| CBS 464.82; ATCC 200945 | Seeds of Alnus sp. | Netherlands | G.S. de Vries | HM148102, HM148346, HM148591 | |
| CBS 484.80 | Cortaderia sp. | Colombia | — | HM148103, HM148347, HM148592 | |
| CPC 11818 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148104, HM148348, HM148593 | |
| CPC 13150 | Puccinia bromina ssp. symphyti-bromarum | Germany | K. Schubert | HM148105, HM148349, HM148594 | |
| CPC 14190 | Outside air | Netherlands | M. Meijer | HM148106, HM148350, HM148595 | |
| CPC 14191 | Outside air | Netherlands | M. Meijer | HM148107, HM148351, HM148596 | |
| CPC 14241 | Fruit of Sambucus nigra | Netherlands | P.W. Crous | HM148108, HM148352, HM148597 | |
| CPC 14368; BA1735 | Dust, school | Denmark | B. Andersen | HM148109, HM148353, HM148598 | |
| Cladosporium iranicum | CBS 126346*; CPC 11554 | Leaf of Citrus sinensis | Iran | W. Gams | HM148110, HM148354, HM148599 |
| Cladosporium licheniphilum | CBS 125990*; CPC 13224 | Phaeophyscia orbicularis and Physcia sp. | Germany | W. von Brackel | HM148111, HM148355, HM148600 |
| Cladosporium lycoperdinum | CBS 126347; CPC 12102 | Galls of Apiosporina morbosa on Prunus sp. | Canada | K.A. Seifert | HM148112, HM148356, HM148601 |
| CBS 126348; CPC 11833 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148113, HM148357, HM148602 | |
| CBS 274.80C | Puya sp. | Colombia | W. Gams | HM148114, HM148358, HM148603 | |
| CBS 574.78C; VKM F-2759 | Aureobasidium caulivorum | Russia | — | HM148115, HM148359, HM148604 | |
| Cladosporium myrtacearum | CBS 126349; CPC 13689; NSM 734672 | Eucalyptus placita | Australia | B.A. Summerell | HM148116, HM148360, HM148605 |
| CBS 126350*; CPC 14567 | Corymbia foelscheana | Australia | B.A. Summerell | HM148117, HM148361, HM148606 | |
| Cladosporium oxysporum | CBS 125991; CPC 14371; BA 1738 | Soil, near the terracotta army | China: Xi'an, Shaanxi | B. Andersen | HM148118, HM148362, HM148607 |
| CBS 126351; CPC 14308; BA 1707 | Indoor air | Venezuela | B. Andersen | HM148119, HM148363, HM148608 | |
| Cladosporium paracladosporioides | CBS 171.54*; ATCC 11278, 200943; IFO 6369; IMI 049626; MUCL 917; NCTC 4097 | — | — | — | HM148120, HM148364, HM148609 |
| Cladosporium perangustum | CBS 125996*; CPC 13815 | Cussonia sp. | South Africa | P.W. Crous | HM148121, HM148365, HM148610 |
| CBS 126364; CPC 14532 | Erythrophleum chlorostachys | Australia | B.A. Summerell | HM148122, HM148366, HM148611 | |
| CBS 126365; CPC 11820 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148123, HM148367, HM148612 | |
| CBS 167.54; ATCC 11276; IMI 049624 | — | — | — | HM148124, HM148368, HM148613 | |
| CPC 11046 | Margarine | Australia | N. Charley | HM148125, HM148369, HM148614 | |
| CPC 11133 | Eucalyptus sp. | India | W. Gams | HM148126, HM148370, HM148615 | |
| CPC 11526 | Acacia mangium | Thailand | W. Himaman | HM148127, HM148371, HM148616 | |
| CPC 11609 | Musa sp. | India | M. Arzanlou | EF679356, EF679431, EF679508 | |
| CPC 11663 | Oncoba spinosa | New Zealand | C.F. Hill | HM148128, HM148372, HM148617 | |
| CPC 11806 | Strelitzia sp. | South Africa | W. Gams | HM148129, HM148373, HM148618 | |
| CPC 11815 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | U.S.A.: Washington | D. Glawe | HM148130, HM148374, HM148619 | |
| CPC 11819 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | U.S.A.: Washington | D. Glawe | HM148131, HM148375, HM148620 | |
| CPC 11821 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | U.S.A.: Washington | D. Glawe | HM148132, HM148376, HM148621 | |
| CPC 11831 | Chasmothecia of Phyllactinia guttata on leaves of Corylus sp. | U.S.A.: Washington | D. Glawe | HM148133, HM148377, HM148622 | |
| CPC 11856 | Acacia mangium | Thailand | W. Himaman | HM148134, HM148378, HM148623 | |
| CPC 12216 | Morus rubra | Germany | N. Ale-Agha | HM148135, HM148379, HM148624 | |
| CPC 12792 | Musa sp. | Polynesia | I. Budenhagen | HM148136, HM148380, HM148625 | |
| CPC 12793 | Musa sp. | Polynesia | I. Budenhagen | HM148137, HM148381, HM148626 | |
| CPC 13686 | Eucalyptus placita | Australia | B.A. Summerell | HM148138, HM148382, HM148627 | |
| CPC 13727 | Teratosphaeria maculiformis | South Africa | P.W. Crous | HM148139, HM148383, HM148628 | |
| CPC 13730 | Protea caffra | South Africa | P.W. Crous | HM148140, HM148384, HM148629 | |
| CPC 13774 | Protea caffra | South Africa | P.W. Crous | HM148141, HM148385, HM148630 | |
| CPC 13870 | Teratosphaeria fibrillosa | South Africa | P.W. Crous | HM148142, HM148386, HM148631 | |
| CPC 14004; MRC 03367 | Oats | South Africa | — | HM148143, HM148387, HM148632 | |
| CPC 14008; MRC 10135 | Wheat | South Africa | — | HM148144, HM148388, HM148633 | |
| CPC 14247 | Magnolia sp. | U.S.A. | P.W. Crous | HM148145, HM148389, HM148634 | |
| CPC 14256 | Leaves of pecan tree | U.S.A. | P.W. Crous | HM148146, HM148390, HM148635 | |
| CPC 14566 | Corymbia foelscheana | Australia | B.A. Summerell | HM148147, HM148391, HM148636 | |
| CPC 14911 | Strelitzia sp. | South Africa | P.W. Crous | HM148148, HM148392, HM148637 | |
| CPC 15192 | Protea cynaroides | South Africa | L. Mostert | HM148149, HM148393, HM148638 | |
| Cladosporium phyllactiniicola | CBS 126352*; CPC 11836 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148150, HM148394, HM148639 |
| CBS 126353; CPC 11823 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148151, HM148395, HM148640 | |
| CBS 126354; CPC 11825 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148152, HM148396, HM148641 | |
| CBS 126355; CPC 11830 | Chasmothecia of Phyllactinia guttata on leaves of Corylus avellana | U.S.A.: Washington | D. Glawe | HM148153, HM148397, HM148642 | |
| Cladosporium phyllophilum | CBS 125992*; CPC 11333 | Taphrina sp. on Prunus cerasus | Germany | K. Schubert | HM148154, HM148398, HM148643 |
| CPC 13873 | On Teratosphaeria proteae-arboreae on Protea arborea | South Africa | P.W. Crous | HM148155, HM148399, HM148644 | |
| Cladosporium pini-ponderosae | CBS 124456*; CPC 13980; CIEFAP 322 | Pinus ponderosa | Argentina | A. Greslebin | FJ936160, FJ936164, FJ936167 |
| Cladosporium pseudocladosporioides | CBS 117134 | Cloud water | — | M. Sancelme | HM148156, HM148400, HM148645 |
| CBS 117153 | Living leaves of Paeonia sp. | Germany | R. Kirschner | HM148157, HM148401, HM148646 | |
| CBS 125993*; CPC 14189 | Outside air | Netherlands | M. Meijer | HM148158, HM148402, HM148647 | |
| CBS 126356; CPC 14278 | Leaves | France | P.W. Crous | HM148159, HM148403, HM148648 | |
| CBS 126390; CPC 13499 | Myrothecium inundatum | Germany | M. Grube | HM148160, HM148404, HM148649 | |
| CBS 149.66 | Triticum aestivum | U.S.A. | C.W. Hesseltine | HM148161, HM148405, HM148650 | |
| CBS 176.82 | Pteridium aquilinum | Romania | — | HM148162, HM148406, HM148651 | |
| CBS 574.78A; VKM F-422 | Mycophilic, Melampsoridium betulae | Russia | — | HM148163, HM148407, HM148652 | |
| CBS 574.78B; VKM F-2759 | Mycophilic, Melampsoridium betulae | Russia | — | HM148164, HM148408, HM148653 | |
| CBS 667.80; IHEM 3705 | Malus sylvestris | Italy | — | HM148165, HM148409, HM148654 | |
| CBS 673.69 | Air | Netherlands | — | EF679353, EF679428, EF679505 | |
| CPC 11392 | Chrysanthemum coronarium var. spatiosum | South Korea | H.D. Shin | HM148166, HM148410, HM148655 | |
| CPC 11605 | Agrimonia pilosa | South Korea | H.D. Shin | HM148167, HM148411, HM148656 | |
| CPC 11841; Hill 730; ICMP 14870 | Leaves of Phalaris aquatica | New Zealand | C.F. Hill | HM148168, HM148412, HM148657 | |
| CPC 12850 | Pruned wood | U.S.A. | K.A. Seifert | HM148169, HM148413, HM148658 | |
| CPC 13339 | Eucalyptus molucana | Australia | — | HM148170, HM148414, HM148659 | |
| CPC 13488 | Vernonia sp. | Brazil | O. Pereira | HM148171, HM148415, HM148660 | |
| CPC 13529 | Sagittaria graminea | Italy | W. Gams & K.A. Seifert | HM148172, HM148416, HM148661 | |
| CPC 13683; NSW 734672 | Eucalyptus placita | Australia | B.A. Summerell | HM148173, HM148417, HM148662 | |
| CPC 13992 | Coffee tree | U.S.A. | P.W. Crous | HM148174, HM148418, HM148663 | |
| CPC 13998; CAMS 001160 | Aloe dichotoma | South Africa | — | HM148175, HM148419, HM148664 | |
| CPC 14001; MRC 03240 | Oats | South Africa | — | HM148176, HM148420, HM148665 | |
| CPC 14002; MRC 03245 | Oats | South Africa | — | HM148177, HM148421, HM148666 | |
| CPC 14003; MRC 03366 | Oats | South Africa | — | HM148178, HM148422, HM148667 | |
| CPC 14005; MRC 03850 | Oats | South Africa | — | HM148179, HM148423, HM148668 | |
| CPC 14006; MRC 03978 | Wheat | South Africa | — | HM148180, HM148424, HM148669 | |
| CPC 14007; MRC 03979 | Oats | South Africa | — | HM148181, HM148425, HM148670 | |
| CPC 14010; MRC 10183 | Sorghum sp. | South Africa | — | HM148182, HM148426, HM148671 | |
| CPC 14013; MRC 10221 | Wheat | South Africa | — | HM148183, HM148427, HM148672 | |
| CPC 14014; MRC 10232 | Wheat | South Africa | — | HM148184, HM148428, HM148673 | |
| CPC 14020; MRC 10814 | Wheat | South Africa | — | HM148185, HM148429, HM148674 | |
| CPC 14193 | Outside air | Netherlands | M. Meijer | HM148186, HM148430, HM148675 | |
| CPC 14230 | Pine needles of Pinus sp. | Netherlands | P.W. Crous | HM148187, HM148431, HM148676 | |
| CPC 14295; BA 1694 | Soil | Chile: Easter Island | B. Andersen | HM148188, HM148432, HM148677 | |
| CPC 14357; BA1678 | Food, coffee leaf | Uganda: Mubende | B. Andersen | HM148189, HM148433, HM148678 | |
| CPC 14382 | Acer macrophyllum | Canada | B. Callan | HM148190, HM148434, HM148679 | |
| CPC 14975a; HJS 1038 | Rosa canina | Slovenia | H.J. Schroers | HM148191, HM148435, HM148680 | |
| CPC 14992 | Eucalyptus sp. | Indonesia | M.C. Wingfield | HM148192, HM148436, HM148681 | |
| CPC 5100; ATCC 66669 | Creosote-treated southern pine pole | U.S.A.: New York | — | AY251070, HM148437, HM148682 | |
| Cladosporium rectoides | CBS 125994*; CPC 11624 | Vitis flexuosa | South Korea | H.D. Shin | HM148193, HM148438, HM148683 |
| CBS 126357; CPC 11405 | Plectranthus sp. | South Korea | H.D. Shin | HM148194, HM148439, HM148684 | |
| Cladosporium scabrellum | CBS 126358*; CPC 14976; HJS 1031 | Ruscus hypoglossum | Slovenia | H.J. Schroers | HM148195, HM148440, HM148685 |
| Cladosporium subuliforme | CBS 126500*; CPC 13735 | Chamaedorea metallica | Thailand | I. Hidayat & J. Meeboon | HM148196, HM148441, HM148686 |
| Cladosporium tenuissimum | CBS 125995*; CPC 14253 | Lagerstroemia sp. | U.S.A.: Louisiana | P.W. Crous | HM148197, HM148442, HM148687 |
| CBS 126359; CPC 12794 | Musa sp. | Polynesia | I. Budenhagen | HM148198, HM148443, HM148688 | |
| CBS 126501; CPC 14410 | Musa sp. | Ivory Coast | K. Daouda | HM148199, HM148444, HM148689 | |
| CBS 117.79 | Fruit | Burundi | J. Rammelo | HM148200, HM148445, HM148690 | |
| CBS 262.80 | Fruit | Nigeria | — | HM148201, HM148446, HM148691 | |
| CPC 10538 | Musa sp. | Mozambique | A. Viljoen | HM148202, HM148447, HM148692 | |
| CPC 10539 | Musa sp. | Mozambique | A. Viljoen | HM148203, HM148448, HM148693 | |
| CPC 10882 | Gnaphalium affme | South Korea | H.D. Shin | HM148204, HM148449, HM148694 | |
| CPC 11555 | Citrus sinensis | Iran | W. Gams | HM148205, HM148450, HM148695 | |
| CPC 11612 | Musa sp. | Indonesia | M. Arzanlou | HM148206, HM148451, HM148696 | |
| CPC 11805 | Strelitzia sp. | South Africa | W. Gams | HM148207, HM148452, HM148697 | |
| CPC 12223 | Rust | Brazil | U. Braun | HM148208, HM148453, HM148698 | |
| CPC 12795 | Musa sp. | Polynesia | I. Budenhagen | HM148209, HM148454, HM148699 | |
| CPC 13222 | Callistemon viminalis | Australia | P.W. Crous | HM148210, HM148455, HM148700 | |
| CPC 14250 | Magnolia sp. | U.S.A.: Louisiana | P.W. Crous | HM148211, HM148456, HM148701 | |
| Cladosporium tenuissimum Lineage 1 | CPC 11130 | Dalbergia sp. | India | W. Gams | HM148212, HM148457, HM148702 |
| CPC 11132 | Citrus sp. | India | W. Gams | HM148213, HM148458, HM148703 | |
| CPC 11521 | Acacia mangium | Thailand | W. Himaman | HM148214, HM148459, HM148704 | |
| CPC 11929 | Acacia mangium | Thailand | W. Himaman | HM148215, HM148460, HM148705 | |
| CPC 13252 | Rock | Australia | P.W. Crous | HM148216, HM148461, HM148706 | |
| CPC 13732 | Shorea siamensis | Laos | P. Phengsintham | HM148217, HM148462, HM148707 | |
| CPC 14196 | Basella alba | Laos | P. Phengsintham | HM148218, HM148463, HM148708 | |
| CPC 14311; BA1710 | Decaying branch under water | Venezuela: Mochima Bay | B. Andersen | HM148219, HM148464, HM148709 | |
| CPC 14312; BA1711 | Sediment, red mangrove | Venezuela: Mochima Bay | B. Andersen | HM148220, HM148465, HM148710 | |
| CPC 14370; BA1737 | Soil, near the Gua Lawah/Bat Cave | Bali: Pasinggahan | B. Andersen | HM148221, HM148466, HM148711 | |
| Cladosporium uredinicola | CPC 5390; ATCC 46649 | Hyperparasite on Cronartium fusiforme f. sp. quercum on Quercus nigra leaves | U.S.A.: Alabama | — | AY251071, HM148467, HM148712 |
| Cladosporium varians | CBS 126360; CPC 11327 | Ulmus sp. | Germany | K. Schubert | HM148222, HM148468, HM148713 |
| CBS 126361; CPC 11134 | Leaf debris | India | W. Gams | HM148223, HM148469, HM148714 | |
| CBS 126362*; CPC 13658 | Catalpa bungei | Russia | V.A. Melnik | HM148224, HM148470, HM148715 | |
| CPC 14975b; HJS 1038 | Rosa canina | Slovenia | H.J. Schroers | HM148225, HM148471, HM148716 | |
| Cladosporium verrucocladosporioides | CBS 126363*; CPC 12300 | Rhus chinensis | South Korea | H.D. Shin | HM148226, HM148472, HM148717 |
| Cladosporium vignae | CBS 121.25; ATCC 200933; MUCL 10110 | Vigna unguiculata | U.S.A. | M.W. Gardner | HM148227, HM148473, HM148718 |
| Cladosporium xylophilum | CBS 113749 | Bing cherry fruits | U.S.A. | F.M. Dugan | HM148228, HM148474, HM148719 |
| CBS 113756 | Bing cherry fruits | U.S.A. | F.M. Dugan | HM148229, HM148475, HM148720 | |
| CBS 125997*; CPC 12403 | Dead wood of Picea abies | Russia | D.A. Shabunin | HM148230, HM148476, HM148721 | |
| CBS 126588; CPC 13512 | Twigs of Salix viminalis | Italy | W. Gams | HM148231, HM148477, HM148722 | |
| CPC 12101 | Galls of Apiosporina morbosa | Canada | K.A. Seifert | HM148232, HM148478, HM148723 | |
| CPC 14281 | Leaves | France | P.W. Crous | HM148233, HM148479, HM148724 | |
| CPC 14364; BA1725 | Indoor air | Denmark | B. Andersen | HM148234, HM148480, HM148725 |
ATCC: American Type Culture Collection, Virginia, U.S.A.; ATHUM: ATHUM Culture Collection of Fungi, National and Kapodistrian University of Athens, Greece; BA: Personal culture collection of Birgitte Andersen, Denmark; CAMS: SERA's Centre for Applied Mycological Studies, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CECT: Coleccion Espanola de Cultivos Tipo, Universidad de Valencia, Spain; CIEFAP: Centro de Investigación y Extensión Forestal Andino Patagónico, Argentina; CPC: Culture collection of Pedro Crous, housed at CBS; Hill: Personal culture collection of Frank Hill, New Zealand; HJS: Personal culture collection of Hans-Josef Schroers, Slovenia; ICM: Istituto cantonale di microbiologia, Bellinzona and Univ. of Geneva, Switzerland; ICMP: International Collection of Micro-organisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; IFO: Institute for Fermentation, Osaka, Japan; IHEM: BCCM/IHEM, Scientific Institute of Public Health, Brussels, Belgium; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; JCM: Japan Collection of Microorganisms, RIKEN BioResource Center, Saitama, Japan; LCP: Fungal Strain Collection, Laboratory of Cryptogamy, Museum National d'Histoire Naturelle, Paris, France; MRC: Medical Research Council, Cape Town, South Africa; MUCL: Mycotheque de l'Universite catholique de Louvain, Laboratoire de Mycologie Systematique et Appliquee, Universite catholique de Louvain, Louvain-la-Neuve, Belgium; NBRC: NITE Biological Resource Center, National Institute of Technology and Evaluation, Chiba, Japan; NCTC: National Collection of Type Cultures, PHLS Central Public Health Laboratory, London, U.K.; VKM: All-Russian Collection of Microorganisms, Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Moscow region, Russian Federation; VTT: VTT Culture Collection, VTT Technical Research Centre of Finland, Finland.
ACT: partial actin gene, TEF: partial translation elongation factor 1-alpha gene, ITS: internal transcribed spacer regions with 5.8S rRNA gene.
Ex-type cultures.
DNA isolation, amplification and sequence analysis
Fungal colonies were established on agar plates, and genomic DNA was isolated as described in Crous et al. (2009f, 2009f). Partial gene sequences were determined as described by Crous et al. (2006b) and Schubert et al. (2007b) for actin (ACT), translation elongation factor 1-α (TEF), and part of the nuclear rDNA operon spanning the 3' end of the 18S rRNA gene, the first internal transcribed spacer, the 5.8S rRNA gene, the second internal transcribed spacer and the 5' end of the 28S rRNA gene (ITS). The primer EF-2 (O'Donnell et al. 1998) can be used as alternative reverse primer for amplification of the TEF region. The nucleotide sequences were generated using both PCR primers to ensure good quality sequences over the entire length of the amplicon. Sequence data obtained from Schubert et al. (2007b) and Zalar et al. (2007) were used as reference data for the alignments (Table 1). Subsequent sequence alignment followed Crous et al. (2006b). MrModeltest v. 2.2 (Nylander 2004) was used to determine the best nucleotide substitution model for each locus using the Akaike information criterion (AIC). The phylogenetic analyses were performed with MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2003, Ronquist & Huelsenbeck 2005) and the Markov Chain Monte Carlo (MCMC) analysis of 4 chains started from random tree topology and lasted 18 000 000 generations. Trees were saved each 1 000 generations, resulting in 18 001 saved trees in each of the two run files. Burn-in was set at 5 000 000 generations after which the likelihood values were stationary. Neighbour-joining analyses using the HKY85 substitution model were applied to each data partition to check the stability and robustness of each species clade under the different partitions (data not shown). The ITS region has limited resolution for many species in Cladosporium, therefore results for the ACT and TEF regions were used for comparison of clade stability. Gaps longer than 10 bases were coded as single events for the phylogenetic analyses (TEF alignment). Novel sequence data were lodged in GenBank (Table 1) and the alignment and tree in TreeBASE (www.treebase.org).
Morphology
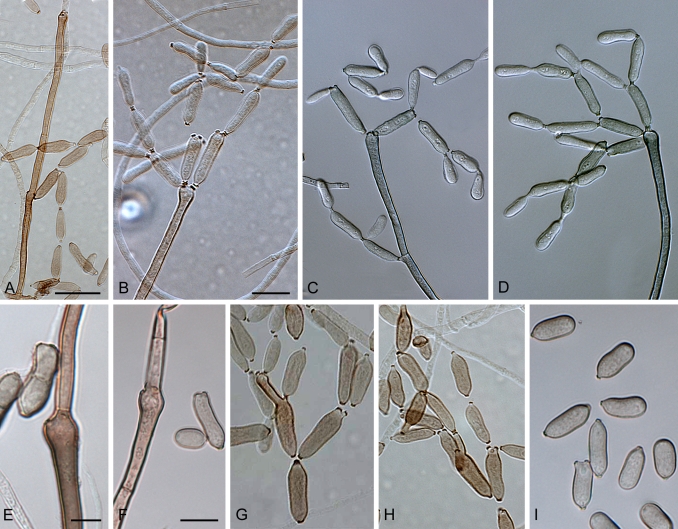
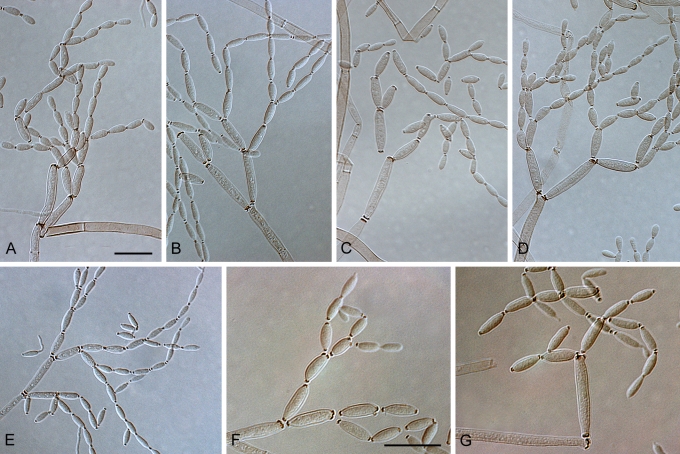
Light microscopy (LM). Microscopic observations of isolates were made from colonies cultivated for 7 d under continuous near ultraviolet light at 25 °C on SNA. Preparations were mounted in Shear's solution (Crous et al. 2009f, Crous et al. 2009f). To study conidial development and branching patterns of conidial chains, squares of transparent adhesive tape (Titan Ultra Clear Tape, Conglom Inc., Toronto, Canada) were placed on conidiophores growing in the zone between the colony margin and 2 cm inwards, and mounted between two drops of Shear's solution under a glass cover slip. Conidial terminology follows Schubert et al. (2007b). Wherever possible, 50 measurements (× 1 000 magnification, differential interference contrast microscopy, Zeiss Axioscope 2 PLUS) were made of conidia with outliers given in parentheses. For cultural characteristics colonies were cultivated on PDA, SNA, OA and MEA for 14 d at 25 °C in the dark, after which the surface and reverse colours were rated using the charts of Rayner (1970).
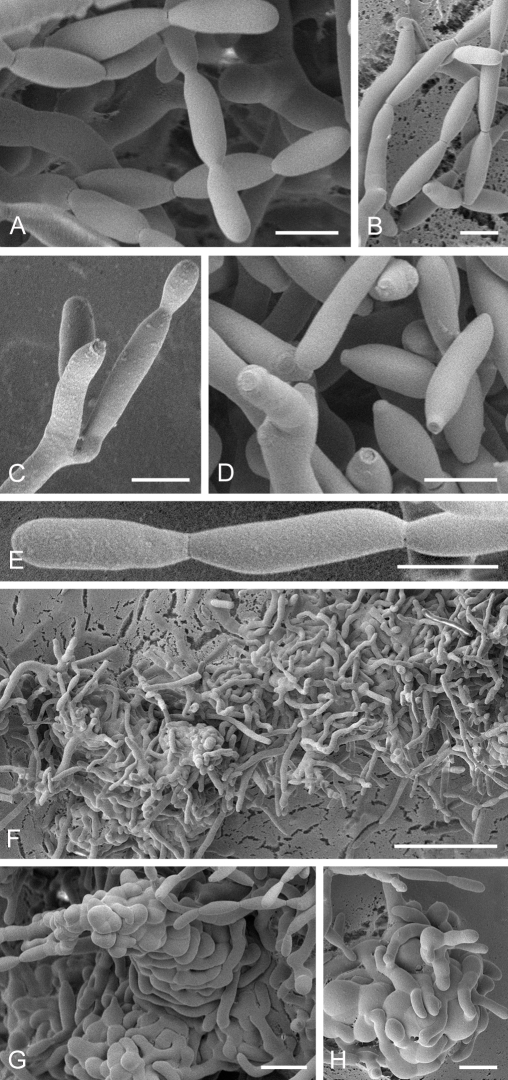
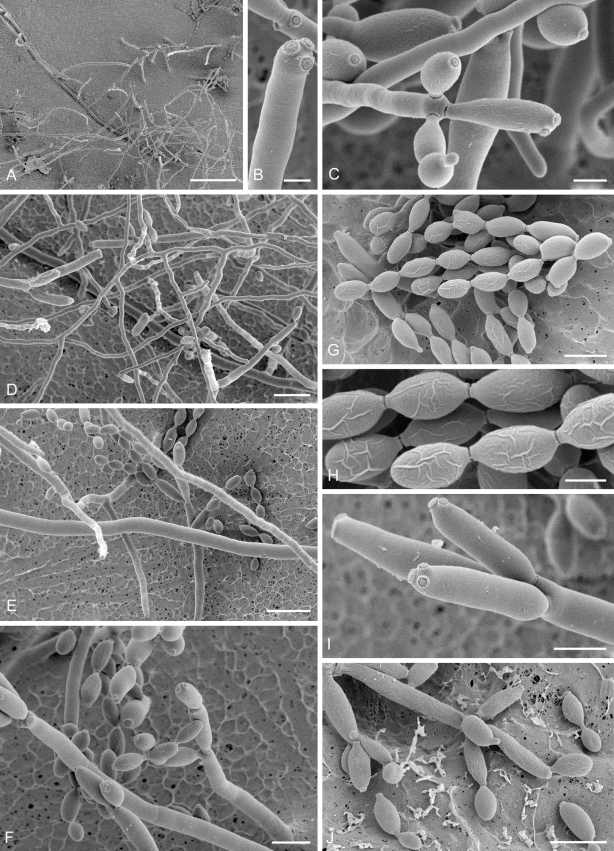
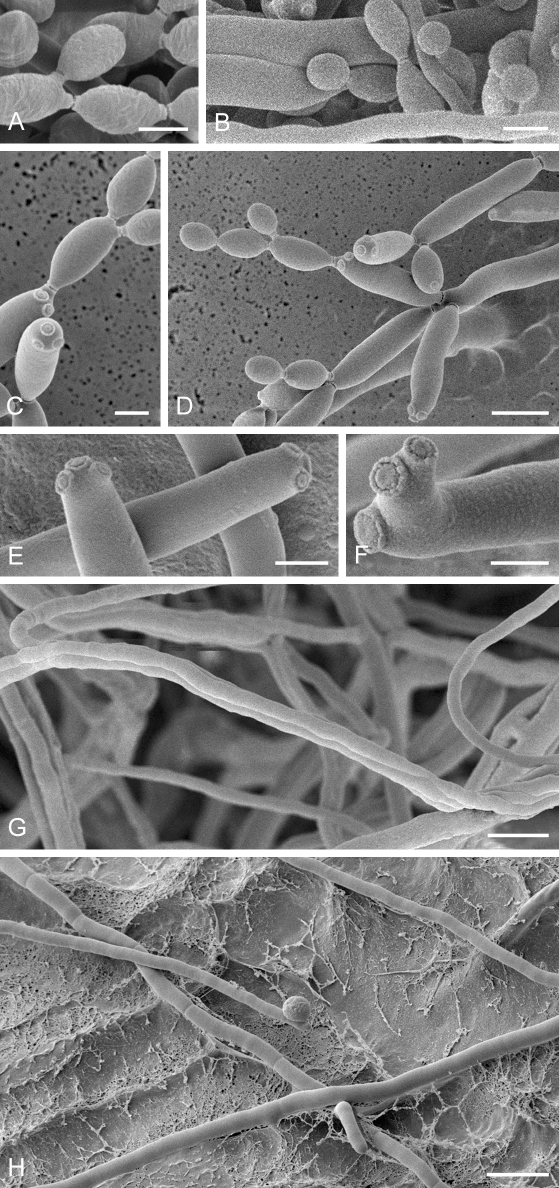
Low-temperature scanning electron microscopy (SEM). Isolates of Cladosporium spp. were grown on SNA with 30 g agar/L for 3–4 d at room temperature under black light. Relevant parts of the small colonies with conidiophores and conidia were selected under a binocular (× 10–50 magnification, Nicon SMZ 1500), excised with a surgical blade as small agar blocks (3 × 3 mm), and transferred to a copper cup for snap-freezing in nitrogen slush. Agar blocks were glued to the copper surface with frozen tissue medium (KP-Cryoblock, Klinipath, Duiven, Netherlands) mixed with 1 part colloidal graphite (Agar Scientific, Stansted, U.K.). Samples were examined in a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan) equipped with an Oxford CT1500 Cryostation for cryo-electron microscopy (cryoSEM). Electron micrographs were acquired from uncoated frozen samples, or after sputter-coating by means of a gold/palladium target for 3 times during 30 s. Micrographs of uncoated samples were taken at an acceleration voltage of 3 kV, and consisted of 30 averaged fast scans (SCAN 2 mode), at 5 kV in case of the coated samples (PHOTO mode).
RESULTS
DNA phylogeny
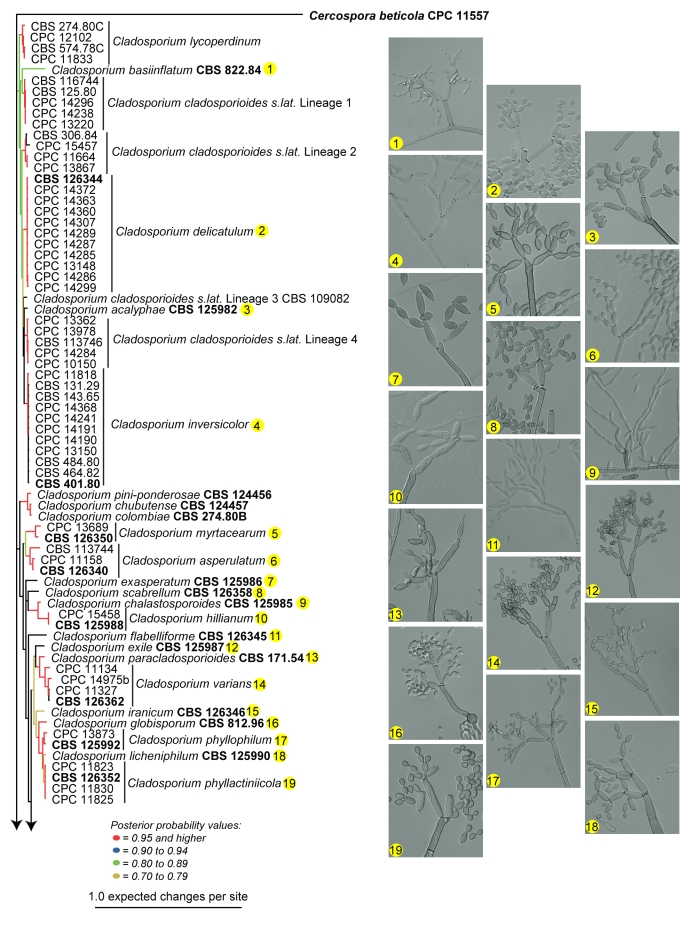
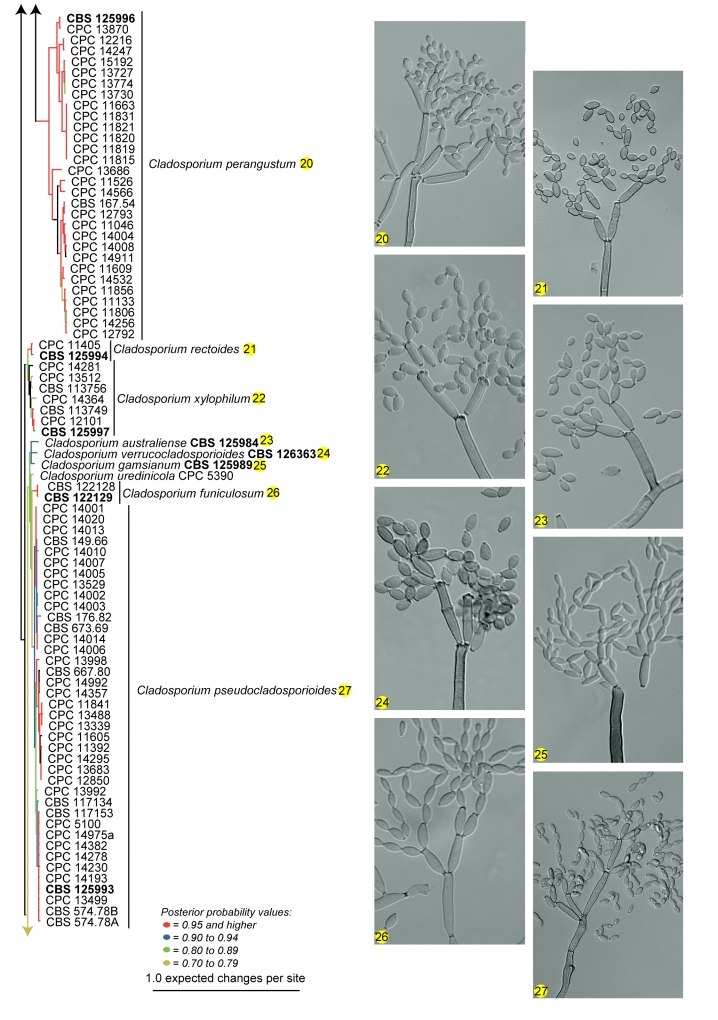
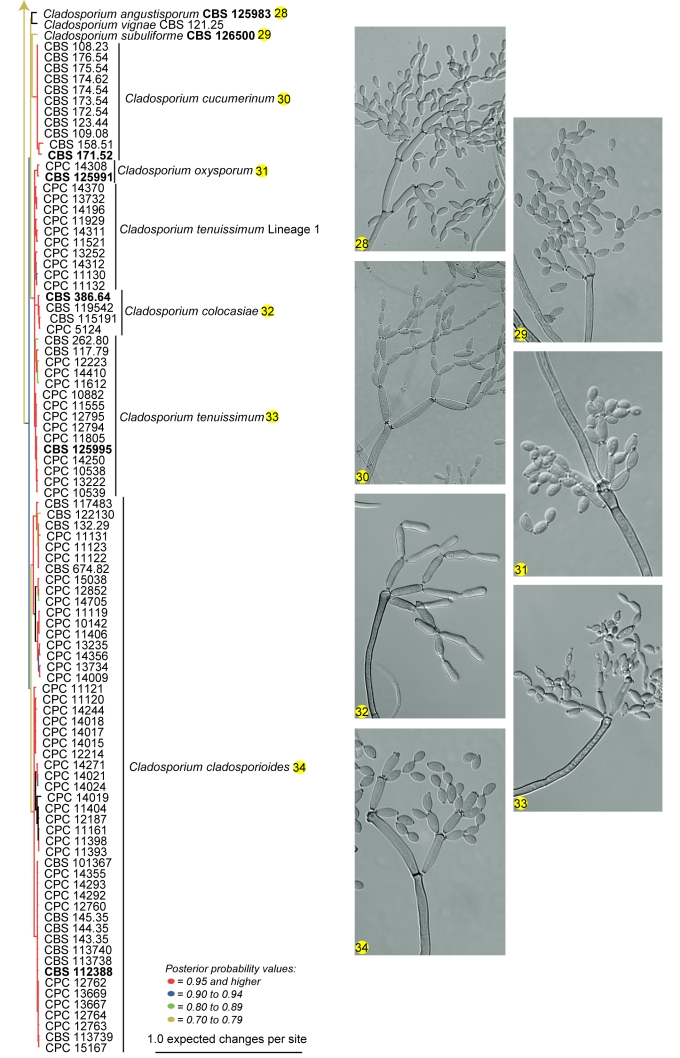
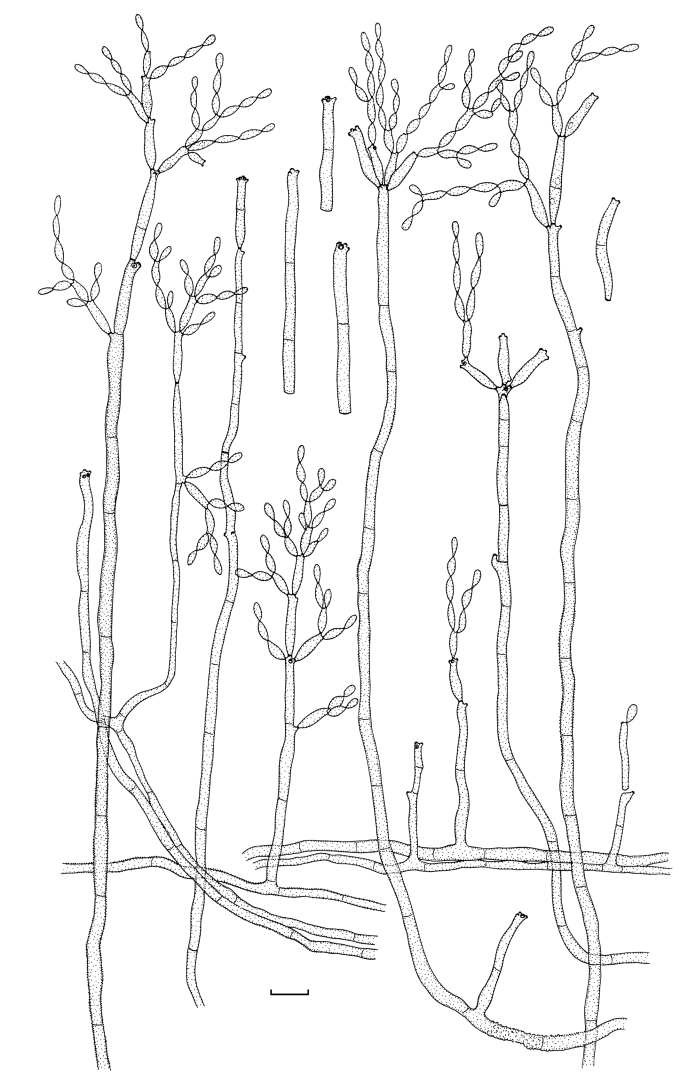
Amplification products and gene sequences of similar size to those reported previously (Crous et al. 2006b, Schubert et al. 2007b) were obtained. The resulting alignment contained 253 taxa (including the outgroup taxon) and 497, 193 and 373 characters (including alignment gaps) were used in the ITS, ACT and TEF partitions, respectively. The best model selected for ITS was a general time-reversible (GTR) substitution model with a proportion of the sites invariable and the state frequencies set at fixed (SYM+I model); and for both ACT and TEF a general time-reversible (GTR) substitution model with inverse gamma rates and the state frequencies set at Dirichlet (GTR+I+G model). For the Bayesian analysis, 26 002 trees were obtained from which the consensus tree and posterior probabilities were calculated (Fig. 1). Based on the phylogenetic and morphological results, 22 novel species are described. Further phylogenetic results are discussed under the species notes below where applicable.
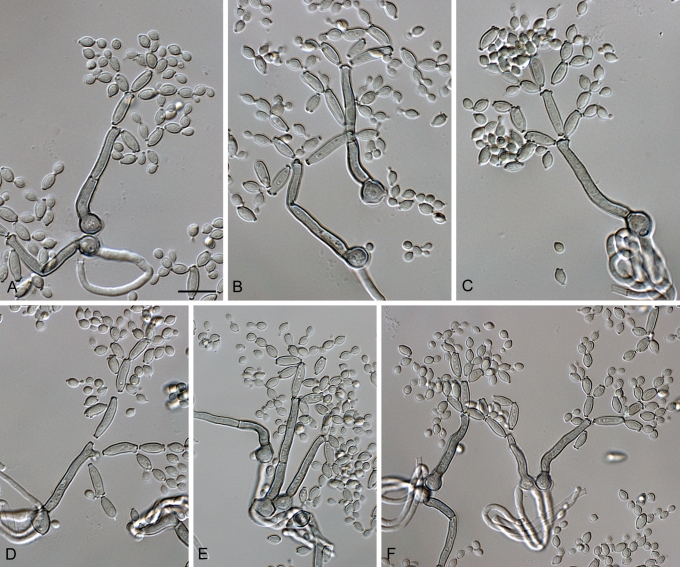
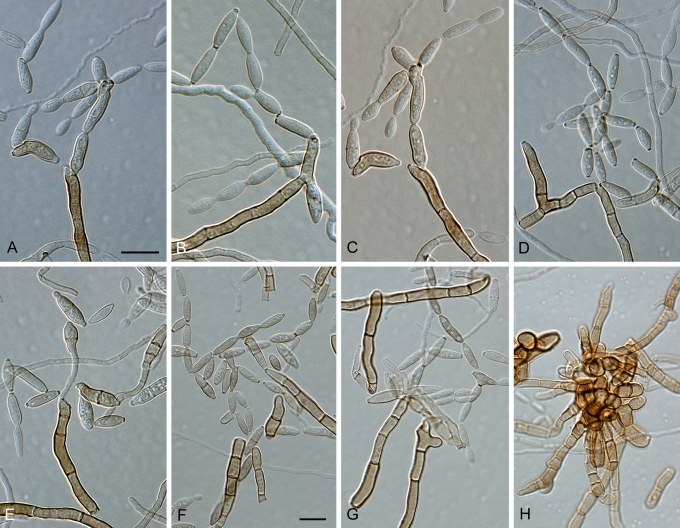
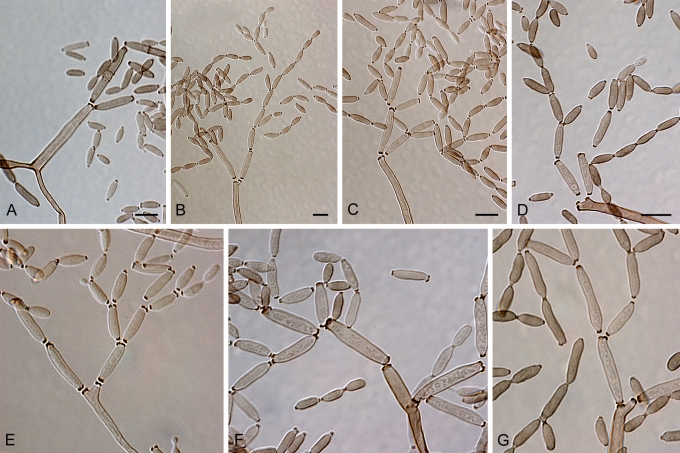
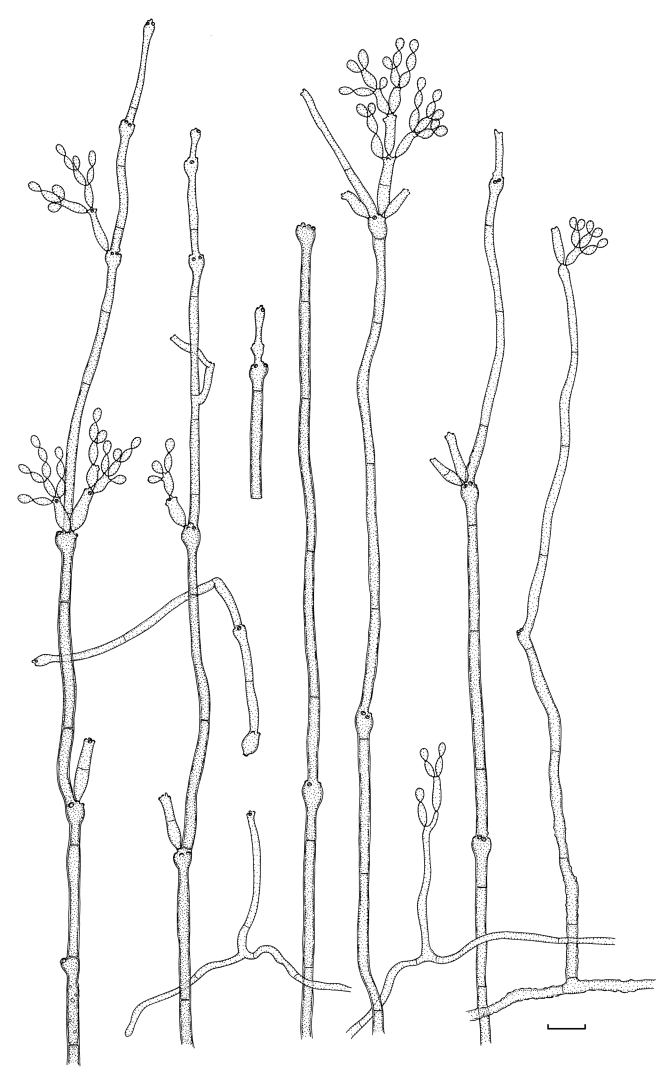
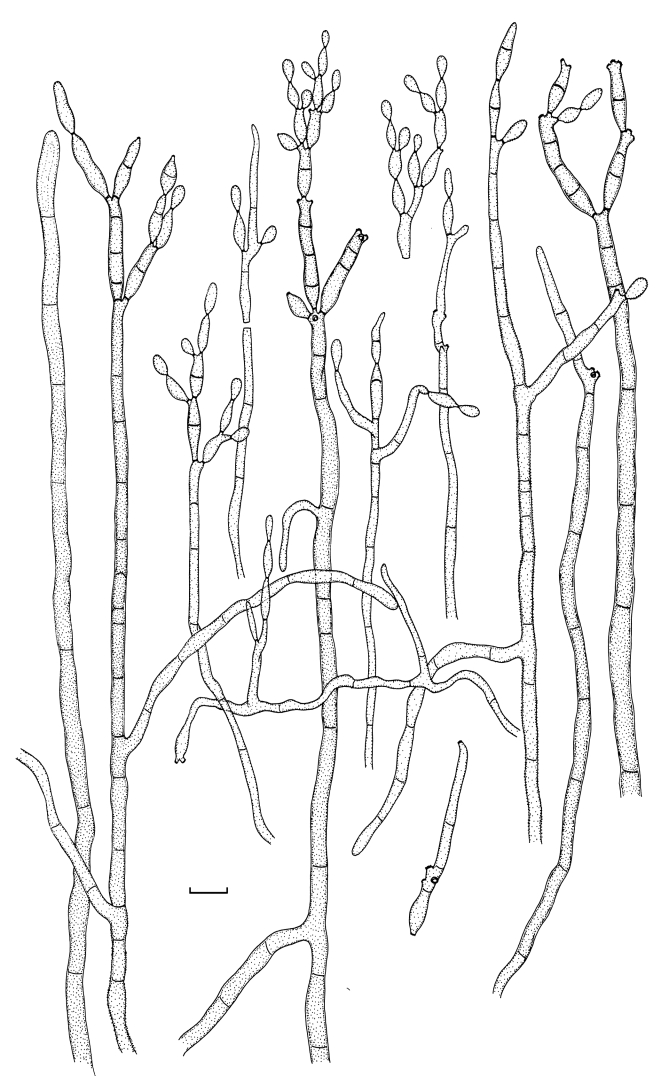
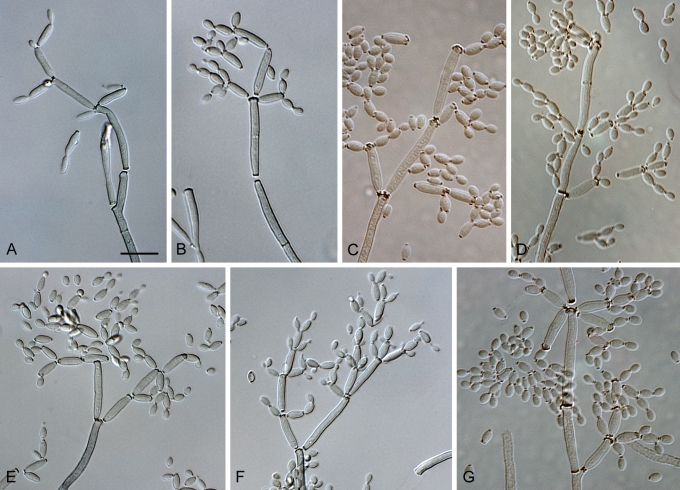
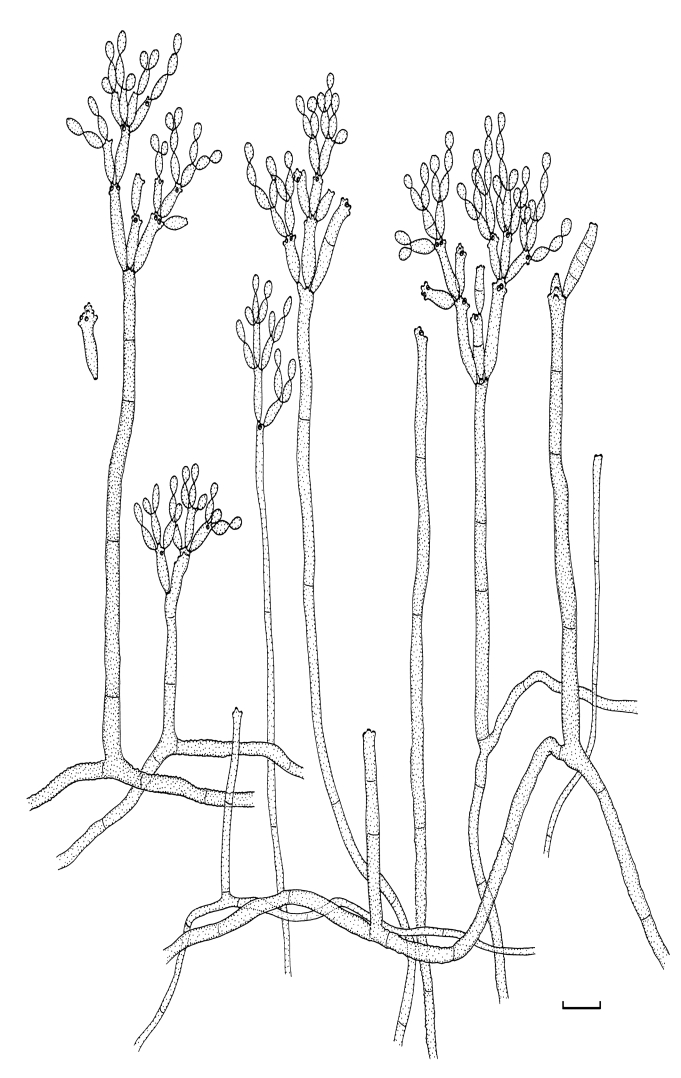
Fig. 1.
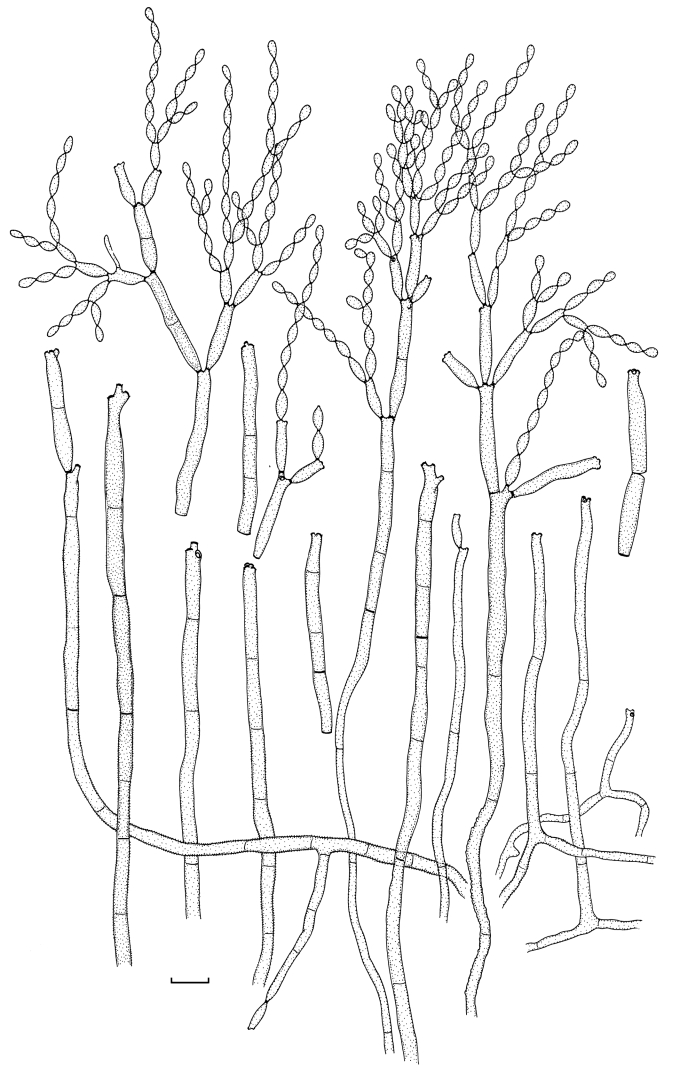
(Parts a–c) Consensus phylogram of 26 002 trees resulting from a Bayesian analysis of 253 sequences in a combined ITS, ACT and TEF alignment. Bayesian posterior probabilities are colour-coded as indicated in the legend. Conidiophores are illustrated for all species treated in this study except for C. uredinicola and C. vignae which did not sporulate. The tree was rooted to sequences of Cercospora beticola strain CPC 11557 (GenBank accession numbers AY840527, AY840458, AY840494, respectively for ITS, ACT and TEF).
Taxonomy
Key to the Cladosporium species treated
Morphological features used in the key to distinguish the species treated in this study were determined by light microscopy after 7 to 9 d growth at 25 °C on SNA, and cultural characteristics after 14 d incubation on PDA. Cladosporium uredinicola and C. vignae are not included in the key since isolates of these species did not sporulate during the course of the present examinations, and measurements given in literature were made only on PDA and are therefore only partly comparable. Terminology used for conidial types, scars and surface ornamentation follow Schubert et al. (2007b). To reflect the morphological variability of some of the species, especially with regard to surface ornamentation of conidia, these species are listed twice or up to three times in the key, e.g. C. exile. The C. cladosporioides s. lat. complex (see couplet 35 in the key), includes species that are morphologically close to C. cladosporioides but still distinguishable due to a combination of subtle features as well as C. cladosporioides s. str. and morphologically indistinguishable but phylogenetically distinct lineages of the latter species.
