Abstract
Hypothesis
An objective cochlear framework, for evaluation of the cochlear anatomy and description of the position of an implanted cochlear implant electrode, would allow the direct comparison of measures performed within the various sub-disciplines involved in cochlear implant research.
Background
Research on the human cochlear anatomy in relation to tonotopy and cochlear implantation is conducted by specialists from numerous disciplines such as histologists, surgeons, physicists, engineers, audiologists and radiologists. To allow accurate comparisons between and combinations of previous and forthcoming scientific and clinical studies, cochlear structures and electrode positions must be specified in a consistent manner.
Methods
Researchers with backgrounds in the various fields of inner ear research as well as representatives of the different manufacturers of cochlear implants (Advanced Bionics Corp, Med-El, Cochlear Corp) were involved in consensus meetings held in Dallas, March 2005 and Asilomar, August 2005. Existing coordinate systems were evaluated and requisites for an objective cochlear framework were discussed.
Results
The consensus panel agreed upon a 3-dimensional, cylindrical coordinate system of the cochlea using the “Cochlear View” as a basis and choosing a z-axis through the modiolus. The zero reference angle was chosen at the centre of the round window, which has a close relationship to the basal end of the Organ of Corti.
Conclusions
Consensus was reached on an objective cochlear framework, allowing the outcomes of studies from different fields of research to be compared directly.
Introduction
Over the years researchers from different fields have been particularly interested in unraveling the anatomic substrates of the tonotopic organization of the cochlea, most recently in regard to cochlear implantation. Several techniques for estimation of the exact intracochlear position of a cochlear implant electrode array have been described and applied. Histopathologists, surgeons and radiologists used different methods, each meeting the specific needs in their field of research. However, the existing methods posed several problems regarding objectivity, influence of cochlear size and shape and combined applicability for histological, surgical as well as psychophysical studies. This has traditionally led to different descriptions of electrode positions across and even within sub-disciplines. Therefore there was a need for an objective cochlear framework.
To address these problems two consensus meetings were held in Dallas, March 2005 and in Asilomar, August 2005. A panel of researchers with backgrounds in the various fields (C Boëx, JJ Briaire, LT Cohen, CC Finley, JHM Frijns C James, PA Leake, PS Roland, T Roland, MW Skinner, BM Verbist), as well as representatives of the different manufacturers of cochlear implants (MA Faltys (Advanced Bionics, Sylmar, California, USA), JF Patrick (Cochlear, Sydney, Australia), C Jolly (Med El, Innsbruck, Austria)), discussed the need for a universal, objective coordinate system.
From a frequency analysis point of view the organ of Corti (OC) is the most important cochlear anatomic structure. It has been used as point of reference in many studies on cochlear anatomy and function. The tonotopic organization of the cochlea, for instance, has been mathematically characterized by Greenwood. He studied the frequency-position relation for acoustic stimulation in various mammalian species. The exponential formula he postulated gives characteristic frequencies of acoustic sensitivity (in Hz) as function of fractional length of the OC (measured from the helicotrema) [1-3]. These same frequency distributions are now used in cochlear implants to map the frequency distribution as a function of length along the array, implying that the insertion depth of the electrode array and the position of the individual contacts relative to the OC will determine the perceived pitch in cochlear implant patients. Numerous studies on pitch sensation have, however, shown that the cochlear frequency-position map does not accurately predict the pitch sensation produced by electrical stimulation of an electrode, even if high electrical stimulation rates are used to rule out any influence of the stimulation rate [4-8]. Boëx et al [7] showed that pitch sensations were found to be more than 1 octave (using insertion angle) or more than 2 octaves (using insertion lengths) lower than predicted by Greenwood's frequency position.
One possible factor contributing to the down shift of pitch in relation to Greenwood's function is the site of neural activation. Peri-modiolar cochlear implants are designed to stimulate the auditory nerve at the level of the spiral ganglion instead of the peripheral process [9;10] It has been reported that there is an angular offset between the position of the basilar membrane and that of the associated ganglion cells, particularly in the apical third of the cochlea [11;12]. Sridhar et al [10] determined the spiral ganglion map by tracing the fiber trajectories in temporal bones and found that the fibers in the extreme base and apex do not follow a radial trajectory. To overcome the shorter length of the spiral ganglion in relation to the OC there is a compression of the frequency map at low frequencies [10;13]. Thus the shift would become pronounced only at a deep insertion (beyond 450 degrees insertion depth in CView© (see below), personal communication Cohen, based on analysis of data provided by Pat Leake).
Since the length of the OC cannot be directly determined in most temporal bone and imaging studies an objective cochlea framework closely related to the OC is needed. This framework should be easily applicable for all subspecialties involved in cochlear research and patient care. By introducing such a cochlear coordinate system, results from a given field could be more easily translated to or combined with results from other fields of hearing research. The consensus panel discussed the requirements for such a framework and evaluated existing coordinate systems in this context.
Rationale of the various components of a cochlear framework
Angular or linear measurement
Cochlear implant insertion depth has been described either in terms of linear distance (in mm) or insertion angle (in degrees).
From the surgeon's and histopathologist's view much is to be said for the use of linear measurements. Such a measurement provides the surgeon with an immediate estimate of the result, e.g., in terms of the number of contacts inserted into the cochlea. Results in a longitudinal system can potentially be correlated to a fixed structure such as the OC or the electrode array. Yet, as described above, difference in stimulation site and compression of the spiral ganglion relative to the OC would possibly require corrections, especially at the apex and extreme base. Moreover, it is necessary to have reliable measures of both the length of the OC and the intracochlear position of the array. However, as mentioned before, the length of the OC cannot be determined in most temporal bone and imaging studies and there is a great intersubject variability of cochlear length as well as differences in OC length. [12;14-19]. The actual values differ between authors. Sato reported a mean cochlear length of 37.1 +/- 1.6 mm in males and of 32.3 +/- 1.8mm in females [20]. The inter-gender variability reported by Sato was not confirmed in Ketten's and, later, in Skinner's and Stakhovskaya's studies [13;16;17]. Moreover, the intracochlear trajectory of an electrode array differs between straight designs and perimodiolar designs. Two implants with the same insertion depth measured in mm along the array but with a different position in relation to the outer wall of the scala tympani, will potentially reach considerably different characteristic frequencies. If, however, the insertion length can be correlated to the OC, a much better result will be obtained: thus longitudinal insertion depth expressed as percentage length along the OC is a viable alternative approach.
