International collaborative research in developing countries raises difficult ethical issues in the setting of severe diseases and complex costly treatments. Discussion of two matters has characterised the debate on this type of research. First, what standard of care should be provided to participants in intervention studies, particularly those in control groups?1–4 Second, what level of benefits should be provided to individuals and communities during a study and after completion, particularly with respect to treatments proven effective through research?4–6 Here, we focus on a third issue, investigators’ responsibilities for meeting participants’ needs for ancillary care.
Ancillary-care needs have been identified as distinct only in the past few years.7,8 Richardson and Belsky demarcated such care as “that which is not required to make a study scientifically valid, to ensure a trial’s safety, or to redress research injuries”.7,8 Examples include treatment of schistosomiasis detected in the urine of participants in a malaria study and management of HIV acquired in a preventive HIV vaccine trial.7 Requirements for ancillary care can arise in any study in which people with unmet health needs are enrolled, but no policies or guidelines exist with respect to the researchers’ duty to meet such needs. After briefly describing Richardson and Belsky’s framework to set out ancillary-care responsibilities, we discuss two cases that raise frequently faced but important challenges, which we believe have not received sufficient attention in existing published work.
Richardson and Belsky propose a two-question framework to establish duties of ancillary care. First, does treatment for a particular health need fall within the so-called scope of entrustment between participant and investigator? Second, how strong is the individual’s claim to that care? Richardson and Belsky argue that the investigator-participant relation is determined by the subset of health needs that participants entrust to the investigator when agreeing to take part in a clinical research study. Investigators’ responsibility for provision of care is restricted to care addressing that subset of participants’ health needs. Such needs are the ones for which researchers have some responsibility for provision of care. The scope of entrustment is thus “fixed by the subset of the permissions obtained during the consent process that are required for the research team to carry out the study validly and safely”7 and typically includes caring for the disease under study and “following up on any clinically relevant information or diagnoses generated”.8 If a specific form of care is within the scope of entrustment, the participant does have some claim to that care. The strength of the individual’s claim is then ascertained by the degree of the individual’s vulnerability and dependence, the depth of the relation between investigator and participant, the extent of gratitude owed to the individual for accepting uncompensated burden, and the importance of reasons against providing care. Such reasons might include, for example, scientific considerations, cost, duration of treatment, or deficient infrastructure.7,8
Richardson and Belsky stress the need for ongoing dialogue to provide answers for these two questions in particular studies. The cases we discuss next, both funded by US sponsors, contribute to that process. The first case, however, also poses a challenge, suggesting that the range of ancillary-care responsibility is defined by more than entrustment of specific health needs and could be wider than Richardson and Belsky propose.
The first case, which illustrates the challenge of establishing the investigators’ scope of responsibilities, is the Mother-Offspring Malaria Study (MOMS), a 5-year observational study focused on the pathogenesis and clinical outcomes of severe malarial disease in children. Participants were mother-infant pairs at the Muheza Designated District Hospital (MDDH) in Tanzania and were enrolled around the time of delivery.9 The protocol included obtaining peripheral, placental, and umbilical-cord blood at delivery, capillary blood at 2-week intervals for the first year of life then every month until age 4 years, and venous blood every 6 months.
Children in MOMS received all care (including treatment for malaria) from MDDH, with all costs paid by the project. Additionally, a coinvestigator reviewed case records for enrolled children during the study to ensure that they were receiving appropriate inpatient and outpatient care. Despite the high prevalence of HIV in the local population, the hospital could not afford antiretroviral drugs and did not routinely provide prophylaxis for HIV-associated opportunistic infections. The annual government expenditure on health care was only US$12 per person.
During the first year of MOMS, several children with HIV died from opportunistic infections. Study investigators then developed a plan with the hospital to refer all children with persistent HIV-positive antibody status to a hospice clinic and to provide co-trimoxazole to all offspring of HIV-infected mothers. At least two types of ancillary care were thus provided: 1) monitoring to ensure proper malaria care; and 2) hospice referral for HIV-related treatment, including co-trimoxazole prophylaxis.