1. Conidia finely verruculose to coarsely verrucose or irregularly rough-walled...................................................................................... 2 1. Conidia smooth or almost smooth..................... 14
2. Conidia distinctly ornamented, verruculose to coarsely verrucose or irregularly rough-walled........................................................... 3 2. Conidia less ornamented, almost smooth to asperulate or minutely verruculose, sometimes irregularly rough-walled...................... 7
3. Conidia frequently septate, with 0–3 septa; surface with coarse verrucae up to 1 μm high................... C. verrucocladosporioides 3. Conidia mainly 0–1-septate, occasionally with a second septum; verrucae lower, only up to 0.5 μm high........................................ 4
4. Conidiophores up to 430 μm long, smooth; small terminal conidia globose, subglobose or obovoid, broad, 4.5–6 μm wide.......................................................... C. acalyphae 4. Conidiophores shorter, up to 200 μm long, usually shorter, almost smooth to minutely verruculose or irregularly rough-walled; small terminal conidia obovoid, ellipsoid, ovoid, rarely subglobose, 2.5–4.5 μm wide................................................................................. 5
5. Conidiophores macronematous, (2.5–)3.5–5.5 μm wide, walls thickened, 0.5–1(–1.5) μm wide, sometimes even appearing to be two-layered; small terminal conidia 5–6 μm long................................................................................................. C. pini-ponderosae 5. Conidiophores macro-, semimacro- and micronematous, narrower, (1.5–)2.5–4 μm wide, walls unthickened or only slightly thickened, about 0.5 μm wide; small terminal conidia longer, 4–9 μm long......................................................................................................... 6
6. Ramoconidia 4–5 μm wide, aseptate; conidia finely verruculose to usually verruculose, occasionally distinctly verrucose; conidiogenous loci and hila (0.5–)0.8–2(–2.2) μm diam...................................................................................................................... C. chubutense 6. Ramoconidia narrower, 2.8–4 μm, 0–2-septate; conidia mostly distinctly verruculose-rugose or irregularly rough-walled; conidiogenous loci and hila narrower, 0.5–1.5 μm diam.................................................................................................................... C. exasperatum
7(2). Terminal unbranched part of the branched conidial chains usually very long with up to 8(–10), sometimes up to 17 conidia............ 8 7. Conidia in densely branched chains, terminal unbranched part of the chains much shorter with 1–4 conidia.................................. 11
8. Conidia inversely coloured with small terminal and intercalary conidia being slightly darker than secondary ramoconidia, ramoconidia and conidiophores; small terminal conidia (3–)5–8.5 μm long, intercalary conidia (5–)7–20 μm long; small terminal and intercalary conidia in delicate, loose chains, minutely verruculose or irregularly rough-walled, rugose, secondary ramoconidia and ramoconidia smooth........................................................................................... C. inversicolor 8. Conidia not inversely coloured, small terminal and intercalary conidia paler or concolorous with secondary ramoconidia, ramoconidia and conidiophores; small terminal and intercalary conidia shorter, 4–7(–8) μm and (5–)6.5–10(–12) μm, respectively; no differences in ornamentation between smaller conidia and secondary ramoconidia................................................. 9
9. Conidiophores 45–210(–360) μm long, pluriseptate, with up to 12 septa; secondary ramoconidia (7.5–)9–26(–37) × (2.5–)3–5 μm, 0(–1)-septate............................... C. asperulatum 9. Conidiophores shorter, up to 100(–115) μm long, 0–4(–5)-septate; secondary ramoconidia somewhat shorter and narrower, 8–20(–23) × (2.5–)3–4 μm, 0–1(–2)-septate...................... 10
10. Conidiophores macronematous, (2.5–)3.5–4.5(–5) μm wide, thick-walled, walls up to 1 μm wide; conidiogenous cells geniculate, subnodulose with unilateral swellings or occasionally nodulose, with up to six loci crowded at the apex; conidia smooth or almost so to finely verruculose; on Myrtaceae........................................................................................................................... C. myrtacearum 10. Conidiophores macro- and micronematous, slightly narrower, 2–4(–4.5) μm wide, walls slightly thickened, up to 0.5 μm; conidiogenous cells non-nodulose, occasionally geniculate, usually with a single apical scar, sometimes with 2–3 conidiogenous loci at the apex; conidia smooth to minutely verruculose or often irregularly rough-walled; on Cortaderia.............................................. C. colombiae
11(7). Macronematous conidiophores 2.5–5(–6) μm wide; secondary ramoconidia 5–17 (–24) × (2–)3–4.5 μm........... C. phyllactiniicola 11. Macronematous conidiophores narrower, (1.5–)2–4(–5) μm; secondary ramoconidia longer and narrower, 10–30(–34) × 2–3.5(–4) μm..................................................................... 12
12. Conidiophores (1.5–)2–3.5(–4) μm wide, subhyaline, pale olivaceous to pale olivaceous-brown; secondary ramoconidia narrow, 2–3(–3.5) μm wide; conidiogenous loci and hila narrow, (0.8–)1–1.5(–1.8) μm diam.............................................. C. perangustum 12. Conidiophores somewhat wider, (2–)3–4(–5) μm, and darker, pale to medium olivaceous-brown; secondary ramoconidia somewhat wider, 2.5–3.5(–4) μm; conidiogenous loci and hila, 0.5–2 μm diam................................................................................................ 13
13. Ramoconidia 17–41 μm long with a broadly truncate base, 2.5–3 μm wide; small terminal conidia 3.5–5(–5.5) × 2–3 μm, intercalary conidia (4–)5–8(–9) μm long; conidiogenous loci and hila 0.5–2 μm diam............................................................................. C. exile 13. Ramoconidia up to 34 μm, base 2–2.5 μm wide; small terminal conidia 3.5–4.5(–5) × 2–2.2(–2.5) μm, intercalary conidia longer, 5–13 μm; conidiogenous loci and hila slightly narrower, 0.5–1.8 μm diam.................................................................. C. scabrellum
14(1). Macronematous conidiophores nodulose or nodose with swellings usually being quite apart from each other; conidiogenous loci usually restricted to swellings............................ 15 14. Macronematous conidiophores non-nodulose or only occasionally subnodulose due to geniculate proliferation; conidiogenous loci not confined to swellings.......................................... 17
15. Conidia solitary or in short unbranched or branched chains, 5–8(–9) μm wide; phytopathogenic, causing leaf spots on Colocasia........................................................ C. colocasiae 15. Conidia always catenate, usually in densely branched chains, (1.5–)2–4(–5) μm wide; saprobes occurring on numerous substrates............................................................................ 16
16. Conidiophores up to 720 μm or even longer, always nodulose to nodose with conidiogenous loci restricted to swellings (on SNA and in vivo; on PDA and OA conidiophores without swellings.............................................................................................. C. oxysporum 16. Conidiophores up to 310(–460) μm long, often subnodulose or nodulose with a head-like swollen apex and sometimes few additional nodes on a lower level, but most conidiophores neither geniculate nor nodulose, loci often situated on swellings but not restricted to them, in intercalary conidiogenous cells loci often sitting at about the same level round about the stalk, but not connected with swellings as in C. oxysporum; on PDA and OA conidiophores darker, often with swellings....................................... C. tenuissimum
17(14). Conidiophores 4–7(–8) μm wide at the base, attenuated towards the apex, 3–4 μm wide, medium to dark brown, often with a foot-like swollen base................................ C. basiinflatum 17. Conidiophores different, narrower or not distinctly attenuated towards the apex, paler, not dark brown, without a foot-like swollen base................................................................... 18
18. Conidiophores up to 100 μm long, rarely longer and secondary ramoconidia up to 20 μm long, occasionally longer..................... 19 18. Conidiophores up to 330 μm, and secondary ramoconidia up to 30 μm long.................................................................................. 26
19. Conidia in long unbranched or loosely, mostly dichotomously branched chains............................................................................... 20 19. Conidia usually in densely branched chains both at the base of the chain and intercalary............................................................... 23
20. Conidiophores (3–)3.5–4.5 μm wide; conidia 0–3-septate; forming subglobose or globose, dense pseudoparenchymatous conglomerations of swollen hyphal cells........................................................................................................................... C. hillianum 20. Conidiophores 2.5–3.5(–4) μm; conidia 0–1(–2)-septate; without pseudoparenchymatous conglomerations................................. 21
21. Conidial chains flabellate, characteristically spread in a fan-like manner, secondary ramoconidia 11–27 μm long, 0(–1)-septate...................................................... C. flabelliforme 21. Conidial chains not flabellate, secondary ramoconidia 7–19(–23) μm, 0–1(–2)-septate.................................................................. 22
22. Conidial chains very long, with up to 18 conidia; small terminal conidia 5–9 × 2–2.5 μm; cladosporioid scar structure with dome and rim not clearly visible using light microscopy.................................................................................................... C. chalastosporoides 22. Conidial chains shorter, with up to 8(–14) conidia; small terminal conidia shorter and narrower, 2.5–5 × 1.5–2 μm; dome and rim clearly visible............................... C. funiculosum
23(19). Conidiophores with monopodial rejuvenations having a single terminal rather inconspicuous annellation; conidia 1–3(–3.5) μm wide; conidiogenous loci and hila 0.5–1.5(–1.8) μm diam..................................................................................................... C. gamsianum 23. Conidiophores without monopodial rejuvenations; conidia 3–5(–6) μm; conidiogenous loci and hila 0.5–2 μm diam..................... 24
24. Conidiophores mostly 1–3-septate; small terminal and intercalary conidia 2–3 μm wide, secondary ramoconidia 3–4 μm wide; conidiogenous loci and hila 0.8–1.8(–2) μm diam; on Myrtaceae............................................................................. C. myrtacearum 24. Conidiophores pluriseptate; small terminal and intercalary conidia 2–4(–4.5) μm, secondary ramoconidia (2.5–)3–5(–6) μm; conidiogenous loci and hila slightly wider; fungicolous or lichenicolous............................................................................................ 25
25. Mycelium minutely verruculose to irregularly rough-walled; conidiophores 2.5–5(–6) μm wide; conidia smooth or almost so to finely asperulate; fungicolous, occurring on chasmothecia of Phyllactinia....................................................................... C. phyllactiniicola 25. Mycelium dimorphic, fertile hyphae irregularly rough-walled, sterile hyphae smooth; conidiophores narrower, 3–4 μm wide; conidia smooth; on lichens..................... C. licheniphilum
26(18). Conidia 1.5–3(–3.5) μm wide............................ 27 26. Conidia up to 5(–7) μm, mostly 3–4 μm........... 29
27. Conidiophores (8–)12–130(–150) μm long; conidia smooth or almost so to finely verruculose............................... C. perangustum 27. Conidiophores longer, up to 330 μm long; conidia smooth or almost so........................................................................................... 28
28. Conidiophores subulate, formed like an awl with a swollen base and distinctly attenuated towards the apex, 2–3 μm wide at the apex; small terminal conidia 2.5–4.5(–5.5) × 2–2.5 μm........................................................................................................ C. subuliforme 28. Conidiophores not subulate, somewhat wider and not distinctly attenuated towards the apex, (1.5–)2–4 μm wide; small terminal conidia 3–6.5 × 1.5–2 μm...... C. angustisporum
29(26). Conidia 0–3-septate.......................................... 30 29. Conidia 0–1-septate, rarely with an additional septum...................................................................................................................... 34
30. Conidiophores (2–)3–4(–5) μm wide; conidia smooth to sometimes asperulate or minutely verruculose........................................ 31 30. Conidiophores (2.5–)3–6(–6.5) μm; conidia smooth or almost so.................................................................................................... 32
31. Conidia 3–5 μm wide, 0–3-septate, septa often darkened; mycelium dimorphic.......................................... C. paracladosporioides 31. Conidia 2–3.5(–4) μm wide, 0–1(–3)-septate, septa not darkened; mycelium not dimorphic.................................................. C. exile
32(30). Ramoconidia 24–43 × 3–3.5 μm; conidia in long loosely branched chains, often dichotomously branched, up to 10(–14) conidia in the terminal unbranched part of the chain, small terminal conidia 4–8(–10) μm long; phytopathogenic on Cucurbitaceae................................................... C. cucumerinum 32. Ramoconidia longer and wider; conidia in branched chains, branching in all directions, up to five conidia in the terminal unbranched part of the chain, small terminal conidia (2–)3.5–5 μm long............................................................................................................. 33
33. Intercalary conidia (2–)2.5–3(–4) μm wide, secondary ramoconidia (2.5–)3–4(–5) μm wide; conidiogenous loci and hila 0.5–2(–2.5) μm diam; attaining 50–70 mm diam after 14 d on PDA, MEA and OA; occurring on ascomycetes and fruiting bodies of different basidiomycetous fungi.............. C. lycoperdinum 33. Intercalary conidia (2.5–)3–4(–4.5) μm, secondary ramoconidia (2.5–)3–6 μm; conidiogenous loci and hila 0.8–3 μm diam; slower growing on all media, attaining 17–32 mm diam after 14 d; saprobic and possibly endophytic.......................................... C. varians
34(29). Small terminal conidia (2.5–)3–4 μm wide, usually globose or subglobose, secondary ramoconidia (3–)4–5(–6) μm wide..................................................... C. globisporum 34. Small terminal conidia narrower, up to 3 μm wide, subglobose, obovoid, ovoid or limoniform, but not globose, secondary ramoconidia usually narrower................................................ 35
35. Macronematous conidiophores 4–5(–6) μm wide, erect or decumbent; fungicolous, occurring on species of Taphrina..................................................... C. phyllophilum 35. Macronematous conidiophores narrower, usually 2.5–4 μm wide, usually erect, not decumbent; on different substrates (cladosporioides s. lat. complex)................................................... 36
36. Conidia inversely coloured with small terminal and intercalary conidia being slightly darker than secondary ramoconidia, ramoconidia and conidiophores; small terminal conidia (3–)5–8.5 μm long..................................................................................... C. inversicolor 36. Conidia not inversely coloured, small terminal and intercalary conidia paler or concolorous with secondary ramoconidia, ramoconidia and conidiophores; small terminal conidia (2–)3–5(–6) μm.............................................................................................................. 37
37. Conidiophores usually with a head-like swollen apex and sometimes a few additional swellings on a lower level and/or conidiophores slightly to often distinctly sympodially proliferating, growth or branching proceeding in an angle of 45° to almost 90°; in intercalary conidiogenous cells loci sitting at about the same level round about the stalk, garland-like (tenuissimum s. lat.)............................ 38 37. Conidiophores different, without apical or intercalary swellings, at most subnodulose, growth not proceeding in an angle of 45° to almost 90°......................................................... 39
38. Conidiophores usually with a head-like swollen apex, uni- or multilateral, and sometimes with few additional nodules on a lower level; ramoconidia 22–41 μm long; conidia smooth, occasionally irregularly rough-walled................................................. C. tenuissimum 38. Conidiophores without head-like swollen apex; ramoconidia 16–56 μm; the outer walls of small terminal conidia and intercalary conidia often seem to detach, irregular, somewhat refractive........................................................................................... C. rectoides
39(37). Ramoconidia 3–5 μm wide; secondary ramoconidia 10–30(–38) μm long (av. approx. 19–21), small terminal conidia in long unbranched chains, up to 10 conidia in the terminal part of the chain............................................................................................... 40 39. Ramoconidia up to 4 μm wide; secondary ramoconidia shorter, 7–25 μm long (av. approx. 15–16), occasionally few conidia longer, terminal conidial chains shorter, up to six, mainly up to four conidia in the terminal unbranched part of the chain........................... 41
40. Secondary ramoconidia 0–1(–2)-septate, intercalary conidia subrostrate or rostrate....................................................... C. iranicum 40. Secondary ramoconidia usually aseptate, occasionally 1-septate, intercalary conidia not rostrate..................................................................................................... C. cladosporioides (including morphologically indistinguishable but phylogenetically distinct lineages)
41(39). Conidiogenous loci and hila 0.5–1.5(–1.8) μm......................................................................................... C. pseudocladosporioides 41. Conidiogenous loci and hila somewhat wider, 0.5–2 μm.................................................................................................................. 42
42. Conidia almost smooth to often asperulate, loosely verruculose or irregularly rough-walled, especially in small terminal and intercalary conidia............................................................... 43 42. Conidia smooth or almost so............................. 44
43. Intercalary conidia and secondary ramoconidia with numerous distal hila crowded at the apex, in intercalary conidia with 2–4(–6) hila, in secondary ramoconidia with up to 6(–9) hila at the apex, small terminal conidia 2–4 μm long (av. 3.5), intercalary conidia 5–12 μm long (av. 7.9), aseptate.................. C. xylophilum 43. Intercalary conidia and secondary ramoconidia with only few distal hila, in intercalary conidia with 1–2(–3) hila, in secondary conidia with up to three hila, small terminal conidia 3.5–5(–5.5) μm (av. 4.4), intercalary conidia (4–)5–8(–9) μm long (av. 6.3), 0–1-septate................................................................... C. exile
44(42). Mycelium often forming dense ropes, hyphae 1–5 μm wide; conidiophores macronematous, often very long, up to 285 μm; due to the special cell structure conidiophores and conidia often with disto-septa...................................................................... C. australiense 44. Mycelium not forming ropes, hyphae (0.5–)1–3(–4) μm; conidiophores macronematous 50–165 μm long, micronematous 19–75(–100) μm long; conidiophores and conidia without disto-septa.............................................................................................. C. delicatulum
Description of Cladosporium species
The status of numerous isolates identified as C. cladosporioides or C. tenuissimum as well as indeterminate strains included in this study have been subjected to polyphasic analyses, which revealed several novel species. Together with previously described species these new taxa are treated in alphabetical order below.
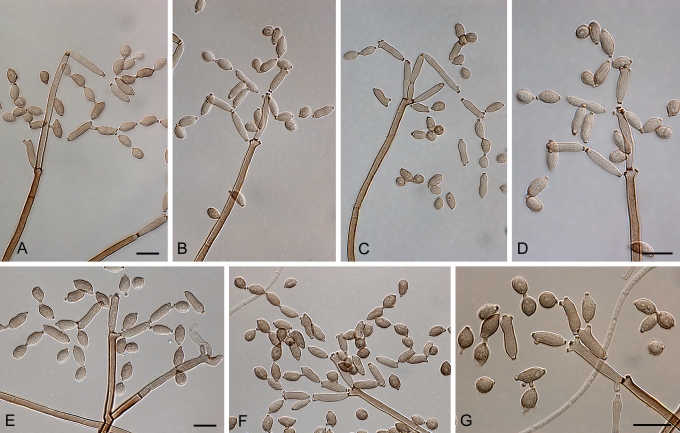
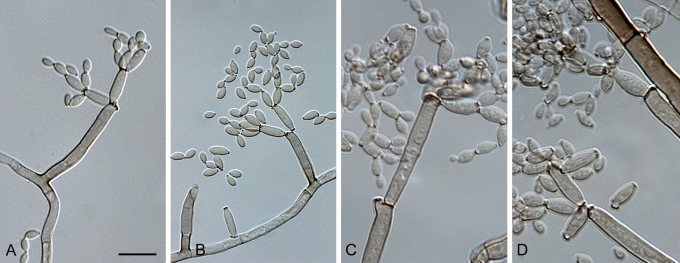
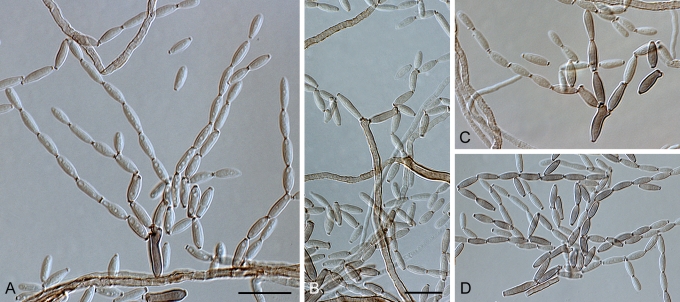
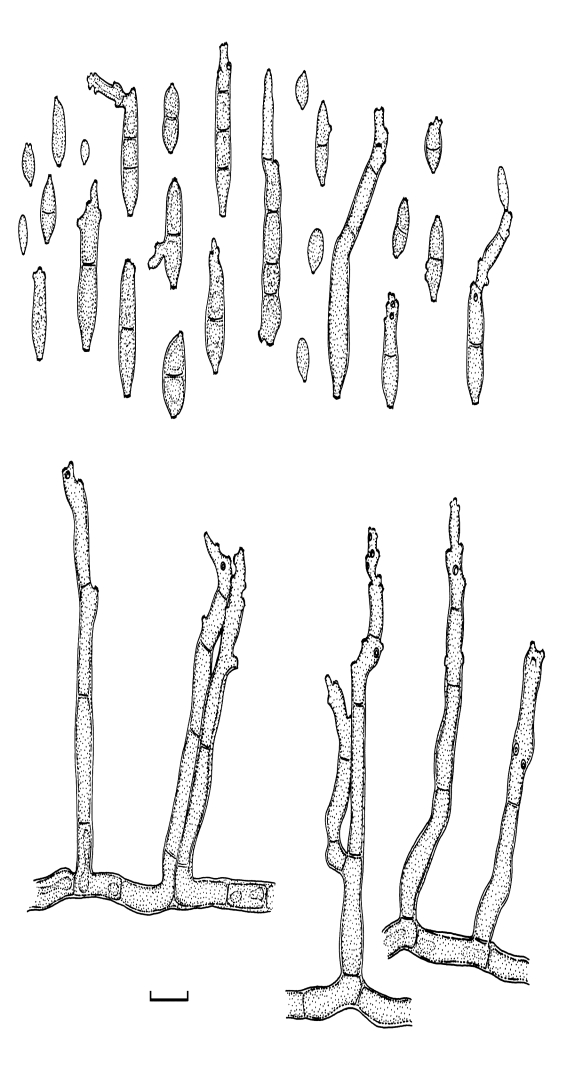
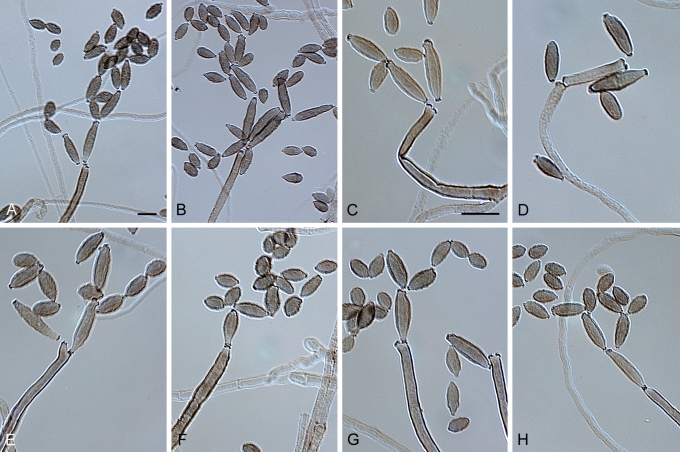
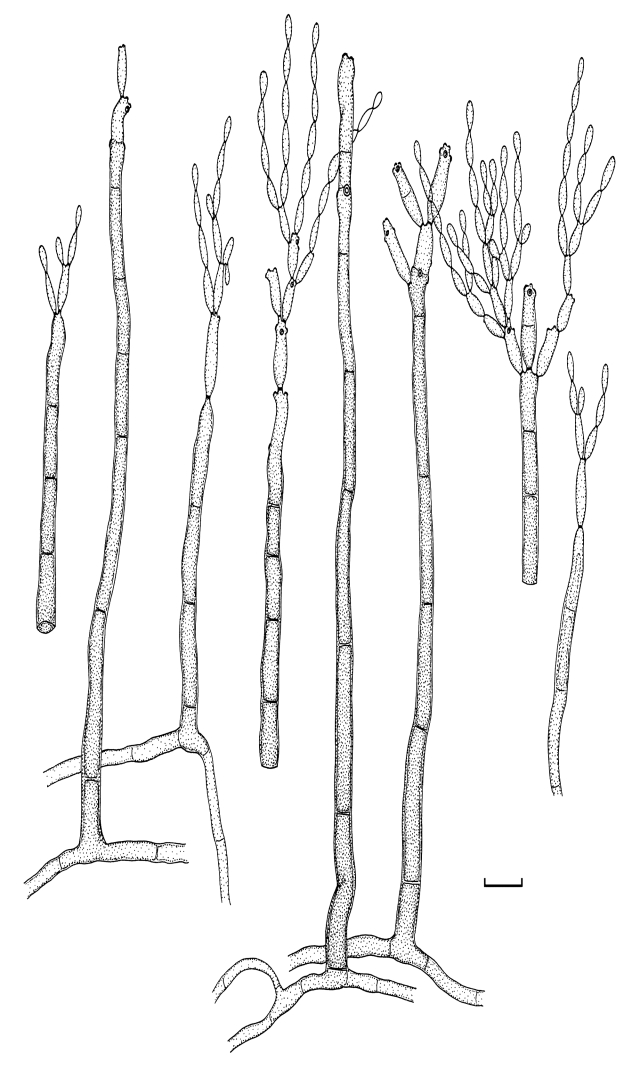
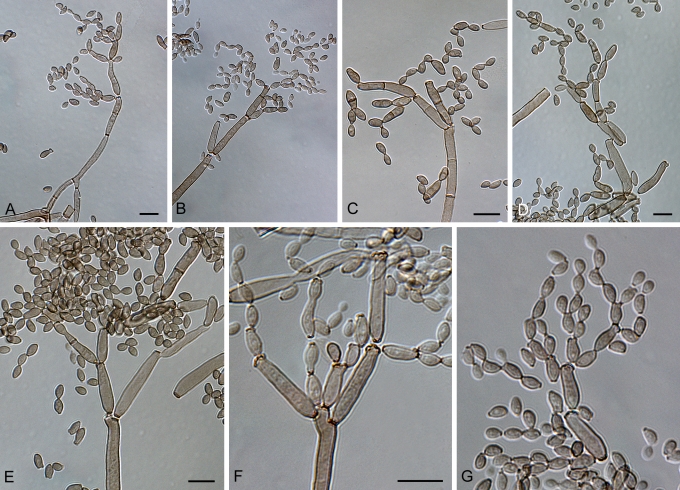
Cladosporium acalyphae Bensch, H.-D. Shin, Crous & U. Braun, sp. nov. MycoBank MB517070. Figs 2, 3, 4.
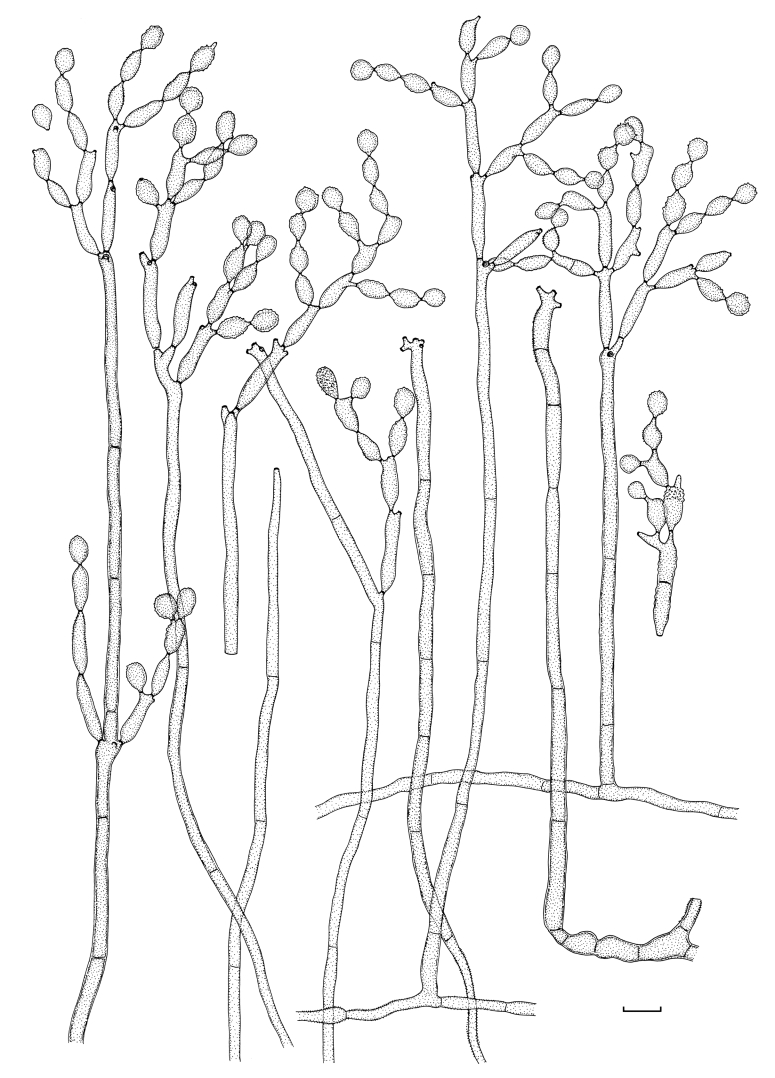
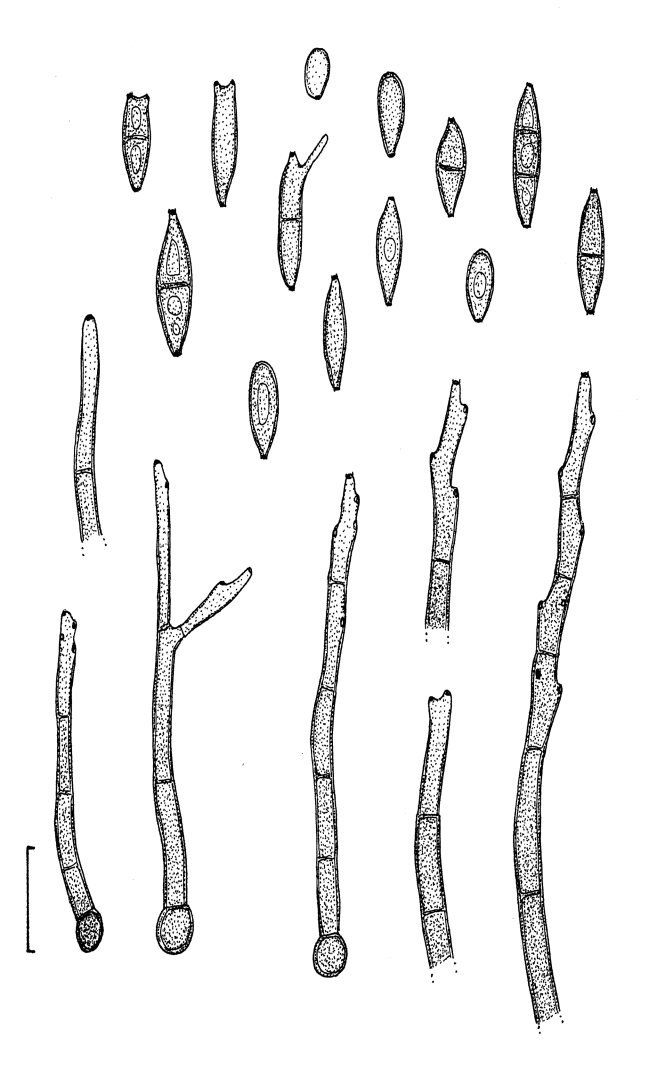
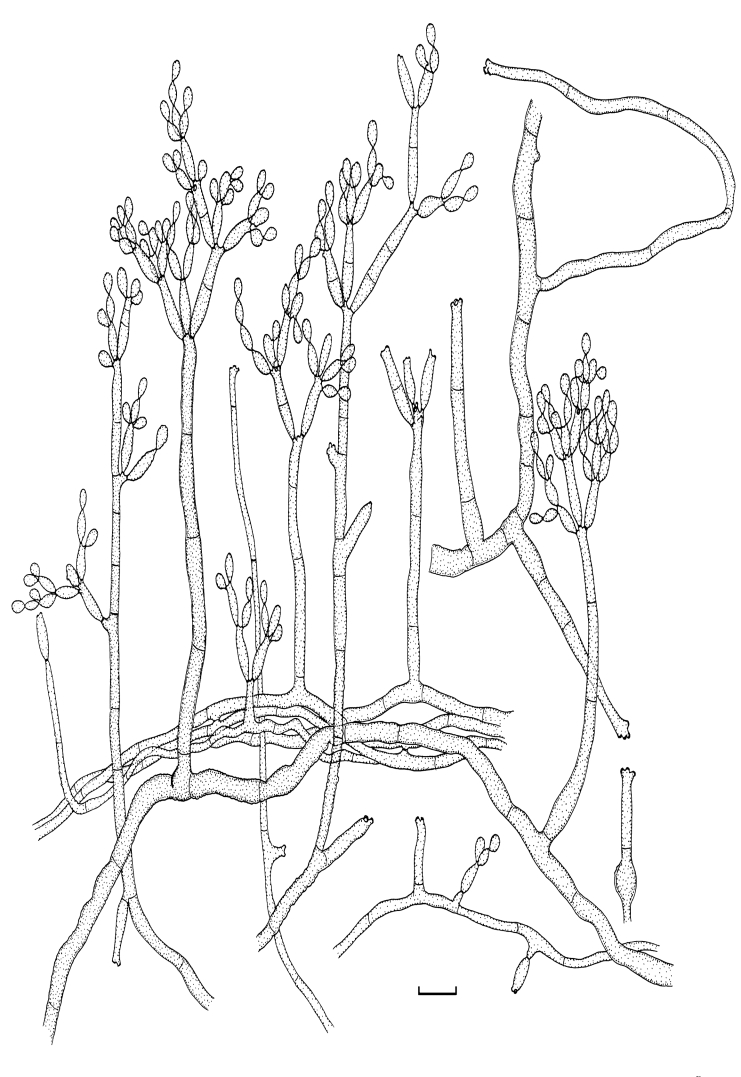
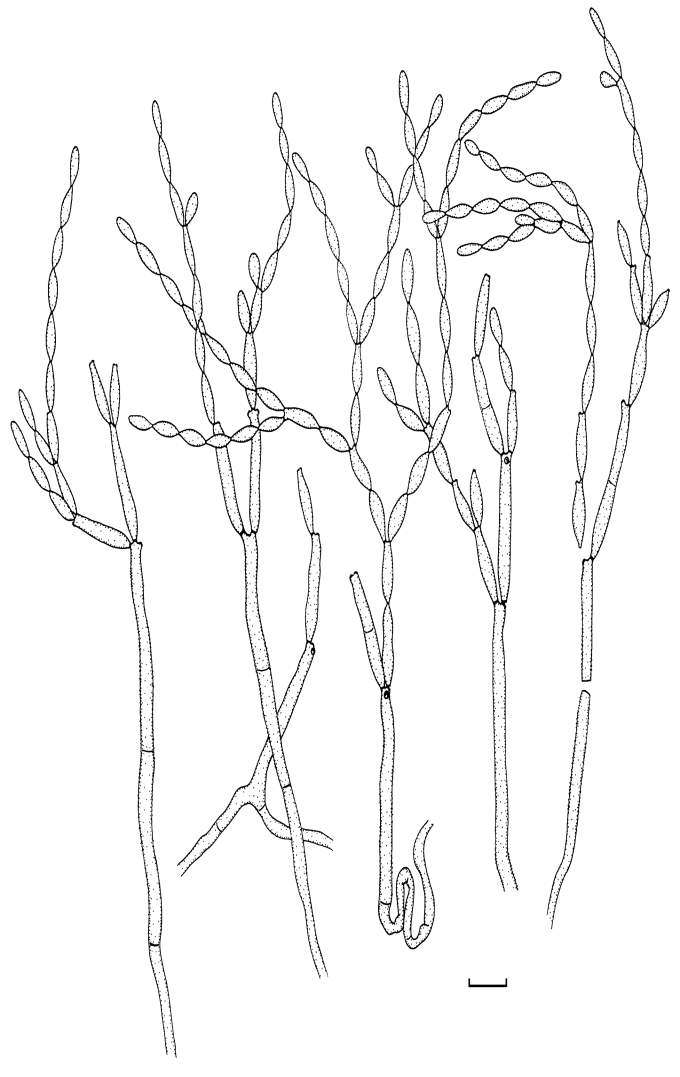
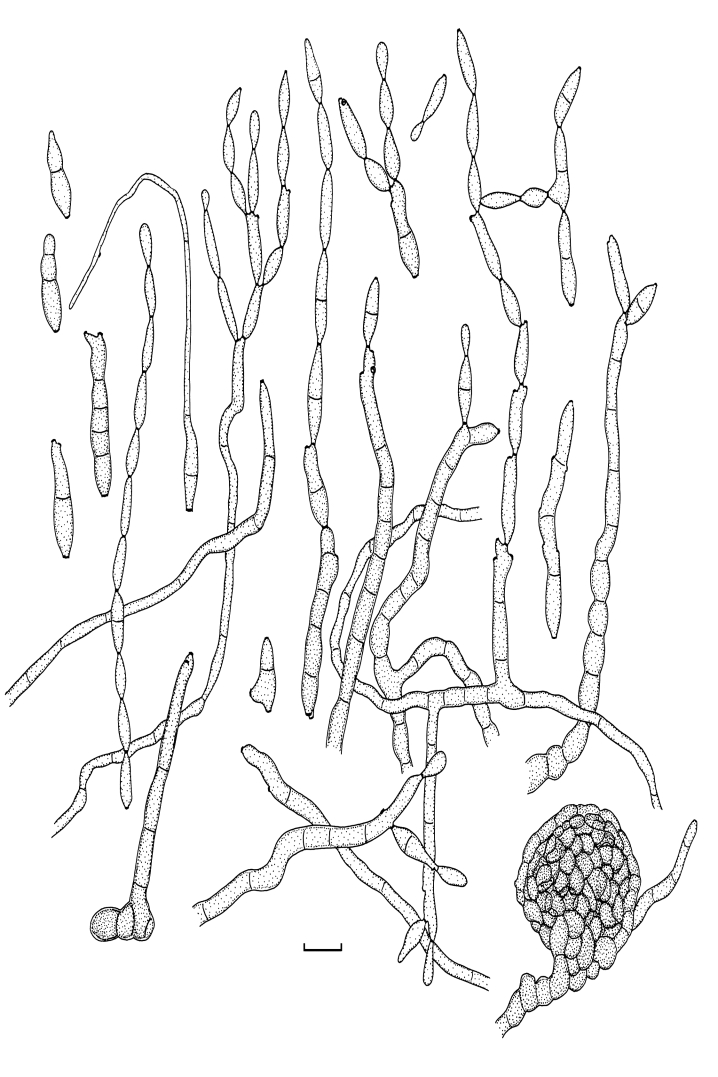
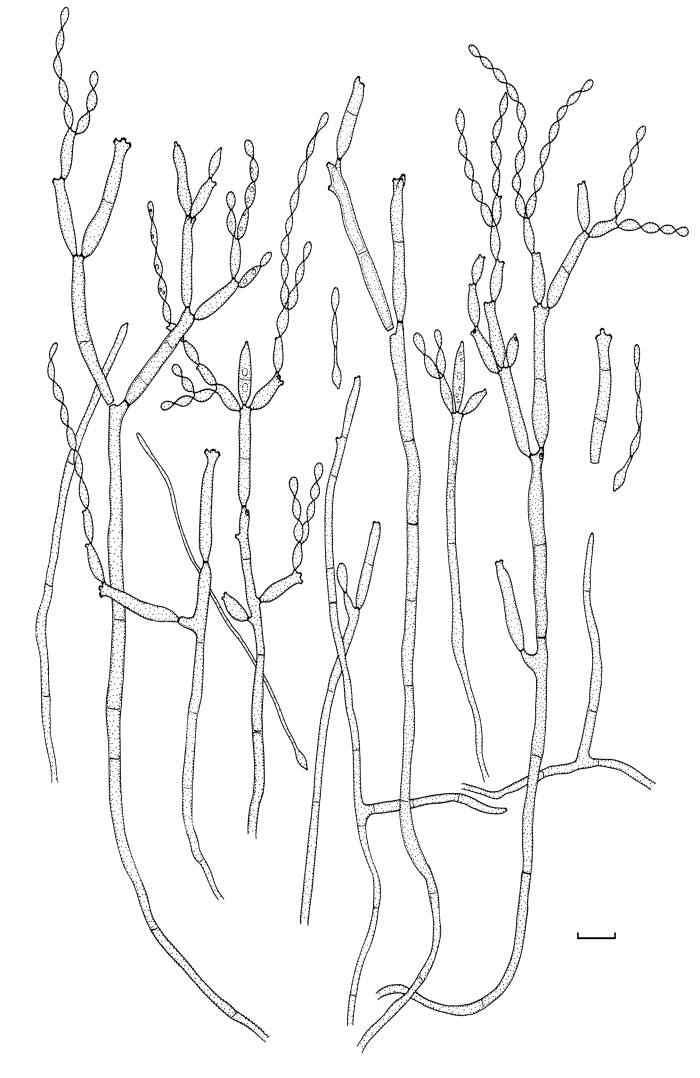
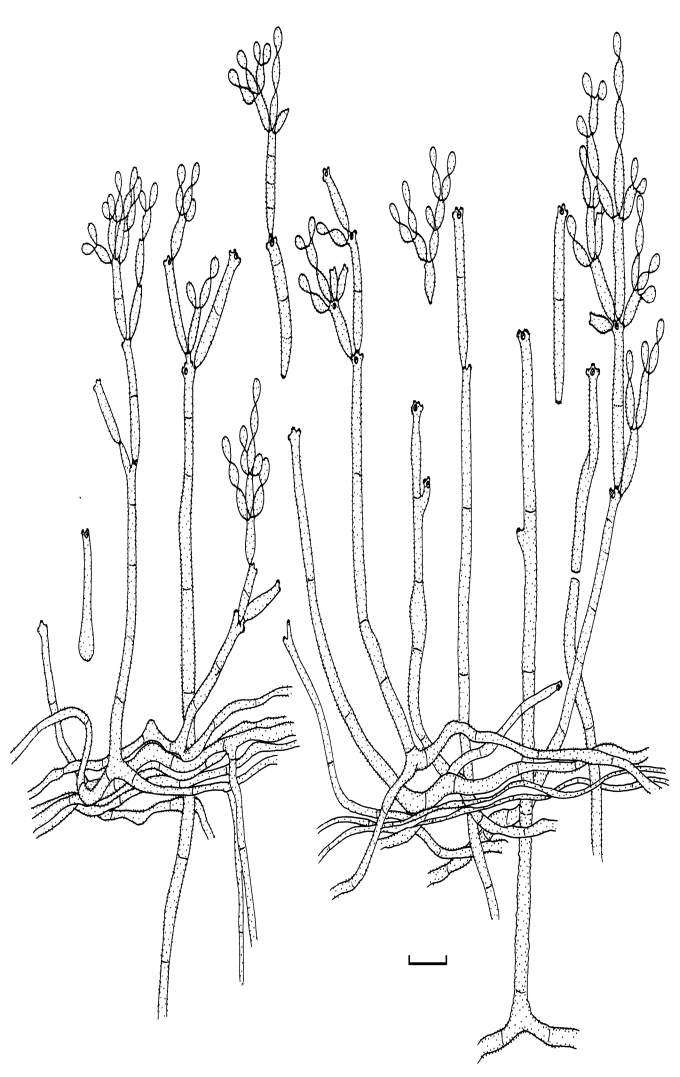
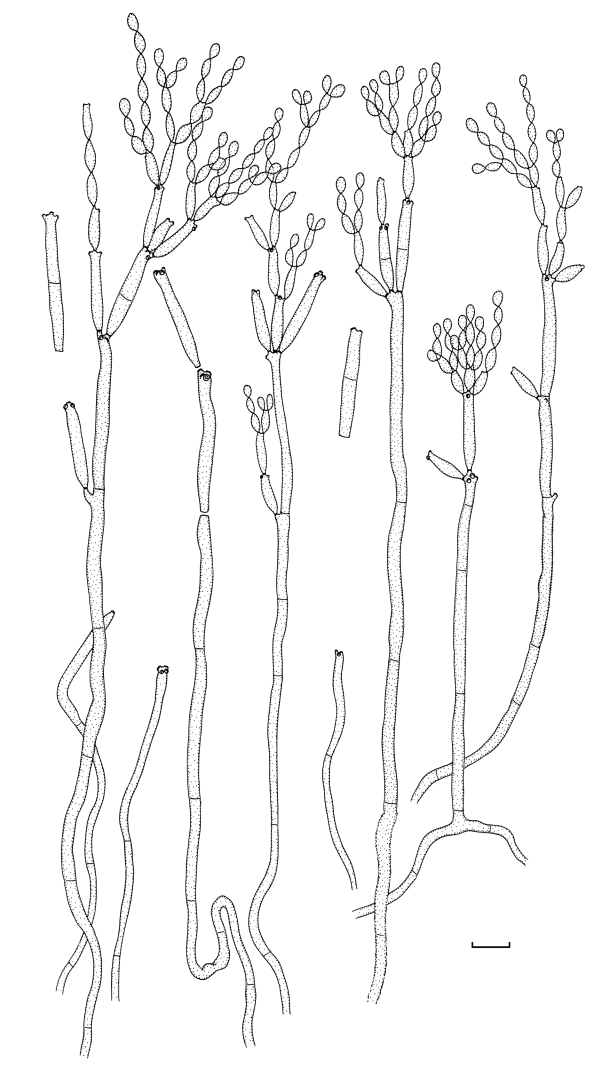
Fig. 2.
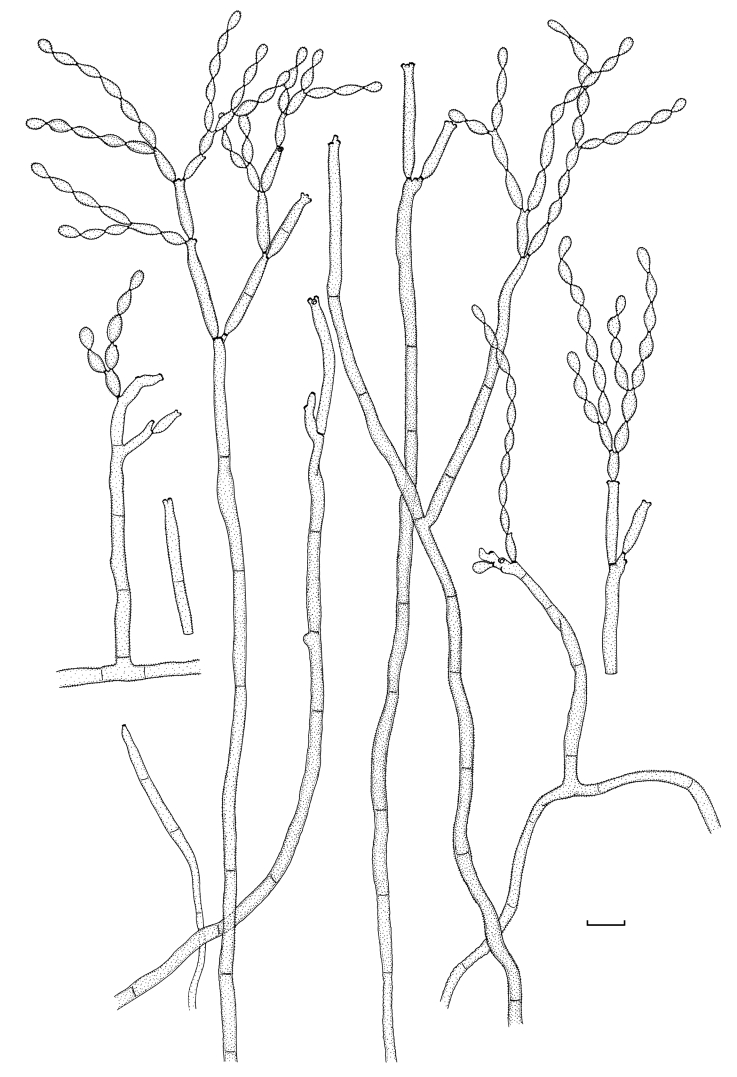
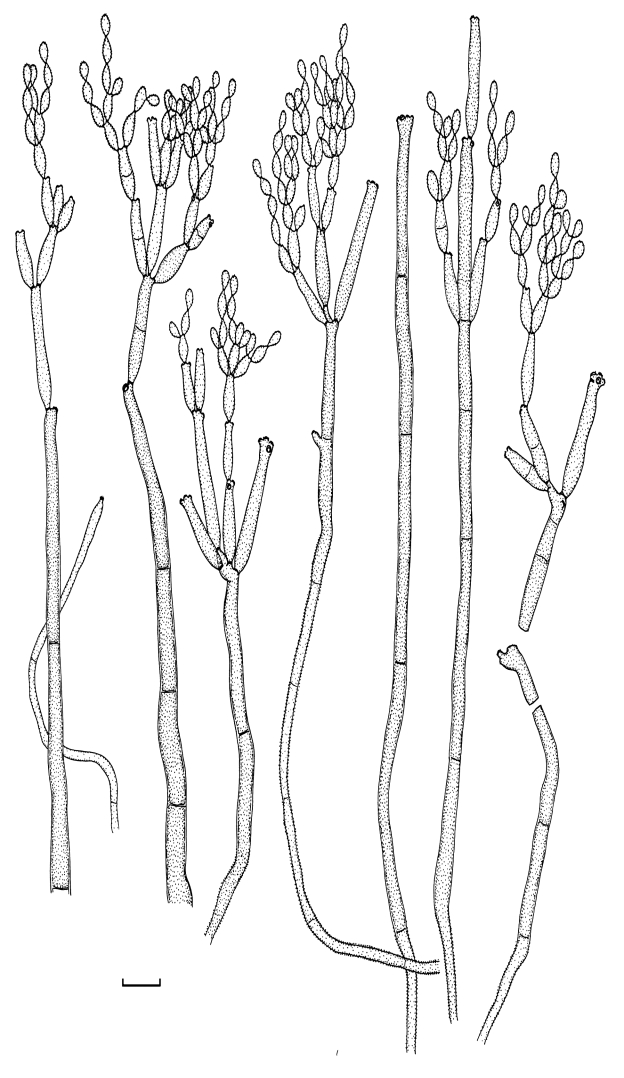
Cladosporium acalyphae (CBS 125982). Macronematous conidiophores, mycelium, ramoconidia and conidial chains. Scale bar = 10 μm.
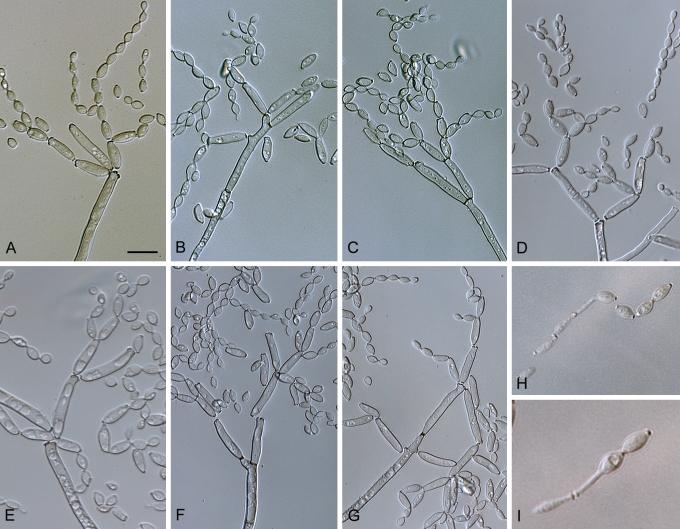
Fig. 3.
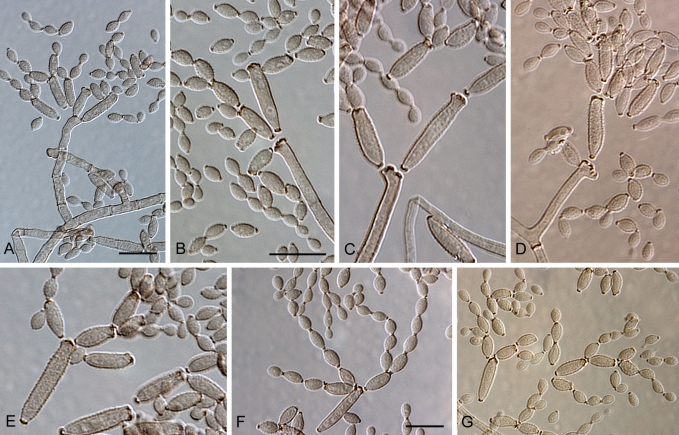
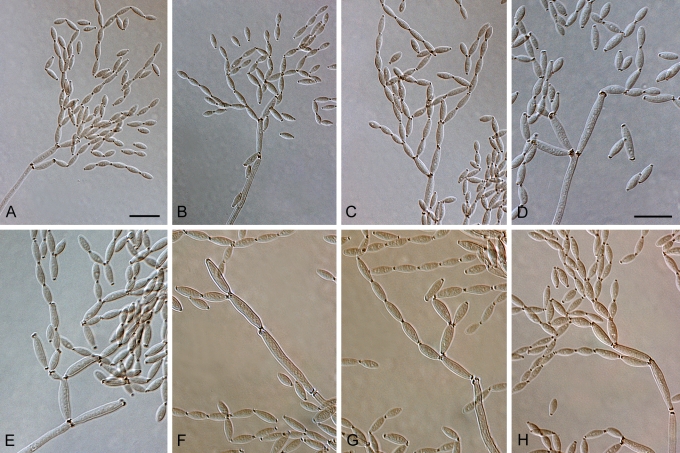
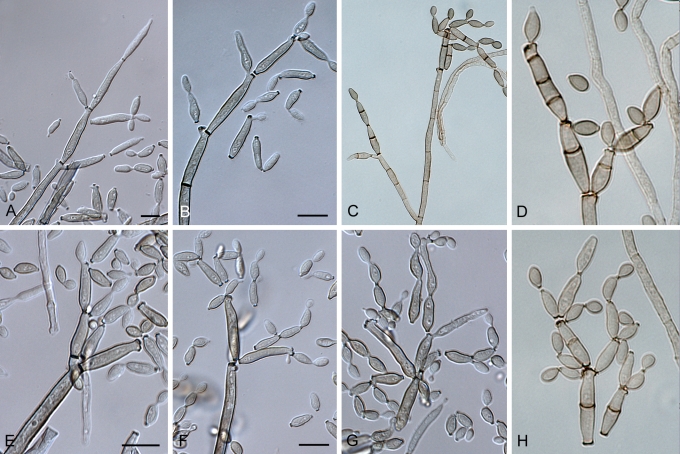
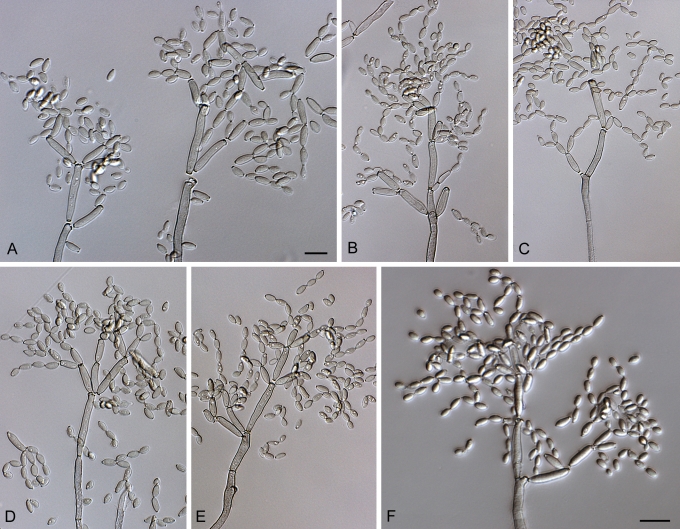
Cladosporium acalyphae (CBS 125982). A–G. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
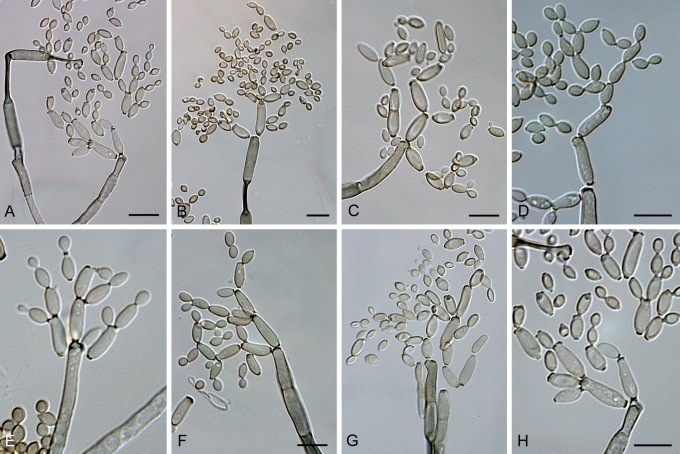
Fig. 4.