Others favour the use of angular measurements in which allowance is made for individual cochlear dimensions as well as intracochlear trajectories of cochlear implant electrodes. Based upon temporal bone studies, Bredberg correlated angular measurements to the distance along the basilar membrane (along the organ of Corti) [14]. He referred to an origin at the helicotrema. According to Kawano et al [12] the use of the helicotrema (= apex modioli) as a reference to define an axis could lead to significant error since the OC is eccentric to the helicotrema. Kawano et al. therefore used only points in the OC coordinate set without an axis of revolution. This resulted in slightly shorter percentage lengths. An estimate of the characteristic frequency associated with the position of each electrode can be derived from the data of Bredberg and the formula of Greenwood. Stakhovskaya et al have shown that measurements of the percentage distance of the OC and spiral ganglion required to reach specific angles of rotation along the cochlea exhibited considerably less intersubject variability than the absolute distances in millimeters [13].
A consensus was reached on a cylindrical coordinate system because of the above-mentioned reasons and the already widely applied angular coordinates. Requisites for a cylindrical coordinate system are a plane of rotation, location of the z-axis, including the locations of zero for the z-axis and zero degrees for the angle.
Plane of rotation
In both histological and radiological studies, approaches have been described for measurement of cochlear volume [13;21] and angle [7;22;23]. These approaches have a plane of rotation along the basal turn of the cochlea in common. In this x-y plane, a polar coordinate system can be applied and angular measurements can be performed.
In conventional X-rays this was achieved by the introduction of the Cochlear View by Marsh and coworkers [23]. This modification of the Stenver's view results in an optimal view of the cochlea, perpendicular to the basal turn and along the modiolar axis. Recent technological advances in the field of computer tomography imaging allow reconstructions to be made in any desired plane without loss of image quality. Multiplanar reconstructions or minimum intensity projections along the basal turn of the cochlea and perpendicular to the modiolus result in the equivalent of the “Cochlear View” [21;24].
Histological cochlear dissections are often performed in the plane of the basilar membrane, for instance to perform fiber tracings [13]: this procedure equals the Cochlear View plane for the basal turn and approximates it for the higher turns, which are separated during the procedure.
The panel agreed upon using the Cochlear View as described by Xu and colleagues [25] as a basis for a grid system for the morphological assessment of the inner ear.
Origin of the z-axis
As long as measurements are performed in a 2-dimensional polar coordinate system, the height of the cochlea is not taken into account, which could lead to underestimation of the insertion depth. To obtain direct information on the vertical trajectory of the cochlear lumen and its contents, the framework has to be extended with the 3rd dimension.
The z-axis, perpendicular to the Cochlear View plane, needs to be placed through the center of the modiolus to prevent locations in the cochlear duct that are very close to the modiolus from jumping by 180°, to the other side of the z-axis. Kós, Boëx et al [26] corrected for misaligned x-rays by shifting the center of rotation for their angular measurements to circumvent these angular jumps, and in this way they mimicked positioning the z-axis through the center of the modiolus.
In volume images, an axis through the center of the modiolus can be directly applied. Skinner and coworkers have found that the best choice of axis position occurs when it is matched to the tightest coiling of the cochlea in the upper 1.5 turns.
Along this z-axis an origin has to be defined. There are two main options for the origin of the z-axis, either at the level of the basal turn or in the most apical turn at the helicotrema. The origin needs to be defined accurately and reproducibly. Because the basal turn is not completely flat and the OC is not visible in all recording techniques, this does not seem to be an easily reproducible option. The location where the defined z-axis leaves the bony modiolus, on the other hand, is well visualized and is presumably located at the height of the helicotrema. This point will be used to set the origin of the z-axis. The direction towards the apex is considered positive.
Extension of the framework into a 3-dimensional cylindrical coordinate system, by adding a z-axis through the centre of the modiolus with its origin at the helicotrema, ensures that all spatial information is represented.
Zero reference angle
The main discussion, and also the point where most existing methods differ, is the zero reference angle. For frequency mapping purposes the zero angle ideally would be placed at the basal end of the OC or the spiral ganglion cells (SG). Histopathologists can directly visualize these structures and they can be identified on high resolution images such as micro CT and orthogonal-plane fluorescence optical sectioning (OPFOS) microscopy. However, with currently available clinical imaging techniques the OC or SG cannot be seen. Therefore, an anatomical structure with a known relationship to the basilar membrane would be a desirable landmark. The round window is very close to the end of the OC. Based on registered micro-CT, OPFOS microscopy and CT images of an isolated temporal bone, Skinner et al measured the distance from the midpoint of the proximal basal turn of the cochlea at the level of the anterior round window to the end of the OC to be 2.7mm or 12°. This corresponds well to the mean length of the hook region (2.5mm) [27].
In order to use an easily visualized landmark in close relationship to the basilar membrane, the panel agreed that the zero angle would be chosen at the level of the centre of the round window. In accordance with the right-hand rule, positive angles will be used in the right ear and negative values in the left ear.