Monitoring of malaria treatment was clearly within the scope of entrustment as articulated by Richardson and Belsky. Malaria was the disease under study, and participants probably expected investigators to take some responsibility for their malaria treatment, particularly because the study had the potential to generate clinically relevant information about their disease. A duty to provide care for HIV-related illness, however, seems to fall outside the scope of entrustment. Knowledge about HIV status is one of many elements of the medical history that were relevant to investigators, but data on HIV were not generated by participation, were not central to the research question, and were available to anyone interacting clinically with participants. Although HIV-related care was thus outside the scope of entrustment as defined by Richardson and Belsky, MOMS investigators felt a sense of duty to ensure hospice referral and prophylaxis for opportunistic infections for sick participants. Was this ancillary care supererogatory—that is, morally praise worthy but optional—or is Richardson and Belsky’s notion of the range of responsibility incomplete?
We believe that the study investigators recognised that their responsibilities to provide ancillary care extended beyond what was entrusted by individuals agreeing to participate. In particular, they recognised that the need for prophylaxis for opportunistic infections was important, that effective treatment was affordable, and that they had an ongoing research relation with participants. These factors play an important part in Richardson and Belsky’s framework by helping to ascertain the strength of responsibilities within the scope of entrustment (ie, malaria-related treatment), but they cannot strengthen a duty that is outside this range (ie, HIV-related treatment). We see no reason why these factors ought not to have a role in establishing the scope as well.
Using Richardson and Belsky’s framework, the way in which investigators planned to use information about HIV infection in the MOMS project is especially relevant to the task of assessing whether some responsibility exists. If adjustment for or stratification by HIV infection were part of the investigators’ analytical plan, for example, HIV-related care might fall within the scope of entrustment. But, if HIV status were gathered only as part of an initial history, such treatment would not be included. Why does relevance of information to the aim of the study indicate the level of responsibility that participants entrust? Have participants disclosing HIV infection as part of the routine medical history taken at the beginning of most research projects really entrusted less than individuals whose HIV status will be central to data analysis?
We agree with Richardson and Belsky that understanding the nature of the investigator-participant relation is essential to locating ancillary-care responsibilities. Furthermore, we concur that such a relation exists in some middle ground between a clinical provider and a detached scientist and varies substantially based on the nature of specific studies. However, there is more to this relation than what is entrusted by people in agreeing to participate in a study. Moreover, what is entrusted seems to depend less heavily on the nature of the study than Richardson and Belsky suggest.
Some researchers might argue that the MOMS project presents a straightforward duty of assistance—or duty of rescue—and that the scope requirement is unhelpful. Singer’s position, for example, suggests that to withhold treatments or services from research participants, which a study can provide and to which individuals would not otherwise have access, would be ethically unjustifiable.10 Richardson and Belsky might even admit a duty of assistance in the MOMS case, asserting that entrustment responsibilities are distinct from duties of assistance and depend only on the nature of research and the investigator-participant relation. We are sympathetic to their desire to locate the responsibilities that inhere in the nature of the relation between investigators and patients, but we are concerned that entrustment provides an incomplete account of the relation. Our analysis thus calls the scope requirement into question and could suggest that what is needed is a deeper context-specific understanding of researchers’ duties of assistance. The boundaries of the range of investigators’ responsibilities remain unclear. At the very least, however, we suggest that some criteria relevant to assessment of the strength of a claim are important determinants of the scope of researchers’ specific duties.
This case is challenging with respect to scope, but the strength of participants’ claims seems strong. The debt of gratitude is small; participants accept few uncompensated risks. However, they have no other source of care and are very vulnerable, and investigators are engaged deeply with the community and individuals. Finally, no strong reasons exist against providing this treatment; it was inexpensive and could be provided through the hospital. As in the second case, however, assessment of strength can, at times, be challenging.
The second case, which illustrates the challenges in determining the strength of participants’ claims, is a population-based prospective study of the effect of short-course directly observed treatment (DOTS) on drug resistance and transmission of tuberculosis between 1995 and 2000 in southern Mexico.11 In this observational study investigators partnered with the existing local DOTS effort and used passive case-finding by community health workers and conventional diagnostic and molecular epidemiological methods to detect study endpoints (figure). Initially, in the local DOTS pro gramme, individuals with new infections were treated with three first-line anti-tuberculosis drugs; those who needed retreatment received an additional fourth agent. In 1998, local health authorities—with the encouragement of the study team and in accordance with WHO recommendations—began treating people with new infections with four drugs and retreatment cases with five agents. Neither the local health department nor the research study provided second-line drug treatment for multi-drug-resistant disease; however, all participants were referred to the national tuberculosis control programme and were assessed by doctors of the National Institute of Respiratory Diseases, the national referral centre.
Figure.