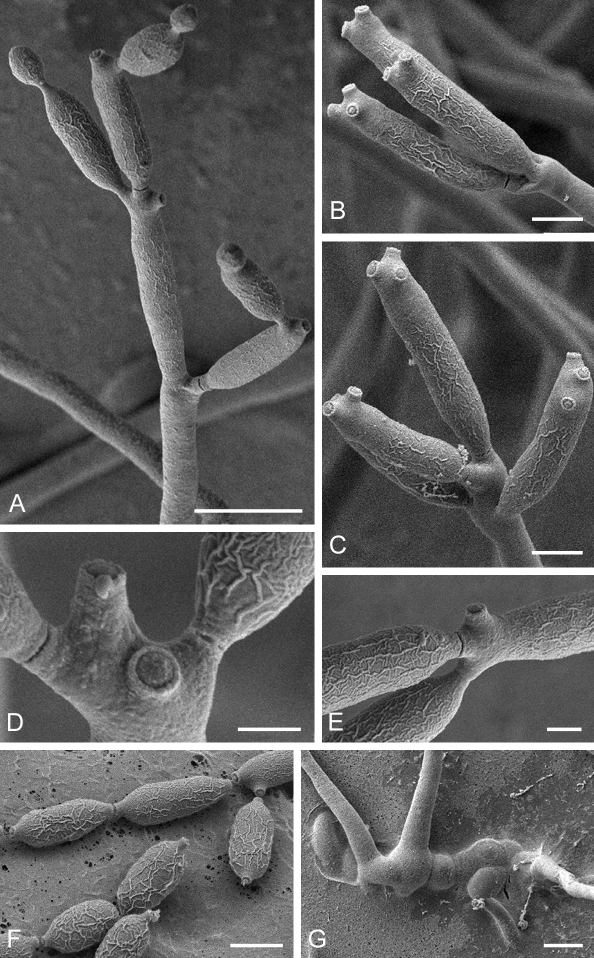
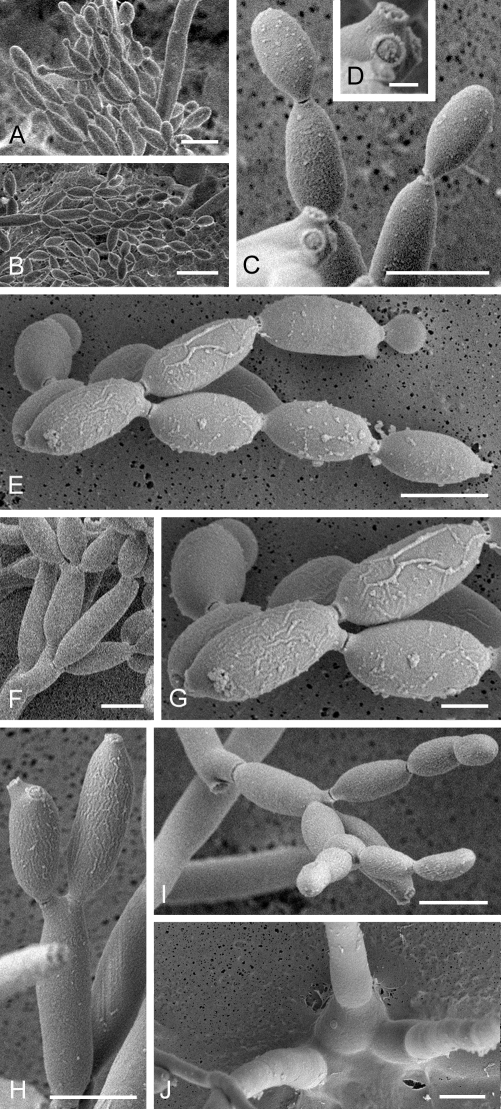
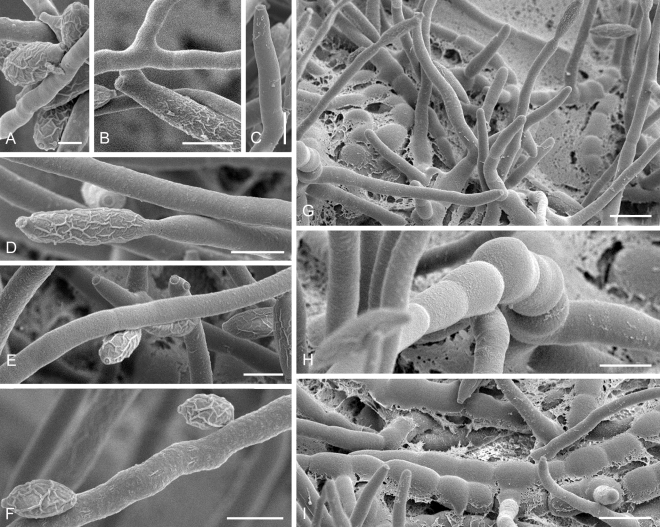
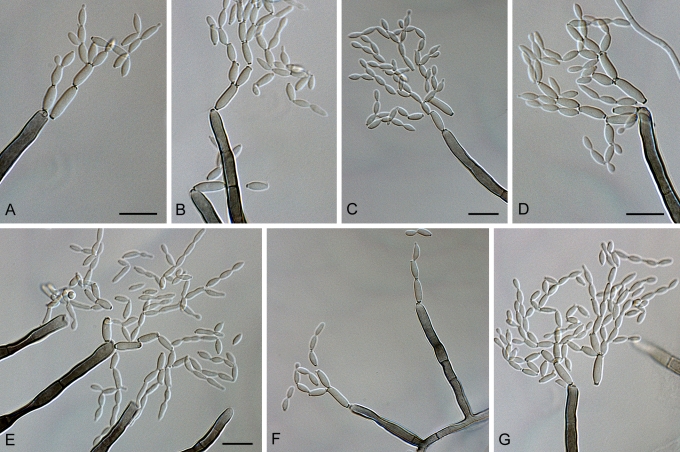
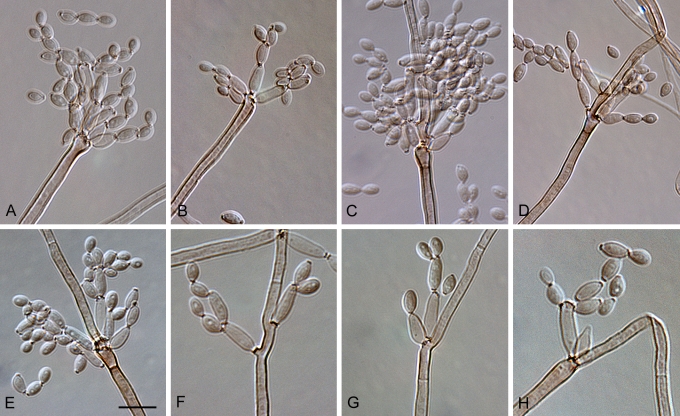
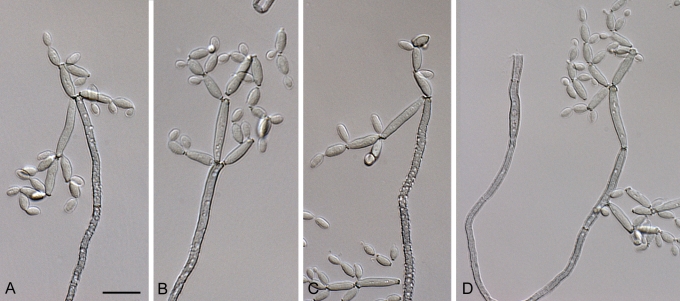
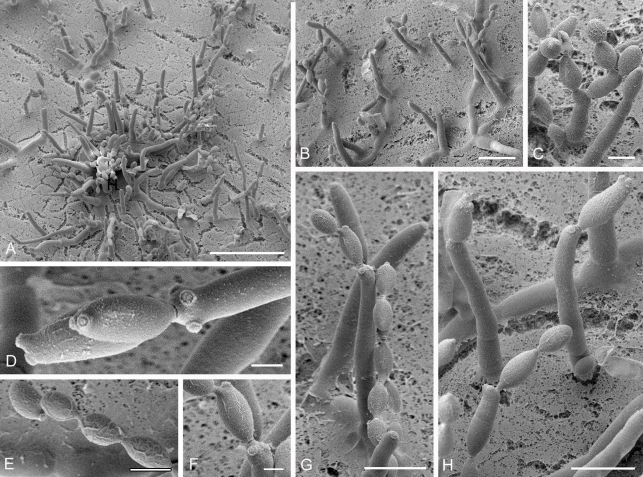
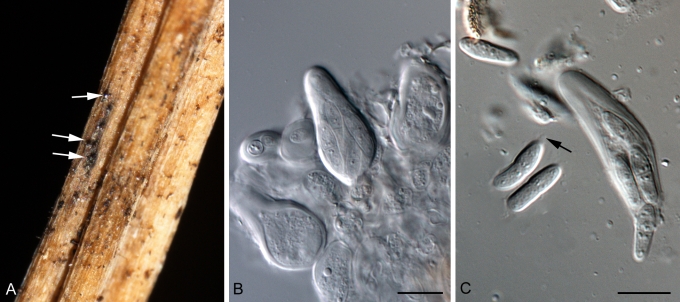
Cladosporium acalyphae (CBS 125982). A. Secondary ramoconidia and conidia on a conidiophore. Note the smooth surface of the conidiophores. B–C. Secondary ramoconidia on smooth conidiophores and patterns of scars. D. Details of scars on a secondary ramoconidium. E. Secondary ramoconidia and scar. F. Conidia as seen with cryoSEM showing a reticulate surface ornamentation. G. SEM micrograph of “meristematic development” on the agar surface and two conidiophores formed. Scale bars = 2 (D, E), 5 (B, C, F), 10 (A, G) μm.
Etymology: Named after Acalypha, the host on which it occurs.
Cladosporii pini-ponderosae et item Cladosporii verrucocladosporioidis simile, sed conidiophoris longioribus, ad 430 μm longis, conidiis minutis terminalibus longioribus et latioribus, saepe globosis, 4.5–9 × 4.5–6 μm.
Mycelium internal and superficial; hyphae unbranched or loosely branched, filiform to cylindrical-oblong, 1–4 μm wide, later up to 7 μm wide, especially towards the base of conidiophores, pluriseptate, not constricted or in wider hyphae slightly constricted at septa, sometimes septa in short succession, smooth or minutely verruculose, walls unthickened or slightly thick-walled. Conidiophores solitary, macronematous, arising terminally and laterally from ascending or plagiotropous hyphae, erect, straight to somewhat flexuous, very long, narrowly cylindrical-oblong, 150–430 × (2.5–)3–4(–5) μm, unbranched or once branched, branches often rather long, appearing like a conidiophore on its own, non-nodulose, sometimes once geniculate, often slightly attenuated towards the apex, pluriseptate, cells rather long, not constricted at septa, medium olivaceous-brown, smooth, walls slightly thick-walled. Conidiogenous cells integrated, terminal and sometimes intercalary, narrowly cylindrical-oblong, non-nodulose, occasionally once geniculate-sinuous, 23–80 μm long, with 1–4 loci at the apex, occasionally few additional loci at a lower level, but mostly above the septum, loci conspicuous, subdenticulate to denticulate, 1.5–2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 21–44(–65) × 3.5–4(–4.5) μm, 0(–1)-septate, base truncate, 2–2.5 μm wide, somewhat refractive. Conidia catenate, in branched chains, branching in all directions or dichotomously, 1–4 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose to obovoid, broad, 4.5–9 × 4.5–6 μm (av. ± SD: 6.9 ± 1.3 × 5.0 ± 0.5), aseptate, apex broadly rounded, base attenuated, hilum often on a short stalk-like prolongation, intercalary conidia ovoid, ellipsoid to subcylindrical, often with rostrate ends, (6–)8–17(–21) × 3.5–5(–6) μm (av. ± SD: 11.3 ± 3.3 × 4.4 ± 0.7), aseptate, attenuated towards apex and base, small terminal and intercalary conidia smooth to loosely verruculose, irregularly verruculose-rugose or rough-walled (LM), surface with irregularly reticulate structure or embossed stripes under SEM probably caused by diminishing turgor and shriveling of young conidia, thin-walled, with 1–3(–4) hila at the apex, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, 12–25(–29) × 3–5 μm (av. ± SD: 18.4 ± 4.5 × 3.9 ± 0.6), aseptate, rarely 1-septate, pale to medium olivaceous-brown, smooth or finely verruculose, walls slightly thickened, hila conspicuous, often situated on small peg-like prolongations, subdenticulate to denticulate, 0.8–2 μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA attaining 60–72 mm diam after 14 d, olivaceous-grey to grey-olivaceous, reverse leaden-grey to iron-grey, powdery to floccose, margins colourless to grey-olivaceous, narrow, feathery, regular, aerial mycelium loose, diffuse to floccose or fluffy, mainly in colony centre, olivaceous-grey, growth effuse, without prominent exudates, sporulation profuse. Colonies on MEA reaching 56–64 mm diam after 14 d, grey-olivaceous to pale olivaceous-grey and iron-grey towards margins, somewhat zonate, reverse iron-grey, powdery to floccose, margins colourless to white, feathery, regular, aerial mycelium diffuse to floccose, pale olivaceous-grey, mainly in colony centre, growth effuse, radially furrowed in the centre, without prominent exudates, sporulation profuse. Colonies on OA attaining 59–67 mm diam after 14 d, dark smoke-grey to brownish, iron-grey towards margins, reverse leaden-grey to iron-grey, powdery to floccose, margins grey-olivaceous, glabrous, regular, aerial mycelium diffuse to floccose, white to pale olivaceous-grey, growth effuse with numerous not very prominent exudates, sporulation profuse.
Specimen examined: South Korea, Hoengseong, N37°32'09” E128°07'07”, isol. from Acalypha australis (Euphorbiaceae), 11 Oct. 2004, coll. H.-D. Shin, isol. P.W. Crous, CBS H-20422, holotype; ex-type culture CBS 125982 = CPC 11625.
Substrate and distribution: On Acalypha australis; South Korea.
Notes: The morphology of C. acalyphae is unique and not comparable with any of the existing species. Surface ornamentation of its conidia is reminiscent of Cladosporium pini-ponderosae (Schubert et al. 2009) and C. verrucocladosporioides (see below) but the small terminal conidia in the latter two species are narrower and the conidiophores are quite different. The biology of this species remains unclear, i.e., it is unknown whether it is saprobic or plant pathogenic. The species clustered as a sister between C. delicatulum and C. inversicolor (Fig. 1, part a) and formed a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
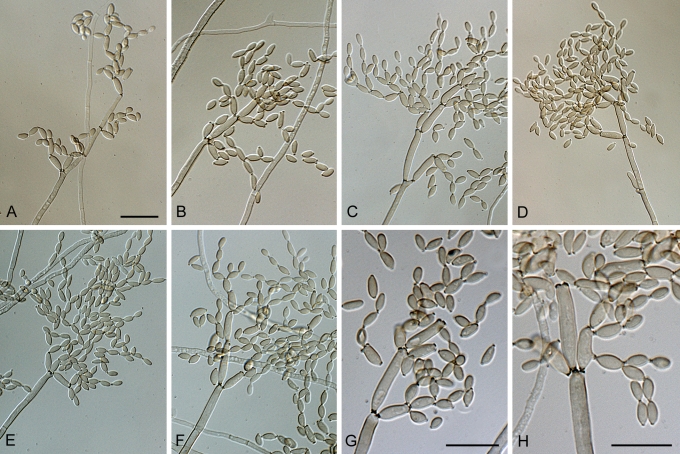
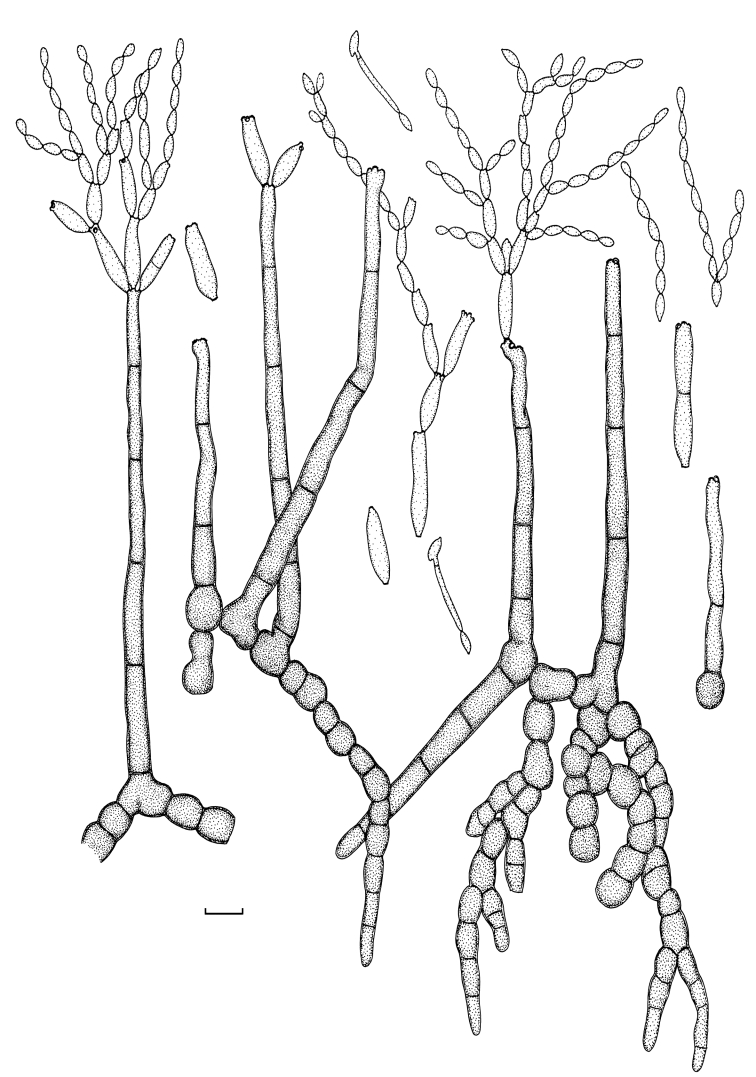
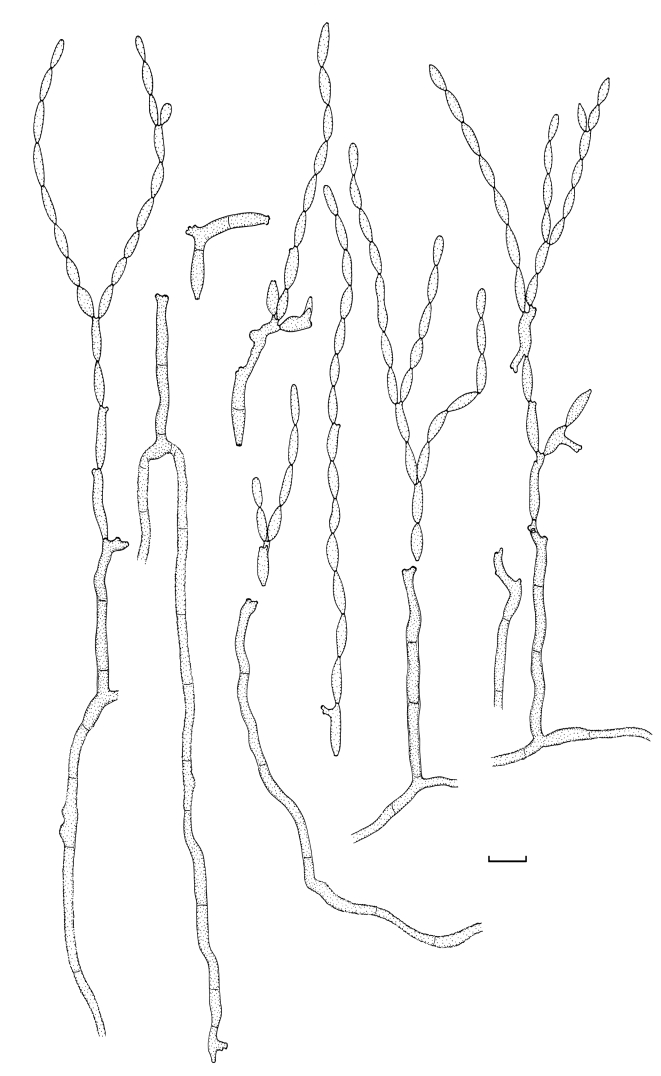
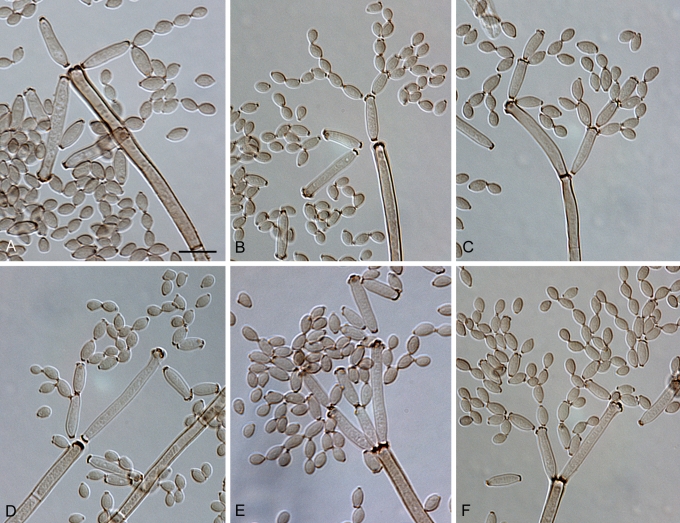
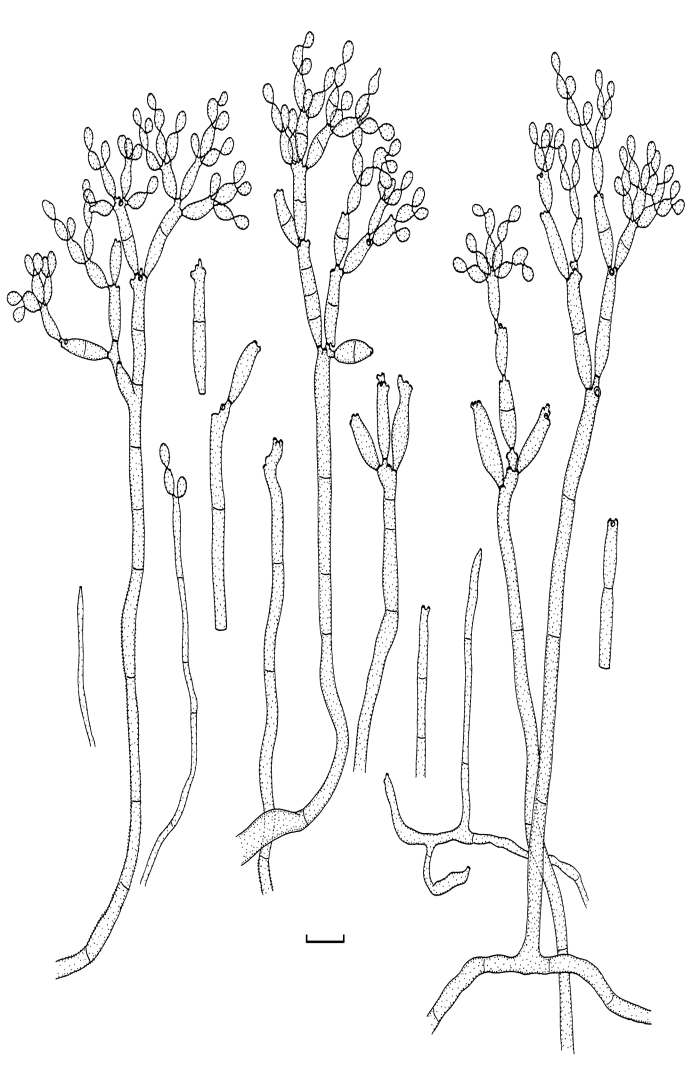
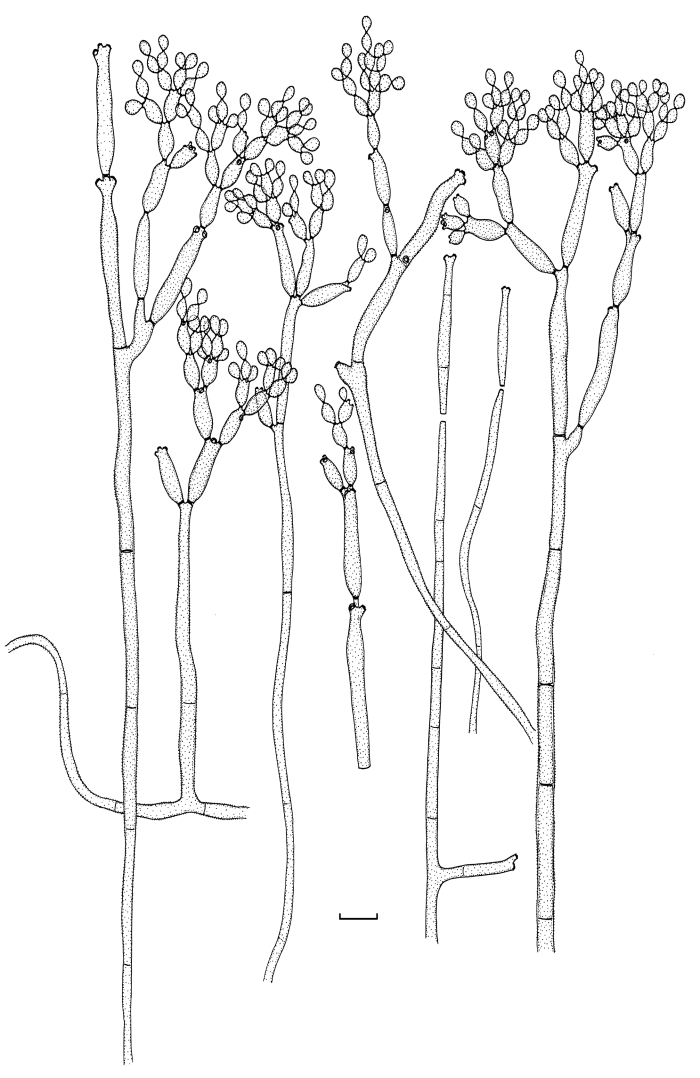
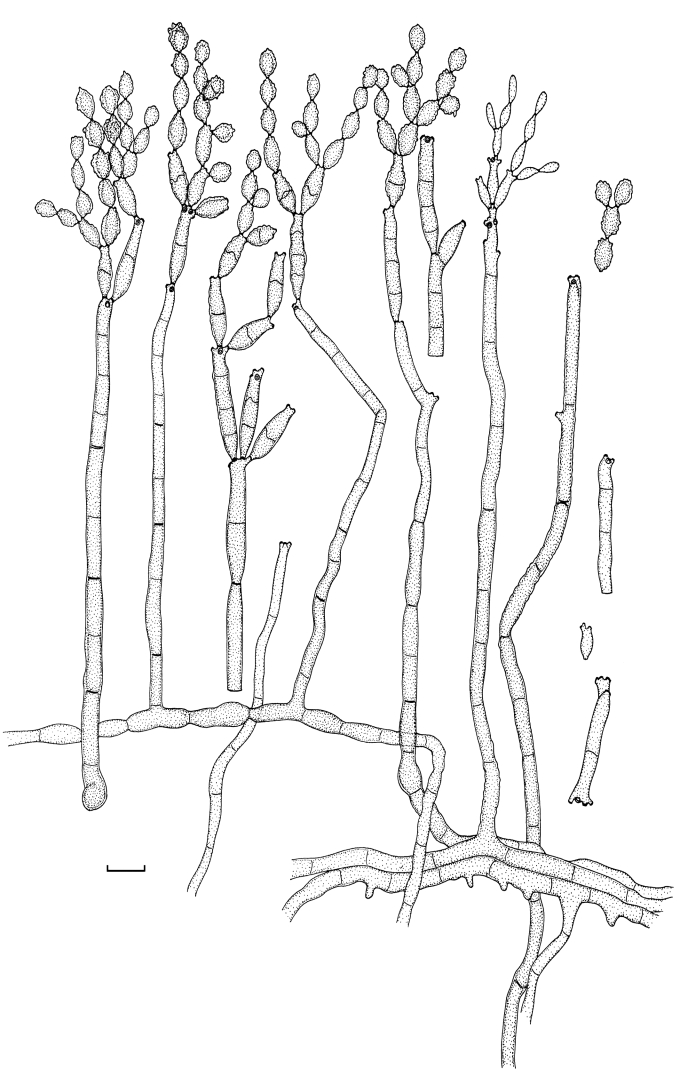
Cladosporium angustisporum Bensch, Summerell, Crous & U. Braun, sp. nov. MycoBank MB517071. Figs 5, 6.
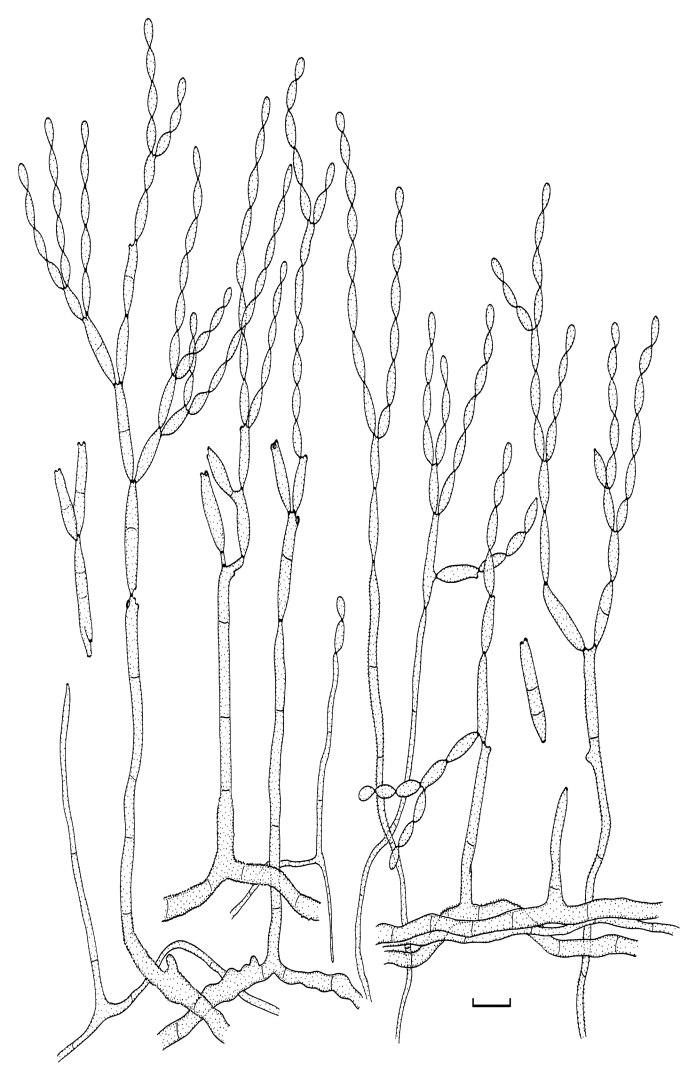
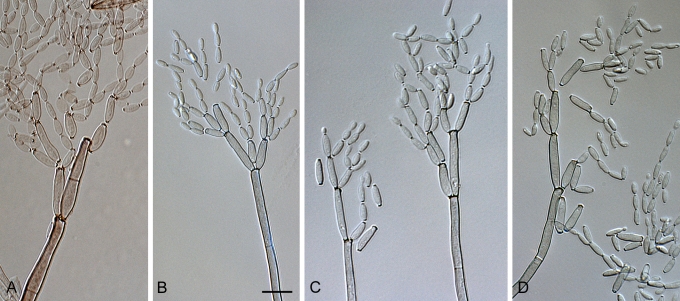
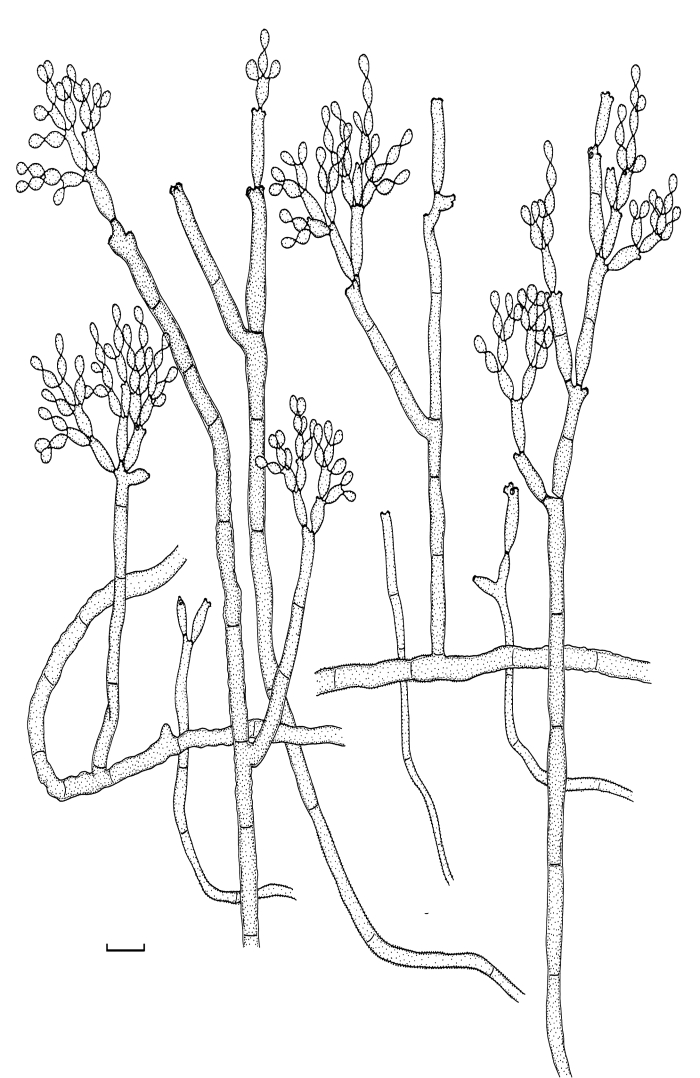
Fig. 5.
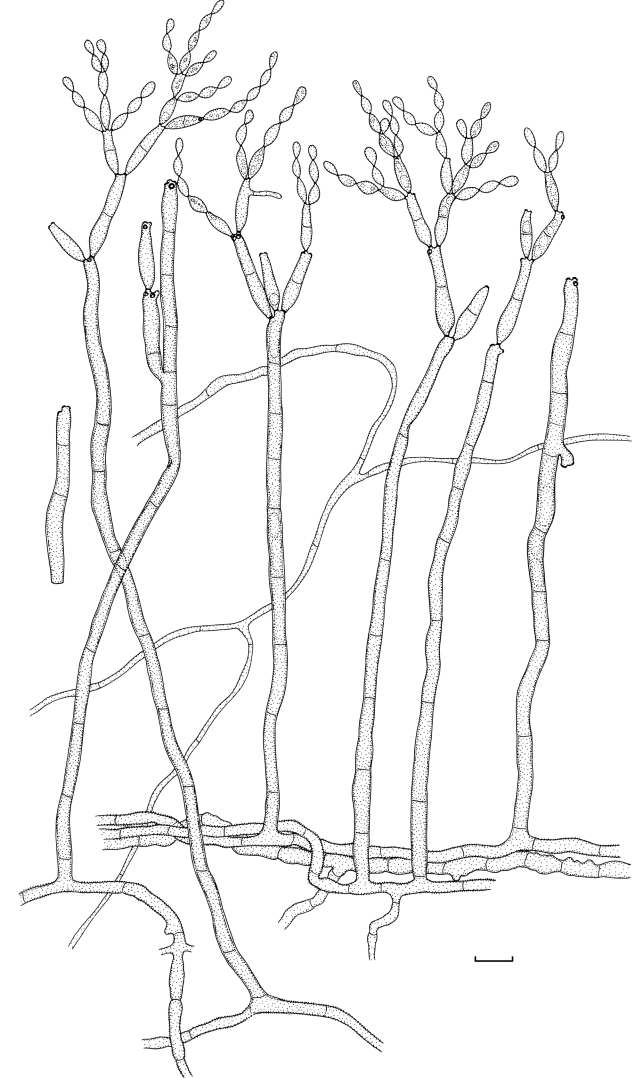
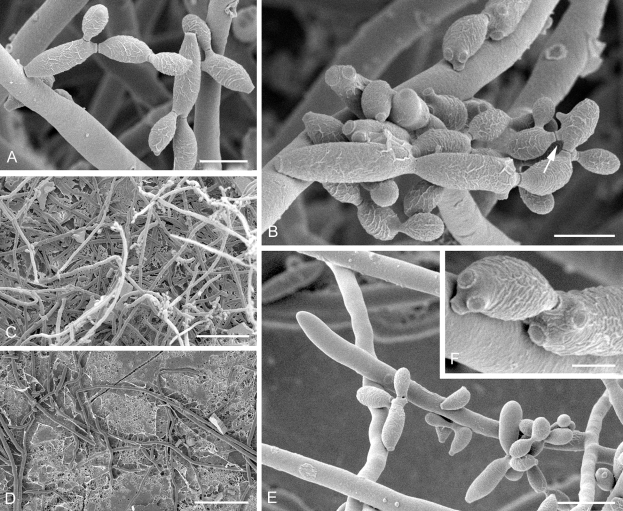
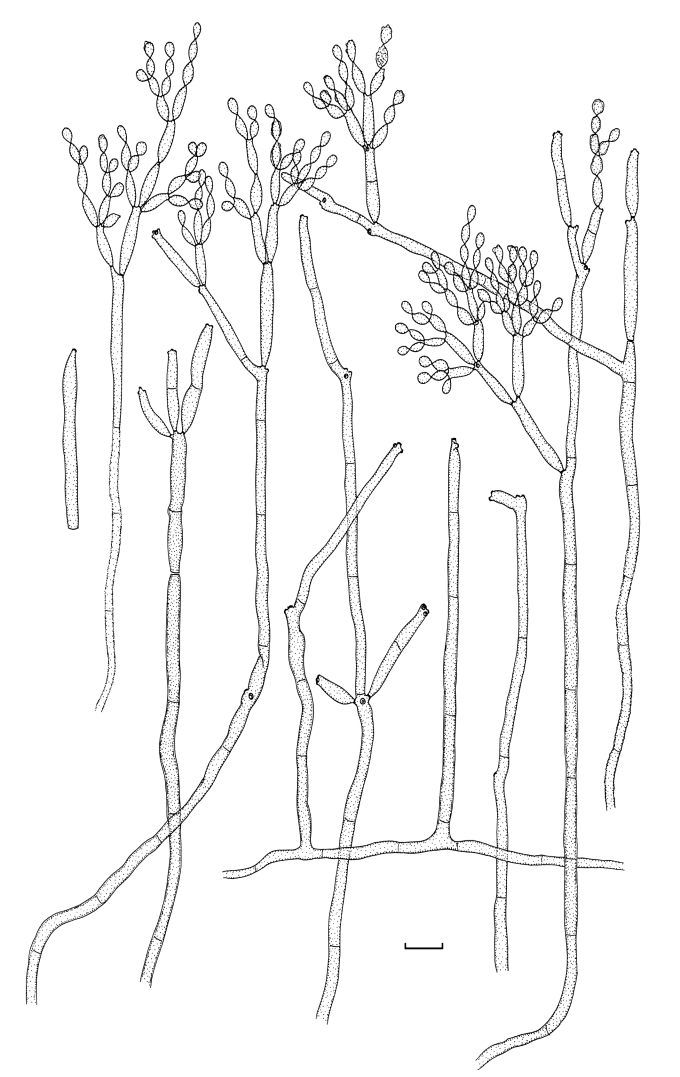
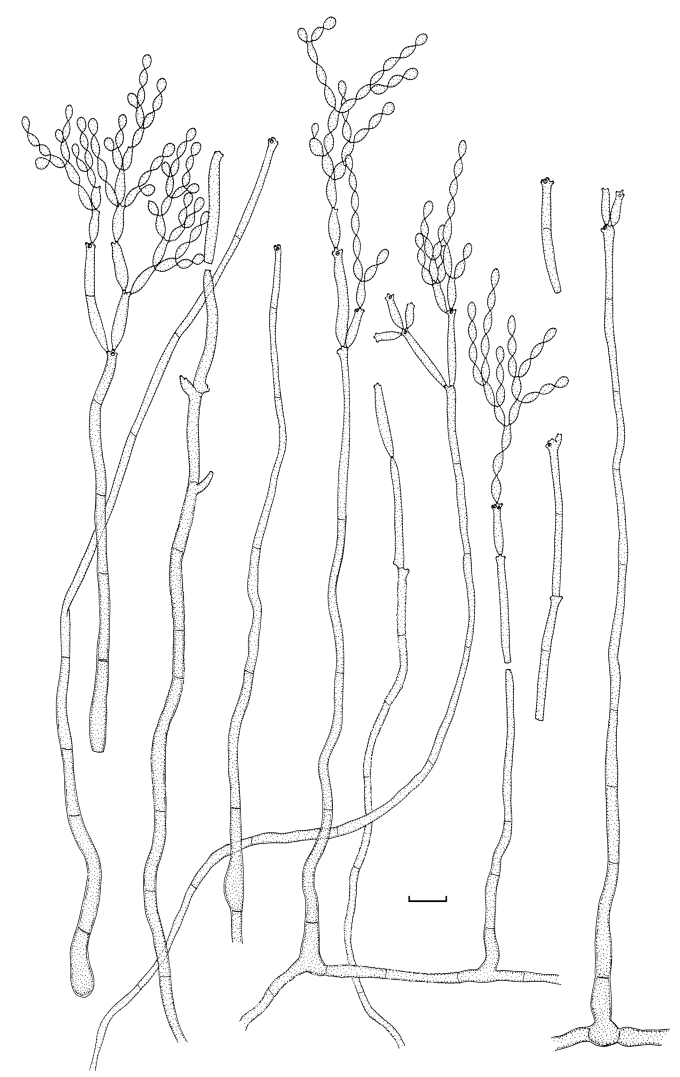
Cladosporium angustisporum (CBS 125983). Macro- and micronematous conidiophores, mycelium often forming ropes, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 6.
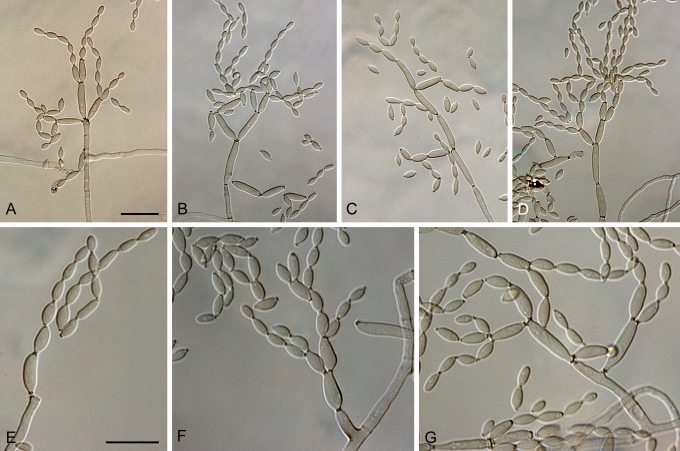
Cladosporium angustisporum (CBS 125983). A–H. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Refers to the narrow conidia.
Cladosporii cladosporioidis simile, sed conidiis angustioribus, 1.5–3 μm latis, conidiophoris dimorphis, longioribus et brevioribus et tamen hyphis in funiculis expansis internoscitur.
Mycelium immersed and superficial; hyphae branched, 1–3 μm wide, septate, mostly not constricted at septa, subhyaline to olivaceous-brown, smooth to verruculose or irregularly rough-walled, walls unthickened, sometimes irregular in outline due to swellings and constrictions, forming expanded hyphal ropes. Conidiophores solitary, macro- and micronematous, erect or ascending, arising terminally from ascending or laterally from plagiotropous hyphae, straight or flexuous, filiform to cylindrical-oblong, non-nodulose, usually not geniculate, two types of conidiophores, short and long ones, 22–280 × (1.5–)2–4 μm, pluriseptate, not constricted at septa, but sometimes irregular in outline due to wider or narrower parts within the stalk, pale to medium olivaceous-brown or pale olivaceous, smooth or verruculose at the base, walls unthickened or slightly thickened. Conidiogenous cells integrated, mainly terminal, sometimes also intercalary, neither nodulose nor geniculate, narrowly cylindrical-oblong, 10–27 μm long, with several loci crowded at the apex, in intercalary conidiogenous cells loci mainly situated on small lateral denticles just below a septum, subdenticulate, conspicuous, 1–1.5(–2) μm diam, thickened and darkened-refractive. Ramoconidia cylindrical, 18–42(–55) μm long, 0–1-septate, concolouress with tips of conidiophores, base broadly truncate, 2.5–3 μm wide, unthickened but sometimes slightly refractive. Conidia catenate, in branched chains, with 1–5 conidia in the terminal unbranched part of the chain, branching in all directions, small terminal conidia obovoid to narrowly ellipsoid, 3–6.5 × 1.5–2 μm (av. ± SD: 4.9 ± 1.0 × 1.8 ± 0.3), aseptate, intercalary conidia narrowly ellipsoid, fusiform, (4–)5.5–11.5(–13) × (1.5–)2–2.5(–3) μm (av. ± SD: 8.1 ± 2.4 × 2.4 ± 0.4), 0(–1)-septate, with 1–3 distal hila, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, (6–)7.5–26 × 2–3 μm (av. ± SD: 14.9 ± 6.1 × 2.7 ± 0.4), 0–1-septate, not constricted at the median septum, pale olivaceous or pale olivaceous-brown, smooth, walls unthickened, somewhat attenuated towards apex and base, with 2–4(–5) distal hila, hila conspicuous, subdenticulate, 0.5–2 μm diam, thickened and darkened-refractive.
Culture characteristics: Colonies on PDA attaining 57–76 mm diam after 1 mo, pale olivaceous-grey to smoke-grey, mouse-grey due to abundant sporulation, glaucous-grey towards margins, reverse greenish-black, fluffy, margin whitish, feathery, broad, aerial mycelium abundant, woolly to fluffy, covering almost the whole colony surface, without prominent exudates, sporulating. Colonies on MEA reaching 45–60 mm diam after 1 mo, smoke-grey, whitish to pale olivaceous-grey due to abundant aerial mycelium, reverse iron-grey to pale greenish-grey, velvety to woolly-fluffy, margin colourless to whitish, feathery, regular, aerial mycelium abundant, dense, fluffy, without prominent exudates, sporulation profuse.
Specimen examined: Australia, North Queensland, Daintree N.P., isol. from Alloxylon wickhamii (Proteaceae), coll. B.A. Summerell, isol. P.W. Crous, CBS H-20423, holotype; ex-type culture CBS 125983 = CPC 12437.
Notes: Cladosporium angustisporum is morphologically very close to C. cladosporioides but its conidia are distinctly narrower, 1.5–3 μm wide, the mycelium usually forms expanded hyphal ropes and the conidiophores are dimorphic, i.e. they occur in two types, short and long ones.
Phylogenetically it is closely allied to Cladosporium vignae and the new species C. subuliforme (see below), but distinct for both TEF and ACT (Fig. 1, part c; distance analyses in TreeBASE). However, the host-specific C. vignae, causal organism of scab, leaf and pod blight of cowpea and leaf blight of Lezpedeza bicolor, differs in having wider, 0–2(–3)-septate conidia (Morgan-Jones & McKemy 1992, Ho et al. 1999) and C. subuliforme described on Chamaedorea metallica from Thailand possesses longer, subulate, slightly to distinctly attenuated conidiophores.
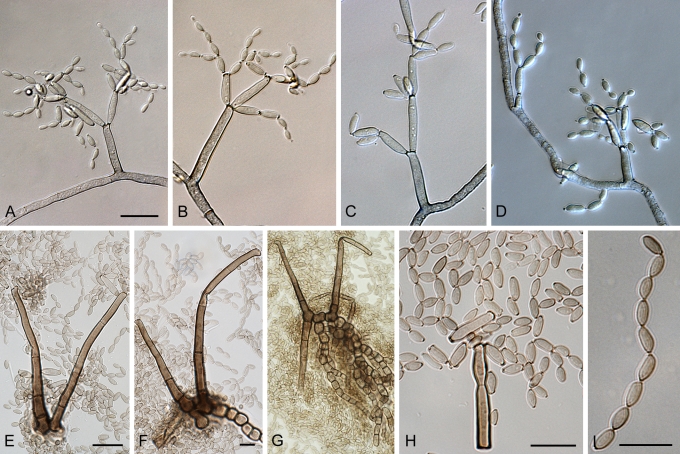
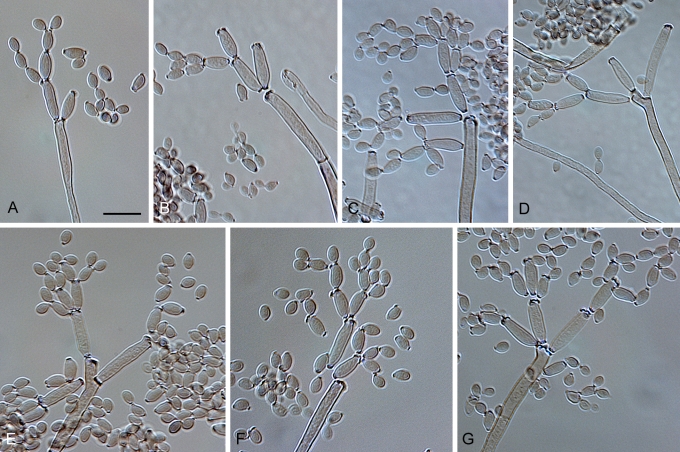
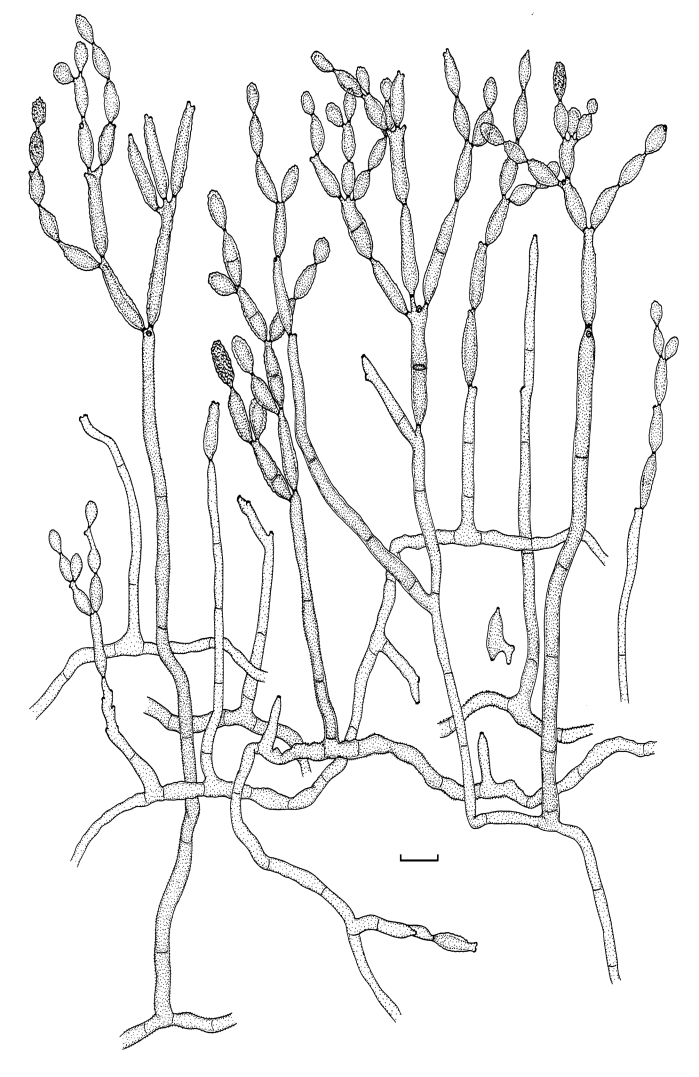
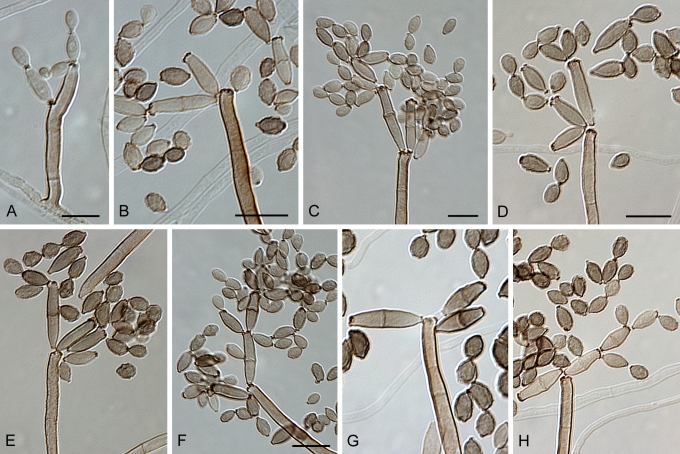
Cladosporium asperulatum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517072. Figs 7, 8, 9.
Fig. 7.
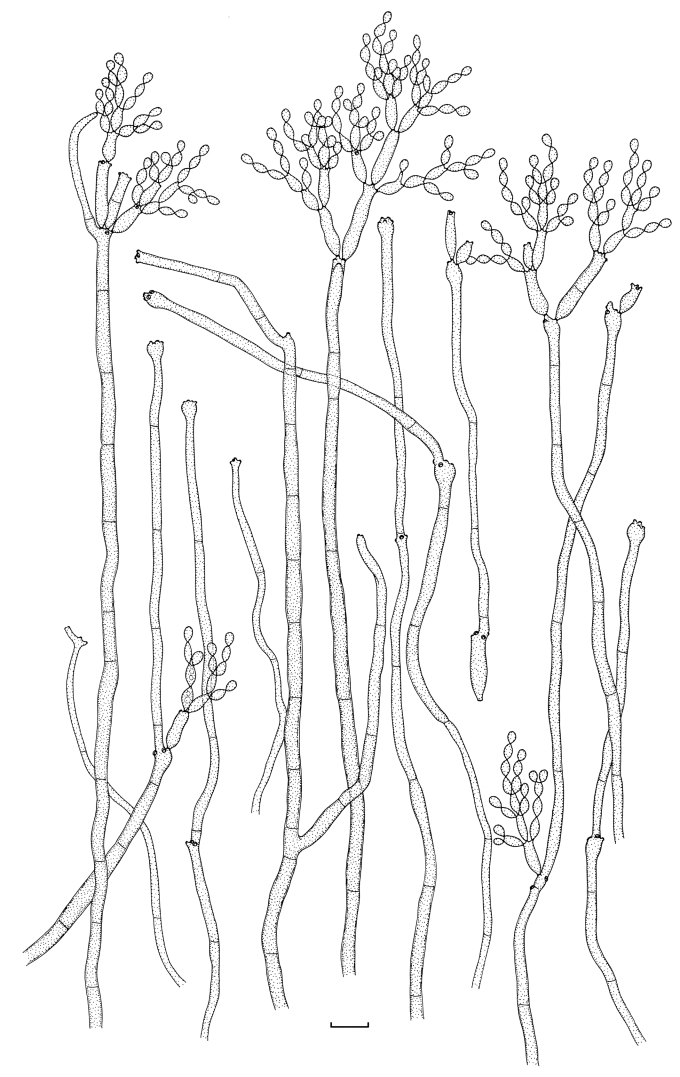
Cladosporium asperulatum (CBS 126340). Macronematous conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 8.
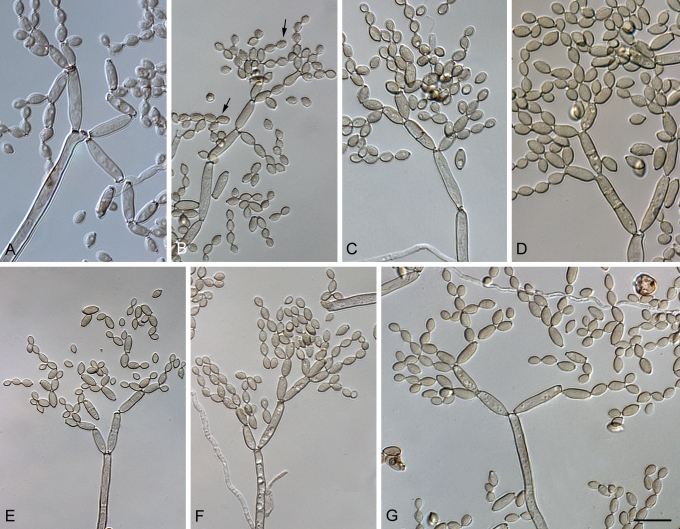
Cladosporium asperulatum (CBS 126340). A–D. Conidiophores and conidia. E–G. Secondary ramoconidia and conidia formed in branched chains. Scale bars = 10 μm.
Fig. 9.
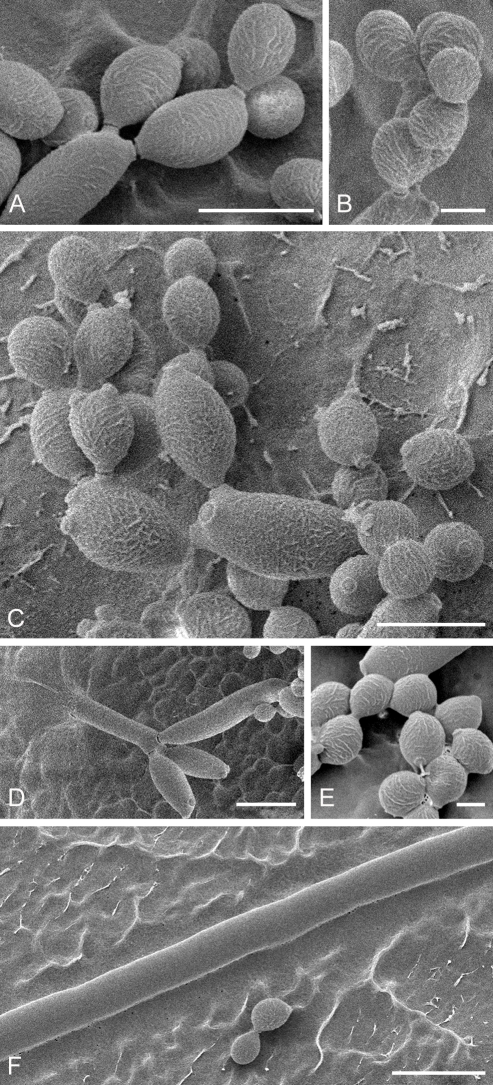
Cladosporium asperulatum (CBS 126340). A–B. Conidiophores and very young conidia (A). C–D. Conidia and details of scars on a secondary ramoconidium. E. Conidia with sparse ornamentation. Note the round conidium-initial. F. Whorl of secondary ramoconidia formed at the tip of a conidiophore. G. Details of ornamentation showing loosely irregularly reticulate structures. H. Secondary ramoconidia. I. Overview of a conidiophore with scars on the tip of the conidiophore. J. Swollen cells at agar surface giving rise to conidiophores. Note the scar on the root structure. Scale bars = 1 (D), 2 (G), 5 (A, C, E–F, H–J), 10 (B) μm.
Etymology: Refers to the asperulate surface ornamentation of its conidia, conidiophores and mycelium.
Cladosporio subtilissimo simile, sed conidiophoris longioribus, ad 210(–360) μm longis, pluriseptatis et item conidiis leniter angustioribus, 2–4(–5) μm latis distinguitur. Differt a Cladosporio cladosporioide conidiophoris et conidiis saepe asperulatis et a Cladosporio perangusto conidiophoris longioribus et leniter latioribus et conidiis latioribus.
Mycelium immersed, sparingly superficial; hyphae unbranched or very sparingly branched, 2–4.5 μm wide, septate, not constricted at septa, subhyaline to pale or medium olivaceous-brown, smooth to minutely verruculose or irregularly verrucose, walls unthickened or almost so, sometimes forming ropes. Conidiophores macro- and micronematous, solitary, arising terminally or laterally from plagiotropous or ascending and erect hyphae, erect, straight to slightly flexuous, cylindrical-oblong, sometimes slightly geniculate towards the apex, non-nodulose, (15–)45–210(–360) × (2–)3–4(–5) μm, sometimes up to 5 μm wide at the base, unbranched, occasionally branched, branches below the apex or at a lower level, usually below a septum, sometimes up to 105 μm long, pluriseptate with 0–12 septa, not constricted, pale to medium olivaceous-brown, paler towards the apex and sometimes attenuated, smooth to asperulate or minutely verruculose, walls slightly thickened; micronematous conidiophores filiform or narrowly cylindrical-oblong, about 2 μm wide, paler and narrower, subhyaline or pale olivaceous-brown, mostly with a single apical scar. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, sometimes slightly geniculate-sinuous towards the apex, 22–38 μm long, smooth or almost so, with 2–4 apical loci, protuberant, subdenticulate, sometimes situated on peg-like prolongations, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 15–50 × 3–4 μm, 0(–1)-septate, concolouress with tips of conidiophores, smooth or almost so, base broadly truncate, (2.2–)2.5–3(–3.2) μm wide, unthickened. Conidia catenate, in branched chains, up to 8(–10) conidia in the terminal unbranched part of the chain, small terminal conidia obovoid, 4.5–7(–8) × 2–3(–3.5) μm (av. ± SD: 5.6 ± 1.0 × 2.6 ± 0.5), intercalary conidia ovoid, fusiform to ellipsoid, 5–11(–13) × 2.5–3(–4) μm (av. ± SD: 8.0 ± 2.1 × 2.8 ± 0.4), aseptate, secondary ramoconidia ellipsoid, fusiform, subcylindrical, (7.5–)9–26(–37) × (2.5–)3–4(–5) μm (av. ± SD: 18.3 ± 6.6 × 3.4 ± 0.6), 0(–1)-septate, very rarely with a second septum, not constricted at septa, subhyaline to pale olivaceous-brown, smooth to minutely verruculose or irregularly rough-walled (LM), under SEM loosely verruculose or surface with irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shriveling of tender conidia, walls slightly thickened, attenuated towards apex and base, hila protuberant, subdenticulate, 0.8–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 48–53 mm diam after 14 d, olivaceous-grey, iron-grey or grey-olivaceous at margins, sometimes zonate, reverse leaden-grey, greyish blue to iron-grey, powdery to fluffy or hairy, margin white, narrow, glabrous, aerial mycelium abundantly formed, dense, fluffy and high in colony centre, growth flat to low convex with somewhat elevated colony centre, without prominent exudates, sporulation profuse. Colonies on MEA reaching 45–64 mm diam after 14 d, olivaceous-grey to pale greenish grey, reverse olivaceous-grey to iron-grey, powdery to fluffy, margin white to smoke-grey, narrow, regular, glabrous to feathery, sometimes radially furrowed, aerial mycelium abundant, several prominent exudates formed appearing blackish, sporulation profuse.