The cochlear coordinate system
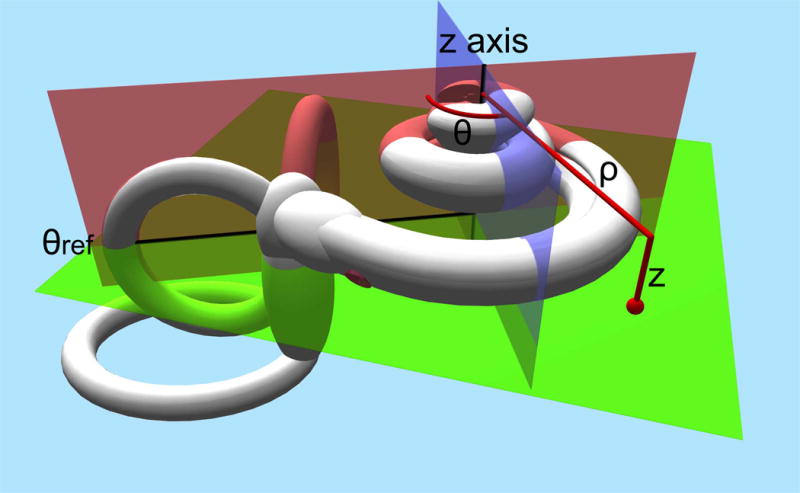
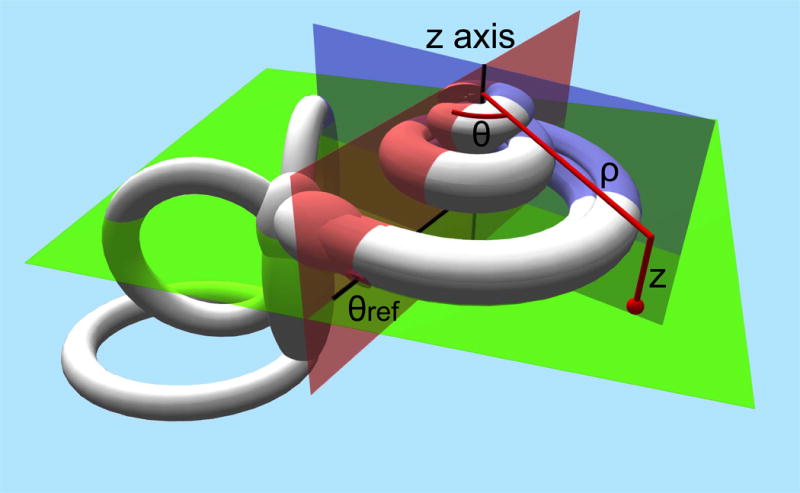
In summary the defined coordinate system uses the Cochlear View, the plane through the basal turn of the cochlea and perpendicular to the modiolus as plane of rotation. The z-axis is placed through the center of the modiolus, with its origin at the level of the helicotrema. As zero reference angle, the centre of the round window is used. (Fig 1) By convention measurements will render positive values in the right ear and negative values in the left ear.
Fig 1.
Schematic drawing of the defined cochlear coordinate system: the plane through the basal turn of the cochlea and perpendicular to the modiolus is chosen as the plane of rotation (green plane). The z-axis is placed through the center of the modiolus (crossing of the red en blue plane), with its origin at the level of the helicotrema. As 0 reference angle (θref) the centre of the round window is chosen (red plane). Measurements will be defined by rotational angle θ and distance to the modiolus ρ.
The coordinate system does not depend on a mathematical function. Even with different approaches to modelling the human cochlea it can be applied in order to provide comparisons of outcomes. Whether a non-parametric method is used, the cochlear spiral is fitted by the Archimedian spiral [16] or with the helico-spiral model – which has been claimed to provide a closer fit [28] the consensus coordinate system still can be applied.
Existing coordinate systems
3D template-based method (Washington University in St. Louis)
Computed tomography images provide much more cochlear anatomic detail than conventional X-rays and allow for direct visualization of the round window and even fine intracochlear anatomic structures, such as the modiolus and the walls of the cochlear duct. For a long time, however, it seemed that image degradation on postoperative imaging after cochlear implantation due to metallic artifacts could not be dealt with succesfully. Skinner, Ketten and co-workers [16;29] were the first to report on assessment of the intracochlear implant position based on spiral computed tomography imaging in vivo. Two methods were used to estimate (linear) insertion depths. First a 3D calculation of length based on individual spiral fits to 2D CT mid-modiolar images was performed. The Archimedian spiral, providing providing a close fit for the midline of the mammalian cochlear canal, was used to model the human cochlea [16]. Second, automated computerized calculations from 3D visualization of the array obtained by segmenting for electrode attenuation properties were employed. Wang's unwrapping algorithm [30] was designed to track the center of mass of a curvilinear structure in predetermined, small steps. Co-registration of a preoperative and a postoperative dataset and segmentation of the electrode array with substantial interactive profiling of individual scans and reconstruction need to be done to perform these measurements. The results proved that significant variations in cochlear anatomy and array distribution among implant patients, which may impact implant performance, can be reliably detected and quantified by using in vivo high-resolution CT and 3-D reconstructions.
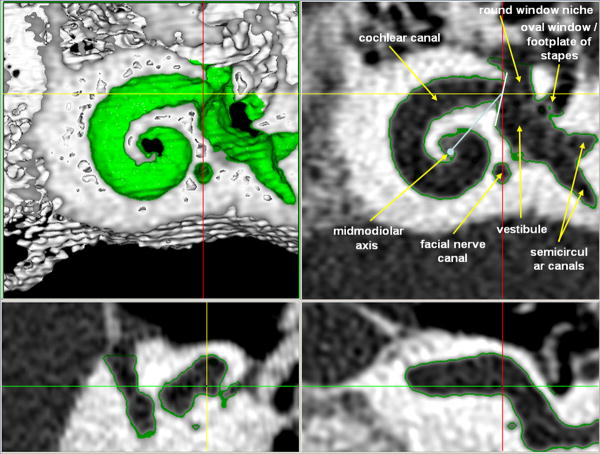
Recently Skinner and Whiting and co-workers [27;31] described a new technique for determining the position of cochlear implants, applied to spiral CT. A preoperative and a postoperative volume CT scan are co-registered. This composite image is then compared with a template from three image volumes (standard clinical spiral CT, micro-CT and OPFOS microscopy) from a single normal-hearing donor cochlea. This improved the selection of the midmodiolar axis and the judgment of the position of each electrode within the cochlea. The angular rotation zero degrees starting point is a line joining the midmodiolar axis with the center of the beginning of the cochlear canal (Fig 2). The distance from this zero degree starting point to the basal tip of the body donor's organ of Corti is 2.7mm (mean length of the hook region 2.55mm).
Fig 2.
3D coordinate system according to Washington University in St. Louis: images from the volume render ortho tool in ANALYZE of the body donor's Volume Zoom 3D volume. Upper left panel is a rendering of the 3D volume thresholded to display only bone with the upper turns of the cochlea cut away; it represents the boundary of the fluid/tissue containing spaces that are surrounded by bone. The other 3 panels are gray scale images of the intersecting orthogonal sections for the selected point on the rendered image, marked by the intersection of the yellow and red lines on the rendered image. The green outline in the other 3 panels shows this boundary between bone and fluid/tissue containing spaces. Upper right panel's image is perpendicular to the modiolar axis (light blue dot) and shows the demarcation of the vestibule and the cochlear canal (white line) as well as the zero degree rotation line (light blue line).
This method is a 3-dimensional coordinate system making use of co-registration of patient CT data and a template of one single body donor ear. The zero degree angle refers to the anterior margin of the round window. The software package used is already extended to include a recording point at the centre of the round window. This additional point provides an individual correction factor to the consensus coordinate system.
CView©
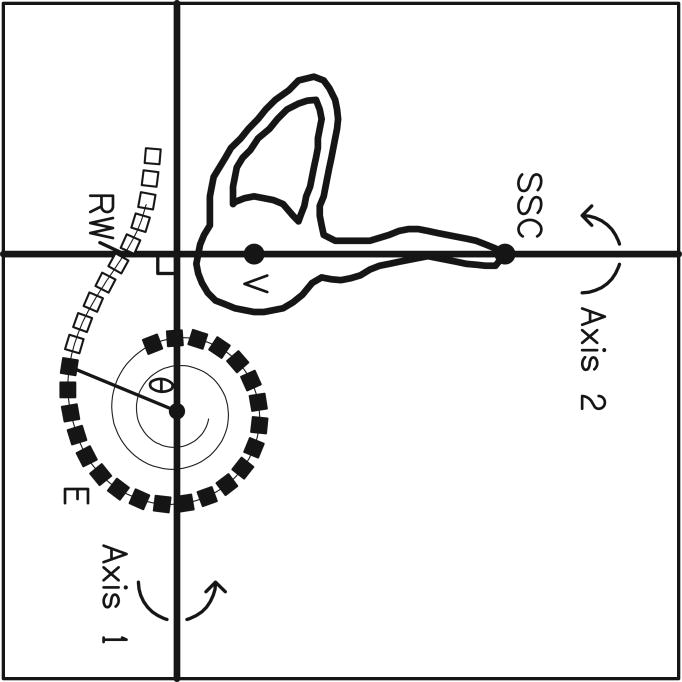
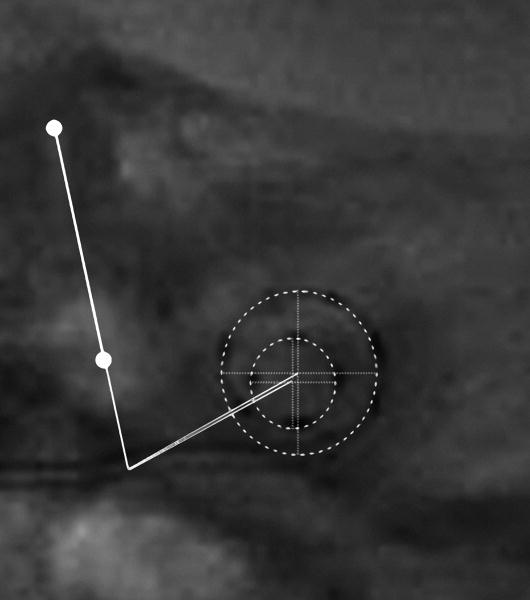
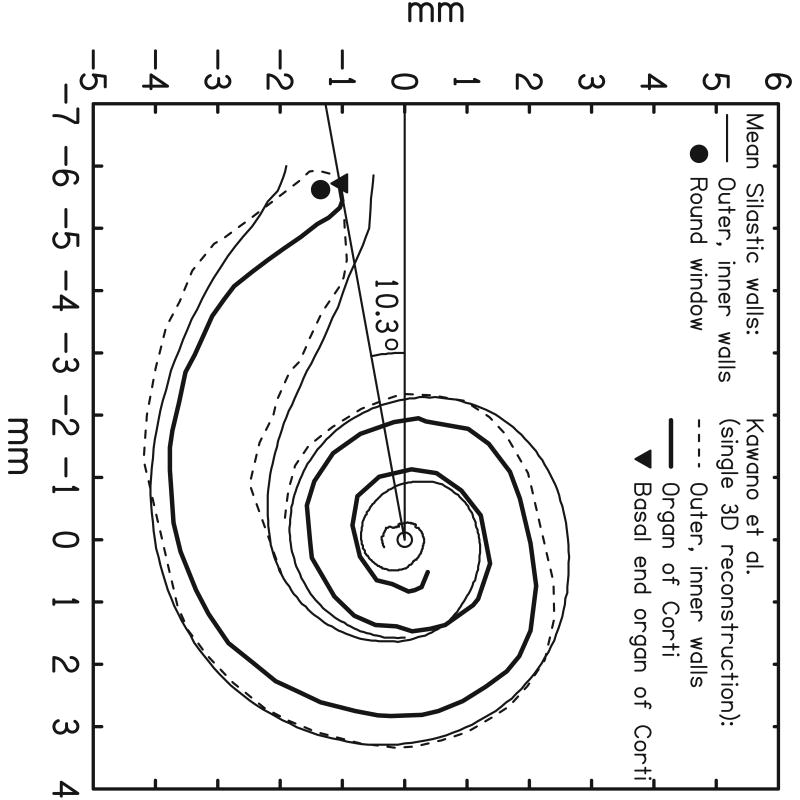
Marsh and coworkers [23] introduced the Cochlear View. On these 2-dimensional images a grid system is applied to allow for angular measurements of insertion depth. The technique was developed by Cohen et al. [22] in order to extract angular position information from digitized X-ray images and to express these angles, approximately relative to the basal end of the organ of Corti. This method was applicable to cochleae of different sizes but assumed an electrode trajectory characteristic of the “straight” Banded Nucleus array. The method was subsequently modified and extended by Cohen et al. [32] in order to become independent of the electrode array and to estimate the distances of electrode bands from the modiolus. These methods as described briefly in Xu et al. [25] and Cohen et al.[22] use a reference line drawn through the apex of the superior semicircular canal (SSCC) and the center of the vestibule, passing close to the round window. Mean spirals of the inner and outer walls of the scala tympani (obtained from Silastic® molds of temporal bones) and of the outer wall of the otic capsule (processed from digitized serial sections of a cochlea) were calculated. The latter can be visualized on both preoperative and postoperative X-rays. By scaling the registered spirals and adjusting X and Y positions, a best fit of the otic capsule outer wall spiral to the image is obtained in a software package called CView©. Therefore, the spiral template reflects the variation of cochlear size. A line is drawn from the estimated spiral center point perpendicular to the reference line through the SSCC. The intersection of the two lines defines the “geometric” zero degree angle. Superposition of Kawano's data [12] after scaling gave an estimation of the angular position of the basal end of the OC. Based upon these measurements, the zero angle for the Cochlear View construction was corrected by 10° to approximate the basal end of the OC [32] (Fig 3 a-c).