Specimens examined: India, isol. from Eucalyptus leaf litter (Myrtaceae), 1 Mar. 2004, coll. W. Gams, isol. P.W. Crous, CBS 126339 = CPC 11158. Portugal, isol. from Protea susannae (Proteaceae), 1 May 2007, P.W. Crous, CBS H-20424, holotype; ex-type culture CBS 126340 = CPC 14040. Cf. asperulatum: U.S.A., isol. from grape bud, F.M. Dugan, 208 db sci 1 = CBS 113744.
Substrate and distribution: On plant material; India, Portugal, U.S.A.
Notes: Cladosporium asperulatum is morphologically comparable with C. subtilissimum but the latter species differs in having 0–4-septate, somewhat shorter conidiophores and somewhat wider conidia [4–32(–37) × 3–5(–6) μm] (Schubert et al. 2007b). Cladosporium cladosporioides is easily distinguishable based on its smooth conidia and somewhat wider conidiophores, and C. perangustum introduced below as a new species possesses shorter and somewhat narrower conidiophores, narrower conidia, (1.5–)2–3(–3.5) μm, and narrower conidiogenous loci and hila, 0.8–1.5(–1.8) μm. The species clustered as a sister to C. myrtacearum (Fig. 1, part a) and formed a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
The isolate from North America (CBS 113744) differs slightly from the other two isolates in lacking mycelial ropes, and having shorter conidiophores with few, often somewhat darkened septa, slightly narrower conidiogenous loci and hila, somewhat shorter globose, subglobose or obovoid small terminal conidia (2.5–5 × 2–3 μm) and somewhat wider intercalary conidia (3–4 μm). Although morphologically and phylogenetically (Figs 1, part a) slightly different, this isolate is tentatively maintained in the new species. Additional isolates are needed to clarify whether these differences are due to intra- or interspecific variation.
Cladosporium australiense Bensch, Summerell, Crous & U. Braun, sp. nov. MycoBank MB517073. Figs 10, 11.
Fig. 10.
Cladosporium australiense (CBS 125984). Conidiophores, ramoconidia and conidial chains, mycelium sometimes forming ropes. Scale bar = 10 μm.
Fig. 11.
Cladosporium australiense (CBS 125984). A–B. Conidiophores and conidial chains. C. Conidiophore with a septate secondary ramoconidium still attached. D. Conidia. Scale bar = 10 μm.
Etymology: Name refers to the country of origin, Australia.
Cladosporio cladosporioide simile, sed conidiophoris et conidiis saepe pseudoseptatis, hyphis in funiculis densis, utique ramoconidiis secundaris 0–1-septatis discernitur.
Mycelium immersed and superficial, abundant, hyphae loosely to densely branched, sometimes anastomosing, filiform to cylindrical-oblong or thicker hyphae irregular in outline due to intercalary swellings and constrictions, 1–5 μm wide, septate, often slightly constricted, subhyaline to pale or medium olivaceous-brown, smooth to verruculose or loosely rough-walled, rugose, walls unthickened or slightly thickened in wider hyphae, rhizoid-like, sometimes cells swollen, up to 7 μm wide, often forming dense ropes with hyphae entwined. Conidiophores macronematous, solitary, arising terminally and laterally from hyphae, erect, slightly flexuous, cylindrical-oblong, often very long, 48–285 × 3–4(–5) μm, seta-like, mostly neither geniculate nor nodulose, occasionally subnodulose and slightly geniculate, unbranched or branched, branches 3–55 μm long, pluriseptate, not constricted at septa, pale to medium olivaceous-brown, smooth, walls somewhat thickened, about 0.5 μm wide, several cells with unusual cell structure having one or few bigger cavities and protoplasm attached at cell walls or forming distosepta. Conidiogenous cells integrated, terminal, occasionally intercalary, cylindrical-oblong, neither geniculate nor nodulose, 6–15(–40) μm long, with 1–4 loci at the apex or 1–3 loci in intercalary cells with loci situated mostly all at more or less the same level, like a garland, conspicuous, subdenticulate, 1–2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, often hardly distinguishable from secondary ramoconidia. Conidia catenate, in branched chains, branching in all directions, up to 2–4(–5) conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose to obovoid, 3–6 × 2–3 μm (av. ± SD: 4.2 ± 0.9 × 2.5 ± 0.4), aseptate, rounded at the apex, intercalary conidia ovoid, ellipsoid to subcylindrical, 5–14(–16) × 2.5–3(–4) μm (av. ± SD: 9.1 ± 3.2 × 3.0 ± 0.4), 0–1-septate, not constricted at septa, with 1–3 distal hila, secondary ramoconidia ellipsoid, subcylindrical to cylindrical, (7–)11–25(–27) × 3–4 μm (av. ± SD: 18.5 ± 5.1 × 3.4 ± 0.3), 0–1(–2)-septate, septum median or often somewhat in the upper half, not constricted at septa, often with additional distosepta, cells with one or few small to large cavities giving the cells a somewhat thick-walled appearance, pale olivaceous to pale olivaceous-brown, smooth, walls unthickened to slightly thickened, slightly attenuated towards apex and base, hila conspicuous, subdenticulate to denticulate, (0.5–)0.8–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 70–75 mm diam after 14 d, pale olivaceous-grey, grey-olivaceous towards margins, reverse greyish-blue to iron-grey, floccose to fluffy, margins colourless to white, regular, somewhat feathery, aerial mycelium abundant, forming loosely to densely floccose or fluffy mats covering the whole colony, smoke-grey to pale olivaceous-grey, growth low convex, without prominent exudates, sporulation sparse. Colonies on MEA reaching up to 80 mm diam after 14 d, olivaceous-grey to pale olivaceous-grey, glaucous-grey due to sporulation, reverse iron-grey, velvety to floccose, margins colourless to white, regular, feathery, aerial mycelium abundant, densely floccose, growth effuse, radially furrowed, without prominent exudates, sporulating. Colonies on OA attaining 65–69 mm diam after 14 d, pale olivaceous-grey to pale greenish-grey, towards margins and few areas grey-olivaceous, with smaller dots of smoke-grey, reverse pale mouse-grey with smaller dots or patches of leaden-grey or iron-grey, velvety to floccose, margins colourless, regular, glabrous, aerial mycelium abundant, dense, forming expanded mats, floccose to fluffy, growth low convex, without prominent exudates, sporulating.
Specimen examined: Australia, New South Wales, Douglas Park, S 34°10'50” E 150°42'18”, isol. from Eucalyptus moluccana (Myrtaceae), 2006, coll. B.A. Summerell, isol. P.W. Crous, CBS H-20425, holotype; ex-type culture: CBS 125984 = CPC 13226.
Substrate and distribution: On Eucalyptus; Australia.
Notes: Cladosporium australiense is compared here with the similar C. cladosporioides with both species having more or less the same conidial shape and dimensions. However, the conidiophores and secondary ramoconidia of the new species possess an unusual cell structure having one or few bigger cavities and protoplasm attached at cell walls or forming distosepta, the mycelium usually forms dense ropes and secondary ramoconidia are 0–1-septate or due to cell structure with additional distosepta. Phylogenetically, both species are distinct (see Fig. 1, parts b & c). The species clusters as a sister to C. xylophilum and C. verrucocladosporioides (Fig. 1, part b) and formed a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium basiinflatum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517074. Figs 12, 13.
Fig. 12.
Cladosporium basiinflatum (CBS 822.84). Mycelium, stromatic hyphal aggregations, conidiophores often with foot-like swollen base and conidial chains. Scale bar = 10 μm.
Fig. 13.
Cladosporium basiinflatum (CBS 822.84). A–D. Young conidiophores arising laterally from hyphae with conidial chains still attached. E–G. Older conidiophores formed in pairs arising from distinctly swollen hyphal cells or stromatic hyphal aggregations and numerous conidia. H. A single ramoconidium and numerous conidia. I. A conidial chain composed of intercalary and small terminal conidia. Scale bars = 10 μm.
Etymology: Name refers to the usually inflated base of conidiophores.
Cladosporii gentianae valde simile, sed conidiis sublaevibus, 0(–1)-septatis, pallidioribus distinguitur.
Mycelium immersed, rhizoid, aerial mycelium sparsely formed; hyphae sparingly branched, 4–7 μm wide, septate, not constricted or often distinctly constricted at septa, medium to dark brown, paler at the tips, at the base of the conidiophores distinctly swollen and constricted at septa, 5–11 μm wide, medium to dark brown, smooth to asperulate, thick-walled, sometimes forming stromatic hyphal aggregations. Conidiophores macronematous, solitary or in groups of two or three, rarely four, arising from swollen hyphal cells or stromatic hyphal aggregations, erect, straight to slightly flexuous, often quite rigid, seta-like, usually non-nodulose, sometimes slightly head-like, unilaterally swollen at the apex, not geniculate, cylindrical-oblong, distinctly attenuated towards the apex, unbranched, 35–140 μm long, at the base 4–7(–8) μm wide, at the apex 3–4 μm wide, 1–6-septate, not constricted at septa, medium to dark brown, paler towards the tip, smooth to minutely verruculose, walls thickened, sometimes two-layered, especially towards the base, about 1 μm wide, often swollen at the base, foot-like. Conidiogenous cells integrated, terminal, cylindrical-oblong, 11–25 μm long, neither nodulose nor geniculate, with 2–3(–4) loci at the apex, subdenticulate, protuberant, 1.2–2 μm diam, thickened and darkened-refractive. Ramoconidia absent or only very rarely formed. Conidia numerous, catenate, in long unbranched or basely branched chains, up to 9(–11) conidia in the unbranched part, straight, small terminal conidia and intercalary conidia obovoid, ovoid to narrowly ellipsoid, 4–6(–7) × (2–)2.5–3 μm, intercalary conidia (6.5–)7–10(–11) × 2.5–3.5(–4) μm (av. ± SD: 8.4 ± 1.3 × 3.0 ± 1.3), aseptate, rounded at the apex or attenuated towards apex and base, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, 10–23(–32) × 3–4.5(–5) μm (av. ± SD: 15.0 ± 4.7 × 4.0 ± 0.4), mainly 4 μm wide, 0(–1)-septate, not constricted at the median septum, pale brown to pale olivaceous-brown, distinctly paler than conidiophores, smooth or almost so, walls unthickened or almost so, slightly attenuated towards apex and base, hila protuberant, often broadly truncate at the base, 1–2(–2.2) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA olivaceous-black to grey-olivaceous due to profuse sporulation and mycelium, reverse iron-grey to dark leaden-grey, felty-woolly, margin narrow, white, feathery, aerial mycelium diffuse, loose, fluffy, without prominent exudates, sporulation profuse, mainly in colony centre. Colonies on MEA greenish olivaceous to grey-olivaceous, reverse olivaceous-grey to iron-grey, powdery to fluffy or felty, margin white, feathery, narrow, without prominent exudates, sporulation profuse. Colonies on OA olivaceous-grey to dull green, with age olivaceous, reverse iron-grey to sky-grey, woolly-felty, margin dull green, outermost margin colourless to white, feathery, aerial mycelium abundant, felty-woolly, loose to dense, without prominent exudates, sporulation profuse.
Specimen examined: Germany, on Hordeum vulgare (Poaceae), CBS H-20426, holotype; ex-type culture CBS 822.84.
Substrate and distribution: On Hordeum; Germany.
Notes: This new species is characterised by having mainly aseptate conidia that are distinctly paler than conidiophores and dark brown, thick-walled conidiophores with usually foot-like swollen basal cells. Cladosporium gentianae is morphologically close to C. basiinflatum but distinguished by having somewhat darker, verruculose or irregularly rough-walled, 0–1(–3)-septate conidia (Lobik 1928, Schubert 2005b). The species clusters as a sister to C. lycoperdinum and C. cladosporioides s. lat. (Fig. 1, part a) and formed a distinct lineage for both TEF and ACT (distance analyses in TreeBASE). It is also somewhat reminiscent of Metulocladosporiella musae (≡ Cladosporium musae), which is Cladosporium-like, and similar with regard to possessing inflated conidiophore bases, but conidiophores have distinctly appressed branches, and the species is phylogenetically quite distinct from Cladosporium (Crous et al. 2006a).
Cladosporium chalastosporoides Bensch, Crous & U. Braun, sp. nov. MycoBank MB517075. Figs 14, 15, 16.
Fig. 14.
Cladosporium chalastosporoides (CBS 125985). Conidiophores, conidia in long unbranched or dichotomously branched chains and microcyclic conidiogenesis. Scale bar = 10 μm.
Fig. 15.
Cladosporium chalastosporoides (CBS 125985). A–B. Conidiophores and conidia. C–D. Conidial chains. Scale bars = 10 μm.
Fig. 16.
Cladosporium chalastosporoides (CBS 125985). A–B. Examples of elongated conidia and chains. C. Branch formation on conidiophores. D. Branched conidiophore, ramoconidia and conidia. E. Elongated conidia. Note the broad areas of connection between the spores. F. Overview of a large cell mass that gives rise to conidiophores and spores. G. Detail of a cell mass showing meristematic growth. H. Differentiation of fungal cells into a cell mass. Scale bars = 5 (A–E), 10 (G–H), 50 (F) μm.
Etymology: Name refers to the conidia and the habit of conidial chains which are reminiscent of the genus Chalastospora.
Cladosporii flabelliformis simile, sed ramoconidiis secundariis brevioribus et latioribus, 10–19 × (2–)2.5–4 μm, catenis longioribus, conidiis usque ad 18 in catenis ramosis et non ramosis internoscitur.
Mycelium immersed and superficial; hyphae unbranched or loosely branched, 1–3 μm wide, septate, not constricted at septa, pale to pale medium olivaceous-brown, almost smooth to usually minutely verruculose, walls unthickened or almost so. Conidiophores solitary, macronematous, arising terminally and laterally from hyphae, erect, straight to somewhat flexuous, cylindrical-oblong, sometimes with constrictions, attenuations and swellings which give the conidiophores an irregular appearance, often slightly to distinctly geniculate-sinuous, usually once, unbranched or once branched, below the apex or at a lower level, 30–80 × (2–)2.5–3.5(–4) μm, 1–4-septate, not constricted at septa, medium olivaceous-brown, smooth, walls unthickened or only very slightly thickened, occasionally slightly attenuated towards the apex. Conidiogenous cells integrated, mainly terminal but also intercalary, cylindrical, often geniculate-sinuous, 6–20 μm long, 1–2(–4) loci at or towards the apex, sometimes situated on small lateral prolongations, subdenticulate to denticulate, 1–1.5 μm diam, central dome and periclinal rim not very conspicuous, loci flat, somewhat thickened and darkened-refractive. Ramoconidia formed, hardly distinguishable from secondary ramoconidia, up to 24 μm long, base 2–3 μm wide, broadly truncate. Conidia catenate, at the base of the chain or intercalary once or twice mostly dichotomously branched, formed in long, unbranched chains of up to 18 conidia, small terminal conidia narrowly obovoid to subcylindrical, 5–9 × 2–2.5 μm (av. ± SD: 7.3 ± 1.1 × 2.1 ± 0.2), aseptate, intercalary conidia fusiform to subcylindrical, 7–14 × 2–3 μm (av. ± SD: 9.4 ± 1.5 × 2.5 ± 0.3), 0(–1)-septate, with 1–2 distal hila, small terminal and intercalary conidia subhyaline, often distinctly paler compared with secondary ramoconidia, secondary ramoconidia fusiform to subcylindrical, 10–19 × (2–)2.5–4 μm (av. ± SD: 15.3 ± 2.4 × 3.1 ± 0.5), 0–1(–)2-septate, not constricted at septa, pale to pale medium olivaceous-brown, smooth or almost so, walls more or less unthickened, slightly attenuated towards apex and base and occasionally constricted in the middle, hila conspicuous, truncate, 0.8–1.8 μm diam, central dome and periclinal rim not very prominent, neither with LM nor SEM, thickened and darkened-refractive; microcyclic conidiogenesis very often occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA attaining 42–49 mm diam after 14 d, olivaceous-grey to iron-grey, olivaceous-black towards margins, reverse iron-grey to olivaceous-black, floccose to fluffy-felty, margins crenate, very narrow, white, feathery, aerial mycelium abundant, dense, covering most of colony surface, floccose to fluffy-felty, growth effuse with elevated colony centre, without exudates, sporulation sparse. Colonies on MEA attaining 38–56 mm diam after 14 d, pale olivaceous-grey to olivaceous-grey, reverse olivaceous-grey, velvety to floccose, margins regular, white, glabrous, aerial mycelium abundant, covering the whole surface, dense, floccose, growth effuse, radially furrowed, without exudates, sporulation sparse. Colonies on OA reaching 38–45 mm diam after 14 d, olivaceous-grey to pale olivaceous-grey, zonate, grey-olivaceous towards margins, colony centre with dots of pale greenish grey aerial mycelium, reverse iron-grey to leaden-grey, velvety to floccose, margin regular to undulate, narrow, glabrous, white, aerial mycelium abundant, covering the whole surface, floccose, flat, without exudates, not sporulating.
Specimen examined: South Africa, Western Cape Province, Jonkershoek Nature Reserve, isol. from fruiting bodies of Teratosphaeria proteae-arboreae (Teratosphaeriaceae) on leaves of Protea nitida [arborea] (Proteaceae), 4 Jan. 2007, P.W. Crous, CBS H-20427, holotype; ex-type culture CBS 125985 = CPC 13864.
Substrate and distribution: On fruiting bodies of Teratosphaeria proteae-arboreae on Protea nitida; South Africa.
Notes: The conidia in this new species are mainly fusiform and arranged in very long mostly unbranched chains, which are reminiscentof the genus Chalastospora (Simmons 2007), especially the species C. gossypii, representing an anamorph lineage in the Pleosporales (Crous et al. 2009a).
The structure of conidiogenous loci and hila is not very prominent; the cladosporioid scar structure characterised by a central dome and a periclinal rim is barely visible using light microscopy and with SEM it is also not very prominent. However, the species clearly clusters within the genus Cladosporium. It clusters as a sister to C. hillianum (Fig. 1, part a) and formed a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium flabelliforme described from Australia on Melaleuca cajuputi and introduced in this paper as a new species is morphologically somewhat similar but distinct in having longer and slightly narrower secondary ramoconidia [11–27 × (2–)2.5–3(–3.5) μm] with conidial chains being characteristically spread in a fan-like manner.
Cladosporium chubutense K. Schub., Gresl. & Crous, Persoonia 22: 116. 2009.
This species was treated in Schubert et al. (2009).
Cladosporium cladosporioides (Fresen.) G.A. de Vries, Contr. Knowl. Genus Cladosporium: 57. 1952. Figs 17, 18, 19. Basionym: Penicillium cladosporioides Fresen., Beitr. Mykol. 1: 22. 1850.
Fig. 17.
Cladosporium cladosporioides (CBS 112388). Macro- and micronematous conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 18.
Cladosporium cladosporioides (CBS 112388). A–F. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Fig. 19.
Cladosporium cladosporioides (CBS 112388). A. Overview of a colony containing running differentiated conidiophore-forming hyphae and many aerial hyphae. B. Scars on a conidiophore. C. Top view of a conidiophore with scars and an aerial structure. D. Branching patterns of aerial hyphae intermingled with spore-forming structures. E. Aerial hyphae, conidiophores and spores. F. Conidiophores sprouting from agar with all types of dispersion structures. G. Conidial chains. H. Detail of conidial chains and ornamentation showing irregularly reticulate structures or embossed stripes probably caused by diminishing turgor and shriveling of tender young conidia. I. Three secondary ramoconidia. J. Secondary ramoconidia J. Secondary ramoconidia and conidia on agar with some irregularly reticulate ornamentation. Scale bars = 2 (B–C, H), 5 (F–G, I–J), 10 (D–E), 50 (A) μm.
For additional synonyms see Dugan et al. (2004) and Schubert (2005b).
Mycelium immersed, rarely superficial; hyphae sparse, unbranched or sparingly branched, (1–)2–4(–5) μm wide, septate, septa occasionally darkened, without any swellings and constrictions, subhyaline, pale olivaceous-brown or pale brown, smooth to minutely verruculose or rough-walled, walls unthickened. Conidiophores solitary, macronematous or semimacronematous, sometimes micronematous, arising terminally from ascending hyphae or laterally from plagiotropous hyphae, straight to somewhat flexuous, narrowly cylindrical to cylindrical-oblong, sometimes filiform, non-nodulose, usually not geniculate-sinuous, occasionally once geniculate, 40–300(–350) × (2.5–)3–4(–5.5) μm, unbranched or occasionally branched, branches usually short, only as peg-like lateral outgrowth just below a septum, occasionally up to 60 μm, mostly in the upper third, pluriseptate, usually not constricted at septa, sometimes slightly constricted and one of the upper septa slightly darkened where ramoconidia are formed, pale to medium olivaceous-brown or brown, smooth to minutely verruculose or verruculose especially towards the base, walls unthickened or slightly thickened, occasionally slightly attenuated towards the apex, base sometimes swollen, up to 7 μm wide; micronematous conidiophores shorter, narrower, paler, unbranched, 9–150 × (1–)1.5–2.5(–3) μm wide. Conidiogenous cells integrated, usually terminal, sometimes intercalary with conidiogenous loci situated on small peg-like or denticle-like lateral outgrowths just below a septum, cylindrical-oblong, not geniculate, non-nodulose, (7–)16–38 μm long, with up to four loci crowded at the apex, subdenticulate to denticulate, protuberant, 1–2(–2.5) μm diam, central dome often not very conspicuous, flat, somewhat thickened and darkened-refractive. Ramoconidia seceding at one of the upper, somewhat darkened septa, straight to slightly curved, cylindrical-oblong, 15–50 × (2.5–)3–5 μm, with up to three septa, pale olivaceous-brown, concolorous with tips of conidiophores, smooth, base not cladosporioid, 2.5–4 μm wide, unthickened or slightly thickened, sometimes slightly refractive. Conidia numerous, catenate, in long branched chains, up to 10 conidia in the upper unbranched part, branching in all directions, small terminal conidia subglobose, obovoid, ovoid to limoniform, 3–6(–7) × (1.5–)2–2.5(–3) μm (av. ± SD: 4.7 ± 0.9 × 2.4 ± 0.3), aseptate, intercalary conidia limoniform, ellipsoid-ovoid, sometimes fusiform or subcylindrical, 5–12(–14.5) × (2–)2.5–3(–4) μm (av. ± SD: 8.1 ± 2.2 × 2.9 ± 0.3), aseptate, with up to 3(–4) distal hila, secondary ramoconidia ellipsoid, subcylindrical to cylindrical-oblong, (7–)10–33(–38) × (2–)2.5–4(–6) μm (av. ± SD: 19.4 ± 6.6 × 3.2 ± 0.5), 0(–1)-septate, occasionally with two septa, not constricted at septa, with up to four distal hila, subhyaline, pale brown or pale olivaceous-brown, smooth, under SEM smooth or surface with somewhat irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shriveling of tender young conidia, thin-walled, sometimes cell structure unusual, with a small cavity in the cells, hila conspicuous, subdenticulate to denticulate, 0.5–2(–2.5) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA grey-olivaceous to dull green or olivaceous-grey, reverse iron-grey, leaden-grey or olivaceous-black, velvety to floccose, margins grey-olivaceous to white, feathery, regular, aerial mycelium sparse, diffuse, or sometimes abundantly formed, dense, floccose-felty, low, forming mats, growth flat to low convex, usually without prominent exudates, occasionally with several small prominent exudates. Colonies on MEA grey-olivaceous to olivaceous or olivaceous-grey, pale olivaceous-grey or whitish due to aerial mycelium, olivaceous-black or olivaceous-buff at margins, reverse olivaceous-black or iron-grey, velvety to floccose, margins white to grey-olivaceous, glabrous to feathery, aerial mycelium sparse, scattered, diffuse to floccose, sometimes abundantly formed, covering almost the whole colony, floccose-felty, whitish, growth flat to effuse, somewhat radially furrowed, without prominent exudates. Colonies on OA grey-olivaceous, towards margins at first greenish olivaceous, then dull-green and again grey-olivaceous, sometimes white, reverse olivaceous-grey to leaden-grey, sometimes pale mouse-grey, velvety to floccose, margins narrow, glabrous, regular, aerial mycelium scattered to sometimes abundant, floccose or felty, loose to somewhat dense, growth flat, no prominent exudates; sporulation usually profuse on all media.
Specimens examined: Sine loco, deposited by C.L. Shear, stored as “C. herbarum”, CBS 132.29. Australia, New South Wales, Mullion Creek, S 33°06'48” E149°08'45”, isol. from Eucalyptus robertsonii ssp. hemisphaerica (Myrtaceae), 15 Jan. 2007, coll. B.A. Summerell, isol. P.W. Crous, NSW 134279, CPC 13667, 13669; Queensland, near Cairns, isol. from Eucalyptus sp. (Myrtaceae), 27 Aug. 2006, P.W. Crous, CPC 13235. Brazil, isol. from soil, CBS 101367. Denmark, isol. from cellulose powder, paint manufacturer, 2007, B. Andersen, BA 1692 = CPC 14293; isol. from soil, pea field, 2007, B. Andersen, BA 1691 = CPC 14292. France, Vallon, Pont d'Arc, isol. from twigs of an unidentified tree, 21 Aug. 2007, P.W. Crous, CPC 14271. Germany, isol. from indoor air, Ch. Trautmann, CBS H-20428, neotype of C. cladosporioides designated here, ex-type culture CBS 112388; isol. from Pisum sativum (Fabaceae), stored as C. cladosporioides f. pisicola, CBS 145.35 = MUCL 926; Essen, botanical garden, 51.45, 7.0167, isol. from leaves of Morus rubra (Moraceae), 2005, N. Ale-Agha, CPC 12214. India, isol. from Dalbergia sp. (Fabaceae), 3 Jan. 2004, coll. W. Gams, isol. P.W. Crous, CPC 11131; isol. from Eucalyptus sp., 3 Jan. 2004, coll. W. Gams, isol. P.W. Crous, CPC 11161. Indonesia, Tele, isol. from Eucalyptus sp., endophyte spots, spots after herbicide, 3 Jan. 2008, coll. M.J. Wingfield, isol. P.W. Crous, as “Neofusicoccum sp.”, CPC 15038. Israel, Jaffa, isol. from Gossypium seeds (Malvaceae), 1967, isol. by M. Gonen, CBS 674.82 = CBS 320.87 = ATCC 38026 = ATCC 200936 = IMI 126640, stored as “C. tenuissimum”. Japan, isol. from bamboo slats, probably authentic strain of C. multigeniculatum, CBS 122130 = ATCC 38012 = IFO 6539 = JCM 10684 = NBRC 6539. Slovenia, Češnjica near Ljubljana, isol. from a living mite inhabiting a strawberry leaf, 4 Apr. 2008, Vojko Škerlavaj, isol. by H.-J. Schroers, HJS 1069 = CPC 15167. South Africa, isol. from Pisum sativum, B.J. Dippenaar, stored as C. cladosporioides f. pisicola, CBS 143.35 = MUCL 10090; Barberton, Laeveld Coop, isol. from wheat, 1988, CPC 14009 = MRC 10150; Eastern Cape, Aiwal North, isol. from wheat, 1989, CPC 14019 = MRC 10813; Free State, Brandfort, isol. from wheat, 1989, CPC 14017 = MRC 10809, CPC 14018 = MRC 10810; Perdespan, isol. from wheat, 1988, CPC 14015 = MRC 10260; Winburg, isol. from wheat, 1989, CPC 14021 = MRC 10827; Gauteng, Pretoria, isol. from pawpaw, 1990, CPC 14024 = MRC 11280. South Korea, Chuncheon, N37°50'10” E127°32'01”, isol. from Celosia cristata (Amaranthaceae), 7 Oct. 2003, coll. H.-D. Shin, isol. P.W. Crous, CPC 11121; Suwon, N37°16'03” E126°59'16”, isol. from chasmothecia of Phyllactinia sp. (Erysiphales) on Fraxinus chinensis subsp. rhynchophylla (Oleaceae), 7 Nov. 2007, coll. H.-D. Shin, isol. P.W. Crous, CPC 14705; Chuncheon, N37°50'10” E127°32'01”, isol. from Phragmidium griseum on Rubus crataegifolius (Rosaceae), 20 Jul. 2004, coll. H.-D. Shin, isol. P.W. Crous, CPC 11398; Jinju, N35°11'24” E128°10'56”, isol. from Phytolacca americana (Phytolaccaceae), 15 Oct. 2003, coll. H.-D. Shin, isol. P.W. Crous, CPC 11122; Jinju, N35°11'24” E128°10'56”, isol. from Plectranthus sp. (Lamiaceae), 1 Jul. 2004, coll. H.-D. Shin, isol. P.W. Crous, CPC 11406; Chuncheon, N37°50'10” E127°32'01”, isol. from Ricinus communis (Euphorbiaceae), 7 Oct. 2003, coll. H.-D. Shin, isol. P.W. Crous, CPC 11119; Yangpyeong, N37°30'12” E127°41'55”, isol. from Rubus coreanus, 23 Jul. 2004, coll. H.-D. Shin, isol. P.W. Crous, CPC 11404; Hongcheon, N37°48'17” E127°51'13”, isol. from leaves of Stellaria aquatica (Caryophyllaceae), 6 Jun. 2005, coll. H.-D. Shin, isol. P.W. Crous, CPC 12187; Hoengseong, N37°32'09” E128°07'07”, isol. from Valeriana fauriei (Valerianaceae), 23 Jun. 2004, coll. H.-D. Shin, isol. P.W. Crous, CPC 11393; Jeongeup, N35°36'05” E126°51'25”, isol. from Vigna unguiculata [= V. sinensis] (Fabaceae), 29 Oct. 2003, coll. H.-D. Shin, isol. P.W. Crous, CPC 11123; Suwon, N37°16'03” E126°59'16”, isol. from Viola mandshurica (Violaceae), 14 Oct. 2003, coll. H.-D. Shin, isol. P.W. Crous, CPC 11120; Hongcheon, N37°48'17” E127°51'13”, isol. from Chenopodium ficifolium (Chenopodiaceae), 10 Mar. 2002, coll. H.-D. Shin, isol. P.W. Crous, CPC 10142. Thailand, Chiang Mai, Mushroom Research Centre, isol. from Areca sp. (Arecaceae), 20 Dec. 2006, coll. I. Hidayat, isol. P.W. Crous, CPC 13734. Uganda, Mubende, isol. from food, coffee leaf, 2000, coll. J.L. Sørensen, isol. B. Andersen, BA 1677 = CPC 14356. U.S.A., 2004, M. Blackwell, CPC 11684 = CBS 117483, as “C. gossypiicola”; California, isol. from Pisum sativum, as C. cladosporioides f. pisicola, CBS 144.35 = ATCC 11284 = IFO 6371 = IMI 049627; Laramie, isol. from food, mouldy pea, 2000, coll. J.L. Sørensen, isol. B. Andersen, BA 1676 = CPC 14355; Louisiana, Baton Rouge, isol. from Magnolia sp. (Magnoliaceae), 8 Sep. 2007, P.W. Crous, CPC 14244; isol. from pruned wood, 2006, K. Seifert, CPC 12852; Washington, isol. from spinach seed, Spinacia oleracea (Chenopodiaceae), 2003, L. du Toit, CBS 126341 = CPC 12763, CPC 12760, 12762, 12764; isol. from grape berry, F.M. Dugan, w99-175a sci 1 = CBS 113740; isol. from grape bud, F.M. Dugan, 113db sci 1 = CBS 113738; isol. from culm node of crested wheat grass, F.M. Dugan, wa2-00 sci 1 = CBS 113739.
Excluded strains within the C. cladosporioides complex (morphologically indistinguishable but phylogenetically distinct, indicated in Fig. 1 as C. cladosporioides s. lat. Lineages 1–4): Argentina, Chubut, Rio Pico, carnelian property, isol. from needles of Pinus ponderosa (Pinaceae), 2007, A. Greslebin, CPC 13978. Denmark, isol. from indoor building material, school, 2007, B. Andersen, BA 1695 = CPC 14296. Germany, isol. from leaves of Acer pseudoplatanus (Aceraceae), L. Pehl, ident. as C. tenuissimum by G.S. de Hoog, CBS 116744; isol. from wheat grain, Triticum sp. (Poaceae), 2007, B. Andersen, BA 1674 = CPC 14284; Bayern, isol. from lichens on leaves of Acer platanoides (Aceraceae), 2006, B. Heuchert, CPC 13220; Tübingen, botanical garden, isol. from Paeonia obovata (Paeoniaceae), Sep. 2006, P.W. Crous, CPC 13362. Netherlands, isol. from seed coat of Cirsium vulgare (Asteraceae), depos. by B.H. van Leeuwen, Jan. 1980, CBS 125.80; Millingerwaard, isol. from fruits of Sambucus nigra (Adoxaceae), 29 Aug. 2007, P.W. Crous, CPC 14238. New Zealand, Auckland, Auckland University campus, Oncoba spinosa (Salicaceae), 9 Jan. 2004, C.F. Hill, Hill 1076-2 = CPC 11664; Waikato, Karapiro, Gorton Road, isol. from imported buds of Prunus avium (Rosaceae), 6 Jan. 2008, J. Rennie, CPC 15457. South Africa, Western Cape Province, Jonkershoek Nature Reserve, isol. from Brunneosphaerella protearum (Mycosphaerellaceae) fruiting bodies, 30 Mar. 2007, P.W. Crous, CPC 13867. South Korea, Pocheon, National Arboretum, N37°45'04” E127°09'55”, isol. from Fatoua villosa (Moraceae), 18 Oct. 2002, coll. H.-D. Shin, isol. P.W. Crous, CPC 10150. U.K., Manchester, isol. from uredospores of Puccinia allii (Pucciniaceae), G.S. Taylor, CBS 306.84; Wales, Pembrokeshire, Skomer Island, isol. from Oberna uniflora [= Silene maritima] (Caryophyllaceae), 22 Aug. 2000, A. Aptroot, CBS 109082. U.S.A., Washington State, isol. from bing cherry fruits, R.G. Roberts, CBS 113746.
Substrates and distribution: On fading and decaying plant material, on living leaves as secondary invader, isolated from air, soil, foodstuffs, water-damaged building materials and numerous other materials; cosmopolitan.
Literature: Yamamoto (1959: 3), Ellis (1971: 319), Subramanian (1971: 285), Domsch et al. (1980: 202), Ellis & Ellis (1985: 290, 468), Wang & Zabel (1990), Braun (1998: 301), Ho et al. (1999: 121), Samson et al. (2000: 108), de Hoog et al. (2000: 583), Samson et al. (2001: 340), Park et al. (2004), Schubert & Braun (2004: 304), Heuchert et al. (2005: 46–47), Pasqualetti et al. (2005).
Notes: Type material of C. cladosporioides, cited by Fresenius (1850) for Penicillium cladosporioides, is not preserved in the Fresenius herbarium at the Senckenberg-Museum in Frankfurt. De Vries (1952) invalidly and erroneously lectotypified the species based on Bisby's dried “standard culture” [isol. fr. Arundo leaves, Bamboo Garden, Kew, 1943 (IMI 25324, 60507, 60509) = CBS 170.54] which, however, proved to belong to the C. herbarum complex. Recent unpublished phylogenetic studies showed this isolate to be conspecific with C. ramotenellum, a saprobic species that occurs more frequently than indicated in Schubert et al. (2007b). The designated neotype, which is a dried plate of a strain isolated from indoor air in Germany, originates from the same country as the authentic material described by Fresenius and agrees well with the description given by Ellis (1971). Together with numerous other isolates from various substrates and geographical regions it forms a well-supported clade (Figs 1, part c). However, C. cladosporioides is still paraphyletic representing a species complex since there are still numerous isolates listed under excluded strains, that are morphologically indistinguishable from C. cladosporioides s. str., but phylogenetically different, clustering apart in various subclades. They are indicated as C. cladosporioides s. lat. in Table 1 and Fig. 1 (part a). The phylogenetic differences between the lineages of C. cladosporioides and C. cladosporioides s. lat. are supported in both the ACT and TEF sequence alignments (distance analyses in TreeBASE).
The conidia in C. cladosporioides, which are among the most ubiquitous bioaerosols found in indoor and outdoor samples (Domsch et al. 1980, Mullins 2001, Park et al. 2004) are usually smooth. Strains with often asperulate or finely verruculose conidia as discussed in Yamamoto (1959), Ellis (1971), de Hoog et al. (2000) and Samson et al. (2000) proved to represent different, phylogenetically distinct species such as C. asperulatum and C. perangustum.
Cladosporium cladosporioides belongs to a species complex with broadly similar conidiophore and conidium morphology, and is close to C. tenuissimum and several foliicolous Cladosporium species, e.g., C. cucumerinum and C. vignae but, besides their pathogenicity to specific host plants, the latter two species differ in some additional aspects as discussed under these species. The two saprobic species C. cladosporioides and C. tenuissimum have often been misidentified and confused. However, the conidiophores in C. cladosporioides are mostly long stipes being usually neither geniculate nor nodulose, but with a single terminal conidiogenous cell. If the conidiogenous cells are formed intercalary, loci are formed as small denticles just below a septum or situated on small lateral outgrowths. In C. tenuissimum the conidiophores often possess a slightly swollen head-like apex and sometimes also have intercalary subnodulose or nodulose swellings, being quite apart from the apical cell and from each other. In intercalary conidiogenous cells the loci are often arranged at about the same level on the stalk, being quite apart from a septum, or the cells are slightly or distinctly geniculate, with loci situated on small lateral shoulders continuing growth sometimes at an angle of 45°. Compared to C. tenuissimum, conidial chains in C. cladosporioides are looser and often much longer in the terminal, unbranched part of the chain with up to 10 conidia. Secondary ramoconidia are somewhat longer in C. cladosporioides.
Several strains were isolated from Pisum sativum and stored at the CBS as C. cladosporioides f. pisicola (≡ Cladosporium pisicola), including CBS 144.35, which is an authentic culture of W.C. Snyder from 1935. The taxonomy and application of the name C. pisicola is, however, rather intricate and depends on typification. Cladosporium cladosporioides is common on the phyllosphere of living and faded or necrotic leaves, stems and pods of peas and often isolated. Snyder (1934) dealt with a plant pathogenic leaf, stem and pod spot disease and introduced the name C. pisicola. However, the latter name was based on heterogeneous elements, viz. cultures of C. cladosporioides and in vivo material with a morphologically distinct leaf spotting Cladosporium. This is not surprising since C. cladosporioides, as most other common saprobic Cladosporium spp., grow very easily in culture, more easily than plant pathogenic taxa. Authentic cultures deposited by Snyder, e.g. CBS 144.35, are, indeed, identical with C. cladosporioides, which has been proven during the course of the present molecular study. Thus, the decision by de Vries (1952) to consider C. pisicola a synonym of C. cladosporioides based on Snyder's authentic culture was correct. But the Cladosporium species causing distinct leaf spots on peas does not agree with the latter species. It is easily distinguishable by its distinctly sympodial (geniculate) conidiophores with terminal as well as intercalary conidiogenous cells and numerous, often crowded conidiogenous loci. Because the conidiophores of C. cladosporioides are rather uniform in vivo as well as in vitro, never changing from filiform, only with a terminal conidiogenous cell, to strongly geniculate with intercalary conidiogenous cells, the causal agent of the leaf spot disease and the cultures agreeing with C. cladosporioides has to be considered two distinct species. The application of the name C. pisicola depends on its typification. Several authentic cultures and samples of W.C. Snyder have been examined, but unfortunately all of them date back to 1935 and 1940 and cannot be considered as type collections since this species was already described in 1934. Therefore, a neotypification is necessary. We prefer to maintain the name C. pisicola for the leaf-spotting Cladosporium on peas by a neotypification with authentic in vivo material. Cultures of the true C. pisicola in this sense and sequence data are not yet available, so that the latter name can currently only be maintained as a distinct morphospecies. A detailed description of this species with a neotypification will be given in the planned monograph of the genus Cladosporium.
Cladosporium colocasiae Sawada, Trans. Nat. Hist. Soc. Taiwan 25: 125. 1916. Fig. 20.
Fig. 20.
Cladosporium colocasiae (A-D, G-H: CBS 386.64 and E-F, I: CBS 119542). A–D. Conidiophores and conidial chains. E–F. Intercalary conidiogenous cells with typical nodes and conidiogenous loci restricted to these swellings. G–H. Microcyclic conidiogenesis with conidia forming secondary conidiophores. I. Conidia. Scale bars = 10 μm.
= Cladosporium colocasiicola Sawada, Special Publ. Coll. Agric. Natl. Taiwan Univ. 8: 195. 1959, nom. inval., syn. nov.
Mycelium immersed and superficial; hyphae loosely branched, 1–4 μm wide, sometimes distinctly swollen, bulboid, about 10 μm wide, septate, not constricted at septa, subhyaline to pale olivaceous-brown, smooth to loosely verruculose, walls unthickened. Conidiophores macronematous, solitary, arising terminally and laterally from hyphae, erect, flexuous, cylindrical-oblong, nodose, with several nodes being quite apart from each other, occasionally branched, very long, up to 1 350 μm or even longer, 3–4(–5) μm wide, nodes multilateral, 5–8 μm diam, pluriseptate, not constricted at septa, pale to medium olivaceous-brown, smooth or sometimes appearing to be reticulate, walls somewhat thickened, about 0.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, nodose with a single node per cell, 15–70 μm long, loci restricted to swellings, usually 2–4 per node, 1–1.8(–2) μm diam. Ramoconidia occasionally formed. Conidia solitary or in short, unbranched or branched chains, more or less straight, broadly ellipsoid-subcylindrical to cylindrical, unbranched terminal conidia 9–16 × 5–7(–8) μm (av. ± SD: 12.5 ± 1.8 × 6.1 ± 0.7), 0–1-septate, catenate conidia 10.5–23(–30) × 5–8(–9) μm (av. ± SD: 17.5 ± 5.2 × 6.0 ± 1.0), 0–1(–2)-septate, septum median or somewhat in the upper or lower half or third, becoming sinuous with age, pale to medium brown, smooth to loosely verruculose or reticulate, walls unthickened or almost so, hila conspicuous, 1–1.8(–2) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis sometimes occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA attaining 56–76 mm diam after 14 d, grey-olivaceous to olivaceous or dull green, reverse olivaceous-black, velvety, pulvinate to floccose, with a narrow white or grey-olivaceous margin, regular to slightly undulate, somewhat feathery, aerial mycelium sparse, locally constricted to few areas, floccose, growth regular, flat to low convex, numerous small but not very conspicuous exudates formed, sporulation profuse. Colonies on MEA reaching 50–68 mm diam after 14 d, pale olivaceous-grey, grey-olivaceous to greenish olivaceous due to abundant sporulation, whitish to smoke-grey due to aerial mycelium, reverse olivaceous-grey to iron-grey, velvety, powdery to floccose or fluffy, with a white regular, glabrous or feathery, narrow margin, aerial mycelium abundantly formed, floccose to fluffy, covering large part of the colony surface, growth effuse, conical, radially furrowed and wrinkled with elevated colony centre, few small conspicuous exudates start to be formed, sporulating. Colonies on OA, 60–65 mm diam after 14 d, grey-olivaceous, whitish or pale olivaceous-grey due to floccose aerial mycelium arranged in tufts, spotted, reverse leaden-grey to iron-grey, pulvinate to floccose, margins grey-olivaceous, glabrous, regular, growth flat, without prominent exudates, sporulation profuse.
Specimens examined: Dominican Republic, intercepted at San Juan, on Colocasia esculenta (= C. antiquorum) (Araceae), 9 Jul. 1985, R. Barbosa, BPI 525147, as C. colocasiicola. Ethiopia, Kaffa Prov., Jimma, on Colocasia esculenta, 10 Nov. 1955, R.B. Stewart, BPI 426383, BPI 426385, NY. Fiji, isol. from Colocasia esculenta, June 2001, C.F. Hill, CBS 115191 = CPC 4323 = STE-U 4323. Japan, Kyoto, on Colocasia esculenta, 2 Oct. 1924, K. Togashi, BPI 426382; Riken, BioResource Centre, isol. from Colocasia esculenta, CBS 119542 = CPC 12726 = ICM 13264; Sendai, 25 Oct. 1918, A. Yasuda, BPI 426381. Puerto Rico, intercepted at San Juan, on Colocasia esculenta, 11 Mar. 1961, H.L. Rubin (BPI 426384). Taiwan, on Colocasia esculenta (= C. antiquorum), 2 June 1910, K. Sawada, PPMH, holotype; ex-type culture CBS 386.64 = ATCC 200944 = MUCL 10084.
Substrate and distribution: On Colocasia esculenta (Araceae); Africa (Ethiopia, Ghana, Guinea, Mauritius, Nigeria), Asia (Brunei, China, Hong Kong, India, Indonesia, Japan, Malaysia, Sabah, Sarawak, Nepal, Pakistan, South Korea, Taiwan), Australasia (American Samoa, Australia, Cook Islands, Federated States of Micronesia, Fiji, French Polynesia, Kiribati, Marshall Islands, New Caledonia, New Zealand, Niue, Papua New Guinea, Samoa, Solomon Islands, Tahiti, Tonga, Vanuatu), Europe (Portugal, Azores), North America (U.S.A.), West Indies (Barbados), Central & South America (Brazil, Dominican Republic, Puerto Rico).
Literature: Bugnicourt (1958), Ellis (1971: 312), Matsushima (1975: 34), David (1988), Ho et al. (1999: 123).
Notes: Type material of this species is in very poor condition and was, therefore, not re-examined. Cladosporium colocasiicola, also recorded on leaves of Colocasia esculenta, was invalidly published, since the author failed to provide a Latin diagnosis. Although type material could not be traced and re-examined, this species is reduced to synonymy with C. colocasiae since the original diagnosis and illustration are almost identical with the latter species.
Cladosporium herbarum, C. oxysporum and C. variabile are morphologically close to C. colocasiae by also possessing nodulose to nodose conidiophores. However, the cosmopolitan C. herbarum, which occurs on numerous substrates, deviates in having verruculose conidia; the conidia of the saprobic C. oxysporum are subglobose, ovoid, limoniform or ellipsoid, narrower, 3–6 μm wide, and 0–1(–2)-septate; and the foliicolous C. variabile, causing leaf spots on spinach, differs in having verruculose to verrucose, wider and usually longer conidia, (6.5–)10–45(–55) × (5–)7–14(–17) μm (in vivo), and wider conidiogenous loci and hila, (1–)2–3(–3.5) μm diam (Schubert 2005b, Schubert et al. 2007b).
Ho et al. (1999) examined C. colocasiae in culture and published a first detailed description of its features in vitro, recording the conidiophores as being much longer than on the natural substratum. Matsushima (1980) mentioned Eucalyptus sp. and Psidium guajava as additional hosts, but these records probably refer to one of the superficially similar, saprobic species discussed above. Zhang et al. (2003) treat C. colocasiicola as a separate species and cite a record on Nelumbo nucifera, which is, however, very doubtful.
Cladosporium colocasiae clusters as a well-supported lineage in the middle of C. tenuissimum (Fig. 1, part c) but keeps its identity as a distinct lineage for both TEF and ACT, clustering between lineages of C. tenuissimum (distance analyses in TreeBASE; also see notes on C. tenuissimum). Although the species is nestled within C. tenuissimum, all isolates of the species always cluster together and it is possible that the gene regions sequenced in this study do not provide sufficient resolution to move the species out of the clades formed by intraspecific sequence variation of C. tenuissimum.
The strain from New Zealand, isolated from Apium graveolens, that clusters with the other isolates of C. colocasiae is very probably a contamination of the latter species. Cladosporium colocasiae is so far known to be host-specific inducing leaf spots only on Colocasia esculenta.
Cladosporium colombiae K. Schub. & Crous, Persoonia 22: 120. 2009.
This species was described and illustrated in Schubert et al. (2009).
Cladosporium cucumerinum Ellis & Arthur, Bull. Agric. Exp. Sta., Indiana 19: 9. 1889. Fig. 21.
Fig. 21.
Cladosporium cucumerinum (CBS 171.52). A–G. Conidiophores and conidial chains. Scale bars = 10 μm.
= Cladosporium cucumerinum var. europaeum Bubák, in herb., syn. nov.
For further synonyms see Dugan et al. (2004) and Schubert (2005b).
Mycelium immersed and superficial; hyphae sparingly branched, 0.5–4 μm wide, later up to 8 μm wide, septate, with age constricted at septa, subhyaline to very pale brown or pale olivaceous-brown, later olivaceous-brown, smooth or almost so, walls unthickened, broader hyphae with slightly thickened walls. Conidiophores solitary, macro- and micronematous, arising terminally from ascending and laterally from plagiotropous hyphae, erect, straight to slightly flexuous, macronematous conidiophores cylindrical-oblong, sometimes once geniculate-sinuous with conidiogenous loci situated laterally on shoulders, non-nodulose, usually unbranched on SNA, sometimes once branched towards the apex (often branched on PDA according to McKemy & Morgan-Jones, 1992), up to 350 μm long, 3–5(–5.5) μm wide, pluriseptate, not constricted at septa, often more densely septate towards the base, pale brown, later medium brown, smooth, walls unthickened or slightly thickened, up to 0.5 μm wide; micronematous conidiophores straight to slightly flexuous, filiform, narrower, 1–2 μm wide, septate, smooth. Conidiogenous cells integrated, usually terminal, filiform to cylindrical-oblong, sometimes once geniculate, up to 47 μm long, 1–2(–3) loci, protuberant, subdenticulate, 1.5–2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, 24–43 × 3–3.5 μm, 0–2-septate, smooth, base truncate, 2.5–3 μm wide, unthickened or only slightly so. Conidia catenate, in long loosely branched chains, often dichotomously branched, up to 10(–14) conidia in the terminal unbranched part of the chain, straight, small terminal conidia obovoid to ellipsoid-ovoid, subglobose, 4–8(–10) × (1–)1.5–3(–3.5) μm (av. ± SD: 6.4 ± 1.2 × 2.2 ± 0.5), aseptate, hila 0.5–0.8(–1) μm diam, intercalary conidia ellipsoid-ovoid to fusiform or subcylindrical, sometimes limoniform, 7–15(–17) × (2–)2.5–4(–5) μm (av. ± SD: 10.9 ± 2.7 × 3.2 ± 0.6), 0(–1)-septate, hila 0.8–1.2(–1.5) μm, secondary ramoconidia ellipsoid, fusiform to cylindrical-oblong, (9.5–)14–30(–40) × (2.5–)3–5(–5.5) μm (av. ± SD: 21.8 ± 5.8 × 3.8 ± 0.7), 0–2(–3)-septate, with age sometimes slightly constricted at septa, septum median or in the upper or lower third, pale brown or very pale brown, later becoming medium brown, smooth, walls unthickened, slightly attenuated towards apex and base, cells sometimes with small cavities, hila 1.5–2.2(–2.5) μm diam, conspicuous, subdenticulate, somewhat thickened and darkened-refractive; conidia often germinating, forming micronematous conidiophores and conidia.
Culture characteristics: Colonies on PDA attaining 50–78 mm diam after 14 d, grey-olivaceous to olivaceous-grey or olivaceous, sometimes dull green towards margins and somewhat zonate, reverse olivaceous-black, velvety to floccose or felty, margin narrow to broad, colourless to white, regular, somewhat feathery, aerial mycelium absent, sparse or sometimes abundantly formed at few parts of the colony or covering almost the whole colony surface, white, loose to dense, woolly, floccose, growth flat, regular, without prominent exudates, sporulation profuse. Colonies on MEA reaching 36–73 mm diam after 14 d, grey-olivaceous to smoke-grey, whitish due to aerial mycelium, reverse iron-grey, velvety to fluffy, margin white, glabrous, regular, aerial mycelium dense, forming mats, covering large parts of the colony surface, growth flat, somewhat wrinkled and folded in colony centre, sometimes with several prominent exudates, sporulation profuse. Colonies on OA attaining 54–79 mm diam after 14 d, pale olivaceous-grey, grey-olivaceous to smoke-grey or whitish, grey-olivaceous or iron-grey at margins, reverse leaden-grey to iron-grey, velvety to floccose, margin regular, colourless to white, glabrous, aerial mycelium absent to abundantly formed, covering most of the colony surface, growth flat, regular, without prominent exudates, sporulation profuse.
Specimens examined: Sine loco, isol. from fruits of Cucumis sativus (Cucurbitaceae), W.W. Gilbert, CBS 108.23; isol. and ident. by J. Westerdijk, 1908, CBS 109.08. Netherlands, isol. from fruits of Cucumis sativus, CBS H-20429, epitype designated here; ex-type culture CBS 171.52 = MUCL 10092; isol. by G.A. de Vries, CBS 158.51 = ATCC 11279 = IFO 6370 = IMI 049628 = VKM F-817; Amsterdam, G.W. van der Helm, isol. by G.A. de Vries, Jan. 1951, CBS 173.54; Berkel, G.W. van der Helm, isol. by G.A. de Vries, CBS 174.54, CBS 175.54; Naaldwijk, isol. by S. Dudok de Wit, CBS 123.44; Jan. 1951, G.W. van der Helm, isol. by G.A. de Vries, CBS 176.54; Sloten, coll. by G.W. van der Helm, isol. by G.A. de Vries, CBS 172.54. U.S.A., Pennsylvania, isol. from painted floor by M.H. Downing, 1951, CBS 174.62 = ATCC 16022 = ATHUM 2861 = CECT 2110 = IFO 31006 = IMI 045534 = MUCL 19019 = QM 489 = VTT D-92188; New York, Geneva, on Cucumis sativus, J.C. Arthur, NY, holotype.
Substrates and distribution: on leaves, stems and fruits of Cucurbitaceae, especially Cucumis sativus, C. melo and Cucurbita pepo, other host genera Citrullus, Lagenaria, Luffa, Momordica, Sechium; cosmopolitan.
Literature: Saccardo (1892: 601), Lindau (1907: 830, 1910: 797), Ferraris (1912: 349), Gonzáles-Fragoso (1927: 206), de Vries (1952: 62), Ellis (1971: 318), Ellis & Holliday (1972), Brandenburger (1985: 403), Ellis & Ellis (1985: 339), von Arx (1987: 193), McKemy & Morgan-Jones (1992), Ho et al. (1999: 125), Zhang et al. (2003: 80–82).
Notes: Original material of the herbarium name C. cucumerinum var. europaeum Bubák agrees very well with the species concept of C. cucumerinum and is therefore reduced to synonymy with the latter species. In de Vries (1952) and McKemy & Morgan-Jones (1992) “Macrosporium cucumerinum Ellis & Everh., Hedwigia 7: 49. 1896.” is cited as a synonym of C. cucumerinum, but in Hedwigia, vol. 7 (published in 1868, not in 1896) there is no reference to this name. In Index Fungorum the original citation of Macrosporium cucumerinum is given as “Proc. Acad. Nat. Sci. Philadelphia 1895: 440. 1895.” and “Alternaria cucumerina (Ellis & Everh.) A. Elliott, Amer. J. Bot. 4: 472. 1917.” is given as current name. De Vries (1952) listed Chloridium polysporum (≡ Acladium polysporum) as an additional synonym and stressed that this name antedated C. cucumerinum. McKemy & Morgan-Jones (1992) discussed this “synonymy” and stated that the two species are not identical and do not even belong in the same genus. Hughes (1958), who examined type material of Wallroth's species, reduced A. polysporum to synonymy with Botrytis cinerea.
Cladosporium cucumerinum, belonging to a species complex with broadly similar conidiophore and conidium morphology, is morphologically close to C. cladosporioides and C. vignae. Besides very characteristic symptoms, its pathogenicity to several Cucurbitaceae and its immersed hyphae often possessing a mucoid layer, C. cucumerinum is distinguished from C. vignae in having mostly longer conidiophores, ramoconidia and somewhat longer and wider secondary ramoconidia. Cladosporium cladosporioides, which is saprobic and a secondary invader of diseased plant tissue, has usually narrower, monopodially proliferating, non-geniculate conidiophores in vivo and somewhat narrower conidia in vivo and in vitro. Furthermore, C. cucumerinum and C. cladosporioides are distinguished in vitro by colony appearance, particularly colour, growth rates and degree of ramification of conidiophores. The species clusters as a sister to C. subuliforme (Fig. 1, part c) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium cucumerinum, the causal organism of crown blight and scab or gummosis disease is widespread and especially occurs on Citrullus lanatus, Cucumis melo, C. sativus and Cucurbita pepo. Records from other members of the Cucurbitaceae belong very probably to this species and are, therefore, listed under “substrates and distribution”. Roberts et al. (1986) examined the internal mycoflora of achenes of Helianthus annuus (Asteraceae) and reported C. cucumerinum as isolated from developing sunflower seeds. Morphological data and illustrations have not been provided, and cultures could not be traced, so that a verification of the identity of the fungus concerned was not possible. Hasija (1967) described this species from India on Solanum tuberosum (Solanaceae). In Korea, a Cladosporium species was isolated from leaves of Solanum melongena cultivated in a greenhouse, and identified as C. cucumerinum by Kwon et al. (1999). They carried out inoculation experiments and reported it to cause symptoms in seedlings of watermelon, cucumber, oriental melon and pumpkins. Kwon et al. (2000) reported it to cause a black scab disease on sword bean (Canavalia ensiformis = C. gladiata, Fabaceae) in greenhouses in Korea. Mendes et al. (1998) listed Capsicum annuum as a further host species. These records indicate that C. cucumerinum is possibly not confined to hosts in the Cucurbitaceae. However, molecular examinations of strains from plants other than Cucumis sativus are necessary to confirm the host range of this species. The isolate from a painted floor in the U.S.A. very probably resulted due to contamination with C. cucumerinum. The species was originally described from North America but an epitypification with European material is justified due to its pathogenicity to several hosts of cucurbits and its cosmopolitan distribution.