Fig 3.
CView©: (a) Cochlear View image, (b) schematic drawing of a cochlear coordinate system based upon anatomical landmarks. A reference line is drawn through the superior semicircular canal (SSC) and the centre of the vestibule (V). Based upon Silastic® molds of the scala tympani, inner and outer wall spiral functions were calculated. In addition, from analysis of a temporal bone, a third registered spiral was produced to represent the outer wall of the otic capsule (not shown). This spiral is scaled and shifted on the x-y plane in order to achieve a best fit to the image of the bony outer wall visible on the X-ray, thus determining the centre of the cochlear spiral. A second reference line is drawn from the center point, perpendicular to the first reference line, thus defining a “geometric” zero angle. Kawano's data on the length of the Organ of Corti (OC), superimposed on the figure, enabled estimation of the position of the basal end of the OC (c). This point lay at approximately 10° relative to the “geometric” zero, and was used as the origin for calculation of percentage length along the organ of Corti and, thus, characteristic frequency via the Greenwood equation. (Note, in (b), that although the array tends to penetrate the outer wall of scala tympani, it remains inside the bony outer wall.)
This allowed for direct application of the Bredberg and Greenwood data. Two additional angles were obtained (relative to geometric zero), corresponding to the mean entry points for Banded Nucleus arrays implanted through the round window (13.5°) and through a cochleostomy just apical of the round window (23.8°). The angle representing the “apical” side of the round window would, therefore, be less than 23.8°.
Based on recent data from Skinner et al., Cohen (personal communication) considers that the angular correction relating the “geometric zero” to the basal end of the organ of Corti, in CView, should probably be nearer to five degrees than to ten degrees. However, further data would be required to confirm the appropriateness of such a change.
CView© uses a 2-dimensional coordinate system in the Cochlear View, providing angular measurements approximately from the tip of the OC. Its plane of rotation forms the basis for the universal cochlear framework. From X-ray data it is not possible to visualize the round window directly. Comparison to outcomes of insertion angles measured in 13 patients with this method and with the previously described method showed highly correlated outcomes, reflecting the common conceptual framework of these methods[27].
Postoperative 2D method (Geneva)
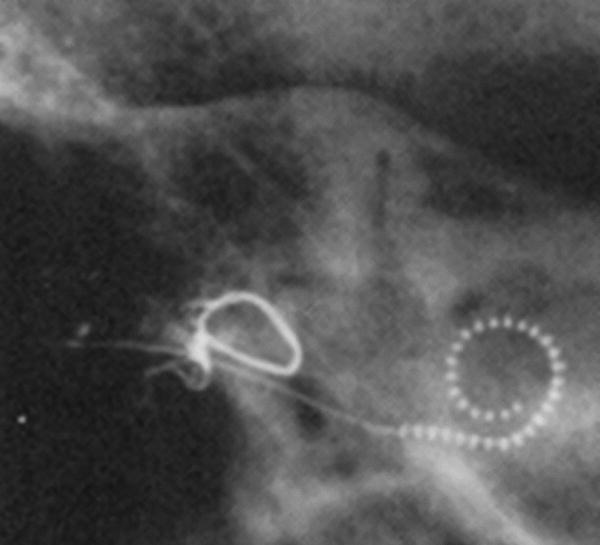
Using the above described Cochlear View Kós, Boëx et al [26] performed both linear and angular measurements of insertion depth. A geometric zero angle was used as a point of reference and the center of the spiral was defined in the first turn (360°) as well as in the second turn (720°) (Fig 4). The geometric zero used by this group was the point at which the electrode array, inserted through the round window, crossed the reference line between SSC and V (as used in CView). It approximates the centre of the round window and differs from the geometric zero used in CView (by approximately 13.5°).
Fig 4.
Angular measurements as performed by Boëx et al: a modified Stenvers (Cochlear View) X-ray is performed (a) and a geometric zero reference was determined by the point at which the electrode array crossed the SSC/V reference line described by Cohen et al and Xu et al. This corresponded approximately to the round window (and differed from the geometric zero of Cohen et al and Xu et al). The line going through this point to the center of the first turn of the spiral made by the electrode array, is used as the zero degree reference line for electrodes belonging to the first turn. For electrodes belonging to the second turn of the spiral of the electrode array a line going through the estimated site of the round window to the center of the second turn of the spiral made by the electrode array, is determined as the 720° line (b).
In this modification of the previous method, the authors attempt to include a z-axis through the center of the modiolus. Consequently, misalignment due to suboptimal positioning of the patient was approximately corrected for. In order to compare results with those obtained with other techniques, an appropriate angular correction factor should be applied.
Histology-based system (UCSF)
Stakhovskaya, Leake and co-workers performed calculations of characteristic frequencies of the OC and SG versus angle of rotation from the round window. [13] In this histopathological study the OC was directly visualized. Surface preparations were obtained of the isolated cochleae through the middle of the modiolus, in a plane oriented as nearly parallel as possible to the radial nerve fibers on each side of the basal turn equal the Cochlear View plane. The zero degree reference point was chosen at 1mm from the basal end of the OC. This point was chosen because the initial portion of the OC curves inferiorly and would project as a continuous zero or close-to–zero degree value and would be difficult to see in imaging studies.
In the histopathological approach the grid system is applied as defined in the consensus and the zero angle is chosen on the directly visualized OC at 1 mm from the basal end of the OC. It is estimated that this approximates the position of the border of the round window adjacent to the vestibule, which can be seen in imaging studies.
CT-based 3D-coordinate system (Leiden)
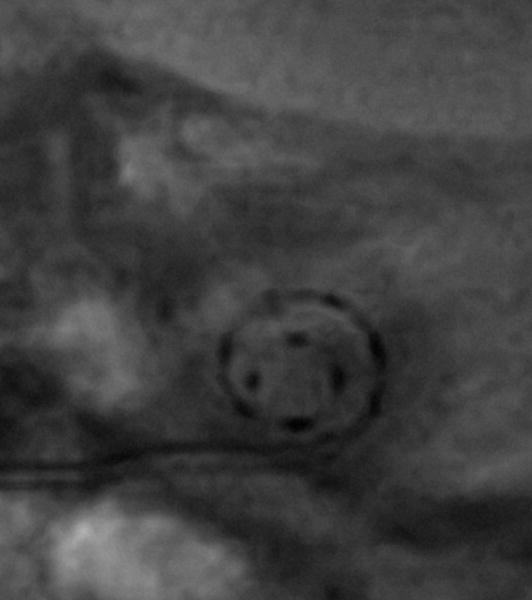
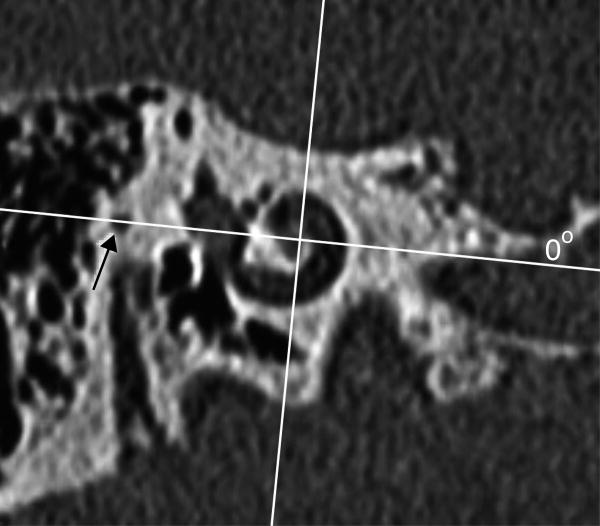
Verbist et al reported an acquisition protocol for multi detector row tomography allowing for visualization of individual electrode contacts and fine anatomic inner ear structures in the human cochlea in a clinical setting.[24] A multiplanar reconstruction through the basal turn of the cochlea is made, rendering a stack of images in the desired plane of rotation. The z-axis is defined through the centre of the modiolus. Since the normal anatomy at the level of the round window can be distorted by surgery (according to the chosen approach for the cochleostomy) the zero angle is chosen at the top of the horizontal semicircular canal (Fig 5). Preliminary results in 25 patients show that taking into account a correction of 34.6° +/-0.8° (2 SD) insertion depth measurements can be made in relation to the centre of the round window.
Fig 5.
CT-derived cochlear coordinates: a) a schematic drawing shows that the x,y,z –axes are applied comparable to fig 1. The zero degree reference angle (θref) is however chosen at the top of the horizontal SCC. (b) 1 reformatted CT image out of a stack of images through the cochlea is shown. The x,y-axes are shown in red and blue respectively. The x-axis is positioned through the most lateral point of the horizontal SCC, which serves as the 0°-angle in the 3 coordinate system.
This grid system is developed to be applied in a clinical situation where only a postoperative scan might be available. The zero degree angle is chosen outside the cochleostomy region, but the relationship to the round window has been established.
Summary and conclusion
Research on the human cochlear anatomy in relation to tonotopy and cochlear implantation conducted by histologists, surgeons, physicists, audiologists, radiologists and others has prompted the need for a universal cochlear coordinate system to achieve comparable measures between histological, surgical and psychophysical findings. To attain a widely accepted standard, researchers with backgrounds in the various fields of inner ear research as well as representatives of the different manufacturers of cochlear implants (Advanced Bionics Corp, Med-El, Cochlear Corp) were involved in consensus meetings held in Dallas, March 2005 and Asilomar, August 2005.