The original diagnosis of Cladosporium dufourii described on decaying fruits of cucurbits from France is very brief: “Dense caespitosum, filamentis ramosis, geniculatis, septatis, olivaceis, e macula circulari atrocaerulea orientibus. Sporidiis rotundis vel oblongis, saepe didymis.” Since type material was not available, it remains unclear whether this species is identical with C. cucumerinum.
Cladosporium delicatulum Cooke, Grevillea 5(33): 17. 1876. Figs 22, 23, 24, 25.
Fig. 22.
Cladosporium delicatulum (K 121551, holotype). Conidiophores and conidia in vivo. Scale bar = 10 μm. U. Braun del.
Fig. 23.
Cladosporium delicatulum (CBS H-20430, reference material). Conidiophores and conidia in vivo. Scale bar = 10 μm.
Fig. 24.
Cladosporium delicatulum (CBS 126344). Macro- and micronematous conidiophores and conidial chains in vitro. Scale bar = 10 μm.
Fig. 25.
Cladosporium delicatulum (CBS 126344). A–G. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
= Cladosporium fasciculatum f. scirpi-lacustris Roum., Fungi Sel. Gall. Exs., Fasc. 17, No. 1688. 1881, syn. nov.
= Cladosporium fasciculatum var. densum Ravenel, in Ravenel & Cooke, Fungi Amer. Exs., Cent. VII, No. 602. 1882, nom. nud., syn. nov.
= Cladosporium tuberum Cooke, Grevillea 12(61): 31. 1883, syn. nov.
Mycelium immersed, rarely superficial; hyphae unbranched or sparingly branched, (0.5–)1–3(–4) μm wide, septate, without swellings and constrictions, subhyaline to pale olivaceous or pale olivaceous-brown, smooth to minutely verruculose, sometimes loosely verrucose. Conidiophores macronematous and micronematous, solitary, arising terminally and laterally from hyphae, erect, straight to somewhat flexuous, cylindrical-oblong, non-nodulose, sometimes slightly geniculate towards the apex, unbranched, occasionally branched, once or several times, often as short peg-like prolongations, 50–165(–200) × 3–4.5(–5) μm, 2–4(–7)-septate, sometimes attenuated at septa, pale olivaceous to pale medium olivaceous-brown, smooth, sometimes loosely minutely verruculose at the base, walls unthickened or almost so, about 0.5 μm wide, sometimes slightly attenuated towards the apex, up to 5.5 μm wide at the base; micronematous conidiophores narrower and pale olivaceous, 19–75(–100) × (1.5–)2–2.5 μm. Conidiogenous cells integrated, terminal, sometimes intercalary, situated on small peg-like prolongations, cylindrical-oblong, sometimes geniculate at or towards the apex, non-nodulose, occasionally the whole cell inflated in shape like a secondary ramoconidium, 11–37 μm long, with (1–)2–3(–4) apical loci, crowded at the apex, conspicuous, subdenticulate to denticulate, sometimes situated on small lateral outgrowths, quite broad, truncate, rim and dome not distinctly visible, 1.5–2.2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 13–46 × 2.5–4(–5) μm, 0–1(–2)-septate, sometimes distinctly constricted at the median septum, base broadly truncate, 2–3 μm wide, neither thickened nor darkened-refractive. Conidia numerous, in densely branched chains, branching in all directions, up to four conidia in the terminal unbranched part of the chain, small terminal conidia obovoid, subglobose or globose, 2.5–4.5(–6) × (1.5–)2–2.5(–3.5) μm (av. ± SD: 3.7 ± 0.8 × 2.4 ± 0.4), aseptate, apex rounded, sometimes irregular due to additional lateral hila, intercalary conidia limoniform to ellipsoid-ovoid or sometimes irregular in outline due to lateral hila, 4–13(–17.5) × 2.5–3.5(–4) μm (av. ± SD: 7.8 ± 3.0 × 3.0 ± 0.4), 0–1-septate, attenuated towards apex and base, with 1–4(–6) distal hila, secondary ramoconidia ellipsoid-ovoid to subcylindrical or cylindrical, (6–)8–23.5(–31) × (2.5–)3–4.5(–5) μm (av. ± SD: 15.6 ± 5.4 × 3.6 ± 0.5), 0–1(–2)-septate, very rarely 3-septate, not constricted at septa, pale olivaceous to pale olivaceous-brown, smooth or almost so, walls unthickened, often only slightly attenuated towards apex and base, with (1–)2–4(–5) distal hila, hila conspicuous, subdenticulate or denticulate, 0.5–2.2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 60–78 mm diam after 14 d, olivaceous-grey, grey-olivaceous to olivaceous and olivaceous-black, reverse olivaceous-black, floccose to villose, margins grey-olivaceous, feathery, regular, aerial mycelium scattered to abundant, covering almost the whole colony surface, floccose to villose, low to rarely high, growth flat, without prominent exudates, sporulation sparse. Colonies on MEA reaching 67–76 mm diam after 14 d, smoke-grey to pale olivaceous-grey, olivaceous-grey or glaucous-grey at margins, reverse olivaceous-grey, floccose, fluffy, margins white, glabrous to feathery, regular, aerial mycelium abundant, covering the whole colony surface, floccose to fluffy, growth flat, radially furrowed and wrinkled in colony centre, without prominent exudates, sporulation sparse or absent. Colonies on OA reaching 55–74 mm diam after 14 d, smoke-grey to pale olivaceous-grey, grey-olivaceous or olivaceous due to abundant sporulation, reverse pale greenish grey to olivaceous-grey, velvety to floccose, margins regular, glabrous, narrow, colourless, aerial mycelium sparse to abundant, covering the whole surface, floccose, loose to dense, low, growth flat, without prominent exudates, sporulation sparse to profuse.
Specimens examined: Collections in vitro: Denmark, isol. from indoor air, 2007, B. Andersen, BA 1679 = CPC 14285, BA 1680 = CPC 14286, BA 1681 = CBS 126342 = CPC 14287; isol. from building material, school, 2007, B. Andersen, BA 1698 = CBS 126343 = CPC 14299; isol. from building material, 2007, B. Andersen, BA 1683 = CPC 14289; Broenshoej, isol. from indoor air, control room, 2000, B. Andersen, BA 1724 = CPC 14363; indoor air sample, in cup board, water damaged room, 2000, B. Andersen, BA 1718 = CPC 14360; beach near Copenhagen, isol. from sea weed, 2007, B. Andersen, BA 1706 = CPC 14307; Valleroed, isol. from dust, school, 2000, B. Andersen, BA 1740 = CPC 14372. Germany, Bayern, München, park of castle Nymphenburg, isol. from Puccinia bromina ssp. symphyti-bromarum var. paucispora, Jul. 2006, K. Schubert, CPC 13148; Sachsen-Anhalt, Halle (Saale), Robert-Franz-Ring, isol. from leaves of Tilia cordata (Tiliaceae), 2 Aug. 2004, K. Schubert, CBS H-20430, CBS 126344 = CPC 11389, reference strain of C. delicatulum. Collections in vivo: France, Lyon, on dead stems of Schoenoplectus lacustris (= Scirpus lacustris) (Cyperaceae), 1880, J. Therry, Roumeguère, Fungi Sel. Gall. Exs. 1688, HBG, and Thüm., Mycoth. Univ. 1767, HBG, HAL, syntypes of C. fasciculatum f. scirpi-lacustris. India, on dead leaves (litter), Colonel Hobsen, No. 23, K 121551, holotype of C. delicatulum; Gorakhpur, on necrotic patches on faded leaves of Dianthus barbatus (Caryophyllaceae), 23 Mar. 1972, Y.N. Srivastava, No. 2, IMI 212469 (originally deposited as C. cladosporioides). U.S.A., California, Marin County, San Rafael, on leaves of Hedera helix (Araliaceae), as secondary invader, 11 Nov. 1935, L. Bonar, Anonymous, Calif. Fungi 427, NY; South Carolina, Aiken, on faded and necrotic leaves of Euonymus japonicus (Celastraceae), Ravenel, Ravenel & Cooke, Fungi Amer. Exs. 602, BPI 426554, syntype of C. fasciculatum var. densum; on tubers of Ipomoea batatas (Convolvulaceae), Ravenel, Ravenel & Cooke, Fungi Amer. Exs. 600, K, lectotype of C. tuberum designated here. Uruguay, on dead pods of Sesbania virgata [= S. marginata] (Fabaceae), June 1932, S. José, Herter, Plantae Urug. Exs. 1496, HBG.
Substrates and distribution: Isolated from air, building material, dust, plant material, sea weed; Europe (Denmark, Germany). Saprobic on dead leaves, fruits, stems, tubers, or occurring as secondary invader on necrotic lesions caused by other fungi in vivo, widely distributed, Asia (India), Europe (France, Germany), North America (U.S.A.), South America (Uruguay).
Notes: Cladosporium delicatulum is the oldest name for a well characterised, widely distributed saprobic species. Type material of this species originating from India, Asia is quite sparse. Since all strains of C. delicatulum included in this study are from Europe we postpone a formal epitypification until Asian cultures will be available. However, the German strain from Tilia cordata can serve as reference strain to fix the application of C. delicatulum and agrees well with the Indian type material (see Fig. 23). The morphology in vitro is very uniform, and clearly different from the closely allied C. inversicolor, which deviates in having longer conidial chains, longer small terminal and intercalary conidia, wider intercalary conidia and secondary ramoconidia, longer ramoconidia with a broader base, with conidia being smooth to loosely verruculose or irregularly rugose. From C. cladosporioides it is distinct due to 0–1-septate intercalary conidia and secondary ramoconidia, only few conidia in the terminal unbranched part of conidial chains, shorter often slightly geniculate conidiophores and shorter secondary ramoconidia. Material in vivo is characterised by having somewhat wider conidiophores (10–200 × 3–7 μm) which are more frequently geniculate-sinuous and conidia that are 4–25(–30) × 2.5–6(–7) μm, and 0–2(–3)-septate (see Figs 22, 23, 24, 25). The species clusters as a sister to C. cladosporioides s. lat. (Fig. 1, part a) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium exasperatum Bensch, Summerell, Crous & U. Braun, sp. nov. MycoBank MB517076. Figs 26, 27, 28.
Fig. 26.
Cladosporium exasperatum (CBS 125986). Macro- and micronematous conidiophores, mycelium and conidial chains. Scale bar = 10 μm.
Fig. 27.
Cladosporium exasperatum (CBS 125986). A–C, E–H. Macronematous conidiophores and conidial chains. D. Ramoconidium seceding at the tip of a conidiophore. Scale bars = 10 μm.
Fig. 28.
Cladosporium exasperatum (CBS 125986). A. Ornamented conidia on aerial structures. Note the small scars with an ornamentation free zone. B. Secondary ramoconidium with a scar and aerial structures. C. Scars on a conidiophore. Note the smooth surface of the conidiophores. D. Ornamented secondary ramoconidium on a conidiophore with visible line of delineation. E. Septate aerial structure with conidiophores, conidia and scars as background. F. Two ornamented globose conidia adhered to an aerial structure showing an irregularly reticulate surface. G. Segmented differentiated hyphae on the agar surface giving rise to numerous conidiophores, conidiophore initials and aerial structures. H. Detail of differentiated substrate hyphae. I. Segmented differentiated hyphae in initials. Scale bars = 2 (A), 5 (B–F, H), 10 (G, I) μm.
Etymology: Name refers to the surface ornamentation of conidia.
Cladosporii acalyphae aliquam simile, sed conidiophoris brevioribus, ad 100 μm longis, locis conidiogenis et hilis angustioribus, 0.5–1.5 μm diam, et tamen conidiis terminalibus non globosis, angustioribus, 3–4.5 μm latis internoscitur. Differt a Cladosporio pini-ponderosae conidiophoris brevioribus et angustioribus, 15–100 × (2–)2.5–4 μm, locis conidiogenis et hilis angustioribus.
Mycelium immersed and superficial; hyphae loosely branched, (1–)1.5–4 μm wide, septate, not constricted at septa, subhyaline to pale or medium olivaceous-brown, smooth to verrucose or irregularly rough-walled, walls unthickened or almost so, sometimes irregular in outline due to swellings, occasionally swollen at the base of conidiophores, up to 6 μm wide. Conidiophores macro- and semimacronematous, solitary, arising laterally or terminally from hyphae, erect, straight to slightly flexuous, cylindrical-oblong, sometimes once, occasionally slightly to distinctly twice geniculate-sinuous below the apex or on a lower level, non-nodulose, unbranched or once branched, 15–100 × (2–)2.5–4 μm, septate, not constricted at septa, pale to usually medium olivaceous-brown, sometimes dark olivaceous-brown, asperulate or irregularly rough-walled, walls unthickened or only very slightly thickened, occasionally slightly attenuated towards the apex. Conidiogenous cells integrated, terminal, cylindrical-oblong, 11–40 μm long, sometimes once, rarely twice geniculate-sinuous, with loci situated on small lateral shoulders, with a single or up to 3(–4) apical loci, conspicuous, subdenticulate to denticulate, 1–1.5 μm diam, periclinal rim and central dome clearly visible, somewhat thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 19–40 × 2.8–4 μm, 0–2-septate, concolorous with tips of conidiophores, smooth, base not cladosporioid, broadly truncate, 2.8–4 μm wide, unthickened and not darkened. Conidia catenate, in branched chains, 2–6 conidia in the unbranched terminal part of the chain, dichotomously branched or branching in all directions, small terminal conidia obovoid to ellipsoid-ovoid, sometimes subglobose, 4–9 × 3–4.5 μm (av. ± SD: 6.7 ± 1.6 × 3.9 ± 0.5), aseptate, apex usually rounded, intercalary conidia ellipsoid-ovoid to fusiform, 7–15 × 3–4.5 μm (av. ± SD: 10.5 ± 2.6 × 3.9 ± 0.4), 0(–1)-septate, not constricted, with 1–2 distal hila, secondary ramoconidia ellipsoid, fusiform to subcylindrical, 9.5–30(–37) × (2.5–)3.5–4.5(–5) μm (av. ± SD: 19.2 ± 6.2 × 3.9 ± 0.6), 0–2-septate, not constricted, sometimes slightly swollen or wider at septa, septa median, somewhat in the upper half or occasionally in the lower third, pale olivaceous to often medium or dark olivaceous-brown, slightly to distinctly irregularly verruculose-rugose (LM), surface with irregularly reticulate structure or embossed stripes under SEM probably caused by diminishing turgor and shriveling of tender conidia, walls thickened, occasionally distinctly constricted in the middle, attenuated towards apex and base, with 1–4 distal hila, hila conspicuous, subdenticulate to denticulate, 0.5–1.5 μm diam, more or less thickened and darkened-refractive; microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA attaining 26–38 mm diam after 14 d, zonate, centre olivaceous-grey, then grey-olivaceous, iron-grey and dull green, reverse iron-grey to dull green, floccose to fluffy, margin regular to undulate, white, glabrous to feathery, aerial mycelium floccose to fluffy, loose to dense, covering large areas of the colony, growth effuse, with elevated colony centre, without prominent exudates, sporulation profuse. Colonies on MEA attaining 12–15 mm diam after 14 d, surface and reverse olivaceous-grey, floccose, margins crenate, very narrow, white, glabrous, aerial mycelium abundant, floccose, dense, covering the whole surface, growth convex but radially furrowed and wrinkled in colony centre, no exudates, sporulating. Colonies on OA reaching 39–54 mm diam after 14 d, olivaceous-grey to grey-olivaceous due to abundant sporulation, reverse olivaceous-grey to pale greenish grey, velvety to floccose, margins regular, colourless to white, glabrous, aerial mycelium abundant, floccose to fluffy, loose to dense, covering large parts of the colony surface, growth flat, without exudates.
Specimen examined: Australia, Northern Territory, Edith Falls, S 14°05'20” E 132°05'12”, isol. from Eucalyptus tintinnans (Myrtaceae), 23 Sep. 2007, coll. B.A. Summerell, isol. P.W. Crous, CBS H-20431, holotype; ex-type culture CBS 125986 = CPC 14638.
Substrate and distribution: On Eucalyptus tintinnans; Australia.
Notes: The conidia of C. exasperatum are characterised by having a unique verruculose-rugose surface ornamentation. Within the Cladosporium cladosporioides complex there are only few species with a somewhat similar surface ornamentation. Cladosporium pini-ponderosae, which was recorded on pine needles of Pinus ponderosa in Argentina possesses longer and somewhat wider, thick-walled conidiophores, 14–190 × (2.5–)3.5–5.5 μm and wider conidiogenous loci and hila, 0.5–2.5 μm diam (Schubert et al. 2009). Cladosporium acalyphae, described on Acalypha australis from South Korea also forms much longer conidiophores (up to 430 μm long), has globose or subglobose, wider small terminal conidia, 4.5–9 × 4.5–6 μm, and somewhat wider conidiogenous loci and hila, and in C. verrucocladosporioides surface ornamentation of conidia is even more pronounced with coarse verrucae being up to 1 μm high and walls sometimes seemingly detaching and appearing to be distinctly thick-walled. Besides these morphological features C. exasperatum forms very slow growing small colonies on all media. The species clusters as a sister to C. scabrellum (Fig. 1, part a) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium exile Bensch, Glawe, Crous & U. Braun, sp. nov. MycoBank MB517077. Figs 29, 30, 31.
Fig. 29.
Cladosporium exile (CBS 125987). Macro- and micronematous conidiophores, mycelium sometimes forming ropes and conidial chains. Scale bar = 10 μm.
Fig. 30.
Cladosporium exile (CBS 125987). A–G. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Fig. 31.
Cladosporium exile (CBS 125987). A. Conidia and secondary ramoconidia with reticulate ornamentation, near long aerial hyphae or conidiophores. B. CryoSEM of different types of conidia on aerial structures. Note a remarkable pattern of blastoconidium formation (backwards) (arrow). C. Numerous hyphae and conidiophores in this overview of a colony of the fungus. D. Hyphae on the agar surface. E. Conidia and aerial structures. F. Detail of conidia and scars. Scale bars = 2 (F), 5 (A–B), 10 (E), 50 (C–D) μm.
Etymology: Name refers to the narrow habit of conidiophores and conidia.
Cladosporio tenello aliquam simile, sed conidiis angustioribus, 2–3.5(–4) μm latis, locis conidiogenis et hilis leniter latioribus, 0.5–2 μm latis distinguitur. Differt a Cladosporio cladosporioide conidiophoris et conidiis saepe asperulatis vel minute verruculosis, ramoconidiis secundariis brevioribus et leniter angustioribus, 0–1(–3)-septatis.
Mycelium immersed and superficial; hyphae sparingly branched, 2–4 μm wide, septate, sometimes constricted at septa, sterile hyphae subhyaline, fertile hyphae pale or medium olivaceous-brown, smooth to minutely verruculose or irregularly rough-walled where conidiophores are formed, walls unthickened to slightly thickened, sometimes forming ropes or swollen at the base of conidiophores, up to 7 μm wide. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from hyphae, erect or ascending, straight to flexuous, cylindrical-oblong, slightly geniculate towards the apex, sometimes subnodulose, unbranched or sometimes once, occasionally twice branched, several long conidiophores up to 305 μm, but mostly shorter, 6–100 μm long, (2–)3–4(–5) μm wide, sometimes wider or even swollen at the base, up to 8 μm wide, pluriseptate, mostly 0–4 septa, long conidiophores with up to nine septa, sometimes slightly constricted at septa, pale brown to medium olivaceous-brown, sometimes paler towards the base, smooth or almost so to minutely verruculose or irregularly rough-walled, walls unthickened or slightly thickened, about 0.5 μm wide. Micronematous conidiophores narrower, shorter and paler, with 1–2 apical loci. Conidiogenous cells integrated, terminal, sometimes intercalary, cylindrical-oblong, sometimes geniculate-sinuous, occasionally subnodulose, 6–34 μm long, with up to six loci per cell, crowded at or towards the apex, sometimes loci situated on small lateral shoulders, subdenticulate to denticulate, (0.7–)1–2 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 17–41 × 2.5–4(–5) μm, 0–1-septate, a single ramoconidium with four septa, base broadly truncate, 2.5–3 μm wide, unthickened, somewhat refractive. Conidia catenate, in branched chains, up to four conidia in the unbranched terminal part of the chain, small terminal conidia subglobose to mostly obovoid, 3.5–5(–5.5) × 2–3 μm (av. ± SD: 4.4 ± 0.7 × 2.4 ± 0.4), intercalary conidia ovoid to ellipsoid, (4–)5–8(–9) × (2–)2.5–3 μm (av. ± SD: 6.3 ± 1.2 × 2.9 ± 0.3), aseptate, with 1–2(–3) distal hila, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, with up to three apical hila, 7–25(–35) × 2.5–3.5(–4) μm (av. ± SD: 15.6 ± 7.7 × 3.1 ± 0.4), 0–1(–3)-septate, not constricted at septa, subhyaline to pale olivaceous-brown, almost smooth to asperulate or minutely verruculose, under SEM surface almost smooth to reticulate or with embossed stripes caused by diminishing turgor and shriveling of tender young conidia, walls unthickened to slightly thickened, slightly attenuated towards apex and base, hila protuberant, subdenticulate to denticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis sometimes occurring.
Culture characteristics: Colonies on PDA olivaceous to glaucous-grey, reverse pale olivaceous-grey to olivaceous-grey, powdery to fluffy, margin white, narrow, somewhat feathery and shiny like metal, aerial mycelium diffuse, loose, fluffy, high, growth low convex, somewhat folded, without exudates, sporulation profuse. Colonies on MEA olivaceous-grey in the centre, greenish grey and glaucous-grey towards margins, zonate, reverse olivaceous-grey, woolly-felty, margin white, narrow, somewhat feathery, radially furrowed, folded and wrinkled, aerial mycelium low to high, loose to more dense, fluffy, without exudates, sporulating. Colonies on OA dark smoke-grey to olivaceous-grey and iron-grey, reverse leaden-grey, powdery to woolly-felty, margin narrow, white or colourless, glabrous, aerial mycelium loose, fluffy to felty, growth flat to low convex, without exudates, sporulating.
Specimen examined: U.S.A., Washington, Seattle, University of Washington campus, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus avellana (Betulaceae), 12 Feb. 2004, D. Glawe, CBS H-20432, holotype; ex-type culture CBS 125987 = CPC 11828.
Substrate and distribution: Isolated from chasmothecia of Phyllactinia guttata on leaves of Corylus; U.S.A.
Notes: Cladosporium tenellum and C. cladosporioides can morphologically be compared with the new species, but C. cladosporioides has usually smooth conidiophores and conidia, and somewhat longer and wider, mainly aseptate secondary ramoconidia, (7–)10–33(–38) × (2–)2.5–4(–6) μm. Belonging to the C. herbarum complex, C. tenellum is phylogenetically quite apart from C. exile and morphologically distinguishable by having wider conidia, 2.5–5(–6) μm, with numerous distal hila and somewhat narrower conidiogenous loci and hila, 0.5–1.5 μm diam (Schubert et al. 2007b). The species clustered as a sister to C. paracladosporioides (Fig. 1, part a) and formed a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium flabelliforme Bensch, Summerell, Crous & U. Braun, sp. nov. MycoBank MB517078. Figs 32, 33.
Fig. 32.
Cladosporium flabelliforme (CBS 126345). Conidiophores and conidia in long often dichotomously branched chains. Scale bar = 10 μm.
Fig. 33.
Cladosporium flabelliforme (CBS 126345). A–H. Conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the flabellate conidial chains spread in a fan-like manner.
Cladosporii chalastosporoidis simile, sed catenis conidiorum flabellatis, brevioribus, conidiis usque ad 7(–9) in catenis terminalibus non ramosis et item ramoconidiis secundariis longioribus et angustioribus, 11–27 × (2–)2.5–3(–3.5) μm discernitur. Differt a Cladosporio cucumerino conidiis intercalaribus et ramoconidiis secundariis angustioribus, 1.5–3(–3.5) μm latis, 0–2(–3)-septatis, locis conidiogenis et hilis angustioribus, 0.5–1.5(–1.8) μm diam.
Mycelium immersed and superficial; hyphae filiform to cylindrical-oblong, unbranched or sparingly branched, 0.5–1 μm wide, at the base of conidiophores wider, 1.5–2.5 μm wide, septate, not constricted, septa often not very conspicuous, subhyaline to very pale olivaceous or pale olivaceous-brown, smooth, walls unthickened, forming dense ropes or filiform hyphae often spirally twisted. Conidiophores macronematous, solitary, arising terminally and laterally from hyphae, erect, straight, cylindrical-oblong, neither nodulose nor geniculate, unbranched, 24–90 × 2–3.5(–4) μm, 0–3-septate, not constricted at septa, very pale olivaceous-brown or olivaceous, smooth or finely verruculose, asperulate, walls unthickened and somewhat irregular towards the base. Conidiogenous cells integrated, terminal, cylindrical-oblong, neither geniculate nor nodulose, 11–42 μm long, with 1–3 loci at the apex, conspicuous, subdenticulate, 1–1.5 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, up to 50 μm long, asperulate as tips of conidiophores, not attenuated towards the base, base about 2.5 μm wide, unthickened and not darkened. Conidia catenate, in long branched chains, often dichotomously branched, up to 7(–9) conidia in the unbranched terminal parts of the chain, conidial chains flabellate (spread in a fan-like manner), small terminal conidia obovoid or ellipsoid, 4.5–8 × 1.5–2.5 μm (av. ± SD: 6.1 ± 1.2 × 2.0 ± 0.3), intercalary conidia fusiform to ellipsoid or subcylindrical, 7–16(–18) × 1.5–3 μm (av. ± SD: 10.0 ± 3.0 × 2.5 ± 0.5), aseptate, occasionally with a single septum, attenuated towards apex and base, with 1–2(–3) distal hila, secondary ramoconidia fusiform to cylindrical-oblong, 11–27 × (2–)2.5–3(–3.5) μm (av. ± SD: 19.2 ± 4.9 × 2.8 ± 0.3), aseptate, very pale olivaceous or olivaceous-brown, smooth, walls unthickened, with 2–3(–4) distal hila, hila conspicuous, subdenticulate, 0.5–1.5 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 50–57 mm diam after 14 d, grey-olivaceous, reverse grey-olivaceous to olivaceous-grey, paler parts olivaceous-buff, floccose to fluffy-felty, margins glabrous, somewhat shiny and slimy, white to olivaceous-buff, aerial mycelium diffuse to somewhat dense, pale olivaceous-grey, floccose to villose, growth low convex to dome-shaped with somewhat elevated colony centre, without conspicuous exudates, sporulation profuse. Colonies reaching 60–80 mm diam after 14 d, smoke-grey to olivaceous, grey-olivaceous towards margins, reverse olivaceous-grey, floccose to fluffy, margins white, glabrous to feathery radially furrowed, aerial mycelium whitish forming dense patches, floccose to felty, growth effuse, without prominent exudates, sporulation profuse. Colonies on OA attaining 55–68 mm diam after 14 d, olivaceous-buff to greenish olivaceous, reverse pale olivaceous-grey to olivaceous-grey, greyish sepia in colony centre, floccose, margins colourless, glabrous, aerial mycelium sparse, diffuse, growth flat, somewhat wrinkled, sometimes concentric zones of higher conidiophores and areas of profuse sporulation, without exudates.
Specimen examined: Australia, Northern Territory, Fogg Dam, S 12°34'01” E 131°17'49”, isol. from Melaleuca cajuputi (Myrtaceae), 23 Sep. 2007, coll. B.A. Summerell, isol. P.W. Crous, CBS H-20433, holotype; ex-type culture CBS 126345 = CPC 14523.
Substrate and distribution: On Melaleuca; Australia.
Notes: Cladosporium flabelliforme is well characterised by its conidial chains spread in fan-like manner. Cladosporium chalastosporoides resembles the new species but differs in having very long unbranched or loosely branched conidial chains with up to 18 conidia in a chain and shorter and wider secondary ramoconidia, 10–19 × (2–)2.5–4 μm; and C. cucumerinum possesses wider intercalary conidia and secondary ramoconidia, (2–)2.5–5(–5.5) μm, being 0–2(–3)-septate and wider conidiogenous loci and hila, 0.5–2.2(–2.5) μm diam. The species clusters as a basal sister to C. exile (Fig. 1, part a) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium funiculosum W. Yamam., Sci. Rep. Hyogo Univ. Agric., Ser. Agric. 4(1): 5. 1959. Figs 34, 35.
Fig. 34.
Cladosporium funiculosum (CBS 122129). Macro- and micronematous conidiophores, mycelium sometimes formed in ropes and conidial chains. Scale bar = 10 μm.
Fig. 35.
Cladosporium funiculosum (CBS 122129). A–E, G. Macronematous conidiophores and conidial chains. F. Micronematous conidiophore with conidial chains. Scale bars = 10 μm.
Mycelium immersed and superficial, hyphae loosely branched, filiform to cylindrical-oblong or irregular in outline due to swellings, 1–3 μm wide, septate, smooth or mostly loosely verruculose to densely verruculose Zasmidium(Stenella)-like, walls unthickened, sometimes forming ropes. Conidiophores micronematous to semimacronematous, solitary, arising terminally and laterally from plagiotropous or ascending hyphae or hyphal strains, filiform to narrowly cylindrical-oblong, neither geniculate nor nodulose, unbranched, occasionally once branched, 10–120 × 2–3(–4) μm, usually rather short, 0–2(–5)-septate, not constricted at septa, subhyaline to pale olivaceous, smooth to minutely verruculose, walls unthickened, sometimes hardly distinguishable from hyphae, sometimes irregular in outline due to swellings and constrictions. Conidiogenous cells integrated, terminal, sometimes intercalary, proliferation often distinctly sympodial, but neither geniculate nor nodulose, 10–33 μm long, with 1–3 loci at the apex, sometimes few additional loci at a lower level, subdenticulate, 1–1.5 μm diam, somewhat thickened and darkened-refractive. Ramoconidia not formed. Conidia catenate, in long unbranched or basely, often dichotomously branched chains, up to 8(–14) conidia in the unbranched terminal part, straight, small terminal conidia subglobose, obovoid, narrowly ovoid, ellipsoid, sometimes narrowly obclavate, 2.5–5 × 1.5–2 μm (av. ± SD: 4.3 ± 1.0 × 1.7 ± 0.3), aseptate, intercalary conidia narrowly ellipsoid, fusiform to subcylindrical, 5–16 × (1.5–)2–3 μm (av. ± SD: 9.3 ± 3.3 × 2.6 ± 0.4), 0–1-septate, with 1–2 distal hila, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, 7–23(–27) × 2.5–3.2(–4) μm (av. ± SD: 15.6 ± 5.1 × 2.9 ± 0.3), 0–1(–2)-septate, not constricted at septa, septum often somewhat in the upper half, with (1–)2–3 distal hila, often with a second hilum near the base forming additional conidia “backwards”, subhyaline to pale olivaceous, smooth, walls unthickened, slightly to distinctly attenuated towards apex and base, cell structure granular, hila conspicuous, subdenticulate, 0.5–1.5 μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 65–78 mm diam after 14 d, glaucous-grey or iron-grey to black, olivaceous towards margins, reverse greenish grey to grey-olivaceous or dark mouse-grey to black, floccose, felty-woolly to shiny, margin white to olivaceous, feathery, regular, aerial mycelium abundant, floccose to villose, low to high, mainly in colony centre, growth effuse to low convex, somewhat wrinkled, with numerous small to large prominent exudates, sometimes coalescing, forming slimy ring-like structures in colony centre, not sporulating. Colonies on MEA reaching up to 80 mm diam after 14 d, pale olivaceous-grey to buff or rosy-buff, reverse olivaceous-grey, brick to dark brick towards margins, zonate, floccose to felty, margin white, glabrous to feathery, narrow, regular, aerial mycelium abundant, covering most of the colony surface, floccose to felty, dense, low, growth effuse, radially furrowed and wrinkled, without prominent exudates, not sporulating. Colonies on OA attaining 58–67 mm diam after 14 d, white to smoke-grey, pale olivaceous-grey or olivaceous-grey, colony centre buff or rosy-buff, reverse pale olivaceous-grey to fawn, floccose to felty, margins colourless to white, glabrous, regular, aerial mycelium abundant, covering the whole surface, floccose to felty, growth flat, with numerous small prominent exudates, not sporulating.
Specimen examined: Japan, isol. from leaves of Vigna umbellata (= Phaseolus chrysanthos; Fabaceae), probably authentic strain of C. funiculosum; ex-type culture: CBS 122129 = ATCC 38010 = IFO 6537 = JCM 10683.
Substrate and distribution: On Phaseolus; Japan.
Notes: Conidiophore measurements and the species epithet “funiculosum” introduced in Yamamoto (1959) very probably refer to hyphal strains and not conidiophores since these are often hardly distinguishable from hyphae or hyphal strands.
Two strains of C. funiculosum are included in this study. One of these (CBS 122128 = ATCC 16160 = IFO 6536 = JCM 10682) was named C. coralloides and should represent an authentic strain of the latter species but this seems to be a different species. The two strains are both phylogenetically (Fig. 1, part b as sister to C. pseudocladosporioides; distance analyses in TreeBASE) and morphologically identical whereas C. coralloides, an invalidly published name isolated from Ficus carica and Oryza sativa, is to be excluded from the genus Cladosporium since the scar structure is not cladosporioid. A dried specimen from BPI was re-examined agreeing with the species description published in Yamamoto (1959) and shows metulocladosporiella-like structures. The “lectotype” of C. coralloides designated in Ho et al. (1999) was incorrect since it was not an element from the protologue of the original description.
The sequence data of DNA from ATCC 16160 that we received from Shari Lupien clustered with C. cladosporioides lineage 2 isolates (not shown here) and not with the other C. funiculosum sequences. It seems as if the different isolates stored in the different culture collections represent different species. CBS strains of C. funiculosum are subcultures of authentic strains deposited at the IFO culture collection.
Cladosporium gamsianum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517079. Figs 36, 37.
Fig. 36.
Cladosporium gamsianum (CBS 125989). Conidiophores and conidial chains. Scale bar = 10 μm.
Fig. 37.
Cladosporium gamsianum (CBS 125989). A–G. Conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Dedicated to Prof. dr Walter Gams for numerous valuable isolates and to honour his contribution to mycology.
Cladosporii cladosporioidis aliquam simile, sed conidiophoris monopodialiter proliferatibus, conidiis brevioribus et angustioribus, 3–14.5 × 1–3(–3.5) μm, locis conidiogensis et hilis angustioribus, 0.5–1.5(–1.8) μm diam. Differt a Cladosporio angustisporo conidiophoris brevioribus et leniter latioribus, 10–146 × 3–5 μm, conidiis minutis terminalibus angustioribus, 1–1.5(–2) μm latis, ramoconidiis secundariis brevioribus, usque ad 14 μm longis.
Mycelium sparingly branched, 1–3.5 μm wide, at the base of conidiophores wider, up to 5 μm wide, septate, subhyaline to pale olivaceous or pale olivaceous-brown, with age hyphae becoming dark brown, smooth, sometimes constricted at septa and minutely verruculose towards the base of conidiophores, walls unthickened, forming ropes. Conidiophores solitary or in small groups of four or sometimes fasciculate, macronematous, arising terminally and laterally from hyphae or hyphal ropes, erect, straight or slightly flexuous, cylindrical-oblong, not geniculate, sometimes slightly swollen at the apex, sometimes with monopodial rejuvenations (monopodially proliferating without conidiogenesis) and a single rather inconspicuous annellation, unbranched, 10–146 × 3–5 μm, pluriseptate, sometimes slightly constricted at septa, medium olivaceous-brown, paler towards or at the uppermost apex, with age dark brown, more thick-walled and two-layered, walls about 1 μm wide, slightly attenuated towards the apex, base sometimes up to 6.5 μm wide. Conidiogenous cells integrated, sympodially proliferating, usually terminal, with age intercalary, cylindrical-oblong, not geniculate, with age slightly swollen, subnodulose at or towards the apex with loci situated at these lateral shoulders, 9–18 μm long, rupturing the outer wall layer around some of the scars, resulting in a lateral displacement of scars, leaving more or less conspicuous circumferential annular fringes of the torn wall, lateral scars in face view conspicuous, flat, non-protuberant or only slightly so, slightly thickened and darkened, with 1–4 loci at the apex, with age up to eight loci crowded towards the apex, loci conspicuous, subdenticulate, 1–1.5(–1.8) μm diam, thickened and darkened-refractive. Conidia catenate, in branched chains, branching dichotomously or in all directions, narrow, straight, small terminal conidia obovoid, 3–6 × 1–1.5(–2) μm (av. ± SD: 5.0 ± 0.9 × 1.4 ± 0.3), aseptate, intercalary conidia fusiform to narrowly ellipsoid-ovoid, 4.5–12 × 1.5–2.5 μm (av. ± SD: 7.4 ± 2.0 × 1.9 ± 0.4), aseptate, with 1–3(–5) distal hila, secondary ramoconidia narrowly ellipsoid to subcylindrical, (6–)7–14.5 × (1.2–)1.5–3(–3.5) μm (av. ± SD: 10.3 ± 2.4 × 2.6 ± 0.6), 0(–1)-septate, sometimes constricted at the median septum, subhyaline to pale olivaceous or pale olivaceous-brown, smooth, walls unthickened or almost so, attenuated towards apex and base, with 1–5 distal hila, conspicuous, subdenticulate, 0.5–1.5(–1.8) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA olivaceous-grey to iron-grey, grey-olivaceous towards margins, somewhat zonate, reverse olivaceous-grey to greyish-blue, grey-olivaceous towards margins, woolly-felty, margin white, glabrous, aerial mycelium abundant, covering almost the whole colony surface, woolly-felty, sometimes high, growth flat, numerous small or large prominent exudates formed, sporulation profuse. Colonies on MEA olivaceous-grey, iron-grey or black towards margins, reverse iron-grey, velvety to woolly, margin white, glabrous, sometimes radially furrowed, aerial mycelium abundant, covering the whole colony surface, growth low convex, without prominent exudates, sporulating. Colonies on OA olivaceous to grey-olivaceous, olivaceous-grey towards margins, reverse leaden-grey to leaden-black, velvety to powdery, margin white, narrow, glabrous, aerial mycelium absent or sparse, growth flat, sporulation profuse.
Specimen examined: South Africa, Pretoria, Walter Sisulu Botanical Garden, 25.706944, 28.229444, isol. from Strelitzia sp. (Strelitziaceae), 17 Feb. 2005, coll. W. Gams, isol. P.W. Crous, CBS H-20434, holotype; ex-type culture CBS 125989 = CPC 11807.
Substrate and distribution: On Strelitzia; South Africa.
Notes: The mode of rejuvenation with more or less conspicuous annular structures not being connected with conidiogenesis and conspicuous lateral conidiogenous loci is unique within the genus Cladosporium and hitherto confined to C. gamsianum. It is reminiscent of the genus Annellosympodia (McTaggart et al. 2007) with its type species A. orbiculata. Conidial chains of C. gamsianum resemble those of C. basiinflatum but the latter species differs in having longer and wider conidia, 4–23(–32) × (2–)2.5–4.5(–5) μm, and wider conidiophores with usually distinctly inflated basal cells. Cladosporium cladosporioides and C. angustisporum are also somewhat similar, but C. cladosporioides has much longer and wider conidia, 3–33(–38) × (1.5–)2–4(–6) μm, with wider conidiogenous loci and hila, 0.5–2(–2.5) μm diam; and in C. angustisporum the conidiophores are longer and somewhat narrower without having annular structures, small terminal conidia are somewhat wider, 1.5–2 μm, and secondary ramoconidia are up to 26 μm long. The species clusters as a sister to C. verrucocladosporioides (Fig. 1, part b) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium globisporum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517080. Figs 38, 39, 40.
Fig. 38.
Cladosporium globisporum (CBS 812.96). Macro- and micronematous conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 39.
Cladosporium globisporum (CBS 812.96). A–B. Delicately ornamented conidia showing a somewhat irregularly reticulate surface or slightly embossed stripes probably caused by diminishing turgor and shriveling of tender young conidia. C. Conidia, secondary ramoconidia and scars. D. Conidiophore with secondary ramoconidia. E. Globose ornamented small terminal conidia. F. Running hyphae on agar and conidia. Scale bars = 2 (B, E), 5 (A, C), 10 (D, F) μm.
Fig. 40.
Cladosporium globisporum (CBS 812.96). A–F. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Etymology: Name refers to the shape of small terminal and intercalary conidia which is mainly globose or subglobose.
Cladosporii cladosporioidis simile, sed conidiis minutis terminalibus saepe globosis, latioribus, (2.5–)3–4 μm latis, conidiis intercalaribus latioribus, 3–4(–5) μm latis, et tamen ramoconidiis secundariis latioribus, (3–)4–5(–6) μm latis internoscitur.
Mycelium mainly immersed, sparingly branched, 2–5 μm wide, septate, not constricted at septa, pale brown, smooth to minutely verruculose, walls unthickened. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from ascending or plagiotropous hyphae, erect, straight to slightly flexuous, cylindrical-oblong to filiform, non-nodulose, sometimes geniculate, unbranched to once branched, branches as short denticle-like lateral outgrowth, later becoming longer, 17–165 × 3–5 μm, micronematous conidiophores (1–)2–2.5(–3) μm wide, 0–4-septate, cells quite long, not constricted at septa, septa often darkened, pale to pale medium brown, slightly paler towards the apex, minutely verruculose, asperulate, walls unthickened or slightly thickened, up to 1 μm wide. Conidiogenous cells integrated, often distinctly sympodially proliferating, terminal, usually non-nodulose, sometimes slightly geniculate, filiform to cylindrical-oblong, somewhat flexuous, 17–55 μm long, with up to three apical loci, sitting close together at the apex, conspicuous, subdenticulate to denticulate, (1.2–)1.5–2(–2.2) μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 19–41(–56) × 3–4(–5) μm, 0(–2)-septate, base broadly truncate. Conidia catenate, in densely branched chains, straight to slightly curved, small terminal conidia globose, subglobose to obovoid, 2.5–6(–8) × (2.5–)3–4 μm (av. ± SD: 4.5 ± 1.6 × 3.2 ± 0.5), broadly rounded at the apex, intercalary conidia subglobose, broadly ellipsoid-ovoid, (4–)5–9(–14) × 3–4(–5) μm (av. ± SD: 6.6 ± 2.2 × 3.7 ± 0.5), aseptate, with up to 3(–5) distal hila, often distinctly denticulate, secondary ramoconidia ellipsoid to subcylindrical, 9–25(–30) × (3–)4–5(–6) μm (av. ± SD: 16.6 ± 5.6 × 4.3 ± 0.5), 0(–1)-septate, with 3–4 distal hila, sometimes hila not only distal but also lateral in the middle of the cell, pale brown, smooth or almost so, under SEM surface reticulate or with somewhat embossed stripes caused by diminishing turgor and shriveling of tender young conidia, walls unthickened or only slightly so, attenuated towards apex and base, hila conspicuous, often distinctly denticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA grey-olivaceous to olivaceous, reverse leaden-grey, velvety to powdery, margin colourless to white, feathery, aerial mycelium sparse, loose, fluffy, only few areas covered, growth flat, without exudates, sporulation profuse. Colonies on MEA grey-olivaceous, pale olivaceous-grey towards margins, reverse olivaceous-grey, velvety, due to aerial mycelium several white patches, fluffy, loose to dense, without exudates, sporulation profuse. Colonies on OA grey-olivaceous to pale olivaceous due to profuse sporulation or olivaceous-buff, reverse leaden-grey to iron-grey, velvety to powdery, glittering due to numerous small, not very prominent exudates (like little water drops), margin colourless, feathery, aerial mycelium absent or sparse, growth flat.
Specimen examined: Sweden, isol. from meat stamp, 1986, M. Olsen, No. M291, CBS H-20435, holotype; ex-type culture CBS 812.96.
Substrate and distribution: Isolated from meat stamp; Sweden.
Notes: Conidiophore morphology of Cladosporium globisporum closely resembles that of C. cladosporioides but the latter species is easily distinguishable by having narrower, obovoid, ovoid or limoniform small terminal conidia, (1.5–)2–2.5(–3) μm wide, narrower and somewhat longer intercalary conidia and narrower secondary ramoconidia, (2–)2.5–4(–6) μm wide.
With its numerous terminal and intercalary globose or subglobose conidia C. globisporum is reminiscent of species belonging to the C. sphaerospermum complex, but these conidia are smooth or almost so in C. globisporum, and not minutely verruculose to verrucose as in C. sphaerospermum, C. halotolerans and C. langeronii. Conidia of these three species are usually narrower and more frequently septate. Cladosporium psychrotolerans also differs in having 0–1(–2)-septate, somewhat narrower secondary ramoconidia, (2–)2.5–3(–5) μm wide (Zalar et al. 2007). The species clusters as a basal sister to C. phyllophilum (Fig. 1, part a) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium hillianum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517081. Figs 41, 42.
Fig. 41.
Cladosporium hillianum (CBS 125988). Mycelium, hyphal conglomeration, conidiophores and conidia. Scale bar = 10 μm.
Fig. 42.
Cladosporium hillianum (CBS 125988). A–G. Conidiophores and conidia. H. Hyphal conglomeration. Scale bars = 10 μm.
Etymology: Dedicated to Frank Hill (recently deceased), who collected numerous valuable Cladosporium species from New Zealand.
Cladosporio delicatulo aliquam simile, sed hyphis latioribus, 1–4.5(–6) μm latis, glomis pseudoparenchymatibus formantibus, conidiis minutis terminalibus obovoidibus et ellipsoidibus, longioribus et latioribus et item locis conidiogenis et hilis angustioribus, (0.5–)0.8–1.5 μm diam distinguitur. Differt a Cladosporio chalastosporoide conidiophoris latioribus, (3–)3.5–4.5 μm latis, conidiis minutis terminalibus latioribus, 0–1-septatis, ramoconidiis secundariis 0–3(–4)-septatis.
Mycelium internal and superficial; hyphae branched, 1–4.5 μm wide, becoming swollen with age, up to 6 μm wide, single cells 9 μm wide, pluriseptate, narrower hyphae usually not constricted at septa, but wider ones often slightly to distinctly constricted, due to swellings, branchings and constrictions often irregular in outline, sometimes septa in short succession, pale to medium olivaceous-brown, smooth, sometimes slightly rough-walled, walls unthickened or somewhat thickened, forming subglobose to globose dense conglomerations (teleomorph initials?), pseudoparenchymatous, textura angularis, 29–55 μm diam, composed of somewhat swollen hyphal cells, 3.5–6 μm wide, medium brown or olivaceous-brown, slightly thick-walled. Conidiophores solitary, semimacronematous or micronematous, often hardly distinguishable from superficial hyphae, arising terminally and laterally from plagiotropous or ascending hyphae, erect or ascending, straight to slightly flexuous, cylindrical-oblong, sometimes once geniculate-sinuous and subnodulose, (12–)25–100 × (3–)3.5–4.5 μm, pluriseptate, 2–8-septa, sometimes slightly constricted at septa, septa often not very conspicuous, pale olivaceous-brown or pale brown, smooth, walls slightly thickened, cell structure somewhat unusual, guttulate. Conidiogenous cells integrated, terminal, occasionally also intercalary, cylindrical, 8–24 μm long, sometimes geniculate-sinuous or subnodulose, with a single locus or often up to four loci at or towards the apex, situated on small lateral shoulders, not very conspicuous, 1–1.5(–2) μm diam, slightly thickened, not darkened but sometimes slightly refractive. Ramoconidia occasionally formed. Conidia catenate, in long unbranched or basely branched chains, branching often dichotomously, up to 7(–11) conidia in the unbranched terminal part, small terminal conidia obovoid to ellipsoid, (5–)6.5–11 × (2–)2.5–4 μm (av. ± SD: 8.0 ± 1.8 × 3.2 ± 0.7), 0–1-septate, septum median or somewhat in the upper half, intercalary conidia ellipsoid to fusiform or irregular, 7.5–14(–17.5) × 2.5–3.5(–4.5) μm (av. ± SD: 11.0 ± 2.9 × 3.2 ± 0.5), 0–1(–2)-septate, septum median or somewhat in the upper half, secondary ramoconidia fusiform, ellipsoid to subcylindrical or irregular, 10–20(–30) × (2.5)3–4.5(–5) μm (av. ± SD: 16.1 ± 4.8 × 3.7 ± 0.7), 0–3(–4)-septate, sometimes slightly to distinctly constricted at septa and becoming sinuous with age, subhyaline to pale brown or pale to medium olivaceous-brown, smooth, walls unthickened or slightly thickened, with 1–2(–4) distal hila, subconspicuous to conspicuous, (0.5–)0.8–1.5 μm diam, slightly thickened, somewhat darkened-refractive; microcyclic conidiogenesis often occurring forming secondary conidiophores and conidia, often germinating.
Culture characteristics: Colonies on PDA attaining 13–17 mm diam after 14 d, olivaceous-grey to iron-grey, reverse iron-grey to olivaceous-black, fluffy, margins narrow, colourless to white, somewhat feathery, slightly undulate, aerial mycelium fluffy, loose, high, pale olivaceous-grey, growth low convex. Colonies on MEA reaching 24–25 mm diam after 14 d, olivaceous-grey with patches of white due to aerial mycelium, reverse olivaceous-grey to iron-grey, velvety, margin narrow, white, crenate, radially furrowed, glabrous, aerial mycelium whitish to pale olivaceous-grey, dense, growth low convex to convex with elevated and wrinkled colony centre. Colonies on OA attaining 25–30 mm diam after 14 d, iron-grey with patches of smoke-grey due to aerial mycelium and sporulation, reverse leaden-grey to olivaceous-grey, margins white, regular, glabrous, aerial mycelium loose to dense, fluffy, smoke-grey or whitish, growth flat, sporulating on all media, without prominent exudates.
Specimen examined: New Zealand, Auckland, St. Johns, Auckland University campus, artificial pond, isol. from leaf mold of Typha orientalis (Typhaceae), 29 May 2008, R. Beever NZ 2008/2765b, CBS H-20436, holotype; ex-type cultures CBS 125988 = CPC 15459, CPC 15458.
Substrate and distribution: On Typha; New Zealand.
Notes: Strain CPC 15458 did not sporulate on any medium, with colonies being somewhat wider and paler with abundant aerial mycelium. The scars of C. hillianum are often not very prominent but distinctly cladosporioid. The formation of pseudoparenchymatous subglobose or globose dense hyphal conglomerations on SNA plates is unique and not yet known for any other Cladosporium species known from culture.
David (1997) described C. heleophilum from Typha latifolia, which belongs to the C. herbarum complex, forming typically herbarum-like verruculose conidia, which make it easy to distinguish it from C. hillianum. Cladosporium chalastosporoides introduced as a new species in this paper is phylogenetically (Fig. 1, part a) and morphologically allied but forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE) and deviates in having narrower conidiophores, (2–)2.5–3.5(–4) μm, aseptate narrower small terminal conidia, 2–2.5 μm wide, and 0–1(–2)-septate secondary ramoconidia. Cladosporium delicatulum differs in forming numerous subglobose, globose or obovoid, shorter and narrower small terminal conidia, 2.5–4.5(–6) × (1.5–)2–2.5(–3.5) μm, wider conidiogenous loci and hila, 0.5–2.2 μm diam and narrower hyphae not forming pseudoparenchymatous conglomerations of textura angularis.
Cladosporium inversicolor Bensch, Crous & U. Braun, sp. nov. MycoBank MB517082. Figs 43, 44.
Fig. 43.
Cladosporium inversicolor (CBS 401.80). Conidiophores and conidial chains with intercalary conidia and small terminal conidia sometimes verruculose or irregularly rough-walled, rugose. Scale bar = 10 μm.
Fig. 44.
Cladosporium inversicolor (CBS 401.80). A–G. Conidiophores and conidial chains with intercalary conidia and small terminal conidia somewhat darker than ramoconidia and secondary ramoconidia. Scale bars = 10 μm.
Etymology: Name refers to the reverse pigmentation of conidia and conidiophores with small terminal conidia and intercalary conidia slightly darker than ramoconidia, secondary ramoconidia and conidiophores.
Cladosporii cladosporioidis aliquam simile, sed conidiis saepe minute regulariter verruculosis vel irregulariter verruculosis, conidiis minutis terminalibus et intercalaribus leniter longioribus et latioribus, inverse coloratis et tamen ramoconidiis secundariis leniter brevioribus, usque ad 24(–29) μm longis, discernitur. Differt a Cladosporio delicatulo catenis conidiorum longioribus, saepe dichotome ramosis, usque ad 17 conidiis in catenulis terminalibus non-ramosis, conidiis minutis terminalibus et intercalaribus longioribus, laevibus vel laxe verruculosis vel irregulariter verruculosis, saepe atrioribus quam ramoconidia et conidiophora.
Mycelium immersed and sparingly superficial; hyphae mainly unbranched, 1.5–3(–4.5) μm wide, septate, not constricted at septa, without swellings, pale olivaceous to pale olivaceous-brown, smooth to often minutely verruculose, walls unthickened. Conidiophores macronematous, solitary, arising terminally and laterally from hyphae, erect, straight to somewhat flexuous, cladosporioides-like, cylindrical-oblong, somewhat geniculate-sinuous towards or at the apex, non-nodulose, unbranched or once branched, 15–225 × 2.5–4(–5) μm, aseptate or with few septa, not constricted at septa, subhyaline to very pale olivaceous-brown, smooth, sometimes rough-walled at the base; occasionally also micronematous, about 1.5 μm wide. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, non-nodulose, sometimes geniculate at or towards the apex due to sympodial proliferation, 15–66 μm long, with (1–)2–3 loci, conspicuous, subdenticulate, 1–2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, 17–42 × 3–3.5 μm, aseptate, occasionally with up to three septa, base (1.8–)2–3 μm wide, unthickened. Conidia numerous, catenate, in branched chains, often dichotomously branched, sometimes in more directions, terminal unbranched parts of the chains often very long, up to eight conidia, sometimes even up to 17 conidia, small terminal conidia obovoid to ellipsoid, sometimes subglobose, (3–)5–8.5 × 2–3(–3.5) μm (av. ± SD: 5.9 ± 1.6 × 2.6 ± 0.4), aseptate, apex rounded, attenuated towards the base, intercalary conidia ovoid, fusiform to ellipsoid, (5–)7–20 × (2–)2.5–3.5(–4) μm (av. ± SD: 10.3 ± 3.5 × 2.9 ± 0.4), aseptate, attenuated towards apex and base, with 1–3(–4) distal hila, secondary ramoconidia subcylindrical, 10.5–24(–29) × (2.2–)2.8–4(–4.2) μm (av. ± SD: 16.9 ± 4.0 × 3.3 ± 0.5), 0–1(–2)-septate, but mainly aseptate, not constricted at septa, pale to olivaceous-brown, small terminal conidia and intercalary conidia slightly darker than ramoconidia, secondary ramoconidia and conidiophores, smooth to loosely minutely verruculose or irregularly rough-walled, rugose, verruculose-rugose surface ornamentation especially in small terminal and intercalary conidia, conidia slightly attenuated towards apex and base, with (1–)2–4(–6) distal hila, walls unthickened or almost so, hila conspicuous, subdenticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 60–75 mm diam after 14 d, olivaceous-grey, grey-olivaceous towards margins, leaden-grey to olivaceous-black reverse with grey-olivaceous margins, floccose, margins regular, white or colourless, feathery, aerial mycelium sparse to abundant, diffuse to floccose, loose to dense, growth effuse, without prominent exudates, sporulation profuse. Colonies on MEA 62–65 mm diam after 14 d, grey-olivaceous to olivaceous-grey or olivaceous, reverse iron-grey to black, velvety, floccose to felty, margins colourless or white, glabrous to feathery, regular, aerial mycelium whitish to smoke-grey, felty-floccose, growth effuse, sometimes radially furrowed in colony centre, without exudates, sporulation profuse. Colonies on OA 50–65 mm diam after 14 d, grey-olivaceous to greenish olivaceous, olivaceous, olivaceous-grey or olivaceous-buff, reverse pale greenish grey to olivaceous-grey, leaden-grey or iron-grey, velvety to floccose, margins glabrous, olivaceous-grey, narrow, aerial mycelium smoke-grey to pale olivaceous-grey, felty, growth flat, without exudates, sporulation profuse.
Specimens examined: Sine loco, isol. from Triticum aestivum (Poaceae) by F.T. Bennett, 1929, deposited as C. cladosporioides, CBS 131.29 = ATCC 200942 = ATCC 11275 = IMI 049623 = LCP 52.404. Colombia, isol. from Cortaderia sp. (Poaceae), CBS 484.80. Denmark, Usseroed, isol. from school dust, 2000, B. Andersen, BA 1735 = CPC 14368. Germany, Bayern, München, park of castle Nymphenburg, isol. from Puccinia bromina ssp. symphyti-bromarum var. paucispora, Jul. 2006, K. Schubert, CPC 13150. Netherlands, isol. from a leaf of Tilia sp. (Tiliaceae), deposited Jan. 1965 as C. cladosporioides, isol. by A.A. Verhorst, ident. by G.A. de Vries, CBS 143.65; isol. from a leaf of Triticum aestivum, deposited Jul. 1980 as C. cladosporioides, isol. by N.J. Fokkema, ident. by G.A. de Vries, CBS H-20437, holotype; ex-type culture CBS 401.80 = ATCC 200941; Baarn, de Vuursche, isol. from seeds of Alnus sp. (Betulaceae), 14 Mar. 1982, G.S. de Vries, No. 4110, CBS H-1604, CBS 464.82 = ATCC 200945, deposited as C. laxicapitulatum; Millingerwaard, isol. from a fruit of Sambucus nigra (Adoxaceae), 29 Aug. 2007, P.W. Crous, CPC 14241; Zwolle, isol. from outside air, 7 Jan. 2007, M. Meijer, CPC 14190, 14191. U.S.A., Seattle, University of Washington campus, 47.6263, 122.3331, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus avellana (Betulaceae), 16 Sep. 2004, D. Glawe, CPC 11818.
Substrates and distribution: On plant material, isol. from air and food, also mycophilic; Europe (Denmark, Germany, Netherlands), North America (U.S.A.), South America (Colombia).
Notes: Phylogenetically C. inversicolor is closely related to C. delicatulum (Fig. 1, part a; distance analyses in TreeBASE). Morphologically it differs in having often dichotomously branched, very long conidial chains with sometimes up to 17 conidia in the terminal unbranched part of the chain and longer small terminal conidia and intercalary conidia which are smooth or loosely minutely verruculose or irregularly rough-walled and usually slightly darker than ramoconidia, secondary ramoconidia and conidiophores, although the terminal conidia are usually paler in Cladosporium spp. This inverse coloration of conidia has not yet been observed in any Cladosporium species known from culture. Cladosporium cladosporioides is similar to C. inversicolor but distinct in that it has usually smooth conidia with small terminal conidia and intercalary conidia being somewhat shorter and not inversely pigmented, longer secondary ramoconidia and wider ramoconidia.
Cladosporium iranicum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517083. Figs 45, 46.
Fig. 45.
Cladosporium iranicum (CBS 126346). Macro- and micronematous conidiophores, ramoconidia and conidial chains, intercalary conidia sometimes subrostrate. Scale bar = 10 μm.
Fig. 46.
Cladosporium iranicum (CBS 126346). A–D, F–G. Macronematous conidiophores and conidial chains. E. Conidia and conidiophore showing the often guttulate cell structure. H–I. Subrostrate intercalary conidia. Scale bar = 10 μm.
Etymology: Named after the country of origin, Iran.
Cladosporii cladosporioidis simile, sed ramoconidiis secundariis 0–1(–2)-septatis, conidiis intercalaribus saepe subrostratis vel rostratis internoscitur.
Mycelium mainly immersed; hyphae sparingly branched, 1–5 μm wide, septate, sometimes with swellings and constrictions, therefore irregular in outline, almost hyaline to pale or medium olivaceous-brown, smooth to minutely verruculose, walls unthickened to slightly thickened, forming ropes. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from plagiotropous or ascending hyphae, erect or ascending, straight to slightly flexuous, filiform to cylindrical-oblong, neither nodulose nor geniculate, unbranched or once branched, branches mostly only as short lateral prolongations just below a septum, 40–180(–325) × (2–)2.5–4(–5) μm, pluriseptate, mostly 2–5-septate, not constricted at septa, few septa darkened (viz. those septa at the base of potential ramoconidia), subhyaline to pale, sometimes medium olivaceous-brown, smooth or almost so at the apex and finely asperulate towards the base, walls unthickened to slightly thickened. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, neither geniculate nor nodulose, 6–44 μm long, smooth, with up to three apical loci, sometimes one or two loci at a lower level, denticulate, protuberant, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia sometimes formed, cylindrical-oblong, 31–42 × 3.5–5 μm, often 1-septate, base broadly truncate, 2.2–3 μm wide, not thickened, somewhat refractive. Conidia catenate, in long unbranched and branched chains, chains often dichotomously branched, up to 10 conidia in the unbranched terminal part, straight to slightly curved, small terminal conidia and intercalary conidia in long unbranched chains, obovoid, limoniform to narrowly fusiform or ellipsoid, 4–10 × (1–)1.5–3 μm (av. ± SD: 6.2 ± 1.7 × 2.3 ± 0.6), aseptate, often subrostrate to rostrate, secondary ramoconidia ellipsoid to subcylindrical or cylindrical-oblong, 10–33 × (2–)3–4 μm (av. ± SD: 21.0 ± 6.8 × 3.4 ± 0.5), 0–1(–2)-septate, not constricted at septa, septum median, sometimes in the lower third, often not very conspicuous, almost hyaline to pale olivaceous-brown, smooth or almost so, walls unthickened or slightly thickened, slightly to distinctly attenuated towards apex and base, cell structure 1–2 guttulate, with up to three distal hila, hila protuberant, denticulate, 0.5–2 μm diam, thickened and darkened-refractive; conidia often germinating especially terminal ones, germ tubes up to 30 μm long; no microcyclic conidiogenesis.
Culture characteristics: Colonies on PDA attaining 53–60 mm diam after 1 mo, olivaceous-grey to grey-olivaceous towards the margins, reverse iron-grey to leaden-grey, powdery to fluffy, margin regular, white, broad, glabrous, aerial mycelium abundantly formed, fluffy, especially in the colony centre, no exudates formed, sporulation profuse. Colonies on MEA reaching 47 mm diam after 1 mo, smoke-grey or whitish due to aerial mycelium, pale olivaceous-grey towards margins, reverse olivaceous-grey to buff, fluffy-felty, margin regular, white, glabrous, radially furrowed, aerial mycelium abundant, fluffy, covering the whole surface, no exudates, sporulation profuse.
Specimen examined: Iran, isol. from a leaf of Citrus sinensis (Rutaceae), on scale insect, 2004, W. Gams, CBS H-20438, holotype; ex-type culture CBS 126346 = CPC 11554.
Substrate and distribution: On Citrus; Iran.
Notes: The morphology of conidiophores and conidia of C. iranicum is very close to C. cladosporioides, but its secondary ramoconidia are often 1(–2)-septate and intercalary conidia often subrostrate or rostrate. Phylogenetically this new species clusters quite apart from C. cladosporioides (Fig. 1, part a vs. c; distance analyses in TreeBASE). Growth rates of these two species are also quite different with C. iranicum growing more slowly.
Cladosporium sphaerospermum, originally described by Penzig (1882) on chlorotic leaves and branches of Citrus sp. from Italy is quite distinct from C. iranicum by having small globose, verrucose conidia and more frequently septate secondary ramoconidia and ramoconidia (Zalar et al. 2007). Cladosporium corrugatum occurring on Citrus aurantium in Australia differs by having narrower conidiogenous loci and hila and shorter, 0–2(–3)-septate conidia (Schubert 2005b).
Cladosporium licheniphilum Heuchert & U. Braun, Herzogia 19: 12. 2006. Fig. 47.
Fig. 47.
Cladosporium licheniphilum (CBS 125990). A–H. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Mycelium immersed and superficial, dimorphic, sparingly branched, 2–5 μm wide, septate, not constricted at septa, hyaline to subhyaline or olivaceous-brown, sterile hyphae smooth, fertile hyphae giving rise to conidiophores irregularly rough-walled, Zasmidium(Stenella)-like, often irregular in outline, walls unthickened to somewhat thickened. Conidiophores macronematous, sometimes also micronematous, arising laterally or terminally from plagiotropous or ascending hyphae, solitary, sometimes in loose groups of two or three, erect, straight to slightly flexuous, cylindrical-oblong, unbranched or once, sometimes twice branched, lateral branches as short outgrowth just below a septum or relatively long, in an angle of 45–90°, 28–90(–145) × 3–4 μm, pluriseptate, not constricted at septa, pale olivaceous to olivaceous-brown, paler towards apices, smooth to somewhat irregularly rough-walled, similar as hyphae, walls thickened, up to 1 μm wide; micronematous conidiophores shorter, narrower and paler, 14–65 × 2–3 μm, septate. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, 5–35 μm long, with 1–5 protuberant, denticle-like loci, often aggregated or sitting on small lateral prolongations, broadly truncate, 1.2–2 μm diam, thickened and distinctly darkened, secondary ramoconidia sitting like a whirl at the tip of conidiophores. Ramoconidia occasionally formed, with a broadly truncate base, not darkened but slightly refractive. Conidia numerous, catenate, in branched chains, often dichotomously branched, especially in the terminal part, small terminal conidia ovoid to obovoid, sometimes subglobose or globose, 3–5 × 2–3 μm (av. ± SD: 4.3 ± 0.8 × 2.6 ± 0.4), aseptate, rounded at the apex, attenuated towards the base, smooth, thin-walled, intercalary conidia ovoid-ellipsoid, 5–8(–12) × 3–4 μm (av. ± SD: 7.6 ± 1.7 × 3.6 ± 0.4), aseptate, distinctly attenuated towards the base, with 1–3 distal hila, sometimes situated on short lateral prolongations at the distal end, secondary ramoconidia ellipsoid to fusiform or subcylindrical, with (1–)2–3(–4) distal hila, 7–18(–23) × (2.5–)3–5(–6) μm (av. ± SD: 12.8 ± 3.9 × 3.9 ± 0.7), 0–1(–2)-septate, not constricted at septa, only very slightly attenuated towards apex and base, base broadly truncate, pale brown or pale olivaceous-brown, smooth, walls unthickened or almost so, hila protuberant, denticulate, 0.5–2 μm diam, thickened, distinctly darkened-refractive; microcyclic conidiogenesis not observed.
Description in vivo: See Heuchert & Braun (2006), von Brackel (2009).
Culture characteristics: Colonies on PDA attaining 8–18 mm diam after 1 mo, pale olivaceous-grey to olivaceous-grey, sometimes olivaceous due to abundant sporulation, reverse olivaceous-grey, velvety to fluffy, margin feathery to glabrous, regular, slow growing, aerial mycelium loose to dense, more abundant at few areas, fluffy, few prominent exudates formed, sporulation profuse. Colonies on MEA reaching 8–18 mm diam after 1 mo, grey-olivaceous to olivaceous-grey, whitish to smoke-grey due to aerial mycelium, reverse olivaceous-grey to iron-grey, velvety to woolly, margin very narrow, white feathery, aerial mycelium dense, high, covering some parts of the colony, abundant, without conspicuous exudates, sporulation profuse.
Specimens examined: Germany, Bavaria, Unterfranken, Kreis Aschaffenburg, parking area at the motorway Würzburg-Frankfurt, south-east of Waldaschaff, isol. from the lichen Phaeophyscia orbicularis and Physcia sp. on stems and bark of Acer platanoides (Aceraceae), 31 May 2006, W. von Brackel, No. 3808, CBS H-20439, epitype of C. licheniphilum designated here; ex-type culture CBS 125990 = CPC 13224. Russia, Altai, Zmeinogorsk Region, Belaya River near Mt. Stanovaya, 51°00'N, 82°44'N, alt. 600 m, Taiga forest, on apothecia of Pertusaria alpina, 12 June 1999, E. A. Davydov, LE, holotype.
Substrate and distribution: On lichens; Europe (Germany, Italy, Norway, Russia), see Heuchert & Braun (2006), von Brackel (2008, 2009).
Notes: The chosen epitype of the recently introduced C. licheniphilum agrees well with the original description given in Heuchert & Braun (2006) taking into consideration that fungal structures are usually slightly narrower in vitro compared with its measurements in vivo. Cladosporium antarticum is the second genuine lichenicolous species known so far in Cladosporium, which is also characterised by forming a dimorphic mycelium. Belonging to the C. herbarum complex, this species was already compared and discussed with C. licheniphilum and several other morphologically similar species in Schubert et al. (2007b). The species clusters as a sister to C. phyllophilum (Fig. 1, part a) and forms a distinct lineage for both TEF and ACT (distance analyses in TreeBASE).
Cladosporium lycoperdinum Cooke, Grevillea 12(61): 32 (1883). Fig. 48.
Fig. 48.
Cladosporium lycoperdinum (CBS 574.78C). A–D. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Mycelium unbranched or loosely branched, filiform to cylindrical-oblong, (0.5–)1–5 μm wide, not constricted at septa, subhyaline to pale or medium olivaceous-brown, smooth or almost so to often minutely verruculose or loosely verrucose, walls unthickened or almost so, occasionally forming ropes. Conidiophores macro- and micronematous, solitary, arising terminally and laterally from hyphae, erect, straight or slightly flexuous, macronematous conidiophores cylindrical-oblong or filiform, non-nodulose, usually not geniculate, occasionally slightly geniculate at or towards the apex due to sympodial proliferation, unbranched or once, rarely twice branched, branches often only as short lateral peg-like prolongations just below a septum, 20–250 × (2.5–)3–6(–6.5) μm, pluriseptate, with septa occasionally in short succession, not constricted at septa, few septa sometimes darkened just below potential ramoconidia or where conidiophores disarticulate into shorter pieces, pale olivaceous to medium olivaceous-brown, smooth to somewhat irregularly rough-walled or minutely verruculose, especially at or towards the base, walls unthickened or almost so, about 0.5 μm wide, sometimes slightly attenuated towards the apex or intercalary somewhat wider; micronematous conidiophores narrower, shorter and paler, 9–105 × 1.5–2.5 μm, filiform, not geniculate, unbranched or once branched, 0–5-septate, subhyaline to pale olivaceous, conidiogenous cells 6.5–50 μm long, loci 0.5–1.2 μm diam. Conidiogenous cells integrated, terminal, intercalary or sometimes pleurogenous, often seceding and forming ramoconidia, cylindrical-oblong, sometimes slightly geniculate due to sympodial proliferation, 10–57 μm long, with (1–)2–4 loci at or towards the apex, sometimes with additional loci situated on a lower level, in intercalary conidiogenous cells loci usually situated on small peg-like lateral outgrowths, loci conspicuous, subdenticulate to denticulate, 1–2 μm diam, thickened and darkened-refractive. Ramoconidia often formed, cylindrical-oblong, 13.5–55 × 3–5(–5.5) μm, 0–3(–6)-septate, not constricted at septa, with 2–4 distal hila, base broadly truncate, 2.2–3(–3.5) μm wide, unthickened or slightly thickened, often somewhat darkened or refractive, without dome and rim. Conidia catenate, in branched chains branching in all directions, up to 5(–7) conidia in the terminal unbranched part of the conidial chains, straight, small terminal conidia subglobose to obovoid or narrowly ellipsoid, (2–)3.5–5 × (1.5–)2–2.5(–3) μm (av. ± SD: 4.2 ± 0.7 × 2.0 ± 0.3), aseptate, intercalary conidia limoniform, ovoid to ellipsoid, 4–14(–16.5) × (2–)2.5–3(–4) μm (av. ± SD: 8.6 ± 3.0 × 2.8 ± 0.5), 0(–1)-septate, with 1–3(–4) distal hila, secondary ramoconidia ellipsoid to cylindrical, sometimes almost doliiform, 8–32(–38) × (2.5–)3–4(–5) μm (av. ± SD: 15.6 ± 6.3 × 3.5 ± 0.5), 0–1(–3)-septate, not constricted at septa, pale olivaceous to pale olivaceous-brown, smooth or almost so, walls unthickened or almost so, with 2–5 distal hila, intercalary conidia and secondary ramoconidia sometimes formed in dense whirls at the conidiogenous cells or secondary ramoconidia, hila conspicuous, subdenticulate, 0.5–2(–2.5) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 50–68 mm diam after 14 d, olivaceous-grey, grey-olivaceous towards margins, reverse leaden-grey to olivaceous-black, floccose to fluffy, margins white to grey-olivaceous, feathery, regular, aerial mycelium abundant, covering the whole colony surface, floccose to fluffy, growth flat to low convex, without prominent exudates, sporulation profuse. Colonies on MEA reaching 50–62 mm diam after 14 d, olivaceous-grey to pale olivaceous-grey, sometimes smoke-grey or white, reverse olivaceous-grey to iron-grey, floccose to felty, margins white, narrow, feathery, regular, aerial mycelium abundant, covering the whole colony surface, growth flat to low convex, sometimes radially furrowed, without prominent exudates, sporulation profuse. Colonies on OA attaining 58–70 mm diam after 14 d, olivaceous to greenish olivaceous, olivaceous-grey at margins, reverse leaden-grey to olivaceous-grey, floccose to felty, margins glabrous, aerial mycelium abundant covering almost the whole colony surface, loose to dense, low to rarely high, growth flat, without prominent exudates, sporulation profuse.
Specimens examined: Canada, Ontario, isol. from galls of Apiosporina morbosa (Venturiaceae) on Prunus sp. (Rosaceae), 2005, coll. K.A. Seifert, isol. P.W. Crous, CBS 126347 = CPC 12102. Colombia, near Cogna, bosque andino, ca. 3000 m alt., isol. from Puya sp. (Bromeliaceae), W. Gams, depos. May 1980, isol. by W. Gams, CBS 274.80C. Russia, Moscow region, isol. from Aureobasidium caulivorum (Dothioraceae), ident. by W. Gams, stored as “C. epichloës”, CBS 574.78C = VKM F-2759. U.S.A., South Carolina, Aiken, on Lycoperdon sp. (Agaricales), Ravenel & Cooke, Fungi Amer. Exs. 595, K 121561, lectotype; isolectotypes: Ravenel & Cooke, Fungi Amer. Exs. 595, e.g., BPI 427244, NY; Washington, Seattle, University of Washington campus, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus avellana (Betulaceae), 12 Feb. 2004, D. Glawe, CBS 126348, CPC 11833.
Substrates and distribution: On ascomycetes and fruiting bodies of different basidiomycetous fungi; Europe (Germany, Russia), North America (Canada, U.S.A.) and South America (Colombia, Uruguay).
Literature: Saccardo (1886: 368), Heuchert et al. (2005).
Notes: Cladosporium lycoperdinum, which was originally described on Lycoperdon sp. from North America (Cooke 1883), seems to be a quite common saprobic species occurring on different fungal fruit bodies of basidiomycetes but also on ascomycetes as already stated in Heuchert et al. (2005). Since none of the strains included in this study have been isolated from a basidiomycete we hesitate to propose an epitype for this species until more isolates are available. However, measurements of conidiophores and conidia of the above listed strains agree very well with the species description of C. lycoperdinum (Heuchert et al. 2005).
The CBS strain 274.80C clusters with the other three isolates identified as C. lycoperdinum (Fig. 1, part a; distance analyses in TreeBASE) but is slightly different in morphology (conidiophores shorter and narrower, shorter and somewhat narrower secondary ramoconidia). It is tentatively maintained in the latter species until additional isolates can be included.
Cladosporium myrtacearum K. Schub., U. Braun & R.G. Shivas, in Braun et al., Australas. Pl. Pathol. 34: 509. 2005. Fig. 49.
Fig. 49.
Cladosporium myrtacearum (CBS 126350). A–H. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Mycelium branched, branches often only as short lateral outgrowths, rhizoid, 1–6 μm wide, septate, with swellings and constrictions, therefore often irregular in outline, subhyaline to medium olivaceous-brown or dingy brown, smooth to often appearing rough-walled, minutely verruculose to irregularly rough-walled, walls unthickened or somewhat thickened, sometimes forming ropes or hyphae twisted. Conidiophores macronematous, sometimes also micronematous, solitary or in loose groups, arising terminally and laterally from ascending, erect and plagiotropous hyphae, erect, straight to flexuous, cylindrical-oblong, geniculate towards the apex, sometimes subnodulose towards the tip, but also intercalary, with unilateral swellings giving conidiophores a gnarled, knotty appearance, unbranched, occasionally once branched, 9–85(–120) × (2–)3–4.5(–5) μm, (0–)1–3(–5)-septate, not constricted at septa, septa sometimes not very conspicuous, pale to medium olivaceous-brown or dingy brown, sometimes paler towards the apex, smooth or almost so, walls thickened, up to 1 μm wide. Conidiogenous cells integrated, terminal, cylindrical-oblong, geniculate, once or sometimes several times, subnodulose with unilateral swellings due to geniculations, occasionally nodulose, 12–45 μm long, with up to six loci, crowded towards the apex, situated on small lateral shoulders but not confined to swellings, loci conspicuous, subdenticulate to denticulate, 1–1.8 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, 17–30 μm long, about 4 μm wide. Conidia catenate, in branched chains, branching in all directions, up to seven conidia in the unbranched terminal part of the chain, smooth to sometimes verruculose, small terminal and intercalary conidia obovoid to narrowly ellipsoid, mostly fusiform, 5–10(–12) × 2–4 μm (av. ± SD: 8.1 ± 1.8 × 2.9 ± 0.6), aseptate, rarely with a single septum, distinctly attenuated towards apex and base, secondary ramoconidia fusiform, ellipsoid to subcylindrical, (9–)10–20(–25) × 3–4(–4.5) μm (av. ± SD: 14 ± 3.4 × 3.5 ± 0.5), 0–1(–2)-septate, not constricted at septa, septa not very conspicuous, median or somewhat in the upper half, pale or sometimes medium brown or dingy brown, smooth to finely verruculose or somewhat irregularly rough-walled, attenuated towards apex and base, walls unthickened or slightly thickened, with up to five distal hila, sometimes also laterally, hila protuberant, subdenticulate, 0.8–1.8(–2) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 40–57 mm diam after 1 mo, olivaceous-grey, grey-olivaceous towards margins or iron-grey both surface and reverse, buff due to aerial mycelium, towards the margins becoming slimy, not sporulating, appearing zonate, mycelium often aggregated, forming “dread locks”, margin white, regular, glabrous, growth flat with elevated colony centre, aerial mycelium formed, often aggregated, without prominent exudates, sporulating. Colonies on MEA reaching 44–70 mm diam after 1 mo, iron-grey to smoke-grey due to sporulation, whitish towards margins, olivaceous-grey reverse, white to buff towards margins, fluffy, hairy, margin white, glabrous, radially furrowed, aerial mycelium abundantly formed, covering the whole surface, dense, hairy to fluffy, colonies wrinkled, without prominent exudates, sporulation sparse.
Specimens examined: Australia, New South Wales, Bimbadeen Lookout, ca. 10 km of Cessnock, North Coast, isol. from Eucalyptus placita (Myrtaceae), 14 Oct. 2006, coll. B.A. Summerell, isol. P.W. Crous, NSM 734672, CBS 126349 = CPC 13689; Northern Territory, Emerald Springs, S13°37'23, E131°36'40, isol. from Corymbia foelscheana (Myrtaceae), 22 Sep. 2007, coll. B.A. Summerell, isol. P.W. Crous, CBS H-20440, epitype of C. myrtacearum, designated here; ex-type culture: CBS 126350 = CPC 14567; Millingimbi, Townsite, on Corymbia polycarpa, 17 Aug. 1999, A.A. Mitchel, BRIP 26527, holotype, DNAP 26527, isotype.
Substrate and distribution: On Myrtaceae (Corymbia, Eucalyptus); Australia.
Notes: Cladosporium myrtacearum recently described as a leaf-spotting species occurring on Corymbia foelscheana in Australia was known until now only from the type collection (Braun et al. 2005, Schubert 2005b). An additional collection from the same host and country could be examined and included in this study which agrees well with the original description of C. myrtacearum. It is designated here as epitype of this species. However, the biology of C. myrtacearum remains unclear. Although leaf spots similar to those of the type specimen occurred on the infected leaves, sporulation was not connected with these spots.
A second strain isolated from Eucalyptus placita from New South Wales (CPC 13689) clusters with the epitype strain of C. myrtacearum and forms a highly supported subclade (see Fig. 1, part a). Conidiophore and conidial measurements are almost identical but the mycelium in strain CPC 13689 is slightly wider, often formed in ropes or twisted, conidiophores are only macronematous and conidia are somewhat paler and smooth. However, these morphological differences are probably within species variation, but on both ACT and TEF these two isolates are not identical and more strains are needed for clarification.
In vitro C. myrtacearum is morphologically reminiscent of C. antarcticum, but the latter species differs in having dimorphic mycelium, longer and wider, minutely verruculose or verrucose conidia and secondary ramoconidia (Schubert et al. 2007b).
Cladosporium oxysporum Berk. & M.A. Curtis, in Berkeley, J. Linn. Soc. Bot. 10: 362. 1869. Figs 50, 51.
Fig. 50.
Cladosporium oxysporum (CBS 125991). Macro- and micronematous conidiophores, conidia and microcyclic conidiogenesis with conidia forming secondary conidiophores. Scale bar = 10 μm.
Fig. 51.
Cladosporium oxysporum (CBS 125991). A–C. Tips of macronematous conidiophores, being typically nodulose in A and C. D–H. Intercalary conidiogenous cells and conidia. Scale bar = 10 μm.
Mycelium internal and superficial, hyphae loosely branched, 1–4 μm wide, septate, not constricted at septa, but sometimes irregular due to swellings and constrictions, subhyaline to pale olivaceous, darker towards the base of the conidiophores, medium olivaceous-brown, smooth, walls unthickened or slightly thick-walled, occasionally forming ropes. Conidiophores macronematous, sometimes also micronematous, solitary, arising terminally and laterally from hyphae, erect, straight to slightly flexuous, nodose with nodes being quite apart of each other, with a single node at the apex or just below and few additional nodes on a lower level, 1–7 nodes per conidiophore, swellings 3–6 μm wide, unbranched, rarely branched, conidiophores long, 40–720 μm or even longer, 2–4 μm wide, at the base up to 5 μm wide, slightly attenuated towards the apex, pluriseptate, not constricted at septa, pale to medium olivaceous-brown, sometimes dark brown, often paler at the apex, smooth, walls somewhat thick-walled, about 0.5(–1) μm wide; micronematous conidiophores paler, narrower and shorter, also with nodules or only subnodulose or geniculate, often attenuated towards the apex, 30–115 × 1.5–2 μm, nodes 3–4 μm wide. Conidiogenous cells integrated, terminal and intercalary, with a single node, conidiogenous loci confined to these swellings, with 1–4 loci per node, sometimes subnodulose or once geniculate, 14–46 μm long, loci conspicuous, subdenticulate, 0.8–1.5 μm diam, somewhat thickened and darkened-refractive. Ramoconidia rarely occurring. Conidia catenate, in branched chains, up to five conidia in the terminal unbranched part of the chain, branching in all directions, small terminal conidia globose, subglobose to obovoid, 3–5 × 2–3 μm (av. ± SD: 4.2 ± 0.6 × 2.5 ± 0.4), aseptate, apex rounded, intercalary conidia ovoid, limoniform to ellipsoid, (4–) 5–11 × 2.5–3.5(–4) μm (av. ± SD: 7.2 ± 1.9 × 3.0 ± 0.4), aseptate, rarely 1-septate, not constricted at septa, with 2–5(–6) distal hila, attenuated towards apex and base, secondary ramoconidia ellipsoid to subcylindrical, 7–21(–24) × (2.5–)3–4 μm (av. ± SD: 15.0 ± 5.1 × 3.3 ± 0.4), 0–1-septate, not constricted at septa, pale olivaceous to pale olivaceous-brown, smooth, walls unthickened or almost so, with 2–4(–5) distal hila, subdenticulate, 0.5–1.5(–2) μm diam, thickened and darkened-refractive; sometimes microcyclic conidiogenesis occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA attaining 68–78 mm diam after 14 d, smoke-grey to pale olivaceous-grey, reverse leaden-grey, grey-olivaceous at margins both surface and reverse, felty-floccose, margins broad, regular, whitish, glabrous, aerial mycelium abundant, covering most parts of the colony surface, felty-floccose or fluffy, growth low convex, without prominent exudates, sporulating. Colonies on MEA attaining 58–70 mm diam after 14 d, whitish to smoke-grey or pale olivaceous-grey at margins, reverse olivaceous-grey to black, woolly-floccose, margins colourless to whitish, glabrous, regular, radially furrowed, aerial mycelium abundant, covering most of the colony surface, woolly-floccose, whitish to smoke-grey, growth low convex, without prominent exudates, sporulation sparse. Colonies on OA reaching 53–62 mm diam after 14 d, whitish to pale olivaceous-grey or smoke-grey, reverse leaden-grey, pale olivaceous-grey at margins, woolly-floccose, margins colourless, narrow, glabrous, regular, aerial mycelium abundant, white to smoke-grey, densely woolly-floccose, covering most of the colony surface, growth flat to low convex, sometimes several prominent exudates, sporulation sparse.
Specimens examined: China, terracotta army site, isol. from soil, 2000, coll. S. Gravesen, isol. B. Andersen, CBS H-20441, CBS 125991 = CPC 14371, BA 1738, reference strain of C. oxysporum. Venezuela, isol. from indoor air before renovation in lab, 2007, coll. K. Lyhne, isol. B. Andersen, BA 1707 = CBS 126351 = CPC 14308.
Substrates and distribution: Isolates from air, soil, on dead parts of leaves and stems of herbaceous and woody plants and other organic matter; common and widespread, especially in the tropics and subtropics.
Literature: Saccardo (1886: 363), Ellis (1971: 312), McKemy & Morgan-Jones (1991), David (1997: 81), Bagyanarayana & Braun (1999: 13), Ho et al. (1999: 137), de Hoog et al. (2000: 589), Schubert & Braun (2004: 308–309), Heuchert et al. (2005: 48).
Notes: Cladosporium oxysporum, a common and widespread saprobe, especially in the tropics and subtropics on dead parts of leaves and stems of herbaceous and woody plants, occurs also as secondary invader on necrotic leaf lesions caused by other fungi, and has been recorded to induce leaf spots on several host plants (Fisher 1967, Hammouda 1992, Lamboy & Dillard 1997). With its usually long, more or less regularly nodulose or nodose conidiophores and smooth, 0–1(–2)-septate conidia, it is a well-characterised species easily distinguishable from the morphologically allied C. herbarum and C. colocasiae. Type material of C. oxysporum has been re-examined, described and illustrated by McKemy & Morgan-Jones (1991) and David (1997), confirming the interpretation of the species by Ellis (1971) to be accurate. Type material is in poor condition and should therefore not be re-examined. Only few typical conidia and nodulose conidiophores could be observed (Schubert 2005b).
Compared with the high number of isolates identified as C. cladosporioides and C. tenuissimum included in this study, and although C. oxysporum is considered to be common, only two isolates could be assigned to this species. This is maybe due to the sampling, since there are only few isolates from subtropical or tropical areas, or maybe C. oxysporum is not as ubiquitous as some other species of the genus, which was already presumed by McKemy & Morgan-Jones (1991). CBS strain 125991 from China agrees very well with the species concept of C. oxysporum and can therefore serve as reference strain until additional isolates especially from the Carribean Sea are available from which a suitable epitype can be selected.
An unusual phenomenon of the two strains included in this study on PDA is that the conidiophores do not possess the typical swellings, but are unbranched or branched, once or several times geniculate-sinuous, secondary ramoconidia are longer, up to 37 μm long and true ramoconidia are formed.
CBS strain 125.80, which was used as reference strain for C. oxysporum in de Hoog et al. (2000), Wirsel et al. (2002) and Zalar et al. (2007) and illustrated by de Hoog et al. (2000) with nodose conidiophores, is not conspecific with this species. Morphologically it belongs to the C. cladosporioides s. lat. complex, and clusters quite apart from C. oxysporum (see Fig. 1, part a vs. c; distance analyses in TreeBASE) which suggests that it probably got contaminated.
Cladosporium paracladosporioides Bensch, Crous & U. Braun, sp. nov. MycoBank MB517084. Figs 52, 53.
Fig. 52.
Cladosporium paracladosporioides (CBS 171.54). Dimorphic mycelium, macro- and micronematous conidiophores, ramoconidia, conidia and microcyclic conidiogenesis. Scale bar = 10 μm.
Fig. 53.
Cladosporium paracladosporioides (CBS 171.54). A–C, E–F. Macronematous conidiophores and conidial chains. D, H. Conidial chains, septa of secondary ramoconidia distinctly darkened. G. Microcyclic conidiogenesis. Scale bars = 10 μm.
Etymology: Epithet derived from its similar morphology to Cladosporium cladosporioides.
Cladosporii cladosporioidis valde simile, sed conidiis 0–3-septatis et minute asperulatis discernitur. Differt a Cladosporio iranico conidiis terminalibus et intercalaribus latioribus, 3–3.5(–4) μm, ramoconidiis secundariis latioribus, (3–)3.5–5 μm, 0–3-septatis, catenulis terminalibus brevioribus, usque ad 4 conidiis.
Mycelium immersed and abundantly superficial; hyphae sparingly branched, 1.5–5 μm wide, septate, not constricted at septa, pale brown to medium brown or olivaceous-brown, septa mostly quite regular, sometimes 2–3 in short succession, smooth to asperulate or irregularly rough-walled, somewhat dimorphic, many hyphae look like macronematous conidiophores but are not yet sporulating, medium brown and smooth. Conidiophores macro- and micronematous, arising terminally and laterally from ascending or plagiotropous hyphae, erect, straight to mostly flexuous, sometimes hardly distinguishable from hyphae. Macronematous conidiophores cylindrical-oblong, non-nodulose, sometimes distinctly geniculate-sinuous due to sympodial proliferation, once or several times, unbranched or once branched, (17–)50–180(–300) × (2–)3–4 μm, pluriseptate, sometimes slightly constricted at septa, pale to medium brown or olivaceous-brown, smooth to irregularly asperulate or loosely delicately verruculose, walls thickened. Conidiogenous cells integrated, terminal, cylindrical-oblong, sometimes intercalary, occasionally geniculate-sinuous, 8–40 μm long, with 2–4 apical loci or up to five loci per cell, sometimes situated on lateral shoulders or on short lateral prolongations at the apex, protuberant, denticle-like, 1.5–2 μm diam, thickened and darkened-refractive. Micronematous conidiophores narrower and paler, filiform, unbranched, up to 165 μm long or even longer, 2–2.5(–3) μm wide, subhyaline to pale brown. Conidiogenous cells integrated, terminal, narrowly cylindrical-oblong, usually with a single apical locus, sometimes with few loci. Ramoconidia occasionally formed, hardly distinguishable from secondary ramoconidia. Conidia catenate, in branched chains, up to four conidia in the unbranched terminal part of the chain, straight, small terminal conidia obovoid, subglobose, sometimes obpyriform, 4–7(–14) × 3–3.5 μm (av. ± SD: 5.3 ± 1.1 × 3.1 ± 0.2), aseptate, intercalary conidia limoniform, fusiform to ovoid, 6–9(–11) × 3–3.5(–4) μm (av. ± SD: 7.7 ± 1.5 × 3.4 ± 0.3), 0(–1)-septate, with 1–2(–3) distal hila, secondary ramoconidia ellipsoid, fusiform to subcylindrical, with up to four distal hila, 7–26(–30) × (3–)3.5–5 μm (av. ± SD: 16.2 ± 6.2 × 4.0 ± 0.4), sometimes obclavate, up to 36 μm long, 0–3-septate, sometimes slightly constricted at septa, first septum median or often slightly to distinctly in the upper half, sometimes in the lower, with 2–3 septa sometimes irregular, cells of different size, septa darkened, pale brown, smooth or almost so to very finely asperulate, walls somewhat thickened, attenuated towards apex and base, hila protuberant, denticulate, broadly truncate, 0.8–2(–2.5) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occurring, forming secondary conidiophores.
Culture characteristics: Colonies on PDA olivaceous-grey to iron-grey and olivaceous-black, reverse greenish grey to olivaceous-black, woolly-fluffy, margin colourless to white, glabrous to feathery, very narrow, aerial mycelium fluffy, loose, high, colonies somewhat shiny, without prominent exudates, sporulation profuse. Colonies on MEA olivaceous-grey to greenish olivaceous due to profuse sporulation, pale olivaceous-grey towards margins, somewhat zonate, reverse olivaceous-grey, velvety to felty, margin narrow, colourless to white, radially furrowed, colonies folded and wrinkled, aerial mycelium abundant, loose to dense, without conspicuous exudates, sporulating. Colonies on OA olivaceous-grey to iron-grey, smoke-grey due to mycelium and sporulation, reverse greenish black to leaden-grey, powdery to felty, margin colourless to white, narrow, glabrous, aerial mycelium loose, diffuse to dense, felty-fluffy, without exudates, sporulating.
Specimen examined: Isol. by G.A. de Vries, deposited by Raistrick, No. 4079, Sep. 1954, CBS H-20442, holotype; ex-type culture CBS 171.54 = ATCC 11278 = 200943 = IFO 6369 = IMI 049626 = MUCL 917 = NCTC 4097.
Substrate and distribution: Substrate and distribution unknown.
Notes: Cladosporium paracladosporioides is morphologically similar to C. cladosporioides and the newly introduced C. iranicum, but the latter species differs in having narrower terminal and intercalary conidia that are often subrostrate or rostrate and narrower, 0–1(–2)-septate secondary ramoconidia with conidial chains being much longer forming up to 10 conidia in the terminal unbranched part of the chain. Cladosporium cladosporioides is distinct in that the conidia are usually aseptate, smooth and somewhat narrower. Phylogenetically the new species is closely allied to C. varians (see Fig. 1, part a; distance analyses in TreeBASE) but deviates in having longer and wider conidiophores, wider conidiogenous loci and hila and longer intercalary conidia and secondary ramoconidia (Braun et al. 2008b).
Cladosporium perangustum Bensch, Crous & U. Braun, sp. nov. MycoBank MB517085. Figs 54, 55, 56.
Fig. 54.
Cladosporium perangustum (CBS 125996). Macro- and micronematous conidiophores, mycelium often formed in dense ropes, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 55.
Cladosporium perangustum (CBS 125996). A–G. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Fig. 56.
Cladosporium perangustum (CBS 125996). A. Conidia with very gentle surface ornamentation showing irregularly reticulate structures. B. A coherent view on conidiophores, stipes, aerial hyphae and conidia. C. Secondary ramoconidia, conidia and scars. The conidia at the upper right show some cell wall structures. D. Conidiophore with secondary ramoconidia, intercalary and small terminal conidia. Note the disruptions of the cell walls between the conidia. E. Scars on very elongated secondary ramoconidia. F. Scar-pattern at the end of the conidiophores. Note the flattened separation domes. G. Ropes of aerial hyphae. H. Running segmented hyphae that may form conidiophores and not segmented aerial hyphae. Note the blastoconidium on one hypha. Scale bars = 2 (A, C, E–F), 5 (D, G), 10 (B, H) μm.
Etymology: Named after the narrow conidia.
Cladosporio scabrello valde simile, sed conidiophoris et ramoconidiis secundariis leniter angustioribus distinguitur. Differt a Cladosporio exili conidiophoris et ramoconidiis leniter angustioribus, conidiis intercalaribus longioribus, conidiis leniter angustioribus, locis conidiogenis et hilis angustioribus.
Mycelium internal and superficial; hyphae filiform to narrowly cylindrical-oblong, loosely branched, (0.5–)1–4 μm wide, septate, sometimes slightly constricted at septa, sometimes irregular due to intercalary swellings and constrictions, subhyaline to pale olivaceous or pale olivaceous-brown, smooth to usually verruculose or irregularly rough-walled, walls unthickened or almost so, sometimes swollen at the base of conidiophores, sometimes forming dense ropes. Conidiophores solitary, sometimes in pairs, macronematous, semimacronematous or micronematous, arising terminally and laterally from hyphae or from swollen hyphal cells, erect, straight or slightly flexuous, filiform to narrowly cylindrical-oblong, usually neither geniculate nor nodulose, sometimes geniculate-sinuous or unilaterally slightly swollen at the apex, unbranched, occasionally branched, once or several times, branches short, peg-like or up to 30 μm long, conidiophores (8–)12–130(–150) × (1.5–)2–3.5(–4) μm, 0–6 septate, usually not constricted at septa, occasionally septa darkened, subhyaline, pale olivaceous or pale olivaceous-brown, more or less rough-walled, especially towards the base of conidiophores, asperulate-verruculose, at the apex smooth or almost so, walls unthickened or slightly thickened, about 0.5 μm wide, sometimes slightly attenuated towards the apex, at the base sometimes up to 4.5 μm wide. Conidiogenous cells integrated, mainly terminal, sometimes also intercalary or pleurogenous, narrowly cylindrical-oblong, sometimes geniculate-sinuous, non-nodulose, in intercalary cells loci situated on small peg-like lateral prolongations or just below the septum, 7–40 μm long, with 1–4(–5) apically crowded loci, forming clusters of pronounced scars, conspicuous, subdenticulate to denticulate, 0.8–1.5 μm diam, thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 25–45 × 2.5–3(–4.5) μm, aseptate, rarely 1(–2)-septate, base truncate, 2–2.5(–4) μm wide, sometimes slightly darkened or refractive. Conidia numerous, catenate, in branched chains, branching in all directions, 1–4 conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose or ovoid to obovoid, 2–4(–5) × (1.5–)2–2.5 μm (av. ± SD: 3.1 ± 0.6 × 2.1 ± 0.2), apex broadly rounded or slightly attenuated, intercalary conidia ovoid, limoniform to ellipsoid, somewhat fusiform or subcylindrical, 4–16(–19) × 2–3(–3.5) μm (av. ± SD: 8.7 ± 3.8 × 2.5 ± 0.4), 0(–1)-septate, attenuated towards apex and base, with 1–3(–5) distal hila, secondary ramoconidia narrowly ellipsoid to cylindrical-oblong, 6–30(–34) × 2–3(–3.5) μm (av. ± SD: 17.8 ± 7.4 × 2.5 ± 0.4), 0–1(–3)-septate, septum median or often somewhat in the upper half, with 2–4(–7) distal hila, pale olivaceous-brown, smooth or almost so to finely verruculose (LM), under SEM smooth or surface with somewhat irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shriveling of tender conidia, thin-walled, hila conspicuous, subdenticulate to denticulate, (0.8–)1–1.5(–1.8) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring.
Culture characteristics: Colonies on PDA attaining 33–76 mm diam after 14 d, grey-olivaceous to olivaceous, olivaceous-grey or iron-grey, sometimes with patches of smoke-grey or pale greenish grey, reverse dull green, leaden-grey to olivaceous-grey, iron-grey or olivaceous-black, sometimes releasing an olivaceous-buff or orange to luteous soluble pigment into the agar, fluffy, floccose or powdery, margins glabrous to feathery, whitish, olivaceous-buff or pale luteous due to the pigment, broad, regular or somewhat undulate, aerial mycelium diffuse to loosely floccose or felty, growth effuse, usually without prominent exudates, occasionally numerous small to large prominent exudates formed, sporulation profuse. Colonies on MEA reaching 40–72 mm diam after 14 d, pale olivaceous-grey to glaucous-grey or grey-olivaceous, whitish to smoke-grey due to aerial mycelium, reverse olivaceous-grey to iron-grey, occasionally releasing an orange solube pigment into the agar, velvety to floccose, margins white, narrow, regular to undulate, glabrous to somewhat feathery, aerial mycelium abundantly formed, covering most parts of colony surface, loosely to densely floccose or felty, white to pale olivaceous-grey or smoke-grey, growth effuse with sometimes elevated colony centre, radially furrowed, sometimes few small prominent exudates formed, sporulation profuse. Colonies on OA 40–75 mm diam after 14 d, whitish to smoke-grey and pale olivaceous-grey or grey-olivaceous, reverse pale olivaceous-grey, pale greenish grey to olivaceous-grey, leaden-grey or sometimes amber-coloured due to the pigment released into the agar, velvety or fluffy to felty-floccose, margins colourless or greenish olivaceous, glabrous, regular, aerial mycelium abundant, covering large parts of the colony surface, dense, low to high, white, growth effuse, sometimes few prominent exudates formed, sporulating.
Specimens examined: Sine loco, sine dato, isol. by C.H. Hassall, No. 4-1949, ident. by G.A. de Vries as C. cladosporioides, CBS 167.54 = ATCC 11276 = IMI 049624. Australia, isol. from margarine, N. Charley, CPC 11046; isol. from Eucalyptus placita (Myrtaceae), coll. B.A. Summerell, isol. P.W. Crous, CPC 13686; Northern Territory, Emerald Springs, S13°37'23, E131°36'40, isol. from Corymbia foelscheana (Myrtaceae), 22 Sep. 2007, coll. B.A. Summerell, isol. P.W. Crous, CPC 14566; isol. from Erythrophleum chlorostachys (Fabaceae), 9 Jan. 2007, B.A. Summerell, CBS 126364 = CPC 14532. Germany, Essen, botanical garden, 51.45, 7.0167, isol. from Morus rubra (Moraceae), 2005, N. Ale-Agha, CPC 12216. India, isol. from Eucalyptus sp. (Myrtaceae), 3 Jan. 2004, coll. W. Gams, isol. P.W. Crous, CPC 11133; isol. from Musa sp. (Musaceae), 25 Oct. 2004, M. Arzanlou, CPC 11609. New Zealand, Auckland, Auckland University campus, isol. from leaves of Oncoba spinosa (Salicaceae), Sep. 2004, C.F. Hill, Hill 1076-1 = CPC 11663. Polynesia, reserve Pun Kukui in forest, isol. from banana “Eka ulu”, 2006, coll. I. Budenhagen, isol. P.W. Crous, CPC 12792, 12793. South Africa, Alkmar, Laeveld Coop, isol. from wheat, 1988, CPC 14008 = MRC 10135, as C. sphaerospermum; Durban, botanical garden Durban near Reunion, -29.85, 31.0167, isol. from Strelitzia sp. (Strelitziaceae), 2005, coll. W. Gams, isol. P.W. Crous, CPC 11806; Free State, Danielsrus, isol. from oats, 1983, CPC 14004 = MRC 03367; Transkei, Mazeppa Bay, isol. from Strelitzia sp., growing on fruiting structures, 1 June 2008, P.W. Crous, CPC 14911; Pretoria, Walter Sisulu park, isol. from Protea caffra (ascospore isolate) (Proteaceae), 2 Jan. 2007, P.W. Crous, CPC 13730, 13774; isol. from Teratosphaeria maculiformis (Teratosphaeriaceae) on Protea caffra, 2 Jan. 2007, P.W. Crous, CPC 13727; isol. from Cussonia sp. (Araliaceae), 20 Feb. 2007, P.W. Crous, CBS H-20451, holotype; ex-type culture CBS 125996 = CPC 13815; Western Cape Province, Jonkershoek Nature Reserve, isol. from Teratosphaeria fibrillosa (Teratosphaeriaceae), 30 Mar. 2007, P.W. Crous, CPC 13870; Western Cape, Betties Bay, Harold Porter National park, isol. from Protea cynaroides, 4 Dec. 2008, L. Mostert, CPC 15192. Thailand, isol. from Acacia mangium (Fabaceae), 2005, coll. W. Himaman, isol. P.W. Crous, CPC 11526, 11856. U.S.A., Louisiana, Baton Rouge, isol. from Magnolia sp. (Magnoliaceae), 8 Sep. 2007, P.W. Crous, CPC 14247; isol. from leaves of pecan tree, 8 Sep. 2007, P.W. Crous, CPC 14256; Washington, Seattle, University of Washington campus, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus avellana (Betulaceae), 16 Sep. 2004, D. Glawe, CBS 126365 = CPC 11820, CPC 11815, 11819, 11821, 11831.
Substrates and distribution: On plant material, ascomycetes, isolated from food; widely distributed, Africa (South Africa), Asia (India, Thailand), Australasia (Australia, New Zealand, Polynesia), Europe (Germany), North America (U.S.A.).
Notes: Cladosporium exile and C. scabrellum are morphologically comparable with C. perangustum but C. exile differs in having usually longer and somewhat wider conidiophores, slightly wider ramoconidia and conidia, shorter intercalary conidia and somewhat wider conidiogenous loci and hila. In C. scabrellum the conidiophores are mainly macronematous and somewhat wider and secondary ramoconidia slightly wider. Phylogenetically these three species cluster quite apart from each other (see Fig. 1, part b vs. a; distance analyses in TreeBASE).
Due to its numerous subglobose or globose often finely verruculose terminal conidia, C. perangustum is reminiscent of C. sphaerospermum, but the latter species is easily distinguishable by having wider densely septate conidiophores, 0–5-septate ramoconidia and somewhat wider, 0–3(–4)-septate secondary ramoconidia (Zalar et al. 2007). Cladosporium cladosporioides is distinct by having usually smooth aseptate conidia, wider conidiophores, ramoconidia and secondary ramoconidia and wider conidiogenous loci and hila.
Similar to C. cladosporioides and C. pseudocladosporioides, C. perangustum could probably also be a species complex, since there are more phylogenetic differences in that clade for both ACT and TEF than between some of the other more closely related species that we recognise as distinct species. However, the morphology of all isolates is quite uniform and the clade phylogenetically highly supported (posterior probability value 0.99, see Fig. 1, part b; distance analyses in TreeBASE). Therefore, we prefer to treat it as a single species until we can include more isolates or use additional features that allow the recognition of cryptic species.
Cladosporium phyllactiniicola Bensch, Glawe, Crous & U. Braun, sp. nov. MycoBank MB517086. Figs 57, 58.
Fig. 57.
Cladosporium phyllactiniicola (CBS 126352). Macro- and micronematous conidiophores, mycelium and conidial chains. Scale bar = 10 μm.
Fig. 58.
Cladosporium phyllactiniicola (CBS 126352). A–H. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the substrate from which the species was isolated, chasmothecia of Phyllactinia guttata.
Cladosporii uredinicolae simile, sed conidiophoris brevioribus, conidiis intercalaribus brevioribus, 0(–1)-septatis et tamen ramoconidiis secundariis brevioribus, 0–1(–2)-septatis internoscitur. Differt a Cladosporio exili conidiis terminalibus et intercalaribus latioribus, 2–4 μm latis, ramoconidiis secundariis brevioribus et latioribus, 5–17(–24) × (2–)3–4.5 μm.
Mycelium immersed and superficial, plagiotropous, ascending to erect, not dimorphic; hyphae sparingly branched, 1–5(–6) μm wide, septate, not constricted at septa, sometimes swollen, subhyaline to pale brown, minutely verruculose to irregularly rough-walled, especially at the base of conidiophores, sometimes forming ropes, often irregular in outline. Conidiophores macro- and micronematous, arising laterally and terminally from plagiotropous and ascending hyphae, erect, straight to slightly flexuous. Macronematous and semimacronematous conidiophores cylindrical-oblong, non-nodulose, sometimes geniculate towards the apex, unbranched or once branched, 6–105(–120) × 2.5–5(–6) μm, 0–6(–7)-septate, occasionally slightly constricted at septa, pale to pale medium brown or olivaceous-brown, smooth or almost so, walls unthickened in the younger conidiophores, thickened in the older ones, sometimes slightly attenuated towards the apex. Conidiogenous cells integrated, mainly terminal, cylindrical-oblong, sometimes slightly geniculate, 6–25 μm long, with 2–4 conspicuous, subdenticulate to denticulate loci, sometimes forming small clusters or situated on lateral shoulders formed due to sympodial proliferation or on small lateral proliferations, loci protuberant, 1–2 μm diam, thickened and darkened-refractive. Micronematous conidiophores narrowly cylindrical-oblong to mostly filiform, unbranched, non-geniculate and non-nodulose, often only as short lateral outgrowths of hyphae, 8–40 × 2–2.5 μm, with few septa, subhyaline, smooth, walls unthickened. Conidiogenous cells integrated, terminal, 7–15 μm long, with a single locus or up to three apical loci, 1–1.2 μm diam. Ramoconidia occasionally formed, up to 28 μm long, 3.5–4 μm wide, base about 3 μm wide. Conidia catenate, in branched chains, branching in all directions, up to four conidia in the unbranched terminal part, straight, small terminal conidia subglobose to obovoid, 3–6(–7) × 2–4 μm (av. ± SD: 4.2 ± 1.4 × 2.8 ± 0.8), aseptate, attenuated towards the base, broadly rounded at the apex, intercalary conidia limoniform to ellipsoid-ovoid, 5–10 × 3–4(–4.5) (av. ± SD: 6.2 ± 1.1 × 3.4 ± 0.6), 0(–1)-septate, secondary ramoconidia limoniform to usually narrowly to broadly ellipsoid-ovoid to subcylindrical, 5–17(–24) × (2–)3–4.5 μm (av. ± SD: 11.8 ± 4.2 × 3.7 ± 0.7), conidia formed by micronematous and semimacronematous conidiophores shorter and narrower, 0–1(–2)-septate, mainly aseptate, not constricted at septa, subhyaline to pale brown or pale olivaceous-brown, smooth or almost so to finely asperulate, walls unthickened to slightly thickened, often almost not attenuated towards apex and base, hila conspicuous, subdenticulate to denticulate, 0.5–2 μm diam, thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA olivaceous-grey to iron-grey, smoke-grey to pale olivaceous-grey due to aerial mycelium, reverse leaden-grey, felty-woolly, margin narrow, white, somewhat feathery, aerial mycelium sparse to abundant, felty, high, sometimes few small, not very prominent exudates, formed, sporulating. Colonies on MEA olivaceous-grey surface and reverse or grey-olivaceous to greenish grey on surface, with patches of white or smoke-grey due to dense abundant aerial mycelium, fluffy, woolly, margin narrow, white, glabrous to somewhat feathery, greenish olivaceous at margins due to profuse sporulation, wrinkled, sometimes radially furrowed, without exudates, sporulating. Colonies on OA olivaceous-grey to grey-olivaceous or olivaceous, whitish to smoke-grey due to aerial mycelium, reverse leaden-grey, olivaceous-grey to iron-grey, margin narrow, white, glabrous, aerial mycelium absent, diffuse to dense, low to high, fluffy to felty-woolly, without prominent exudates, sporulating.
Specimens examined: U.S.A., Seattle, University of Washington campus, 47.6263 -122.3331, isol. from chasmothecia of Phyllactinia guttata (Erysiphales) on leaves of Corylus avellana (Betulaceae), 2 Dec. 2004, D. Glawe, CBS H-20443, holotype; ex-type culture CBS 126355 = CPC 11830, CBS 126352 = CPC 11836, CBS 126353 = CPC 11823, CBS 126354 = CPC 11825.
Substrate and distribution: Mycophilic occurring on chasmothecia of Phyllactinia guttata; U.S.A.
Notes: Cladosporium phyllactiniicola has to be compared with C. uredinicola and C. exile, the latter introduced in this paper as a new species. Cladosporium uredinicola, usually considered a hyperparasite on rust fungi but also recorded from downy and powdery mildews (Morgan-Jones & McKemy 1990, Heuchert et al. 2005), has longer conidiophores, longer, 0–2-septate intercalary conidia and longer, 0–3(–5)-septate secondary ramoconidia. Our single living isolate (ATCC 46649 = CPC 5390) of C. uredincola, putatively representative, was isolated from Cronartium fusiforme from North America, identified as C. uredinicola by Morgan-Jones & McKemy (1990) who compared it with Spegazzini's type specimen, and was further described by Ho et al. (1999). Unfortunately it is no longer sporulating. Phylogenetically this strain is quite distinct from C. phyllactiniicola (Fig. 1, part a vs. b; distance analyses in TreeBASE). Other strains identified as C. uredinicola proved to belong to species other than that represented by CPC 5390.
Cladosporium exile also isolated from chasmothecia of Phyllactinia guttata differs in having somewhat narrower conidiophores, narrower terminal and intercalary conidia, 2–3 μm wide, and longer and narrower secondary ramoconidia, 7–25(–35) × 2.5–3.5(–4) μm.
Cladosporium phyllophilum McAlpine, Agric. Gaz. New South Wales 7: 153. 1896. Fig. 59.
Fig. 59.
Cladosporium phyllophilum (CBS 125992). A–H. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
= Cladosporium exoasci Ellis & Barthol., in Shear, Fungi Columb., Cent. XV, No. 1493. 1901, nom. nud.
= Cladosporium exoasci Lindau, in Rabenhorst, Krypt.-Fl., ed. 2, 1(8): 808. 1907.
Mycelium immersed to superficial; sparingly branched, 1–4 μm wide, septate, not constricted at septa, pale, almost hyaline to olivaceous-brown or brown, smooth to irregularly rough-walled, verruculose or verrucose with large wart-like structures, walls thin or slightly thickened. Conidiophores semimacronematous or macronematous, solitary, arising laterally or terminally from plagiotropous or ascending hyphae. Macronematous conidiophores erect to decumbent, straight or flexuous, cylindrical-oblong, unbranched or branched, usually once, sometimes twice or up to four times, branches often start as short lateral outgrowth just below a septum, becoming longer with age, up to 65(–90) μm long, often at an angle of 45°, sometimes up to 90°, neither nodulose nor geniculate, 15–180 × 4–5(–6) μm, pluriseptate, not constricted at septa, pale olivaceous to medium olivaceous-brown or brown, smooth to sometimes asperulate, especially towards the apex, walls somewhat thickened, up to 1 μm wide, base sometimes also covered by wart-like structures. Conidiogenous cells integrated, terminal or intercalary, cylindrical-oblong, sometimes slightly geniculate towards the apex, 13–41 μm long, mostly with up to three or four subdenticulate protuberant loci, sitting close together at the apex, 1.5–2 μm diam., thickened and darkened-refractive. Semimacronematous conidiophores paler, smaller and narrower, unbranched or branched once or twice, 15–100 × 2–3 μm, septate, conidiogenous cells 7–19 μm long, with up to seven distal scars, subdenticulate, crowded at the apex, hila 1–1.5 μm diam. Ramoconidia occasionally formed (hardly distinguishable from secondary ramoconidia), cylindrical-oblong, 17–33 × 4 μm, aseptate, sometimes 1-septate, pale olivaceous, smooth, walls unthickened or almost so, base truncate. Conidia numerous, catenate, in branched chains, branching in all directions, 1–3 conidia in the terminal unbranched part of the chain, small terminal conidia obovoid to ovoid, 3–7 × 2–3 μm (av. ± SD: 4.8 ± 1.4 × 2.5 ± 0.3), aseptate, hila 0.5–0.8 μm diam, intercalary conidia ovoid, ellipsoid-ovoid, 6–13 × 3–4 μm (av. ± SD: 8.9 ± 2.1 × 3.2 ± 0.3), aseptate, with up to 4(–5) distal hila, hila 0.8–1.2 μm diam, secondary ramoconidia ellipsoid, subcylindrical to cylindrical, 7–32 × 2.5–4(–5) μm (av. ± SD: 18.7 ± 7.2 × 3.7 ± 0.6), 0(–2)-septate, not constricted at septa, subhyaline, pale olivaceous, smooth or almost so, with 1–4 distal hila, walls unthickened, hila 1–2.2 μm diam, thickened and darkened-refractive; conidia formed by semimacronematous conidiophores shorter, paler and narrower.
Culture characteristics: Colonies on PDA attaining 41–46 mm diam after 1 mo, grey-olivaceous to olivaceous, reverse iron-grey to greyish blue, powdery to felty, margin white, narrow, glabrous, regular, aerial mycelium diffuse to loose, fluffy, mainly in colony centre, growth flat, without prominent exudates, sporulation profuse. Colonies on MEA reaching 49–52 mm diam after 1 mo, smoke-grey to olivaceous-grey or brownish, whitish towards margins, reverse olivaceous-grey, velvety to woolly, margin white, glabrous, radially furrowed, aerial mycelium sparse to more abundantly formed, fluffy, few prominent exudates formed, sporulation profuse. Colonies on OA reaching 44–49 mm diam after 1 mo, grey-olivaceous to olivaceous or olivaceous-grey, reverse leaden-black to leaden-grey, powdery to fluffy, margin white, narrow, glabrous, aerial mycelium sparse, diffuse to more abundantly formed in colony centre, high, fluffy-felty, without prominent exudates, sporulation profuse.
Specimens examined: Australia, Victoria, Armadale, on leaves and twigs of Prunus persica (Rosaceae) infected with and deformed by Taphrina deformans (Taphrinaceae), 16 Feb. 1886, D. McAlpine, VPRI 2490, lectotype of C. phyllophilum, designated by Heuchert et al. 2005). Germany, He-Nassau, Rhön, near Gersfeld, ca. 500 m, on Taphrina pruni (= Exoascus rostrupianus) (Taphrinaceae) on Prunus spinosa (Rosaceae), 31 Jul. 1906, O. Jaap, Jaap, Fungi Sel. Exs. 248, B 70-6327, lectotype of C. exoasci, isolectotypes: Jaap, Fungi Sel. Exs. 248; Sachsen-Anhalt, Halle (Saale), botanical garden, isolated from fruits of Prunus cerasus (Rosaceae) infected with Taphrina sp., 2004, K. Schubert, CBS H-20444, epitype of C. phyllophilum, designated here; ex-type culture CBS 125992 = CPC 11333. South Africa, Western Cape Province, Jonkershoek Nature Reserve, on Teratosphaeria proteae-arboreae (Teratosphaeriaceae) on Protea nitida [= P. arborea] (Proteaceae), 30 Mar. 2007, P.W. Crous, CPC 13873.