Over the years several coordinate systems have been introduced. Since they use different landmarks in their definition of coordinates, the outcomes differ and cannot be easily converted to each other. This is partly due to inherent differences in visualization of soft tissue structures within the cochlea between histopathological studies and different imaging techniques. Therefore the consensus panel formulated an objective framework, applicable in all fields and on both preoperative and postoperative cochleae. The basis of the framework is formed by the Cochlear View, which has been used in all discussed coordinate systems. The extension into a 3-dimensional cylindrical system by placing a z-axis through the center of the modiolus with its origin at the helicotrema ensures optimal spatial information. This can be achieved clinically, in cochlear implant patients, with the use of high resolution imaging. With the evermore widespread availability of multislice CT scanners the requested resolution to apply the 3D framework will come in reach of many clinicians in the near future.[33] The 3D-framework may not seem of direct added value to surgeons for intraoperative feedback. However, if applied to pre- and postoperative imaging, it will render anatomic information for operation planning as well as information of the surgical result. By choosing the round window as the zero degree angle of rotation, a reference point visible in all fields and techniques and in close relationship to the end of the OC has been defined. Accurate comparisons between all methods should now become possible, provided that correction angles to the round window are available.
The value of coordinatesystems for preoperative planning and postoperative evaluation of the precise intracochlear positioning of a cochlear implant has been shown in several reports in the literature. Both 2-dimensional[34;35] and 3-dimensional [13;17;36] coordinate systems provide angular insertiondepths. This information, used in conjunction with the frequencymap developed by Stakhovskaya et al [13], provides a basis for more accurate assignment of frequencybands to stimulation sites in fitting individual cochlear implant users.[34] For assessment of cochlear anatomy and scalar position of cochlear implant electrode contacts 3-dimensional coordinate systems have been applied quite successfully [17;36;37] The z-coordinate reflects the height of a specific anatomic structure or electrode contact in relation to the plane of the basal turn of the cochlea. It therefore provides information on the risk for or the presence of insertion trauma, which has been reported to be correlated with outcome in terms of word recognition scores. [36;38] Thus a 3-dimensional coordinate system can contribute to assessing surgical technique and to correlating the surgical result to psychophysical findings.
As described above the consensus cochlear coordinate system is merely an extension of the existing 2-dimensional CView© method of Xu and Cohen by adding a z-axis, which defines the third dimension. As such it does not differ from and it will provide the same information (insertiondepth and scalar position) as described for other 3-dimensional coordinate systems in literature. Its ultimate role however is to serve as a widely agreed upon coordinate system of reference between the numerous, slightly variable approaches by providing the requisite information to allow the use of the centre of the round window as a 0 degree reference angle.
Acknowledgments
The contribution of UCSF is based on work supported by NIH (#HHS-N-263-2007-00054-C)
Reference List
- 1.Greenwood DD. A cochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood DD. Critical bandwidth and consonance in relation to cochlear frequency-position coordinates. Hear Res. 1991;54:164–208. doi: 10.1016/0378-5955(91)90117-r. [DOI] [PubMed] [Google Scholar]
- 3.Greenwood D. Critical Bandwidth and Frequency Coordinates of Basilar Membrane. Journal of the Acoustical Society of America. 1961;33:1344. [Google Scholar]
- 4.Blamey PJ, Dooley GJ, Parisi ES, Clark GM. Pitch comparisons of acoustically and electrically evoked auditory sensations. Hearing Research. 1996;99:139–150. doi: 10.1016/s0378-5955(96)00095-0. [DOI] [PubMed] [Google Scholar]
- 5.Tong YC, Clark GM. Absolute Identification of Electric Pulse Rates and Electrode Positions by Cochlear Implant Patients. Journal of the Acoustical Society of America. 1985;77:1881–1888. doi: 10.1121/1.391939. [DOI] [PubMed] [Google Scholar]
- 6.Collins LM, Zwolan TA, Wakefield GH. Comparison of electrode discrimination, pitch ranking, and pitch scaling data in postlingually deafened adult cochlear implant subjects. Journal of the Acoustical Society of America. 1997;101:440–455. doi: 10.1121/1.417989. [DOI] [PubMed] [Google Scholar]
- 7.Boex C, Baud L, Cosendai G, Sigrist A, Kos MI, Pelizzone M. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. J Assoc Res Otolaryngol. 2006;7:110–124. doi: 10.1007/s10162-005-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorman MF, Spahr T, Gifford R, et al. An electric frequency-to-place map for a cochlear implant patient with hearing in the nonimplanted ear. J Assoc Res Otolaryngol. 2007;8:234–240. doi: 10.1007/s10162-007-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briaire JJ, Frijns JH. The consequences of neural degeneration regarding optimal cochlear implant position in scala tympani: a model approach. Hear Res. 2006;214:17–27. doi: 10.1016/j.heares.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Sridhar D, Stakhovskaya O, Leake PA. A frequency-position function for the human cochlear spiral ganglion. Audiol Neurootol. 2006;11 1:16–20. doi: 10.1159/000095609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariyasu L, Galey FR, Hilsinger R, Byl FM. Computer-Generated 3-Dimensional Reconstruction of the Cochlea. Otolaryngology-Head and Neck Surgery. 1989;100:87–91. doi: 10.1177/019459988910000201. [DOI] [PubMed] [Google Scholar]
- 12.Kawano A, Seldon HL, Clark GM. Computer-aided three-dimensional reconstruction in human cochlear maps: Measurement of the lengths of organ of corti, outer wall, inner wall, and Rosenthal's canal. Annals of Otology Rhinology and Laryngology. 1996;105:701–709. doi: 10.1177/000348949610500906. [DOI] [PubMed] [Google Scholar]
- 13.Stakhovskaya O, Sridhar D, Bonham BH, Leake PA. Frequency map for the human cochlear spiral ganglion: implications for cochlear implants. J Assoc Res Otolaryngol. 2007;8:220–233. doi: 10.1007/s10162-007-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredberg G. Cellular pattern and nerve supply of the human organ of Corti. Acta Otolaryngol. 1968;Suppl [PubMed] [Google Scholar]
- 15.Wright A, Davis A, Bredberg G, Ulehlova L, Spencer H. Hair cell distributions in the normal human cochlea. Acta Otolaryngol Suppl. 1987;444:1–48. [PubMed] [Google Scholar]
- 16.Ketten DR, Vannier MW, Skinner MW, Gates GA, Wang G, Neely JG. In vivo measures of cochlear length and insertion depth of nucleus cochlear implant electrode arrays. Annals of Otology Rhinology and Laryngology. 1998;107:1–16. [PubMed] [Google Scholar]
- 17.Skinner MW, Ketten DR, Holden LK, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in nucleus-22 recipients. Jaro. 2002;3:332–350. doi: 10.1007/s101620020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulehlova L, Voldrich L, Janisch R. Correlative study of sensory cell density and cochlear length in humans. Hear Res. 1987;28:149–151. doi: 10.1016/0378-5955(87)90045-1. [DOI] [PubMed] [Google Scholar]
- 19.Hardy M. The length of the organ of Corti in man. Am J Anat. 1968;62:291–311. [Google Scholar]
- 20.Sato H, Sando I, Takahashi H. Sexual Dimorphism and Development of the Human Cochlea - Computer 3-D Measurement. Acta Oto-Laryngologica. 1991;111:1037–1040. doi: 10.3109/00016489109100753. [DOI] [PubMed] [Google Scholar]
- 21.Escude B, James C, Deguine O, Cochard N, Eter E, Fraysse B. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiol Neurootol. 2006;11 1:27–33. doi: 10.1159/000095611. [DOI] [PubMed] [Google Scholar]
- 22.Cohen LT, Xu J, Xu SA, Clark GM. Improved and simplified methods for specifying positions of the electrode bands of a cochlear implant array. American Journal of Otology. 1996;17:859–865. [PubMed] [Google Scholar]
- 23.Marsh MA, Xu J, Blamey PJ, et al. Radiologic Evaluation of Multichannel Intracochlear Implant Insertion Depth. American Journal of Otology. 1993;14:386–391. [PubMed] [Google Scholar]
- 24.Verbist BM, Frijns JHM, Geleijns J, van Buchem MA. Multisection CT as a valuable tool in the postoperative assessment of cochlear implant patients. American Journal of Neuroradiology. 2005;26:424–429. [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Xu SA, Cohen LT, Clark GM. Cochlear view: Postoperative radiography for cochlear implantation. American Journal of Otology. 2000;21:49–56. [PubMed] [Google Scholar]
- 26.Kos MI, Boex C, Sigrist A, Guyot JP, Pelizzone M. Measurements of electrode position inside the cochlea for different cochlear implant systems. Acta Oto-Laryngologica. 2005;125:474–480. doi: 10.1080/00016480510039995. [DOI] [PubMed] [Google Scholar]
- 27.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl. 2007;197:2–24. [PubMed] [Google Scholar]
- 28.Yoo SK, Wang G, Rubinstein JT, Vannier MW. Three-dimensional geometric modeling of the cochlea using helico-spiral approximation. Ieee Transactions on Biomedical Engineering. 2000;47:1392–1402. doi: 10.1109/10.871413. [DOI] [PubMed] [Google Scholar]
- 29.Skinner MW, Ketten DR, Vannier MW, Gates GA, Yoffie RL, Kalender WA. Determination of the position of nucleus cochlear implant electrodes in the inner ear. Am J Otol. 1994;15:644–651. [PubMed] [Google Scholar]
- 30.Wang G, Vannier MW, Skinner MW, Kalender WA, Polacin A, Ketten DR. Unwrapping Cochlear implants by spiral CT. IEEE Trans Biomed Eng. 1996;43:891–900. doi: 10.1109/10.532123. [DOI] [PubMed] [Google Scholar]
- 31.Whiting BR, Holden TA, Brunsden BS, Finley CC, Skinner MW. Use of Computed Tomography Scans for Cochlear Implants. J Digit Imaging. 2007 doi: 10.1007/s10278-007-9045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen LT, Xu J, Tycocinski M, Saunders E, Raja D, Cowan R. Evaluation of an X-ray analysis method: comparison of electrode position estimates with information from phase contrast X-ray and histology. 5th European Symposium on Paediatric Cochlear Implantation; Antwerp, Belgium. 2000. [Google Scholar]
- 33.Verbist BM, Joemai RMS, Teeuwisse WM, Veldkamp WJH, Geleijns J, Frijns JHM. Evaluation of 4 multisection CT systems in postoperative imaging of a cochlear implant: A human cadaver and phantom study. American Journal of Neuroradiology. 2008;29:1382–1388. doi: 10.3174/ajnr.A1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebscher SJ, Hetherington A, Bonham B, Wardrop P, Whinney D, Leake PA. Considerations for design of future cochlear implant electrode arrays: Electrode array stiffness, size, and depth of insertion. Journal of Rehabilitation Research and Development. 2008;45:731–747. doi: 10.1682/jrrd.2007.08.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor SEJ, Bell DJ, O'Gorman R, Fitzgerald-O'Connor A. CT and MR Imaging Cochlear Distance Measurements May Predict Cochlear Implant Length Required for a 360 degrees Insertion. American Journal of Neuroradiology. 2009;30:1425–1430. doi: 10.3174/ajnr.A1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbist BM, Ferrarini L, Briaire JJ, et al. Anatomic considerations of cochlear morphology and its implications for insertion trauma in cochlear implant surgery. Otol Neurotol. 2009;30:471–477. doi: 10.1097/MAO.0b013e3181a32c0d. [DOI] [PubMed] [Google Scholar]
- 38.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]