Substrates and distribution: On species of Taphrina, including T. cerasi, T. communis, T. deformans and T. pruni on Prunus s. lat. species; Asia (Armenia, Georgia, Kazakhstan, Uzbekistan), Australia, Europe (Czech Republic, France, Germany, Romania, Switzerland), North America (U.S.A.).
Literature: McAlpine (1902: 100), Saccardo (1906: 575), Lindau (1910: 796), Braun (2001: 53), Heuchert et al. (2005: 36–40).
Notes: Cladosporium phyllophilum proved to be an older name for the fungicolous C. exoasci Lindau (Heuchert et al. 2005). The chosen epitype agrees well with the description given in Braun (2001) and Heuchert et al. (2005), although conidiophore and conidial measurements are slightly narrower in vitro than on the natural host. Since it is ecologically specialised to Taphrina species and C. exoasci was originally described from Germany the epitypification with European material seems to be justified.
The epitype strain clusters together with an isolate from Teratosphaeria proteae-arboreae, which morphologically slightly differs in having shorter, mostly unbranched conidiophores and shorter, less-septate, smooth to asperulate conidia. Since there are not enough isolates for a final conclusion it is tentatively maintained in C. phyllophilum until additional strains can be included to clarify its status. On ACT, the two strains are 98 % identical (217/220 bases), whereas on TEF they are 96 % identical (374/387 bases and 4 gaps) (distance analyses in TreeBASE).
Phylogenetically C. phyllophilum is allied to C. licheniphilum and C. phyllactiniicola (Fig. 1, part a; distance analyses in TreeBASE) but differs from the latter species in that conidiophores are longer and often branched, small terminal conidia narrower and secondary ramoconidia longer. Cladosporium licheniphilum deviates in having shorter and narrower conidiophores and shorter intercalary conidia and secondary ramoconidia.
Cladosporium pini-ponderosae K. Schub., Gresl. & Crous, Persoonia 22: 118. 2009.
This species was described and illustrated in Schubert et al. (2009).
Cladosporium pseudocladosporioides Bensch, Crous & U. Braun, sp. nov. MycoBank MB517087. Figs 60, 61.
Fig. 60.
Cladosporium pseudocladosporioides (CBS 125993). Macro- and micronematous conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 61.
Cladosporium pseudocladosporioides (CBS 125993). A–F. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Etymology: Name refers to its morphological similarity to Cladosporium cladosporioides.
Cladosporii cladosporioidis valde simile, sed ramoconidiis secundariis leniter brevioribus et angustioribus, 0–1(–2)-septatis, locis conidiogenis et hilis angustioribus, 0.5–1.5(–1.8) μm diam distinguitur. Differt a Cladosporio paracladosporioide ramoconidiis secundariis angustioribus, 0–1(–2)-septatis, locis conidiogenis et hilis angustioribus.
Mycelium immersed and superficial; hyphae unbranched or sparingly branched, (0.5–)1–4 μm wide, septate, sometimes constricted at septa, especially in wider ones, subhyaline to pale olivaceous or pale olivaceous-brown, smooth or almost so, walls sometimes slightly thickened, about 0.5 μm wide, sometimes irregular in outline due to swellings and constrictions, sometimes forming small ropes of few hyphae, sometimes cells swollen, up to 6.5 μm wide, fertile hyphae minutely verruculose, mainly at the base of conidiophores. Conidiophores macronematous, sometimes also micronematous, solitary or in small loose groups, arising terminally and laterally from hyphae or swollen hyphal cells, erect, straight to slightly flexuous, cylindrical-oblong, non-nodulose, sometimes once geniculate-sinuous or slightly swollen at the apex, unbranched or branched once or twice, occasionally three times, branches often only as short denticle-like lateral outgrowth just below a septum, 15–155 μm long, 2–4 μm, sometimes attenuated towards apex, 0–5-septate, sometimes slightly constricted at septa, pale to pale medium olivaceous-brown, sometimes paler towards the apex, smooth or almost so, at the base asperulate or finely verruculose like fertile hyphae, walls slightly thickened, about 0.5 μm wide or unthickened; micronematous conidiophores filiform, narrower, not attenuated, about 1.8 μm wide. Conidiogenous cells integrated, terminal, sometimes intercalary, slightly attenuated, narrowly cylindrical-oblong, sometimes once geniculate, non-nodulose, (6.5–)9–33 μm long, with 1–4 loci at the apex, occasionally with up to seven loci crowded at or towards the apex, in intercalary cells loci situated on small lateral peg-like outgrowths, 1–2(–3) loci, conspicuous, subdenticulate, 1–1.5(–1.8) μm diam, somewhat thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 19–48 × 3–4 μm, 0–2(–3)-septate, pale olivaceous-brown, smooth, base broadly truncate, 2–3 μm wide, unthickened or slightly thickened, sometimes slightly refractive. Conidia very numerous, catenate, in branched chains, branching in all directions with 3–6(–9) conidia in the terminal unbranched part of the chain, small terminal conidia obovoid, ovoid to limoniform or ellipsoid, sometimes subglobose, 3–5.5 × (1–)1.5–2.5 μm (av. ± SD: 4.1 ± 0.7 × 2.1 ± 0.3), apex rounded or attenuated towards apex and base, intercalary conidia ovoid, limoniform to ellipsoid or subcylindrical, 4.5–13(–19) × (1.8–)2–3 μm (av. ± SD: 8.8 ± 3.9 × 2.6 ± 0.3), 0(–1)-septate, slightly attenuated towards apex and base, with 1–4(–5) distal hila, secondary ramoconidia ellipsoid-ovoid to subcylindrical or cylindrical-oblong, (6.5–)8–23(–29) × (2–)2.5–3.5(–4) μm (av. ± SD: 16.1 ± 5.1 × 2.9 ± 0.3), 0–1(–2)-septate, septum median or often somewhat in the lower half, pale olivaceous to pale olivaceous-brown, smooth or almost so, sometimes slightly rough-walled, walls unthickened, with (1–)2–4(–6) distal hila, conspicuous, subdenticulate, 0.5–1.5(–1.8) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 65–78 mm diam after 14 d, olivaceous-grey to grey-olivaceous, reverse leaden-grey to olivaceous-black, felty-floccose, margins regular, glabrous to feathery, grey-olivaceous, aerial mycelium felty-floccose, growth effuse to low convex, few small prominent exudates formed, sporulation profuse. Colonies on MEA attaining 52–75 mm diam after 14 d, smoke-grey to dark smoke-grey or grey-olivaceous, reverse iron-grey, floccose, margins white, narrow, glabrous to somewhat feathery, aerial mycelium white, floccose, abundant, dense, growth effuse and somewhat radially furrowed, mostly without prominent exudates, sporulation profuse. Colonies on OA reaching 55–73 mm diam after 14 d, olivaceous to grey-olivaceous or olivaceous-buff, pale olivaceous-grey to greenish grey towards margins, reverse pale greenish grey, leaden-grey to iron-grey, floccose, margins colourless, glabrous, regular, aerial mycelium floccose to felty, sometimes covering large parts of colony surface, growth effuse with few prominent exudates, sporulation profuse.
Specimens examined: Sine loco, isol. from cloud water, coll. & isol. by M. Sancelme, ident. as C. tenuissimum by G.S. de Hoog, CBS 117134. Australia, Bimbadeen Lookout, ca. 10 km of Cessnock, North Coast, isol. from Eucalyptus placita (Myrtaceae), 10 Nov. 2006, coll. B.A. Summerell, isol. P.W. Crous, NSW 734672, CPC 13683; New South Wales, Douglas Park, isol. from E. moluccana, 31 Aug. 2006, coll. B.A. Summerell, isol. P.W. Crous, CPC 13339, 13340. Brazil, Vicosa, Parque National de Serra du Brigadeiro, isol. from Vernonia sp. (Asteraceae), 27 Jul. 2006, O. Pereira, CPC 13488. Canada, Ontario, Pearth, River Tay, isol. from Sagittaria graminea (Alismataceae), 9 Jan. 2006, W. Gams & K.A. Seifert, CPC 13529; British Colombia, Victoria, isol. from Acer macrophyllum (Aceraceae), 9 Jun. 2007, coll. B. Callan, isol. P.W. Crous, CPC 14382. Chile, Easter Island, isol. from soil, 2007, B. Andersen, BA 1694 = CPC 14295. France, Caves de Madelaine, isol. from leaves, 21 Aug. 2007, P.W. Crous, CBS 126356 = CPC 14278. Germany, Frankfurt am Main, Botanical Garden, isol. from Paeonia sp. (Paeoniaceae), 7 Oct. 2004, R. Kirschner, CBS 117153, stored as Graphiopsis chlorocephala (Fresen.) Trail; Schwäbische Alb, Kuppenalb, isol. from Myrothecium inundatum growing on an old fungal fruit body, 5 Oct. 2006, M. Grube, CBS 126390 = CPC 13499, CPC 13500, 13501. Indonesia, Tele, isol. from Eucalyptus sp. (Myrtaceae), endophyte spots after herbicide, 2008, coll. M.J. Wingfield, isol. P.W. Crous, CPC 14992. Italy, S. Michele all'Adige, isol. from leaves of Malus sylvestris (Rosaceae), depos. by G. de Stanchina, Dec. 1980, ident. by G.S. de Hoog, CBS 667.80 = IHEM 3705. Netherlands, Putten, isol. from pine needles of Pinus sp. (Pinaceae), 24 Jul. 2007, P.W. Crous, CPC 14230; Zwolle, isol. from outside air, 7 Jan. 2007, M. Meijer, CBS H-20445, holotype; ex-type cultures: CBS 125993 = CPC 14189, CPC 14193. New Zealand, Auckland, Alfriston, Emblings Bridge, -36.8667, 174.7667, isol. from leaves of Phalaris aquatica (Poaceae), 26 Nov. 2002, C.F. Hill, Hill 730 = ICMP 14870 = CPC 11841. Romania, isol. from Pteridium aquilinum, CBS 176.82. Russia, mycophilic, ident. by W. Gams as C. cladosporioides, CBS 574.78A = VKM F-422; Moscow region, isol. from Melampsoridium betulae (Pucciniastraceae), ident. by W. Gams as C. cladosporioides, CBS 574.78B = VKM F-2759. Slovenia, Gabrovka, isol. from a fruit of Rosa canina (Rosaceae) attached to shrub, 3 Jan. 2008, H.-J. Schroers, HJS 1038 = CPC 14975a. South Africa, Eastern Cape, Aiwal North, isol. from wheat, 1989, CPC 14020 = MRC 10814; Free state, Amersfoort, isol. from oats, 1984, CPC 14005 = MRC 03850; Danielsrus, isol. from oats, 1983, CPC 14003 = MRC 03366; Hoopstad, isol. from wheat, 1988, CPC 14014 = MRC 10232; Modderpoort, Ladybrand, isol. from wheat, 1983, CPC 14006 = MRC 03978; Slabberts, isol. from oats, 1983, CPC 14001 = MRC 03240, CPC 14002 = MRC 03245; Tugela, isol. from wheat, 1988, CPC 14013 = MRC 10221; Westminster, isol. from oats, 1983, CPC 14007 = MRC 03979; Northern Cape, Prieska, isol. from Aloe dichotoma (Asphodelaceae), 2005, CPC 13998 = CAMS 001160; unknown location, isol. from Sorghum sp. (Poaceae), 1988, CPC 14010 = MRC 10183, as C. cucumerinum. South Korea, Hoengseong, N37°32'09” E128°07'07”, isol. from Agrimonia pilosa (Rosaceae), 4 Aug. 2004, H.-D. Shin, CPC 11605; Namyangju, N37°34'59” E127°13'52”, isol. from Chrysanthemum coronarium var. spatiosum (Asteraceae), 30 Sep. 2004, H.-D. Shin, CPC 11392. Uganda, Mubende, isol. from coffee leaf, 2000, coll. J.L. Sørensen, isol. B. Andersen, BA 1677 = CPC 14357. U.S.A., Illinois, Peoria, isol. from Triticum aestivum (Poaceae), 1966, C.W. Hesseltine, NRRL A-14110, CBS H-10342, culture CBS 149.66; Louisiana, Baton Rouge, isol. from prunes wood, 2006, K.A. Seifert, CPC 12850; New York, Binghamton, isol. from creosote-treated southern pine pole, ATCC 66669 = CPC 5100; Virginia, Front Royal, isol. from pods of Kentucky coffee tree, 2007, P.W. Crous, CPC 13992.
Substrates and distribution: On plant material and fungal fruiting bodies, isolated from air, soil, water and food; widely distributed, Africa (Uganda, South Africa), Asia (Indonesia, South Korea), Australasia (Australia, New Zealand), Europe (France, Germany, Italy, Netherlands, Romania, Russia, Slovenia), North America (Canada, U.S.A.), South America (Brazil, Chile).
Notes: Cladosporium pseudocladosporioides is morphologically very close to C. cladosporioides but the secondary ramoconidia in the latter species are longer and somewhat wider, (7–)10–33(–38) × (2–)2.5–4(–6) μm, usually aseptate, conidiogenous loci and hila are wider, 0.5–2(–2.5) μm diam, and hyphae usually do not form ropes. However, a distinction between these two species only based on morphology is not always easy; in such cases an unclear strain can be referred to as belonging to the C. cladosporioides complex. Phylogenetically, both species are allied but distinct (Fig. 1, part b vs. c; distance analyses in TreeBASE). Cladosporium paracladosporioides differs in having wider, 0–3-septate secondary ramoconidia, wider conidiogenous loci and hila and is phylogenetically distinct (see Fig. 1, part b).
Cladosporium pseudocladosporioides probably represents a species complex maybe including some cryptic species since there is some variation within the isolates (observed in both ACT and TEF alignments; distance analyses in TreeBASE). Few isolates form numerous loci and hila at apices of conidiogenous cells and secondary ramoconidia with conidia formed in dense whirls, but besides this measurements and morphology are quite conserved within all the isolates. Therefore, it is treated as a single species until additional isolates can be included.
Cladosporium rectoides Bensch, H.-D. Shin, Crous & U. Braun, sp. nov. MycoBank MB517088. Figs 62, 63.
Fig. 62.
Cladosporium rectoides (CBS 125994). Conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 63.
Cladosporium rectoides (CBS 125994). A–G. Macronematous conidiophores and conidial chains; small terminal conidia sometimes with surface ornamentation indicated by the arrows in B. Scale bar = 10 μm.
Etymology: Name refers to the shape of conidiophores which are commonly geniculate with growth proceeding at an angle of 45–90°.
Cladosporii rectangularis simile, sed ramoconidiis formantibus, conidiis angustioribus, ramoconidiis secundariis 0–1(–2)-septatis et tamen locis conidiogenis et hilis latioribus secernitur.
Mycelium sparingly developed to more abundantly superficial, hyphae unbranched or sparingly branched, 1–4.5 μm wide, sometimes up to 6 μm wide, septate, not constricted at septa, pale olivaceous or pale olivaceous-brown, smooth to minutely verruculose or verruculose, sometimes verrucose, walls unthickened, sometimes forming ropes. Conidiophores solitary, macronematous, occasionally micronematous, arising terminally and laterally from hyphae, erect, straight to slightly flexuous, narrowly cylindrical-oblong, once or twice slightly to often distinctly geniculate-sinuous or slightly nodulose but often neither geniculate nor nodulose, with few apical loci, unbranched or branched, branches sometimes quite long, up to 80 μm, conidiophores 19–210 × (2–)2.5–4 μm, pluriseptate, not constricted at septa, sometimes growth proceeding at an angle of 45–90°, geniculations mostly intercalary, quite apart of the apex, pale to pale medium olivaceous-brown, smooth, walls slightly thick-walled, about 0.5 μm wide. Conidiogenous cells integrated, terminal and intercalary, distinctly sympodially proliferating, cylindrical-oblong, sometimes geniculate or right-angled, 12–47 μm long, sometimes few additional loci at a lower level, often arranged like a garland round about the stalk, in intercalary cells loci often situated on small lateral shoulders, conspicuous, subdenticulate, 1–2 μm diam, somewhat thickened and darkened-refractive. Ramoconidia cylindrical-oblong, 16–56 × 3–4 μm, 0–1-septate, commonly formed, base truncate, 2.2–3 μm wide, not thickened, sometimes slightly refractive. Conidia catenate, in branched chains, branching in all directions, up to 4(–5) conidia in the unbranched terminal part of the chain, small terminal conidia globose, subglobose to obovoid, (2.5–)3–4(–5) × 2–3 μm (av. ± SD: 3.8 ± 0.7 × 2.5 ± 0.4), aseptate, subhyaline, smooth, apex rounded, the outer wall often seems to detach, somewhat refractive, appearing to be like a halo and walls appearing to be thick-walled, intercalary conidia ovoid, ellipsoid to subcylindrical, 5–15(–17) × (2.5–)3–3.5 μm (av. ± SD: 9.2 ± 3.2 × 3.0 ± 0.3), 0–1-septate, not constricted at septum, with 1–3(–4) distal hila, the outer wall also seems to detach as in small terminal conidia, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, 8–28 × (2.5–)3–4 μm (av. ± SD: 17.4 ± 5.3 × 3.4 ± 0.3), 0–1(–2)-septate, pale olivaceous-brown, smooth, walls unthickened or slightly thick-walled, slightly attenuated towards apex and base, cells with one or more cavities, with 2–3(–4) distal hila, hila conspicuous, subdenticulate, 0.5–2 μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 68–82 mm diam after 14 d, olivaceous-grey to iron-grey, whitish to pale olivaceous-grey due to aerial mycelium, reverse olivaceous-black, velvety to floccose or fluffy, margins feathery, colourless, regular, aerial mycelium mainly in colony centre, fluffy-floccose, growth effuse, deep into the agar, without prominent exudates, sporulation profuse. Colonies on MEA reaching 65–80 mm diam after 14 d, smoke-grey, grey-olivaceous to brownish, whitish to pale olivaceous-grey due to aerial mycelium, reverse iron-grey, powdery to fluffy-floccose, margins regular, feathery, colourless, aerial mycelium mainly in colony centre, loose, diffuse to densely fluffy-floccose, growth effuse, somewhat furrowed or wrinkled in colony centre, without prominent exudates, sporulation profuse. Colonies on OA attaining 60–77 mm diam after 14 d, pale olivaceous-grey to smoke-grey, greenish grey, iron-grey or grey-olivaceous at margins, reverse sky-grey to olivaceous-grey, velvety to woolly-floccose, margins colourless, glabrous, regular, aerial mycelium mainly in colony centre, white to smoke-grey, loosely to densely floccose, growth effuse, without prominent exudates, sporulation profuse.
Specimens examined: South Korea, Jinju, N35°11'24” E128°10'56”, isol. from Vitis flexuosa (Vitaceae), 18 Oct. 2004, coll. H.-D. Shin, isol. P.W. Crous, CBS H-20446, holotype; ex-type culture CBS 125994 = CPC 11624; Jinju, N35°11'24” E128°10'56”, isol. from Plectranthus sp. (Lamiaceae), 1 Jul. 2004, coll. H.-D. Shin, isol. P.W. Crous, CBS 126357 = CPC 11405.
Substrate and distribution: On different plants; Asia (South Korea).
Notes: The conidiophores of C. rectoides are very characteristic and reminiscent of C. rectangulare, a species described on Epidendrum from North America, which differs, however, in lacking ramoconidia and in having wider conidia (2–5(–6) μm wide) with secondary ramoconidia being mostly aseptate and narrower conidiogenous loci and hila, 0.5–1.5 μm diam (Schubert & Braun 2004). Cladosporium tenuissimum is morphologically also somewhat similar but different in that conidiophores are usually longer, intercalary conidia narrower and conidiogenous loci and hila somewhat narrower. Phylogenetically both species cluster quite distant from each other (see Fig. 1, part b vs. c; distance analyses in TreeBASE).
Cladosporium scabrellum Bensch, Schroers, Crous & U. Braun, sp. nov. MycoBank MB517089. Figs 64, 65, 66.
Fig. 64.
Cladosporium scabrellum (CBS 126358). Macro- and micronematous conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 65.
Cladosporium scabrellum (CBS 126358). A–D (after 3 days). Macronematous conidiophores arising solitary or in small loose groups from hyphae or swollen hyphal cells and short conidial chains. E–H (after 7 days). Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Fig. 66.
Cladosporium scabrellum (CBS 126358). A. Globose conidia with nearly smooth surface. B. Overview of conidial chains. C. Elongated conidiophores. D. Secondary ramoconidium with large scars. E. Whorls of secondary ramoconidia and conidia. Note the presence of four scars on the top ramoconidium and the reticulate ornamentation of one of the conidia. F. Secondary ramoconidia of which one elongated. G. Conidiophore with secondary ramoconidia and large scar. Scale bars = 2 (A), 5 (D–G), 10 (B), 50(C) μm.
Etymology: Name refers to the surface ornamentation of mycelium, conidiophores and conidia.
Cladosporio tenuissimo valde simile, sed conidiophoris brevioribus, conidiis minutis terminalibus et ramoconidiis secundariis leniter angustioribus distinguitur. Differt a Cladosporio cladosporioide conidiophoris brevioribus, ramoconidiis secundariis leniter angustioribus, 0–1-septatis, conidiis tenuibus, minute verruculosis vel indistincte asperulatis.
Mycelium immersed and superficial, hyphae unbranched or loosely branched, 0.5–4 μm wide, mostly 1–3 μm wide, septate, sometimes constricted at septa, subhyaline to pale medium olivaceous-brown, smooth to minutely verruculose or loosely verruculose, walls unthickened or almost so, often with intercalary swellings and constrictions, forming loose to usually dense long characteristic ropes, somewhat interlaced, hyphae which give rise to conidiophores solitary and not in ropes, sometimes slightly wider at the base of conidiophores. Conidiophores macronematous, occasionally micronematous, solitary, arising laterally and terminally from hyphae, erect, straight, cylindrical-oblong, neither nodulose nor geniculate, unbranched, occasionally once branched with quite short branches just below a septum, 40–115(–185) × 3–4 μm, at the base up to 5 μm wide, septate, not constricted at septa, medium olivaceous-brown, smooth or finely verruculose, walls only slightly thickened, about 0.5 μm wide. Conidiogenous cells integrated, mainly terminal, but also intercalary, cylindrical-oblong, neither geniculate nor nodulose, 25–53 μm long, with up to four loci at the apex, conspicuous, subdenticulate, 1–1.8 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, up to 34 μm long, 4 μm wide, base 2–2.5 μm wide, smooth or finely verruculose. Conidia very numerous, catenate, in densely branched chains, branching in all directions, up to four conidia in the unbranched terminal part, small terminal conidia subglobose to obovoid, 3.5–4.5(–5) × 2–2.2(–2.5) μm (av. ± SD: 4.1 ± 0.5 × 2.1 ± 0.2), aseptate, intercalary conidia limoniform, fusiform, ellipsoid to subcylindrical, 5–13 × 2–3(–3.5) μm (av. ± SD: 8.2 ± 2.9 × 2.7 ± 0.4), 0–1-septate, not constricted, slightly attenuated towards apex and base, with 1–4 distal hila, secondary ramoconidia ellipsoid to subcylindrical or cylindrical, 10–25 × 2.5–3.5(–4) μm (av. ± SD: 17.2 ± 4.6 × 3.1 ± 0.4), 0–1-septate, not constricted, with 3–4(–5) distal hila, pale to pale medium olivaceous-brown, smooth or almost so to often indistinctly asperulate or loosely minutely verruculose, more obvious in small terminal and intercalary conidia, under SEM smooth or surface reticulate or with embossed stripes caused by diminishing turgor and shriveling of tender young conidia, walls unthickened, hila conspicuous, subdenticulate, 0.5–1.8 μm diam, somewhat thickened and darkened-refractive; without microcyclic conidiogenesis.
Culture characteristics: Colonies on PDA grey-olivaceous to iron-grey, reverse iron-grey to olivaceous-black, grey-olivaceous towards margins, velvety to floccose, margins white, glabrous to feathery, aerial mycelium scattered, floccose, smoke-grey, growth flat, few small to large prominent exudates, sporulation profuse. Colonies on MEA olivaceous-grey to smoke-grey in colony centre due to aerial mycelium and lack of sporulation, most of the colony grey-olivaceous due to abundant sporulation, reverse olivaceous-grey to iron-grey, velvety to felty-floccose, margins white, glabrous to feathery, growth effuse with elevated colony centre, tip of the colony immersed, sometimes radially furrowed, without exudates. Colonies on OA olivaceous, greenish olivaceous towards margins and with stripes and triangles of iron-grey, reverse iron-grey to pale greenish grey, floccose to felty-villose, margins colourless, narrow, glabrous, aerial mycelium mainly in colony centre, floccose to felty-villose, pale olivaceous-grey, growth flat, without exudates, sporulation profuse.
Specimen examined: Slovenia, Boštanj near Sevnica, on wilted part of leaf of Ruscus hypoglossum (Ruscaceae), 2 Jan. 2008, H.-J. Schroers, CBS H-20447, holotype; ex-type culture CBS 126358 = CPC 14976 = HJS 1031.
Substrate and distribution: On wilted leaves of Ruscus; Europe (Slovenia).
Notes: Mycelium of C. scabrellum often forms long dense ropes and the smooth or mostly indistinctly asperulate conidia are very numerous. Morphologically it has to be compared with C. cladosporioides and C. tenuissimum. Cladosporium cladosporioides is distinct in having usually longer conidiophores and smooth conidia with secondary ramoconidia being mostly aseptate and somewhat wider and longer, while C. tenuissimum also possesses longer conidiophores and somewhat wider small terminal conidia and secondary ramoconidia. Phylogenetically C. scabrellum is quite distinct from these two species (see Fig. 1, part a vs. c; distance analyses in TreeBASE).
Cladosporium subuliforme Bensch, Crous & U. Braun, sp. nov. MycoBank MB517090. Figs 67, 68.
Fig. 67.
Cladosporium subuliforme (CBS 126500). Subulate conidiophores, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 68.
Cladosporium subuliforme (CBS 126500). A–C. Tips of conidiophores with conidial chains. D. Subulate conidiophore with terminal and intercalary conidiogenous cell and conidia. Scale bar = 10 μm.
Etymology: Name refers to the characteristic shape of conidiophores, awl-like.
Cladosporio tenuissimo simile, sed conidiophoris subulatis, angustioribus et item ramoconidiis secundariis et ramoconidiis angustioribus distinguitur. Differt a Cladosporio cladosporioide conidiophoris subulatis, ramoconidiis secundariis brevioribus et angustioribus, 0–1-septatis et tamen locis conidiogenis angustioribus.
Mycelium internal and superficial; hyphae sparingly branched, 1–4 μm wide, septate, sometimes slightly constricted at the base of conidiophores, subhyaline to pale olivaceous-brown, smooth to minutely verruculose or verruculose, often somewhat swollen at the base of conidiophores, up to 6 μm wide, sometimes forming ropes. Conidiophores macro- to semimacronematous or micronematous, solitary or in pairs, arising terminally and laterally from hyphae, erect, straight to mostly flexuous, filiform to narrowly cylindrical-oblong, often slightly to distinctly attenuated towards the apex and wider at the base, not nodulose or geniculate, unbranched or branched, branches often only as short peg-like lateral outgrowth just below a septum bearing conidiogenous loci, branches occasionally longer, up to 20 μm, 9–330 × (1.5–)2–3(–3.5) μm, pluriseptate, usually not constricted at septa, pale to medium olivaceous-brown, smooth to sometimes minutely verruculose, parts of the stalk occasionally verrucose, basal cell sometimes swollen up to 8(–10) μm, walls unthickened or only slightly thickened, about 0.5 μm wide. Conidiogenous cells integrated, mainly terminal but also intercalary, narrowly cylindrical-oblong, neither nodulose nor geniculate, 9–40 μm long, with up to five loci crowded at the uppermost apex, in intercalary cells loci often situated on small denticle- or peg-like lateral outgrowths just below a septum, loci conspicuous, subdenticulate, (0.8–)1–1.5(–1.8) μm diam, thickened and darkened-refractive. Ramoconidia commonly formed, cylindrical-oblong, differentiation between ramoconidia and secondary ramoconidia often quite difficult, (14–)17–35 × (1.5–)2–3 μm, 0(–1)-septate, pale olivaceous-brown, smooth, walls unthickened, not attenuated towards the base, base broadly truncate, 2–2.5 μm wide, unthickened, but often somewhat darkened or refractive. Conidia numerous, catenate, in branched chains, up to 5–6 conidia in the unbranched terminal part of the chain, branching in all directions, straight, small terminal conidia obovoid, subglobose, ovoid to limoniform or ellipsoid, 2.5–4.5(–5.5) × 2–2.5 μm (av. ± SD: 4.2 ± 0.9 × 2.2 ± 0.2), aseptate, rounded at the apex, attenuated towards the base, intercalary conidia ellipsoid to subcylindrical, 5.5–12(–13) × 2–3(–3.5) μm (av. ± SD: 8.3 ± 2.5 × 2.6 ± 0.4), aseptate, with up to four distal hila, attenuated towards apex and base, secondary ramoconidia ellipsoid to subcylindrical, sometimes cylindrical-oblong, (6–)8–25(–28) × 2–3(–3.5) μm (av. ± SD: 15.1 ± 7.3 × 2.7 ± 0.4), 0–1-septate, not constricted at septa, median or somewhat in the lower half, usually somewhat attenuated towards the base, (2–)3–4(–5) distal hila, pale olivaceous-brown, smooth or almost so, walls unthickened, hila conspicuous, subdenticulate to denticulate, (0.2–)0.5–1.5(–1.8) μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA grey-olivaceous to mainly olivaceous-grey, reverse olivaceous-grey, velvety to floccose, fluffy, margins grey-olivaceous to white, feathery, regular or slightly undulate, aerial mycelium abundant, loose, fluffy, growth effuse to low convex, without exudates, sporulation profuse. Colonies on MEA greenish olivaceous to pale olivaceous-grey and olivaceous-buff, glaucous-grey at margins, reverse olivaceous-grey, floccose to fluffy, margins white, glabrous, regular to somewhat undulate, radially furrowed and wrinkled, effuse, aerial mycelium abundant, fluffy, mainly in colony centre, without exudates, sporulation profuse. Colonies on OA whitish to smoke-grey and pale olivaceous-grey, olivaceous-buff and dull green towards margins, somewhat zonate, grey-olivaceous due to sporulation, reverse leaden-grey, floccose to felty, margins dull green or colourless, regular, glabrous, aerial mycelium abundant, floccose to fluffy-felty, covering large parts of colony surface, growth effuse, without exudates, sporulating.
Specimen examined: Thailand, Chiang Mai, Sansai, Mai Jo, palm nursery, isol. from Chamaedorea metallica (Arecaceae), 26 Dec. 2006, coll. I. Hidayat & J. Meeboon, FIH 401, isol. P.W. Crous, CBS H-20448, holotype; ex-type culture CBS 126500 = CPC 13735.
Substrate and distribution: Isolated from Chamaedorea metallica; Asia (Thailand).
Notes: Its long narrow subulate conidiophores with several loci crowded at the apex and its numerous ramoconidia with narrow loci and hila distinguishes C. subuliforme from the morphologically similar C. tenuissimum, which possesses wider ramoconidia and secondary ramoconidia. Cladosporium cladosporioides deviates in having wider usually not attenuated conidiophores, longer and wider ramoconidia and secondary ramoconidia and somewhat wider conidiogenous loci and hila. Phylogenetically C. subuliforme is close to C. angustisporum (see above; Fig. 1, part c; distance analyses in TreeBASE) but the latter species differs in that small terminal conidia are somewhat longer and narrower, 3–6.5 × 1.5–2 μm, and conidiophores are not attenuated towards the apex in an awl-like manner.
Cladosporium tenuissimum Cooke, Grevillea 6(40): 140. 1878. Figs 69, 70.
Fig. 69.
Cladosporium tenuissimum (CBS 125995). Macro- and micronematous conidiophores usually with a head-like swollen apex and sometimes additional intercalary nodules, conidial chains and microcyclic conidiogenesis. Scale bar = 10 μm.
Fig. 70.
Cladosporium tenuissimum (CBS 125995). A–H. Macronematous conidiophores and conidial chains. I–J. Micronematous conidiophores with conidia. Scale bars = 10 μm.
Mycelium immersed and superficial, hyphae branched, (0.5–)1–5 μm wide, septate, sometimes constricted at septa, subhyaline to pale or medium brown, with swellings and constrictions, often irregular in outline, smooth to sometimes minutely verruculose, sometimes appearing rough-walled, walls unthickened or very slightly thickened, sometimes forming ropes. Conidiophores solitary, macronematous and micronematous, arising terminally and laterally from hyphae; macronematous conidiophores solitary, sometimes in groups of 2–3, erect, straight or slightly flexuous, cylindrical-oblong to almost filiform, sometimes slightly to distinctly geniculate towards the apex, often subnodulose or nodulose with an apical and sometimes a few additional swellings on a lower level, swellings quite distant from the apex and from each other, most conidiophores neither geniculate nor nodulose, unbranched or branched, branching often at an angle of 45–90°, just below the apex or at a lower level, branches sometimes only as short denticle-like prolongations just below a septum, occasionally long, conidiophores 30–310(–460) × 2.5–4 μm (on OA up to 900 μm long), septate, sometimes distinctly constricted at septa, pale to medium brown or olivaceous-brown, smooth, sometimes slightly rough-walled at the base, walls somewhat thickened, sometimes slightly attenuated towards the apex and distinctly swollen at the base, with age conidiophores becoming darker and more thick-walled; micronematous to semimacronematous conidiophores narrower, paler, filiform to narrowly cylindrical-oblong, non-nodulose or only slightly swollen at the apex, unbranched, 17–85 × (1–)2–2.5 μm, with few septa or reduced to conidiogenous cells, pale brown or subhyaline, smooth, walls unthickened or almost so, with a single or up to seven subdenticulate, pronounced loci crowded at the apex. Conidiogenous cells integrated, terminal and intercalary, cylindrical-oblong, sometimes short geniculate at the apex, often nodulose, swellings up to 5 μm wide, cells (4–)10–44 μm long, loci often situated on swellings but not restricted to them, mostly only a single swelling per cell, in terminal cells apex usually head-like uni- or multilaterally swollen with up to eight pronounced, subdenticulate to denticulate loci crowded at the tip, in intercalary conidiogenous cells loci often sitting at about the same level (arranged like a garland round about the stalk) or situated on small lateral shoulders, loci 1–1.5(–2) μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, subcylindrical or cylindrical-oblong, 22–41 × 3–4(–5) μm, 0(–1)-septate, base broadly truncate, 2–3.5 μm wide. Conidia catenate, in densely branched chains, 1–4(–6) conidia in the terminal unbranched part of the chain, branching in all directions, straight, small terminal conidia subglobose, obovoid, limoniform, sometimes globose, (2–)2.5–5(–6) × (1.5–)2–3 μm (av. ± SD: 3.7 ± 1.0 × 2.2 ± 0.4), aseptate, apex broadly rounded, intercalary conidia ovoid, ellipsoid or subcylindrical, 4–12(–17) × (1–)2–3(–4.5) μm (av. ± SD: 8.1 ± 2.7 × 2.8 ± 0.6), aseptate, occasionally 1-septate, with up to 5(–7) distal hila, sometimes cell lumen distinct, secondary ramoconidia ellipsoid, fusiform to subcylindrical or cylindrical, (6–)7–25(–31) × (2–)2.5–4(–5) μm (av. ± SD: 15.0 ± 5.8 × 3.2 ± 0.5), with (1–)2–6(–7) distal hila, sometimes with 1–2 hila at the basal end, 0–1(–2)-septate, sometimes distinctly constricted at septa, with age more frequently septate, pale brown or pale olivaceous-brown, smooth, occasionally irregularly rough-walled, walls unthickened or almost so, attenuated towards apex and base, hila conspicuous, subdenticulate to denticulate, 0.5–1.8(–2) μm diam, thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA attaining up to 84 mm diam after 14 d, smoke-grey to grey-olivaceous or olivaceous-grey, reverse leaden-grey to olivaceous-black, woolly to fluffy, margin glabrous to feathery, grey-olivaceous to white, aerial mycelium abundant, high, fluffy, smoke-grey, dense, without prominent exudates, sporulating. Colonies on MEA reaching 70–80 mm diam after 14 d, smoke-grey to pale olivaceous-grey, pale olivaceous due to abundant sporulation, reverse olivaceous-grey, woolly, fluffy, margins narrow, glabrous to feathery, colourless to white, sometimes radially furrowed and wrinkled, aerial mycelium abundant, fluffy, dense, high, pale olivaceous-grey, covering large parts of the colony surface, growth low convex, few prominent exudates formed, sporulating. Colonies on OA attaining 65–73 mm diam after 14 d, smoke-grey, pale olivaceous-grey to whitish due to aerial mycelium, greenish grey towards margin, reverse olivaceous-grey to iron-grey or leaden-grey, woolly-fluffy to felty, margin colourless to white, narrow, glabrous, aerial mycelium high, abundantly formed, fluffy to felty, whitish, growth flat to low convex, mostly without prominent exudates, sporulating.
Specimens examined: Australia, Cairns, isol. from Callistemon viminalis (Myrtaceae), 18 Aug. 2006, P.W. Crous, CPC 13222. Brazil, Fortaleza, isol. from a rust fungus, 30 Jul. 2005; U. Braun, CPC 12223. Burundi, isol. from a fruit, J. Rammelo, isol. by B.P.R. Vittal, ident. W. Gams, CBS 117.79. Indonesia, isol. from Musa sp. (Musaceae), 2004, M. Arzanlou, CPC 11612. Iran, isol. from Citrus sinensis (Rutaceae), 2004, W. Gams, CPC 11555. Ivory Coast, Abidjan, isol. from Musa sp., 10 Jan. 2005, Kone Daouda, CL1 ra, CBS 126501 = CPC 14410. Mozambique, isol. from Musa sp., coll. A. Viljoen, isol. P.W. Crous, CPC 10538, 10539. Nigeria, isol. from fruit, CBS 262.80. Polynesia, reserve Pun Kukui, in forest, isol. from banana, 2006, coll. I. Budenhagen, isol. P.W. Crous, CPC 12794, 12795. South Africa, Durban, Durban Botanical Garden near Reunion, -29.85, 31.0167, isol. from Strelitzia sp. (Strelitziaceae), 2005, coll. W. Gams, isol. P.W. Crous, CPC 11805. South Korea, Jeju, N33° 27'25” E126°33'40”, isol. from Gnaphalium affine (Asteraceae), 28 Oct. 2005, coll. H.-D. Shin, isol. P.W. Crous, preserved as “Passalora sp.”, CPC 10882. U.S.A., Louisiana, Baton Rouge, isol. from Magnolia sp. (Magnoliaceae), 8 Sep. 2007, P.W. Crous, CPC 14250; isol. from fruits of Lagerstroemia sp. (Lythraceae), 8 Sep. 2007, P.W. Crous, CBS H-20449, epitype of C. tenuissimum, designated here; ex-type culture CBS 125995 = CPC 14253; South Carolina, Aiken, on leaf sheets of Zea mays (Poaceae), H.W. Ravenel, Ravenel & Cooke, Fungi Amer. Exs. 160, NY, lectotype, isolectotypes: Ravenel & Cooke, Fungi Amer. Exs. 160.
Cladosporium tenuissimum s. lat. / Lineage 1: Australia, Queensland, isol. from rock, Chillagoe Mungana Caves National Park, P.W. Crous, CPC 13252. Bali, bat cave, isol. from soil, 2000, coll. J.C. Frisvad, isol. B. Andersen, BA 1737 = CPC 14370. India, isol. from Dalbergia sp. (Fabaceae), 2004, coll. W. Gams, isol. P.W. Crous, CPC 11130; Chandigarh, 30.7372, 76.7872, isol. from Citrus sp. (Rutaceae), 3 Jan. 2004, W. Gams, CPC 11132. Laos, Vientiane Capital, Xaythany District, Xay Villiage, isol. from leaves of Basella alba [= B. rubra] (Basellaceae), 4 Jan. 2007, coll. P. Phengsintham, isol. P.W. Crous, CPC 14196; isol. from Shorea siamensis (Dipterocarpaceae), 22 Jan. 2007, coll. P. Phengsintham, isol. P.W. Crous, CPC 13732. Thailand, isol. from Acacia mangium (Fabaceae), coll. W. Himaman, isol. P.W. Crous, CPC 11521, 11929. Venezuela, Cabruta, Mochimo Bay, isol. from a decayed branch under water, 2007, coll. K. Lyhne, isol. B. Andersen, BA 1710 = CPC 14311; Rojo, Mochimo Bay, isol. from sediment, red mangrove, 2007, coll. K. Lyhne, isol. B. Andersen, BA 1711 = CPC 14312.
Substrates and distribution: On different host plants, also isolated from air, bread and soil; cosmopolitan but especially common in the tropics.
Literature: Saccardo (1886: 365), Oudemans (1919), Ellis (1976: 326), Ho et al. (1999: 140), Heuchert et al. (2005: 50–52).
Notes: Cladosporium tenuissimum and C. cladosporioides are two quite common saprobic species isolated from numerous substrates. They are morphologically very similar and have therefore often been confused. Isolates of both taxa were included in this study and it has been demonstrated that they represent two morphologically, as well as phylogenetically allied but distinct species (see discussion under C. cladosporioides).
On SNA plates conidiophores of C. tenuissimum can become darker and more thick-walled with age and conidia more frequently septate. On OA and PDA the conidiophores are very long and darker, medium to dark olivaceous-brown, surprisingly forming several nodules quite distant from each other which remind one of the closely related C. oxysporum. Cladosporium oxysporum, however, does not form its characteristically nodose conidiophores on OA and PDA, but does so on SNA and in vivo. In contrast, C. tenuissimum usually forms very long, usually straight, dark conidiophores with somewhat thickened walls, but without any swellings or only slightly and unilaterally swollen apices on the natural host (in vivo). Other characters like conidial measurements and width of conidiophores are largely consistent between these two species as already stated by Ellis (1976) and Ho et al. (1999). Phylogenetically they are, however, obviously distinct (Fig. 1, part c; distance analyses in TreeBASE). Also in C. tenuissimum, similar to the situation with C. perangustum, phylogenetic variation is observed which is supported by nucleotide changes in both ACT and TEF gene sequences. However, since the morphology of all isolates is quite uniform we prefer to treat these as intraspecific variation for the moment pending the collection of more strains.
Cladosporium colocasiae clusters between the isolates of C. tenuissimum as discussed under the notes of this species (see above), but is easily distinguishable in always having nodose conidiophores and wider conidia (5–8(–9) μm) formed solitary or in short chains.
Cladosporium stanhopeae, a species described on Stanhopea (Orchidaceae) from Germany (Schubert & Braun 2004, Schubert 2005b) also resembles C. tenuissimum but is tentatively maintained as a separate species until isolates from that host can be included in molecular studies.
Cladosporium uredinicola Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 23: 122–123. 1912.
For descriptions in vitro see Morgan-Jones & McKemy (1990) and Ho et al. (1999); for a description in vivo see Heuchert et al. (2005).
Specimen examined: U.S.A., Alabama, Lee County, Auburn, hyperparasitic on Cronartium fusiforme (Cronartiaceae, Uredinales) on leaves of Quercus nigra (Fagaceae), 20 May 1982, W.D. Kelley, ATCC 46649 = CPC 5390.
Substrates and distribution: Hyperparasitic on telia and uredia of rusts, especially Cronartium and Puccinia, also on downy mildews and powdery mildew fungi; Asia (Hong Kong, India, Iran), Australasia (Australia, New Zealand), Caribbean, Europe (Germany, U.K.), North America (Canada, U.S.A.), South America (Argentina, Brazil).
Literature: Saccardo (1931: 798), Sutton (1973: 40), Ellis (1976: 330), Ellis & Ellis (1985: 571, 1988), Morgan-Jones & McKemy (1990), Ho et al. (1999: 142), Heuchert et al. (2005: 41–46).
Notes: The above strain did not sporulate in the course of these investigations. However, Morgan-Jones & McKemy (1990) and Ho et al. (1999) examined it and provided detailed descriptions of C. uredinicola in vitro. Phylogenetically, the strain is allied to C. funiculosum and C. pseudocladosporioides (Fig. 1, part b; distance analyses in TreeBASE) but morphologically has longer conidiophores and somewhat wider, more frequently septate conidia (Morgan-Jones & McKemy 1990). In the absence of further isolates, particularly from downy and powdery mildew fungi, the identity of such collections remains unclear. Morphologically these collections are indistinguishable and not separable from collections on rust fungi (Morgan-Jones & McKemy 1990, Heuchert et al. 2005). Morgan-Jones & McKemy (1990) examined Spegazzini's type (slides IMI 87162a) as part of their investigations, but Spegazzini's material is from a different rust (Puccinia cestri) and different plant host (Cestrum pubescens) than the material preserved as CPC 5390 (Dugan et al. 2004) and “is in poor condition” (Heuchert et al. 2005). Moreover, isolates from powdery mildew fungi included in the present study proved to represent morphologically as well as phylogenetically distinct species such as C. exile and C. phyllactiniicola (see discussion).
Strain CBS 306.84, isolated from urediniospores of Puccinia allii in the U.K., and identified as C. uredinicola proved to be not conspecific with CPC 5390. Morphologically it belongs to the C. cladosporioides s. lat. complex but phylogenetically it is different from C. cladosporioides s. str. clustering apart from this clade (see Fig. 1, part b vs. c; distance analyses in TreeBASE). Additional isolates, especially from Puccinia, are required so that an epitype for C. uredinicola can be selected. The representative strain given above is from a different rust on a different host than the type (Heuchert et al. 2005), and is presently sterile. Therefore, we refrain from selecting this material as epitype.
Cladosporium varians U. Braun, Melnik & K. Schub., Mikol. Fitopatol. 25: 215. 2008. Fig. 71.
Fig. 71.
Cladosporium varians (CBS 126362). A–F. Macronematous conidiophores and conidial chains. G. Conidia. Scale bars = 10 μm.
= Cladosporium phyllogenum K. Schub., Mikol. Fitopatol. 25: 218. 2008, syn. nov.
Mycelium mainly immersed, rarely superficial; hyphae unbranched or branched, 1.5–7 μm wide, aerial hyphae narrower, 1.5–3 μm wide, septate, not constricted or slightly to distinctly constricted at septa, pale olivaceous, olivaceous-brown or brown, smooth or sometimes minutely verruculose or verruculose, thin-walled or almost so, sometimes hyphal cells distinctly swollen at the base of conidiophores, up to 8 μm wide. Conidiophores macronematous, sometimes also micronematous, terminally or laterally arising from ascending or plagiotropous hyphae, solitary, erect or ascending, straight to flexuous, cylindrical-oblong, non-nodulose, sometimes geniculate-sinuous towards the apex, unbranched or branched, branches often formed as short, denticle-like lateral prolongations just below or at a septum, 25–300(–530) × (2.5–)3.5–6(–7) μm, pluriseptate, upper septa just below potential ramoconidia appear somewhat darker, refractive and thickened, pale olivaceous or olivaceous-brown, smooth to somewhat asperulate, especially towards the base, walls somewhat thickened, 0.5 μm wide; micronematous conidiophores narrower, paler and shorter, flexuous, filiform, unbranched, 17–100 μm long or longer, 2–3(–3.5) μm wide, septate, not constricted at septa, pale olivaceous or pale brown, smooth or asperulate, walls unthickened. Conidiogenous cells integrated, mostly terminal, but also intercalary, cylindrical-oblong, non-nodulose, sometimes geniculate, 8–36(–96) μm long, with a single or often up to three loci at the apex, often situated on denticle-like prolongations, loci truncate or slightly convex, (1.5–)2–3 μm diam, somewhat thickened and darkened-refractive. Ramoconidia subcylindrical or cylindrical, 23–56(–64) × (3–)4–6(–7) μm, 0–2(–3)-septate, concolorous with tips of conidiophores, usually with up to three distal hila, not attenuated towards the base or only slightly so, base broadly truncate, 3–4.5 μm wide, unthickened and somewhat refractive. Conidia polymorphous, numerous, catenate, in branched chains, branching in all directions, up to five conidia in the terminal unbranched part of the chain, small terminal conidia globose, subglobose, ovoid to obovoid, 4–6(–8) × 2.5–3 μm (av. ± SD: 4.8 ± 1.0 × 2.8 ± 0.3), aseptate, intercalary conidia ovoid to ellipsoid, 6–15(–18) × (2.5–)3–4(–4.5) μm (av. ± SD: 9.9 ± 3.2 × 3.5 ± 0.4), 0–1-septate, with up to three distal scars, secondary ramoconidia ellipsoid to subcylindrical or cylindrical-oblong, (8–)11–33(–40) × (2.5–)3–6 μm (av. ± SD: 21.9 ± 7.8 × 4.3 ± 0.7), 0–2(–5)-septate, mainly 1-septate, sometimes slightly to distinctly constricted at septa, pale olivaceous to pale olivaceous-brown, smooth or almost so, walls unthickened or slightly thickened, slightly attenuated towards apex and base, hila 0.8–3 μm diam; microcyclic conidiogenesis not observed.
Culture characteristics: Colonies on PDA attaining 26–32 mm diam after 14 d, dark green-olivaceous to dark grey-olivaceous, olivaceous or iron-grey, sometimes slightly zonate, reverse grey-olivaceous to olivaceous-grey or leaden-grey, velvety to powdery or floccose, margin white, narrow or broad, regular, entire edge, glabrous to somewhat feathery; aerial mycelium sparse, only at few spots or diffuse, high, hairy to fluffy, pale olivaceous-grey; growth regular, flat to low convex with slightly elevated colony centre, prominent exudates not formed, sporulation profuse, two kinds of conidiophores formed, low and high ones. Colonies on MEA reaching 17–22 mm diam after 14 d, olivaceous-grey, grey-olivaceous to iron-grey surface and reverse, some colonies glaucous-grey at margins, velvety to powdery, margins colourless or white, regular, feathery, narrow to broad, aerial mycelium sparse, diffuse, loosely floccose, growth low convex, radially furrowed, sometimes wrinkled and folded in colony centre, without prominent exudates, sporulation profuse. Colonies on OA attaining 20–22 mm diam after 14 d, olivaceous-grey to iron-grey, grey-olivaceous due to sporulation and pale olivaceous-grey due to aerial mycelium, reverse iron-grey to leaden-grey or olivaceous-grey, surface somewhat zonate, floccose or fluffy, margin white, narrow, glabrous, aerial mycelium sparse, loosely to densely floccose or fluffy, growth flat or low convex, regular, without prominent exudates, sporulating.
Specimens examined: Germany, Sachsen-Anhalt, Halle (Saale), Botanical Garden, on living leaves of Ulmus laevis (Ulmaceae), 9 Jul. 2004, K. Schubert, holotype of C. phyllogenum HAL 1845 F, isotype CBS H-19870 (dried SNA plate); ex-type culture CBS 126360 = CPC 11327. India, Habingirii, isol. from leaf debris, 2004, W. Gams, CBS 126361 = CPC 11134. Russia, St. Petersburg, botanical garden of the academy, isol. from leaves of Catalpa bungei (Bignoniaceae), 15 Jan. 2007, coll. V.A. Melnik, isol. P.W. Crous, LE holotype, HAL 2061 F isotype; ex-type culture CBS 126362 = CPC 13658, CPC 13659, 13660. Slovenia, Gabrovka, isol. from a fruit of Rosa canina (Rosaceae) attached to shrub, 3 Jan. 2008, H.-J. Schroers, HJS 1038 = CPC 14975B.
Substrate and distribution: On plant material, sometimes also endophytic; Asia (India), Europe (Germany, Russia, Slovenia).
Literature: Schubert (2005b: 117–120 and figs 54, 55, pl. 24, figs A–J), Braun et al. (2008b: 215).
Notes: Cladosporium varians and C. phyllogenum have been treated as two distinct species although micromorphological characters of the two species are rather similar. Cladosporium varians was said to differ from C. phyllogenum in having swollen tips on the conidiogenous cells, sometimes unilaterally enlarged, and much shorter ramoconidia, 6–25 × 3–6(–7) μm (Braun et al. 2008b). However, since additional isolates were included in the present study it can be seen that morphological differences between these two species are within the species variation. Therefore, C. phyllogenum is reduced to synonymy with C. varians. The species clusters as a sister to C. paracladosporioides (Fig. 1, part a) and forms a distinct lineage for both TEF and ACT, although some intraspecific variation is present in the individual loci (distance analyses in TreeBASE).
The ecology of Cladosporium varians is not clear. It has been collected as a saprobic fungus on dead, but still attached leaves of Catalpa bungei, but possibly lives also as an endophyte, only growing and sporulating superficially under favourable external conditions with fructification mainly confined to and spread on veins, as demonstrated for the type collection of C. phyllogenum.
Because of small, smooth conidia, this species pertains to the Cladosporium cladosporioides complex (Ellis 1971). It differs from C. cladosporioides in having long, frequently branched conidiophores, arising in vivo from superficial hyphae and often somewhat swollen or unilaterally swollen conidiogenous tips. The ramoconidia possess up to four septa, and subglobose conidia are not abundant. The swollen conidiogenous cells and the formation of subglobose conidia, mixed with cylindrical, ellipsoid and fusiform conidia, are reminiscent of C. tenuissimum (Ellis 1971). In the latter species, the conidiophores are, however, setiform, usually unbranched, often with darker and thicker walls, the ramoconidia are only 0–1-septate, and the conidia range from smooth-walled to verruculose. Due to frequently branched conidiophores and abundant globose conidia, there is also a morphological connection to C. sphaerospermum, which is, however, easily distinguishable by its verruculose conidia. Furthermore, in molecular sequence analyses (based on ITS nrDNA sequence data not shown here) C. varians did not cluster within the C. sphaerospermum complex. Cladosporium diaphanum (Ellis 1976, Schubert 2005b) is superficially also similar, but forms internal mycelium, usually fasciculate conidiophores emerging through stomata, which are not distinctly geniculate, (0–)1–6-septate conidia, and somewhat shorter ramoconidia occur only occasionally.
Cladosporium verrucocladosporioides Bensch, H.-D. Shin, Crous & U. Braun, sp. nov. MycoBank MB517091. Figs 72, 73, 74.
Fig. 72.
Cladosporium verrucocladosporioides (CBS 126363). Macro- and micronematous conidiophores, mycelium sometimes formed in ropes, ramoconidia and conidial chains. Scale bar = 10 μm.
Fig. 73.
Cladosporium verrucocladosporioides (CBS 126363). A–H. Macronematous conidiophores and conidial chains. Scale bars = 10 μm.
Fig. 74.
Cladosporium verrucocladosporioides (CBS 126363). A. CryoSEM of a small colony illustrating the dense middle part with many young conidiophores and some disconnected masses of conidia. B. Stout erect conidiophores sprouting from rounded linearly oriented cells. C, G–H. Details of the fungal colony containing the structures on which conidiophores are formed and the different types of conidia. Note the ornamented conidia and the more or less smooth or only slightly ornamented conidiophores. D. Conidiophore, secondary ramoconidia and scars. E. Rounded conidia in a chain showing the reticulate surface ornamentation. F. Secondary ramoconidium and scars. Note the reduced ornamentation on this cell in comparison with the conidia. Scale bars = 2 (D, F), 5 (C, E), 10 (G–H), 20 (B), 50 (A) μm.
Etymology: Named after its surface ornamentation of conidia, which is similar to that described in the genus Verrucocladosporium.
Cladosporii acalyphae aliquam simile, sed conidiophoris brevioribus, conidiis minutis terminalibus brevioribus, angustioribus, non globosis et tamen ramoconidiis secundariis 0–3-septatis internoscitur. Differt a Cladosporio pini-ponderosae conidiophoris angustioribus, ramoconidiis secundariis 0–3-septatis, locis conidiogenis et hilis angustioribus et a Cladosporio exasperato conidiis minutis terminalibus brevioribus, conidiis intercalaribus latioribus, ramoconidiis secundariis 0–3-septatis, verrucis ad 1 μm longis.
Mycelium immersed and superficial; loosely branched, 1–4.5 μm wide, septate, mostly not constricted at septa, sometimes distinctly constricted and due to swellings and intercalary constrictions irregular in outline, subhyaline to pale or medium olivaceous-brown, smooth to minutely verruculose or verruculose, walls unthickened or almost so, at the base of conidiophores sometimes wider, up to 5 μm, sometimes anastomosing and forming ropes of few hyphae. Conidiophores macro-, sometimes also micronematous, arising terminally and laterally from hyphae, solitary, erect, straight to flexuous, cylindrical-oblong, sometimes once distinctly geniculate-sinuous, non-nodulose, unbranched, 18–130(–175) × (2.5–)3–4 μm, pluriseptate, septa often in short succession and darkened, especially just below potential ramoconidia, sometimes distinctly constricted at septa, pale to medium olivaceous-brown, smooth to verruculose-verrucose or irregularly rough-walled, at the apex or the whole conidiophore with surface ornamentation, walls only slightly thickened, base often somewhat swollen, up to 5(–7) μm wide, sometimes slightly attenuated towards the apex. Conidiogenous cells integrated, mostly terminal, sometimes also intercalary, cylindrical-oblong, sometimes once geniculate, 7–30 μm long, with 1–3(–4) loci at the apex, occasionally up to eight loci crowded at the apex, sometimes situated on small lateral prolongations, subdenticulate, protuberant, 1–1.8 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, 19–36(–45) × 3–4(–5) μm, 0–2(–3)-septate, not constricted at septa, base unthickened, broadly truncate, 2–3.5 μm wide. Conidia catenate, in branched chains, branching in all directions, up to 4(–5) conidia in the unbranched terminal part of the chain, small terminal conidia obovoid, subglobose, 3–6.5(–7.5) × 2.5–4.5(–5.5) μm (av. ± SD: 5.0 ± 1.4 × 3.4 ± 1.0), aseptate, rarely 1-septate, apex usually broadly rounded, intercalary conidia ovoid, limoniform to ellipsoid-ovoid, 6–13.5(–15) × 3–6 μm (av. ± SD: 9.5 ± 3.0 × 4.2 ± 0.8), 0–1(–2)-septate, not constricted at septa, with 1–3 distal hila, apex and base often rounded or attenuated, secondary ramoconidia ellipsoid-ovoid, subcylindrical to cylindrical-oblong or somewhat irregular, (7–)8.5–30 × (2.8–)3–4(–5) μm (av. ± SD: 18.9 ± 6.0 × 3.7 ± 0.5), 0–3-septate, not constricted at septa, with 2–6 distal hila crowded at the apex and situated on small lateral prolongations at the apex giving conidia an irregular appearance, younger ones pale olivaceous, later usually medium, sometimes dark olivaceous-brown, almost smooth (younger conidia) to mostly irregularly rough-walled, surface ornamentation variable, coarsely verruculose-rugose to verrucose (LM), irregular in outline, coarse verrucae up to 1 μm high, sometimes outer wall with surface ornamentation seemingly detaching, under SEM surface with irregularly reticulate structure or embossed stripes probably caused by diminishing turgor and shriveling of tender conidia, walls almost unthickened or often appear to be distinctly thickened, up to 1 μm wide, hila more or less conspicuous (sometimes not very conspicuous due to surface ornamentation), subdenticulate to denticulate, 0.5–1.8 μm diam, thickened and darkened-refractive; sometimes germinating, occasionally microcyclic conidiogenesis occurring.
Culture characteristics: Colonies on PDA attaining 47–57 mm diam after 14 d, olivaceous-grey to iron-grey, reverse olivaceous-black, grey-olivaceous towards margins, felty-floccose to fluffy, margin white, regular, glabrous, aerial mycelium abundant, loose to dense, without prominent exudates, sporulation profuse. Colonies on MEA reaching 52–73 mm diam after 14 d, pale olivaceous-grey to olivaceous-grey or whitish, reverse iron-grey, velvety to floccose, margins white, glabrous to feathery, regular, aerial mycelium loose to dense, low, growth effuse, radially furrowed, with wrinkled and folded colony centre, often somewhat immersed, without prominent exudates, sporulating. Colonies on OA attaining 47–54 mm diam after 14 d, smoke-grey to grey-olivaceous or olivaceous-grey, reverse pale mouse-grey to leaden-grey, floccose to fluffy-felty, margin colourless, glabrous, regular, aerial mycelium fluffy to felty-floccose, abundant, covering large parts of the colony, growth effuse to low convex, without exudates, sporulation profuse.
Specimen examined: South Korea, Hongcheon, N37°48'17” E127°51'13”, isol. from leaves of Rhus chinensis (Anacardiaceae), 11 Sep. 2005, coll. H.-D. Shin, isol. P.W. Crous as “Pseudocercospora rhoina”, CBS H-20450, holotype; ex-type culture CBS 126363 = CPC 12300.
Substrate and distribution: On Rhus; Asia (South Korea).
Notes: The conidial surface ornamentation is reminiscent of the recently introduced genus Verrucocladosporium (Crous et al. 2007b), which is a sister taxon to Cladosporium s. str. Within the C. cladosporioides complex there are only a few species characterised by having a similar verruculose or irregularly rough-walled surface ornamentation, but C. acalyphae, C. exasperatum and C. pini-ponderosae are comparable. Phylogenetically, all of them are quite distinct (see Fig. 1, part b vs. a; distance analyses in TreeBASE). Morphologically, C. acalyphae differs by much longer conidiophores, longer and wider, often globose terminal conidia and usually aseptate secondary ramoconidia. Cladosporium pini-ponderosae described on Pinus from Argentina is distinct in that the conidiophores, conidiogenous loci and hila are wider and secondary ramoconidia somewhat wider, 0–1(–2)-septate (Schubert et al. 2009). Cladosporium exasperatum has longer, small terminal conidia, narrower intercalary conidia and 0–2-septate secondary ramoconidia with irregularly short conical verrucae being not as high as in C. verrucocladosporioides.
Cladosporium vignae M.W. Gardner, Phytopathology 15(8): 457. 1925.
For descriptions in vitro see Morgan-Jones & McKemy (1992) and Ho et al. (1999); for a description in vivo see Schubert (2005).
Specimens examined: U.S.A., Indiana, LaFayette, on Vigna unguiculata [= V. sinensis] (Fabaceae), M.W. Gardner, BPI 427608, lectotype, designated here; Sep. 1924, M.W. Gardner, BPI 427604, isolectotype; 25 Aug. 1925, M.W. Gardner, BPI 427602, topotype; authentic strain CBS 121.25 = ATCC 200933 = MUCL 10110.
Substrates and distribution: On Lespedeza and Vigna spp.; widely distributed, Africa (South Africa, Zimbabwe), Asia (China), Australasia (Australia), North America (U.S.A.), South America (Brazil).
Literature: De Vries (1952: 99), Morgan-Jones & McKemy (1992), Ho et al. (1999: 144), Schubert (2005b).
Notes: This species, which is a seed-borne parasite, is the causal agent of scab, leaf and pod blight on Vigna unguiculata and Lespedeza bicolor. Gardner (1925), who introduced this species, stated that only young growing tissues are susceptible. Inoculation experiments were carried out to prove the pathogenicity of C. vignae. Under favourable conditions infections occurred with great rapidity and virulence, and visible lesions already causing crinkling of the leaves may be present within 48 h of inoculation. Attempts to infect field pea seedlings (Pisum sativum) with the cowpea fungus have been unsuccessful. Records of C. vignae on Pisum spp. (e.g., Winstead et al. 1960) are, therefore, doubtful and probably misidentifications. Da Silva & Minter (1995) recorded this species from Brazil on Vigna unguiculata subsp. cylindrical [= V. catjang (“Vigna cajanga”)].
De Vries (1952) examined an isolate of C. vignae sent to the CBS by M.W. Gardner in 1925, but found sporulation to be poor. On the basis of what could be observed, he concluded that this species was similar to C. cladosporioides and that it would probably have to be considered as a forma specialis of that species once better isolates were studied. The same isolate examined by de Vries is still preserved in the CBS culture collection but forms only sterile mycelium. Morgan-Jones & McKemy (1992) and Ho et al. (1999) examined C. vignae in culture, provided detailed descriptions of its features in vitro and discussed its morphological similarity with C. cladosporioides treating them as two separate species. Besides its pathogenicity to Vigna and Lespedeza spp. and its very characteristic symptoms, C. vignae is distinguished from C. cladosporioides in having somewhat wider conidiophores with several to numerous often somewhat crowded conidiogenous loci. Cladosporium cucumerinum, causal agent of crown blight and scab or gummosis disease of Cucurbitaceae, is morphologically also close to C. vignae but separated by its mostly longer conidiophores, its somewhat longer and wider ramoconidia and its immersed hyphae often possessing a slime coat. Phylogenetically C. vignae is quite distinct from the morphologically similar C. cladosporioides and C. cucumerinum (see Fig. 1, part c; distance analyses in TreeBASE).
Cladosporium lupiniphilum known from Byelorussia on Lupinus luteus has somewhat wider, 0–3-septate conidia and terminal conidiogenous cells with only few conidiogenous loci. Cladosporium robiniae on Robinia pseudoacacia, originally described as a species of Heterosporium, possesses fasciculate, nodulose conidiophores and conidia that are verrucose to echinulate, wider and more frequently septate (23–37 × 8.5–13.5 μm, 1–6-septate) (David 1997). Cladosporium psoraleae on Cullen corylifolium [≡ Psoralea corylifolia] (Fabaceae, tribus Phoraleae) is tentatively maintained as a separate species since the conidiogenous loci are somewhat wider and the conidia are usually somewhat longer and wider, subglobose and obovoid terminal conidia are usually lacking (Schubert 2005b). Additional collections, cultures and molecular data are needed to clarify whether this species is distinct from C. vignae or not.
Cladosporium xylophilum Bensch, Shabunin, Crous & U. Braun, sp. nov. MycoBank MB517092. Figs 75, 76.
Fig. 75.
Cladosporium xylophilum (CBS 125997). Macro- and micronematous conidiophores, mycelium sometimes formed in ropes and conidial chains. Scale bar = 10 μm.
Fig. 76.
Cladosporium xylophilum (CBS 125997). A–G. Macronematous conidiophores and conidial chains. Scale bar = 10 μm.
Etymology: Refers to a favourable substrate from which it was isolated, wood.
Cladosporii cladosporioidis simile, sed conidiophoris brevioribus et angustioribus, ramoconidiis secundariis brevioribus, 0–1(–2)-septatis, conidiis saepe irregulariter verruculosis vel verrucosis discernitur. Differt a Cladosporio sphaerospermo ramoconidiis sparsis, 0(–1)-septatis, ramoconidiis secundariis 0–1(–2)-septatis.
Mycelium immersed and superficial; hyphae unbranched or loosely branched, (0.5–)1–4(–5) μm wide, septate, not constricted at septa, sometimes with irregular swellings and outgrowths, subhyaline to pale or medium olivaceous-brown, smooth to asperulate, minutely verruculose or irregularly verrucose and rough-walled, sometimes with wart-like structures on the surface, walls unthickened or almost so, occasionally swollen at the base of conidiophores, up to 8 μm wide, sometimes forming ropes or rhizoids, branched at the base of conidiophores. Conidiophores macro-, semimacro- to sometimes micronematous, solitary, arising terminally and laterally from hyphae, erect, straight to slightly flexuous, cylindrical-oblong, usually neither nodulose nor geniculate, sometimes subnodulose at the uppermost apex, occasionally once geniculate-sinuous, unbranched, sometimes once branched, 7–155(–190) × 2–4(–5) μm, 0–6-septate, sometimes slightly constricted at septa, pale to medium olivaceous-brown, smooth or almost so, sometimes somewhat irregularly rough-walled or verruculose, especially towards the base, sometimes wider at the base, up to 5.5 μm wide, or slightly attenuated towards the apex, walls unthickened or slightly thickened; micronematous conidiophores paler, subhyaline to pale olivaceous-brown, smooth or almost so. Conidiogenous cells integrated, usually terminal, cylindrical-oblong, usually neither nodulose nor geniculate, sometimes subnodulose at the uppermost apex with loci situated on small lateral shoulders due to sympodial proliferation, 6–36 μm long, with (1–)2–4(–6) apically crowded loci forming clusters of pronounced scars, sometimes with few additional loci at a slightly lower level, protuberant, subdenticulate to denticulate, (0.8–)1–2 μm diam, thickened and darkened-refractive. Ramoconidia occasionally formed, cylindrical-oblong, 19–35 μm long, 0(–1)-septate, smooth, base broadly truncate, 2.5–3 μm wide. Conidia numerous, catenate in densely branched chains, branching in all directions, mostly 2–4(–5) conidia in the unbranched terminal part of the chains, straight, small terminal conidia subglobose, obovoid, sometimes globose, 2–5(–6) × 2–2.5 μm (av. ± SD: 3.9 ± 0.9 × 2.3 ± 0.3), aseptate, slightly attenuated towards apex and base, apex broadly rounded, intercalary conidia ovoid, limoniform to ellipsoid or subcylindrical, sometimes irregular in outline especially towards the distal end due to numerous hila arranged in sympodial clusters of pronounced scars, 5–11(–13) × (2–)2.5–3 μm (av. ± SD: 7.7 ± 2.2 × 2.6 ± 0.3), 0–1-septate, septum median, not constricted, with 2–7(–10) distal hila, crowded at the apex, sometimes situated on small lateral prolongations, small terminal conidia and intercalary conidia almost smooth to often irregularly rough-walled, loosely verruculose to verrucose, attenuated towards apex and base, secondary ramoconidia ellipsoid, subcylindrical to cylindrical-oblong or irregular in outline, (5.5–)7–23(–32) × (2–)2.5–4(–5) μm (av. ± SD: 14.5 ± 5.1 × 3.1 ± 0.5), 0–1(–3)-septate, septum median or somewhat in the upper half, not constricted, with (2–)3–7(–10) distal hila, crowded at the apex or situated on small lateral prolongations, pale olivaceous to pale medium olivaceous-brown, smooth or almost so, walls unthickened or almost so, hila conspicuous, subdenticulate to denticulate, 0.5–2 μm diam, somewhat thickened and darkened-refractive; microcyclic conidiogenesis occasionally occurring with conidia forming secondary conidiophores.
Culture characteristics: Colonies on PDA attaining 52–74 mm diam after 14 d, olivaceous-grey to grey-olivaceous, reverse iron-grey to olivaceous-black, floccose to fluffy, margins grey-olivaceous, feathery, aerial mycelium abundant, floccose to fluffy, loose to dense, growth effuse, without exudates, sporulation profuse. Colonies on MEA reaching 47–74 mm diam after 14 d, olivaceous-grey, whitish due to aerial mycelium, reverse olivaceous to iron-grey, velvety to floccose-felty, margins feathery, aerial mycelium felty, whitish to pale olivaceous-grey, loose to dense, growth effuse with sometimes papillate surface, sometimes with numerous small prominent exudates, sporulation profuse. Colonies on OA reaching 47–58 mm diam after 14 d, pale olivaceous-grey to olivaceous-grey, olivaceous-buff, greenish olivaceous to grey-olivaceous due to sporulation, reverse pale olivaceous-grey to olivaceous-grey, velvety, floccose to fluffy-felty, margins grey-olivaceous, glabrous to feathery, regular, aerial mycelium abundant, fluffy to floccose, felty, low to high, loose to dense, growth effuse, without exudates, sporulation profuse.
Specimens examined: Canada, Ontario, isol. from galls of Apiosporina morbosa (Venturiaceae) on twigs of Prunus sp. (Rosaceae), 2005, coll. K.A. Seifert, isol. P.W. Crous, CPC 12101. France, caves de Madelaine, isol. from leaves, 21 Aug. 2007, P.W. Crous, CPC 14281. Italy, isol. from twigs of Salix viminalis (Salicaceae), Sep. 2006, coll. W. Gams, isol. P.W. Crous, CBS 126588 = CPC 13512, CPC 13513, 13514. Russia, Leningrad Oblast, Roshino district, Pionerskoje Forestry, isol. from dead wood of Picea abies (Pinaceae), 2005, D.A. Shabunin, CBS H-20452, holotype; ex-type cultures CBS 125997 = CPC 12403. U.S.A., Washington, isol. from bing cherry fruits (Rosaceae), isol. by F.M. Dugan, CBS 113749 = cv 10-53 sci1, CBS 113756 = st5-25 sci 1.
Substrates and distribution: On wood and plant material; Europe (France, Italy, Russia), North America (Canada, U.S.A.).
Notes: With its numerous subglobose, globose and ovoid, verruculose or verrucose terminal conidia C. xylophilum resembles C. sphaerospermum but differs in having usually 0–1(–2)-septate ramoconidia and conidia. Cladosporium cladosporioides is distinct in that the conidiophores are longer and somewhat wider, (2.5–)3–5(–5.5) μm wide, conidia are smooth and secondary ramoconidia usually aseptate and much longer.
Strain CPC 14364, isolated from indoor air in Denmark, clusters with the isolates of C. xylophilum given above but deviates in morphology in that small terminal conidia and intercalary conidia are smooth, secondary ramoconidia shorter (6–17(–19) μm) and conidiogenous loci and hila slightly narrower (0.5–1.5(–1.8) μm diam). Based on ACT sequence data this single strain clusters outside the C. xylophilum subclade, but with TEF data it sits within these isolates (Fig. 1, part b; distance analyses in TreeBASE). Also for the other strains in the species some intraspecific variation exists and therefore it is tentatively maintained in C. xylophilum until additional isolates can be included to clarify the status of this strain.
DISCUSSION
Cladosporium cladosporioides is an intricate complex demonstrated here to contain several cryptic species, some of which are named herein, with others remain unnamed. Numerous species recognised in this complex are morphologically very similar and often only morphologically distinguished by careful observation and with detailed biometric data. As far as possible, species were distinguished herein based on a combination of morphologic and biometric features, culture characteristics and molecular data. In some cases, strains that are clearly genetically distinct were morphologically indistinguishable. Some groups of taxa are tentatively treated as a species complex until analyses of more extensive sets of isolates can generate clearer taxonomic differentiation. For species or phylogenetic lineages between which morphological differences are lacking or ambiguous, correct determination is only possible using molecular techniques. Documented morphological variability within a species can increase with the availability of greater numbers of isolates, or can be attributed to environmental conditions, age of the cultures, etc., all of which render attempts at identification on morphological criteria even more difficult. For this reason we recommend that identification of these species be based on a polyphasic approach using a range of morphological and molecular markers.
The presented results are a conservative approach to define species entities, in that new species were only introduced when justified by sufficient morphological and phylogenetic differences. Similar to the C. herbarum complex (Schubert et al. 2007b), a huge diversity of species and genotypes has been found on diverse substrates isolated in a wide range of habitats. Most of the species seem to be saprobic, but some are phytopathogenic, host-specific taxa are also involved, e.g., C. cucumerinum, C. colocasiae and C. vignae. Many of the new species are described from and collected in Australia, and it can be expected that more will be discovered, especially from hosts belonging to the Myrtaceae (Eucalyptus, Corymbia, Melaleuca) (Crous et al. 2009d). Members of this plant family appear to be favourable hosts for numerous plant pathogenic and saprobic fungi. The biology of the Cladosporium species described on Myrtaceae is still unclear but thus far, fungi occurring on hosts of this plant family have proven to be largely host family specific (Cheewangkoon et al. 2009).
The mycelium in most of the Cladosporium species treated here is more or less Zasmidium (Stenella-)-like in vitro, being verruculose or verrucose to irregularly rough-walled, an observation not previously documented from the natural hosts since the fungal hyphae are usually intercellular in host tissue. In addition to the more Cercospora-like conidia in Stenella, Deighton (1979) considered the verruculose superficial mycelium of Stenella as a basic character for the discrimination between Stenella and Cladosporium. Deighton's view was not supported in our results here, nor, in analogous studies on the C. herbarum complex (Schubert et al. 2007b). However, the scar structure of Stenella-like hyphomycetes is distinct by being not cladosporioid. The type species of Stenella, S. araguata, proved to be an anamorph of Teratosphaeria (Teratosphaeriaceae) (Crous et al. 2007b). Recent phylogenetic studies showed that the Stenella-like morphology type is polyphyletic within the Mycosphaerellaceae, and paraphyletic within the Capnodiales (Crous et al. 2009e). Within the Mycosphaerellaceae, Zasmidium proved to be the oldest name for Stenella-like hyphomycetes (Arzanlou et al. 2007) and differs from Stenella s. str. in having planate, cercosporoid loci (versus pileate conidiogenous loci in Stenella) (Crous et al. 2009c, d; Braun et al. 2010). In several species expanded superficial hyphal ropes are formed, e.g. in C. angustisporum, C. australiense and C. perangustum.
The conidiophores in most species treated in this study are straight to somewhat flexuous, narrowly cylindrical to cylindrical-oblong or sometimes filiform, non-nodulose, usually not or only once geniculate-sinuous, unbranched or occasionally branched with branches being often short, only formed as peg-like lateral outgrowths just below a septum. Nodose conidiophores with distinct, regular, more pronounced swellings, clearly separated and distant from each other, are formed in C. colocasiae, C. oxysporum and partly also in C. tenuissimum. The process of conidiogenesis within these species has been described in detail by McKemy & Morgan-Jones (1991). Conidiophores become temporarily determinate, linear apical growth ceases. The conidiophores swell appreciably at the extreme apex and a few conidia are formed in close proximity to one another at the surface of the inflated portion. Following conidiation, apical meristematic terminal growth resumes giving rise initially to a narrow, hypha-like extension above the fertile node. This grows to varying lengths, depending upon growing conditions. The extended distal portion usually becomes separated from the node below by a transverse septum and then ceases growth. Terminal swellings and conidiation then ensue at the higher level and the sequence of events is repeated a number of times to give rise to the characteristic nodose morphology. In the C. herbarum complex the process of conidiogenesis differs in that the conidiophores often possess multilateral swellings round about the stalks, these swellings usually formed in quick succession giving conidiophores a somewhat gnarled or knotty appearance (Schubert et al. 2007b).
Surface ornamentation of conidia in the C. cladosporioides complex is quite variable ranging from smooth or almost so to irregularly verruculose-rugose, verrucose or rough-walled in some species. This is comparable with the C. sphaerospermum complex in which species with both smooth-walled as well as ornamented conidia are included (Zalar et al. 2007), whereas all species in the C. herbarum complex possess ornamented conidia with the ornamentation ranging from minutely verruculose to verrucose, echinulate or spiny (Schubert et al. 2007b). The most prominent surfaces within the C. cladosporioides complex are formed by C. acalyphae, C. exasperatum and C. verrucocladosporioides. Under SEM the surface of their irregularly verruculose-rugulose conidia show irregularly reticulate structures or embossed stripes. This phenomenon was also described and illustrated for powdery mildew anamorphs (Cook et al. 1997, Braun et al. 2002). Cladosporium cladosporioides usually forms smooth conidia (LM) but under SEM such wrinkled structures or embossed stripes are also visible. They are not as prominent as in C. acalyphae or C. exasperatum and therefore not to be seen when using light microscopy and seem to occur more commonly in older conidia. Several species are characterised by irregular ornamentation on the small terminal and intercalary conidia, whereas secondary ramoconidia are smooth or almost so, as in C. inversicolor, C. acalyphae and C. rectoides. Combined with additional taxonomic features, this characteristic can be used for species delimitations.
CryoSEM provides opportunity to study organisation of the fungal colony at relatively high magnifications and fine details of the conidiophore, (ramo)conidia and scars, and reveals additional features that can be used to distinguish the different species as already stated in the C. herbarum complex (Schubert et al. 2007b). CryoSEM was used to study morphological criteria of potential significance for the C. cladosporioides complex. The most basic architecture of fungal morphology is an interconnected fungal mycelium that gives rise to aerial hyphae. Initially, the fungal spore germinates and forms a germ tube that accelerates in growth (see for instance, Köhli et al. 2008) and differentiates into a prostrate hypha that extends parallel to or penetrates into the agar substrate. After reaching a certain length, a branch hypha forms behind a septum. Subsequently, hyphae branch away from the substrate into the air. At first sight the aerial hyphae appear similar to the hyphae adhering to the substrate (“substrate hyphae”), but aerial hyphae must be different, as no nutrients can be taken up directly by these cells, and therefore they are dependent on mycelium in direct contact with the substrate for water and nutrients (Dijksterhuis 2010). However, aerial hyphae may play an important role in boosting the respiration of a fungal colony (Rahardjo et al. 2002), and therefore biomass and enzyme production (Te Biesebeke et al. 2006). The aerial hyphae may form different types of fruiting bodies and sexual spores (as the ascomata of the Eurotiales, or apothecia and perithecia in other groups of fungi) or other structures eventually forming asexual spores for dispersion by water or air (see van Leeuwen et al. 2010). The latter structures can be very simple (as the simple spore-releasing phialides in Fusarium), or elaborate (as the ramified structures from Cladosporium and terverticillate Penicillia) or even closed pycnidial conidiomata that resemble ascomata.
Growth of the substrate hyphae in Cladosporium was documented in a number of cases at the margin of colonies (C. chalastosporoides, C. exile, C. globisporum, C. perangustum and C. scabrellum). These hyphae grew on the agar surface and penetrated into the agar. In several species the hyphae differentiated relatively quickly into regularly segmented, broad hyphae that gave rise to conidiophores (C. chalastosporoides, C. cladosporioides, C. exasperatum, C. exile, C. globisporum, C. perangustum, C. scabrellum and C. verrucocladosporioides). In other cases the individual hyphal cells appeared swollen and pigmented, and were not elongated. In C. acalyphae, C. asperulatum, C. chalastosporoides and C. perangustum these cells developed into three-dimensional “meristematic” or “parenchymatic” structures, e.g. in C. chalastosporoides, they are the basis of the structure of the fungal colony on which many conidiophores are formed (see Fig. 16F–H). These structures are diagnostic for this species. Cladosporium perangustum and C. chalastosporoides exhibited both the segmented “broad hyphae” type as well as the meristematic type of structures. In the first species they appeared to co-exist, while in the second species the hyphae were observed to fuse and form these elaborated structures. In C. exasperatum the segmented hyphae could also protrude above the agar surface (see Fig. 28H). In C. asperulatum a scar was observed directly on the swollen cell of the three-dimensional structure suggesting the potential of these cells to form (ramo)conidia directly (Fig. 9J).
These observations suggest that in the C. cladosporioides clade, the substrate hyphal stage might be ephemeral, and quickly develops into segmented, pigmented and/or meristematic structures on which conidiophores are formed. There is clearly variation in the amount of hyphae present under these growth conditions. For instance, C. acalyphae, C. verrucocladosporioides and C. asperulatum form little of any hyphae and only exhibit conidiophores sprouting from clumps of fungal cells. In C. exile, hyphae develop into a very regular segmented pattern, and cells between certain “islands” disappear, presumable due to lysis (apoptosis?), which is unique for this species in this study.
Aerial hyphae were observed in several species, but it is difficult to predict if aerial structures will develop into stipes of conidiophores or if they can be regarded as very elongated conidiophores (as with C. exile and C. perangustum). Cladosporium exasperatum produced structures that formed loops and anastomosed in mid air, while C. cladosporioides formed numerous aerial hyphae with side branches.
The secondary ramoconidia and conidia of the C. cladosporioides clade are easily dislodged from the conidiophores during snap freezing, or as a result of electrostatic forces expelled from the electron beam due to the fragile connections that exist between the spores. This was more prominent than with fungi of the C. herbarum clade that are discussed in Schubert et al. (2007b), and might suggest that there is variation in the strength of the connection between the cells or in other properties of the spores (as electrostatic properties of the conidial cell wall) that result in different forces on these spores resulting in their release.
Using microscopy, chains of conidia were observed to be intact, and it was clear that these chains could extend to 7–8 or even more cells. Cladosporium asperulatum especially formed delicate, long chains containing elongated spores. There is a marked variety in the shape of the conidia, from nearly globose (very marked in C. globisporum and also C. exasperatum; C. scabrellum, C. perangustum and C. verrucocladosporioides) to highly elongated (most extreme in C. chalastosporoides and to a lesser extent C. asperulatum and C. acalyphae). The size of the conidium gradually diminishes throughout the chain. If the conidia of a chain are only marginally connected and no free cytoplasmic contact is possible, which is suggested by the SEM-pictures, novel cells must have been formed from the nutrient sources present in the former conidium. It might be hypothesised that there is a maximal length of a chain based on the ability of the more terminal conidia to obtain nutrients.
Novel for the species of the C. cladosporioides clade is the reticular ornamentation or embossed stripes on the cell walls of conidia and ramoconidia in most species. This is most prominent in C. acalyphae, C. exile, C. verrucocladosporioides and C. exasperatum as well as the neotype of C. cladosporioides in some micrographs (Fig. 19H). Cladosporium chalastosporoides did not exhibit ornamentation on the spores. The other species had more subtle patterns present on the spores (especially C. globisporum has a delicate, but subtle ornamentation). The nature of this ornamentation is not clear; it could be a mucus-like material. Conidiophores or ramoconidia were in all cases more or less smooth structures. In general, secondary ramoconidia are ornamented in C. acalyphae, C. exile, C. perangustum, C. asperulatum and C. exasperatum.
Scars are prominent in all species and tend to be become increasingly larger as their position in the conidial chain becomes progressively basal. Ramoconidia and secondary ramoconidia can bear markedly wider scars (see for example C. exile, Fig. 31B; C. perangustum, Fig. 56F; C. asperulatum, Fig. 9I). They normally show a distinct rim and a more or less flattened dome. Cladosporium exile, C. perangustum and C. chalastosporoides show notable scars but a precise analysis of these structures is complex due to the differences between the cells.
During the development of the conidiophore its complexity increases and the overall structure becomes obscured due to numerous branches and conidia. One can ask how the conidiophores of Cladosporium do develop when the chains of conidia must have a restricted length (e.g. eight cells?) as a result of the lack of an open connection of cytoplasm that delivers building blocks for the continuous production of new spores as is the case within the genera Penicillium and Aspergillus. It has been observed that conidiophores and spores are formed by C. cladosporioides within 24 h after seeding of spores (J. Dijksterhuis, unpubl. data). This might indicate that rapid and abundant spore formation is an important ecological strategy for this fungus.
When is a Cladosporium conidiophore completed? Ramoconidia and to a lesser extent secondary ramoconidia can be regarded as the powerhouses of spore formation, because of their capability to give rise to four whorls of “downstream” ramoconidia that each form several conidial chains. Cladosporium perangustum, C. asperulatum and C. scabrellum form markedly long ramoconidia and it would be of interest to evaluate whether these species form more spores than other species in the clade. Cladosporium chalastosporoides shows a characteristic way of chain formation with short conidiophores originating from a mass of meristematic cells. Here, the conidiophore and ramoconidia show a marked bend before producing the last set of chains (whorl) of spores (see Fig. 16A). An overview of a small colony of C. verrucocladosporioides (Fig. 74A) suggests that continually more conidiophores are formed between older ones, which also leads to a further increase in the number of spores.
Most species in this study were avid spore formers on the growth media tested, and showed apparent differences in the stages of colony formation and not only at the level of conidiophore morphology. Characteristic differences appeared in the timing and appearance of formation and differentation of substrate hyphae and aerial hyphae, including of segmented hyphae and three-dimensional structures within the colony. Other marked differences were observed in the formation of conidiophores, conidia and conidial ornamentation.
Within the C. cladosporioides complex we failed to induce Davidiella teleomorphs as we successfully did in the C. herbarum complex. All Davidiella states readily forming in the latter complex were homothallic (Schubert et al. 2007b). In the absence of nettle stems we used pine needles in the course of the present examinations, but it seems that they are a less useful growth medium for inducing Cladosporium teleomorphs. However, some members of the C. cladosporioides complex appear to have Davidiella states. For instance, CBS strain 109082 represents an ascospore isolate, collected on Silene maritima, Skomer Island, Pembrokeshire, Wales, U.K. by A. Aptroot, as Mycosphaerella tassiana var. arthopyrenioides. Morphologically the CBS strain is almost indistinguishable from C. cladosporioides, but represents one of the unnamed, distinct lineages in the tree (C. cladosporioides s.lat. Lineage 3; see Fig. 1, part a). The Davidiella teleomorph (in vivo; CBS H-19874) (Fig. 77) is characterised by numerous small, dark brown, submerged ascomata, 120–150 μm diam, with a central periphysate ostiole, 5–10 μm diam. Asci are obovoid, (25–)30–32(–35) × (8–)10–13(–14) μm. Ascospores are fusoid-ellipsoidal, thick-walled, constricted at the median septum, containing angular lumina (Aptroot 2006), turning brown once discharged, and some contain remnants of a mucoid layer, (10–)11–13(–14) × (3–)3.5(–4) μm. With this knowledge in mind, recent collections have revealed Davidiella states for other taxa in the cladosporioides complex (P.W. Crous, unpubl. data). They have thus far largely been overlooked, as the ascomata are usually submerged, around 100 μm in diameter, and thus rather inconspicuous compared to the erumpent, large ascomata observed in the herbarum complex (Schubert et al. 2007b). The fact that the cladosporioides clade includes taxa with a sexual cycle could help explain all the cryptic lineages observed in the present study. Further studies assessing whether these fungi are heterothallic or homothallic, using molecular-based tools to determine the nature and presence of mating genes may help to resolve these issues.
Fig. 77.
Davidiella state (CBS H-19874) of Cladosporium cladosporioides-like strain CBS 109082. A. Ascomata on stems of Silene maritima. B–C. Asci and ascospores (arrow denotes mucoid layer). Scale bars = 10 μm.
Several isolates from a single substrate in a single location – chasmothecia of Phyllactinia guttata on leaves of an individual plant, Corylus avellana (Dugan & Glawe 2006; Table 1) were distributed in distinct and widely separated clusters throughout the tree, and represent several completely different species, including the new species Cladosporium exile and C. phyllactiniicola. Other strains clustered with isolates of C. cladosporioides, C. inversicolor, C. lycoperdinum or C. perangustum. Most of these isolates from chasmothecia had previously been assigned to C. uredinicola on the basis of morphology (“fairly variable” in C. uredinicola) and host (powdery mildew) by keys and descriptions in Heuchert et al. (2005). Two other isolates, already included in analysis of the C. herbarum complex, proved to be conspecific with C. macrocarpum and C. tenellum (Schubert et al. 2007b), consistent with a prior determination (Dugan & Glawe 2006). That isolates previously identified as C. uredinicola (Dugan & Glawe 2006; and CBS 306.84) should be more accurately identified in this study by molecular-genetic criteria is not so surprising. Although our putatively representative material (CPC 5390) is now well characterised (here, and Ho et al. 1999, Morgan-Jones & McKemy 1990), Spegazzini's type material of C. uredinicola is in poor condition (Heuchert et al. 2005) and further work, including designation of a neotype, may be necessary. However, that so many taxa should be recovered from a single host (one powdery mildew species) from an individual plant is quite unexpected. There are alternative explanations for the wide range of taxa isolated from these chasmothecia. Use of disinfectants (ethanol, sodium hypochlorite) on objects as small as chasmothecia is problematic (often rendering internal fungi nonviable), so detached chasmothecia were “cleaned” by forcing them through solid agar for 4–5 cm for removal of spores or hyphae adhering to the chasmothecial surface (Dugan & Glawe 2006). Perhaps not all conidia or hyphae adherent to the chasmothecial surface were removed by this method. However, microscopic examination revealed growth of Cladosporium hyphae inside penicillate cells of the chasmothecia, or inside the accompanying gelatinous matrix (Dugan & Glawe 2006). Sometimes hyphae egressed this matrix to sporulate (Dugan & Glawe 2006). Kiss (2003) also noted growth of Cladosporium spp. inside penicillate cells, and discussed impacts on chasmothecial function. The gelatinous matrix probably readily entraps conidia and provides a hospitable environment for growth of multiple Cladosporium species, all components of the aerobiota. The aerobiota comprises numerous genera of fungi, so it remains to be answered why only species in Cladosporium were observed in penicillate cells and matrix by Kiss (2003) and Dugan & Glawe (2006). The phenomenon of co-occurrence of many species on the same lesions on a single host in Mycosphaerella and Teratosphaeria leaf disease complexes has been frequently described and discussed (Crous 1998, Crous et al. 2004b, 2007a, 2008a, b, 2009b, f, f, Crous & Groenewald 2005, Burgess et al. 2007, Arzanlou et al. 2008, Cheewangkoon et al. 2008). Therefore it is not surprising that co-occurring genotypes or species also exist in the related genus Cladosporium (also see Wirsel et al. 2002), suggesting that special care needs to be taken during the isolation and culturing of these taxa.
Acknowledgments
The authors thank the technical staff, Arien van Iperen (cultures), Trix Merkx (deposit of strains) and Marjan Vermaas (photo plates) for their invaluable assistance. Various colleagues collected material used in this study, for which we are very grateful, namely M. Arzanlou (Teheran, Iran), W. von Brackel (Germany), W. Gams (Baarn, Netherlands), D. Glawe (Washington State University, Pullman), B. Heuchert (Martin-Luther-University Halle-Wittenberg, Germany), K.A. Seifert (Agriculture Canada, Ottawa, Canada), and L.J. du Toit (Washington State University, Mount Vernon, U.S.A.).
Taxonomic novelties: Cladosporium acalyphae Bensch, H.D. Shin, Crous & U. Braun, sp. nov., C. angustisporum Bensch, Summerell, Crous & U. Braun, sp. nov., C. asperulatum Bensch, Crous & U. Braun, sp. nov., C. australiense Bensch, Summerell, Crous & U. Braun, sp. nov., C. basiinflatum Bensch, Crous & U. Braun, sp. nov., C. chalastosporoides Bensch, Crous & U. Braun, sp. nov., C. exasperatum Bensch, Summerell, Crous & U. Braun, sp. nov., C. exile Bensch, Glawe, Crous & U. Braun, sp. nov., C. flabelliforme Bensch, Summerell, Crous & U. Braun, sp. nov., C. globisporum Bensch, Crous & U. Braun, sp. nov., C. hillianum Bensch, Crous & U. Braun, sp. nov., C. inversicolor Bensch, Crous & U. Braun, sp. nov., C. iranicum Bensch, Crous & U. Braun, sp. nov., C. paracladosporioides Bensch, Crous & U. Braun, sp. nov., C. perangustum Bensch, Crous & U. Braun, sp. nov., C. phyllactiniicola Bensch, Glawe, Crous & U. Braun, sp. nov., C. pseudocladosporioides Bensch, Crous & U. Braun, sp. nov., C. rectoides Bensch, H.D. Shin, Crous & U. Braun, sp. nov., C. scabrellum Bensch, Schroers, Crous & U. Braun, sp. nov., C. subuliforme Bensch, Crous & U. Braun, sp. nov., C. verrucocladosporioides Bensch, H.D. Shin, Crous & U. Braun, sp. nov., C. xylophilum Bensch, Shabunin, Crous & U. Braun, sp. nov.
References
- Anilkumar TB, Seshadri VS (1975). Cladosporium leaf spot of sunflower. Current Science 44(19): 722. [Google Scholar]
- Aptroot A (2006). Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1–231. [Google Scholar]
- Arx JA von (1987). Plant pathogenic fungi. Beihefte zur Nova Hedwigia 87: 1–288. [Google Scholar]
- Arya C, Arya A (2003). New leaf spot diseases of social forestry trees – II. Journal of Mycology and Plant Pathology 33(2): 320–322. [Google Scholar]
- Arzanlou A, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW (2007). Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater M-F, Buddenhagen IW, Viljoen A, Crous PW (2008). Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20: 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagyanarayana G, Braun U (1999). Phytopathogenic micromycetes from India (II). Sydowia 51(1): 1–19. [Google Scholar]
- Biesebeke R Te, Boussier A, Biezen N van, Braaksma M, Hondel CA van den, Vos WM de, Punt PJ (2006). Expression of Aspergillus hemoglobin domain activities in Aspergillus oryzae grown on solid substrates improves growth rate and enzyme production. Biotechnology Journal 1: 822–827. [DOI] [PubMed] [Google Scholar]
- Brackel W von (2008). Phoma ficuzzae sp. nov. and some other lichenicolous fungi from Sicily, Italy. Sauteria 15: 103–120. [Google Scholar]
- Brackel W von (2009). Weitere Funde von flechtenbewohnenden Pilzen in Bayern – Beitrag zu einer Checkliste IV. Berichte der Bayerischen Botanischen Gesellschaft 79: 5–55. [Google Scholar]
- Brandenburger W (1985). Parasitische Pilze an Gefäßpflanzen in Europa. Fisher Verlag, Stuttgart, New York.
- Braun U (1998). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes). Vol. 2. IHW-Verlag, Eching.
- Braun U (2001). Cladosporium exoasci, C. exobasidii and some allied species. Schlechtendalia 7: 53–58. [Google Scholar]
- Braun U, Cook RTA, Inman AJ, Shin H-D (2002). The taxonomy of the powdery mildew fungi. In: The powdery mildews, a comprehensive treatise (Bélanger RR et al., eds.). APS Press, St. Paul, U.S.A.: 13–55.
- Braun U, Crous PW, Dugan FM, Groenewald JZ, Hoog GS de (2003). Phylogeny and taxonomy of cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s.str. Mycological Progress 2: 3–18. [Google Scholar]
- Braun U, Crous PW, Schubert K (2008a). Taxonomic revision of the genus Cladosporium s. lat. 8. Reintroduction of Graphiopsis (= Dichocladosporium) with further reassessments of cladosporioid hyphomycetes. Mycotaxon 103: 207–216. [Google Scholar]
- Braun U, Crous PW, Schubert K, Shin H-D (2010). Some reallocations of Stenella species to Zasmidium. Schlechtendalia 20: 99–104. [Google Scholar]
- Braun U, Cunnington J, Priest MJ, Shivas RG, Schubert K (2005). Annotated check-list of Ramularia species in Australia. Australasian Plant Pathology 34: 1–7. [Google Scholar]
- Braun U, Hill CF, Schubert K (2006). New species and new records of biotrophic micromycetes from Australia, Fiji, New Zealand and Thailand. Fungal Diversity 22: 13–35. [Google Scholar]
- Braun U, Melnik VA, Schubert K (2008b). Two new species of the hyphomycete genus Cladosporium. Mikologia i Fitopatologia 42(3): 214–220. [Google Scholar]
- Braun U, Schubert K (2007). Taxonomic revision of the genus Cladosporium s. lat. 7. Descriptions of new species, a new combination and further new data. Schlechtendalia 16: 61–76. [Google Scholar]
- Bugnicourt F (1958). Contribution à l'étude de Cladosporium colocasiae Sawada. Revue de Mycologie 23: 233–236. [Google Scholar]
- Burgess TI, Barber PA, Sufaati S, Xu D, Hardy GEStJ, Dell B (2007). Mycosphaerella spp. on Eucalyptus in Asia: New species, new host and new records. Fungal Diversity 24: 135–157. [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-anan C (2008). Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, Hyde KD, To-anun C, Crous PW (2009). Myrtaceae, a cache of fungal biodiversity. Persoonia 23: 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RTA, Inman AJ, Billings C (1997). Identification and classification of powdery mildew anamorphs using light and scanning electron microscopy and host range data. Mycological Research 101(8): 975–1002. [Google Scholar]
- Cooke MC (1883). New American Fungi. Grevillea 12(61): 22–33. [Google Scholar]
- Crous PW (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. [Google Scholar]
- Crous PW, Braun U, Groenewald JZ (2007a). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, Groenewald JZ (2007b). Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Wingfield MJ, Wood AR, Shin H-D, Summerell BA, Alfenas AC, Cumagun CJR, Groenewald JZ (2009a). Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22: 139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004a). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ (2005). Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470. [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ (2004b). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ (2009b). Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood R, Gueidan C, Hoog GS de, Groenewald JZ (2009c). Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schroers H-J, Groenewald JZ, Braun U, Schubert K (2006a). Metulocladosporiella gen. nov. for the causal organism of Cladosporium speckle disease of banana. Mycological Research 110: 264–275. [DOI] [PubMed] [Google Scholar]
- Crous PW, Schubert K, Braun U, Hoog GS de, Hocking AD, Shin H-D, Groenewald JZ (2007c). Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006b). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Groenewald JZ (2009d). Novel species of Mycosphaerellaceae and Teratosphaeriaceae. Persoonia 23: 119–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, Wingfield MJ, Hunter GC, Burgess TI, Andjic V, Barber PA, Groenewald JZ (2009e). Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Mostert L, Groenewald JZ (2008a). Host specificity and speciation of Mycosphaerella and Teratosphaeria species associated with leaf spots of Proteaceae. Persoonia 20: 59–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkleij GJM, Groenewald JZ, Samson RA (eds). (2009f). Fungal Biodiversity. CBS Laboratory Manual Series 1. Centraalbureau voor Schimmelcultures, Utrecht, Netherlands.
- Crous PW, Wingfield MJ, Groenewald JZ (2009f). Niche sharing reflects a poorly understood biodiversity phenomenon. Persoonia 22: 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF (1991). Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632. [Google Scholar]
- Crous PW, Wood AR, Okada G, Groenewald JZ (2008b). Foliicolous microfungi occurring on Encephalartos. Persoonia 21: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JC (1988). Cladosporium colocasiae. Mycopathologia 103: 115–116. [Google Scholar]
- David JC (1997). A contribution to the systematics of Cladosporium. Revision of the fungi previously referred to Heterosporium. Mycological Papers 172: 1–157. [Google Scholar]
- Deighton FC (1979). Studies on Cercospora and allied genera. VII. New species and redispositions. Mycological Papers 144: 1–56. [Google Scholar]
- Dijksterhuis J (2010). The Fungal Cell. In: Fundamentals of mold growth in indoor environments and strategies for healthy living (OCG Adan, Samson RA, eds). Wageningen Academic Press, The Netherlands: in press.
- Domsch KH, Gams W, Anderson TH (1980). Compendium of soil fungi. Vols 1 & 2. Academic Press, London.
- Dugan FM, Braun U, Groenewald JZ, Crous PW (2008). Morphological plasticity in Cladosporium sphaerospermum. Persoonia 21: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan FM, Glawe DA (2006). Phyllactinia guttata is a host for Cladosporium uredinicola in Washington State. Pacific Northwest Fungi 1: 1–5. [Google Scholar]
- Dugan FM, Schubert K, Braun U (2004). Check-list of Cladosporium names. Schlechtendalia 11: 1–103. [Google Scholar]
- Ellis MB (1971). Dematiaceous hyphomycetes. CMI, Kew.
- Ellis MB (1976). More dematiaceous hyphomycetes. CMI, Kew.
- Ellis MB, Ellis JP (1985). Microfungi on land plants. An identification handbook. MacMillan, New York.
- Ellis MB, Ellis JP (1988). Microfungi on miscellaneous substrates. An identification handbook. Croom Helm, London, and Timber Press, Portland, Oregon.
- Ellis MB, Holliday P (1972). Cladosporium cucumerinum. CMI Descriptions of Pathogenic Fungi and Bacteria No. 348.
- El-Morsy EM (2000). Fungi isolated from the endorhizosphere of halophytic plants from the Red Sea Coast of Egypt. Fungal Diversity 5: 43–54. [Google Scholar]
- Ferraris T (1912). Hyphales, Dematiaceae. Flora Italica Cryptogama, Pars I: Fungi, Fasc. 8: 195–534. [Google Scholar]
- Fisher FE (1967). Cladosporium leaf spot of Citrus in Florida. Plant Disease Reporter 51(12): 1070. [Google Scholar]
- Fresenius JBGW (1850). Beiträge zur Mykologie 1. Heinrich Ludwig Brömmer Verlag, Frankfurt.
- Gardner MW (1925). Cladosporium spot on cowpea. Phytopathology 25: 453–462. [Google Scholar]
- Gonzáles-Fragoso DR (1927). Estudio systemático de los Hifales de la Flora Española. Memorias de la Real Academia Ciencias Exactas Físicas y Naturales de Madrid, 2a Serie, 6: 1–377. [Google Scholar]
- Hammouda AM (1992). A new leaf spot of pepper caused by Cladosporium oxysporum. Plant Disease 76(5): 536–537. [Google Scholar]
- Hasija SK (1967). Additions to the fungi of Jabalpur (Madhya Pradesh) – VI. Indian Phytopathology 19(4): 373–377. [DOI] [PubMed] [Google Scholar]
- Heuchert B, Braun U (2006). On some dematiaceous lichenicolous hyphomycetes. Herzogia 19: 11–21. [Google Scholar]
- Heuchert B, Braun U, Schubert K (2005). Morphotaxonomic revision of fungicolous Cladosporium species (hyphomycetes). Schlechtendalia 13: 1–78. [Google Scholar]
- Ho MHM, Castañeda RF, Dugan FM, Jong SC (1999). Cladosporium and Cladophialophora in culture: descriptions and an expanded key. Mycotaxon 72: 115–157. [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2000). Atlas of clinical fungi, 2nd ed. CBS, Utrecht and Universitat rovira I virgili, Reus.
- Hoog GS de, Nishikaku AS, Fernandez Zeppenfeldt G, Padín-González C, Burger E, Badali H, Gerrits van den Ende AHG (2007). Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Studies in Mycology 58: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Hughes SJ (1958). Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 727–836. [Google Scholar]
- Kiss L (2003). A review of fungal antagonists of powdery mildews and their potential as biocontrol agents. Pest Management Science 59: 475–483. [DOI] [PubMed] [Google Scholar]
- Köhli M, Galati V, Boudier K, Roberson RW, Philippsen P (2008). Growth-speed-correlated localization of exocyst and polarisome components in growth zones of Ashbya gossypii hyphal tips. Journal of Cell Science 121: 3878–3889. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Suryanarayanan TS (2002). Endophyte assemblage in young, mature and senescent leaves of Rhizophora apiculata: evidence for the role of endophytes in mangrove litter degeneration. Fungal Diversity 9: 81–91. [Google Scholar]
- Kwon JH, Kang SW, Park CS (1999). Occurrence of eggplant scab caused by Cladosporium cucumerinum in Korea. The Plant Pathology Journal 15(6): 345–347. [Google Scholar]
- Kwon JH, Kang SW, Park CS (2000). Occurrence of sword bean scab caused by Cladosporium cucumerinum in Korea. Mycobiology 28: 54–56. [Google Scholar]
- Lamboy JS, Dillard HR (1997). First report of a leaf spot caused by Cladosporium oxysporum on greenhouse tomato. Plant Disease 81(2): 228. [DOI] [PubMed] [Google Scholar]
- Leeuwen MR van, Doorn T van, Golovina EA, Stark J, Dijksterhuis J (2010). Water- and air-distributed conidia exhibit differences in sterol content and cytoplasmic microviscosity. Applied and Environmental Microbiology 67: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau G (1907). Dr. L. Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. Zweite Auflage. Erster Band: Die Pilze Deutschlands, Österreichs und der Schweiz. VIII. Abteilung: Fungi imperfecti: Hyphomycetes (erste Hälfte), Mucedinaceae, Dematiaceae (Phaeosporae und Phaeodidymae). Leipzig.
- Lindau G (1910). Dr. L. Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. Zweite Auflage. Erster Band: Die Pilze Deutschlands, Österreichs und der Schweiz. IX. Abteilung: Fungi imperfecti: Hyphomycetes (zweite Hälfte), Dematiaceae (Phaeophragmiae bis Phaeostaurosporae), Stilbaceae, Tuberculariaceae, sowie Nachträge, Nährpflanzenverzeichnis und Register. Leipzig.
- Lobik AI (1928). Materialy k mikologicheskoj flore Terskogo okruga. Bolezni Rastenii 17(3–4): 157–199. [Google Scholar]
- Matsushima T (1975). Icones Microfungorum a Matsushima Lectorum. Kobe, Japan.
- Matsushima T (1980). Matsushima Mycological Memoirs No. 1. Saprophytic Microfungi from Taiwan, Part 1. Hyphomycetes. Matsushima Fungus Collection, Kobe, Japan.
- McAlpine D (1902). Fungus diseases of stone-fruit trees in Australia. Department of Agriculture, Victoria, Australia.
- McKemy JM, Morgan-Jones G (1991). Studies in the genus Cladosporium sensu lato IV. Concerning Cladosporium oxysporum, a plurivorous predominantly saprophytic species in warm climates. Mycotaxon 41: 397–405. [Google Scholar]
- McKemy JM, Morgan-Jones G (1992). Studies in the genus Cladosporium sensu lato. VII. Concerning Cladosporium cucumerinum, causal organism of crown blight and scab or gummosis of cucurbits. Mycotaxon 43: 163–170. [Google Scholar]
- McTaggart AR, Shivas RG, Braun U (2007). Annellosympodia orbiculata gen. et sp. nov. and Scolecostigmina flagellariae sp. nov. from Australia. Australasian Plant Pathology 36: 573–579. [Google Scholar]
- Mendes MAS, Silva VL da, Dianese JC, Ferreira MASV, Santos CEN dos, Neto EG, Urben AF, Castro C (1998). Fungos em plantas no Brasil. EMBRAPA. Brasília, D.F.
- Morgan-Jones G, McKemy JM (1990). Studies in the genus Cladosporium sensu lato I. Concerning Cladosporium uredinicola, occurring on telial columns of Cronartium quercuum and other rusts. Mycotaxon 39: 185–202. [Google Scholar]
- Morgan-Jones G, McKemy JM (1992). Studies in the genus Cladosporium sensu lato VI. Concerning Cladosporium vignae, causal organism of leaf and pod spot of cowpea (Vigna unguiculata) and leaf blight of Lespedeza bicolor. Mycotaxon 43: 9–20. [Google Scholar]
- Mullins J (2001). Microorganisms in outdoor air. In: Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control (Flannigan B, Samson RA, Miller JD, eds). Taylor & Francis, London: 3–16.
- Nylander JAA (2004). MrModeltest v2.2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.
- O'Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America 95(5): 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudemans CAJA (1919–1924). Enumeratio Systemica Fungorum. vol. 1 (1919); vol. 2 (1920); vol. 3 (1921); vol. 4 (1923); vol. 5 (index, 1924). Hague comitum, apud M. Nijoff.
- Park HG, Managbanag JR, Stamenova EK, Jong SC (2004). Comparative analysis of common indoor Cladosporium species based on molecular data and conidial characters. Mycotaxon 89: 441–451. [Google Scholar]
- Pasqualetti M, Rambelli A, Mulas B, Tempesta S (2005). Identification key and description of Mediterranean maquis litter microfungi. Bocconea 18: 5–176. [Google Scholar]
- Penzig AJO (1882). Fungi Agromiculi. Michelia 2: 385–503. [Google Scholar]
- Rahardjo YSP, Weber FJ, Paul le Comte E, Tramper J, Rinzema A (2002). Contribution of aerial hyphae of Aspergillus oryzae to respiration in a model solid-state fermentation system. Biotechnology and Bioengineering 78: 539–544. [DOI] [PubMed] [Google Scholar]
- Rayner RW (1970). A mycological colour chart. CMI and British Mycological Society. Kew, Surrey, England.
- Riesen T, Sieber T (1985). Endophytic fungi in winter wheat (Triticum aestivum L.). Swiss Federal Institute of Technology, Zürich.
- Roberts RG, Robertson JA, Hanlin RT (1986). Fungi occurring in the achenes of sunflower (Helianthus annuus). Canadian Journal of Botany 64: 1964–1971. [Google Scholar]
- Ronquist F, Huelsenbeck JP (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Saccardo PA (1886). Sylloge Fungorum vol. 4. Padova, Italy.
- Saccardo PA (1892). Sylloge Fungorum vol. 10. Padova, Italy.
- Saccardo PA (1906). Sylloge Fungorum vol. 18 (Saccardo PA & Saccardo D eds.). Padova, Italy.
- Saccardo PA (1931). Sylloge Fungorum vol. 25 (Trotter A ed.). Avellino, Italy.
- Samson RA, Houbraken JAMP, Summerbell RC, Flannigan B, Miller JD (2001). Common and important species of Actinomycetes and fungi in indoor environments In: Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control (Flannigan B, Samson RA, Miller JD, eds). Taylor & Francis, London: 287–473.
- Samson RA, Reenen-Hoekstra, ES van, Frisvad JC, Filtenborg O (2000). Introduction to food- and airborne fungi, 6th ed. CBS, Utrecht, Netherlands.
- Schoch C, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, BurgessTI, et al. (2009a). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, et al. (2009b). Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Schubert K (2005a). Taxonomic revision of the genus Cladosporium s. lat. 3. A revision of Cladosporium species described by J.J. Davis and H.C. Greene (WIS). Mycotaxon 92: 55–76. [Google Scholar]
- Schubert K (2005b). Morphotaxonomic revision of foliicolous Cladosporium species (hyphomycetes). Ph.D. dissertation. Martin-Luther-University Halle-Wittenberg, Germany. http://sundoc.bibliothek.uni-halle.de/diss-online/05/05H208/index.htm.
- Schubert K, Braun U (2004). Taxonomic revision of the genus Cladosporium s. lat. 2. Cladosporium species occurring on hosts of the families Bignoniaceae and Orchidaceae. Sydowia 56(2): 296–317. [Google Scholar]
- Schubert K, Braun U (2005a). Taxonomic revision of the genus Cladosporium s. lat. 1. Species reallocated to Fusicladium, Parastenella, Passalora, Pseudocercospora and Stenella. Mycological Progress 4(2): 101–109. [Google Scholar]
- Schubert K, Braun U (2005b). Taxonomic revision of the genus Cladosporium s. lat. 4. Species reallocated to Asperisporium, Dischloridium, Fusicladium, Passalora, Pseudoasperisporium and Stenella. Fungal Diversity 20: 187–208. [Google Scholar]
- Schubert K, Braun U (2007). Taxonomic revision of the genus Cladosporium s. lat. 6. New species, reallocations to and synonyms of Cercospora, Fusicladium, Passalora, Septonema and Stenella. Nova Hedwigia 84: 189–208. [Google Scholar]
- Schubert K, Braun U, Groenewald JZ, Crous PW (2007a). Cladosporium leaf-blotch and stem rot of Paeonia spp. caused by Dichocladosporium chlorocephalum gen. nov. Studies in Mycology 58: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K, Braun U, Mułenko W (2006). Taxonomic revision of the genus Cladosporium s. lat. 5. Validation and description of new species. Schlechtendalia 14: 55–83. [Google Scholar]
- Schubert K, Greslebin A, Groenewald JZ, Crous PW (2009). New foliicolous species of Cladosporium from South America. Persoonia 22: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, Dijksterhuis J, Starink MS, Hill CF, Zalar P, Hoog GS de, Crous PW (2007b). Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA, Hughes SJ, Boulay H, Louis-Seize G (2007). Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azalea and the Hormoconis anamorph of Amorphotheca resinae. Studies in Mycology 58: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M da, Minter DW (1995). Fungi from Brazil recorded by Batista and co-workers. Mycological Papers 169: 1–585. [Google Scholar]
- Simmons EG (2007). Alternaria. An identification manual. CBS Biodiversity Series 6: 1–775. [Google Scholar]
- Subramanian CV (1971). Hyphomycetes: an account of Indian species, except Cercosporae. New Delhi.
- Sutton BC (1973). Hyphomycetes from Manitoba and Saskatchewan, Canada. Mycological Papers 132: 1–143. [Google Scholar]
- Vries GA de (1952). Contribution to the knowledge of the genus Cladosporium Link ex Fr. CBS, Baarn.
- Wang CJK, Zabel RA (1990). Identification manual for fungi from utility poles in the eastern United States. ATCC, Rockville, MD.
- Winstead NN, Strider DL, Person LH (1960). Vegetable diseases in North Carolina during 1958–1959. Plant Disease Reporter 44: 491–495. [Google Scholar]
- Wirsel SGR, Runge-Froböse C, Ahrén DG, Kemen E, Oliver RP, Mendgen KW (2002). Four or more species of Cladosporium sympatrically colonize Phragmites australis. Fungal Genetics and Biology 35: 99–113. [DOI] [PubMed] [Google Scholar]
- Yamamoto W (1959). Some species of Cladosporium from Japan. Science Reports of the Hyogo University of Agriculture, Series, Agriculture 4(1): 1–6. [Google Scholar]
- Zalar P, Hoog GS de, Schroers HJ, Crous PW, Groenewald JZ, Gunde-Cimerman N (2007). Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with description of seven new species from hypersaline environments. Studies in Mycology 58: 157–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZY, Liu YL, Zhang T, Li TF, Wang G, Zhang H, He YH, Peng HH (2003). Cladosporium, Fusicladium, Pyricularia. Flora Fungorum Sinicorum 14: 1–297. [Google Scholar]