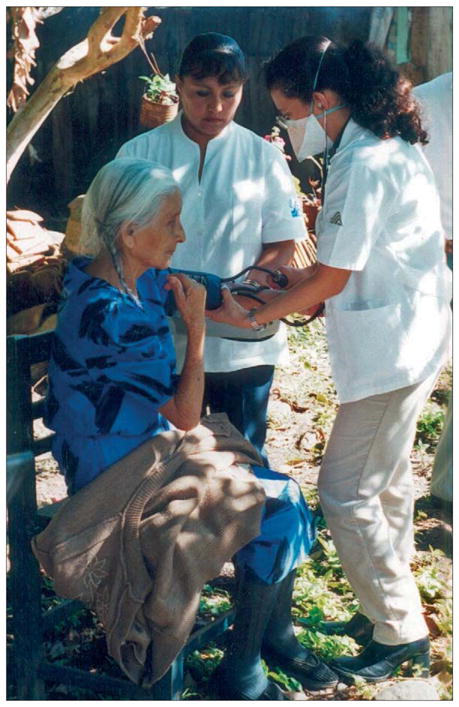
Tuberculosis case finding by community health workers in a rural household in southern Mexico
Over the 5-year study period, researchers noted that, even with moderate levels of multidrug-resistant tuberculosis, DOTS (with only first-line agents) rapidly reduced the incidence of drug-susceptible and drug-resistant infections. In particular, the regimen significantly lowered transmission of multidrug-resistant disease, although it did not decrease deaths in individuals resistant to more than one first-line drug or with multidrug-resistant tuberculosis. The strategy simply rendered these people non-infectious. In total, 41 participants were identified with multidrug-resistant tuberculosis; five died and only eight (19·5%) were judged a treatment success.
In view of the high mortality and failure rates in participants with multidrug-resistant tuberculosis, should investigators have treated these people with second-line anti-tuberculosis drugs? Individuals were treated with the local standard of care and in accordance with available international guidance; WHO did not recommend treatment of multidrug-resistant tuberculosis with second-line drugs at any point during the study. However, the standard of care in the USA at that time was to treat this disease with individualised regimens of second-line agents.12 The cost for such treatment is nearly US$28 000 per patient,13 or $1 148 000 to treat 41 people, far more than the small budget of this observational study. Although, it does not refer explicitly to ancillary-care issues, the Declaration of Helsinki states clearly that the best treatment worldwide must always be provided to research participants, a position not affirmed definitively until October, 2000 (after this study was concluded). More importantly, this position is not shared widely. Other international guidance documents do not include the same requirement, and whether it is ethically defensible is not clear.1
Treatment of multidrug-resistant tuberculosis is arguably within the investigators’ scope of responsibility. They were never entrusted with any health-care decision-making in this observational study. Richardson and Belsky7 propose that the scope of entrustment depends primarily on the nature of the study and is fixed by the subset of the permissions obtained during the consent process. However, investigators had privileged access to individualised information about drug sensitivity, members of the research team were involved in optimisation of the DOTS programme, and investigators were involved in increasing access to treatment for people with susceptible and resistant tuberculosis, both locally and nationwide. The strength of participants’ claims to second-line treatment of multidrug-resistant tuberculosis, however, is even less clear. The participants’ vulnerability and dependence were indisputable since the illness is largely fatal and no other sources of treatment were available. However, individuals assumed no uncompensated risks, and investigators did not have a deep relation with participants; the research team was generally observing the existing DOTS effort. Most importantly, there were strong economic and scientific reasons against providing individualised treatment with second-line drugs. Treating participants at a cost of $1 148 000 was impossible on this study’s budget. Additionally, the study was designed to show the effect of DOTS (with only first-line agents) on multidrug-resistant disease transmission; the finding that DOTS is effective in this respect has important implications for the design of tuberculosis treatment programmes. Had participants been treated with second-line agents, this effect could not have been detected.
Was this study ethically problematic as implemented? The Declaration of Helsinki suggests that it could be improved.4 Furthermore, some commentators would challenge the legitimacy of economic reasons against providing treatment and the true scientific importance of knowing that first-line drugs reduced transmission of multidrug-resistant disease, asserting that both are only relevant as a result of injustice.14 Important inequities are at the root of issues such as tuberculosis in developing countries, but this matter does not invalidate, or make complicit, research aimed at improving health in the presence of such inequities. The Helsinki position is untenable if this important research is to proceed.
After conclusion of this DOTS study, a standardised regimen of second-line drugs was shown to be effective for treatment of multidrug-resistant tuberculosis (although less effective than individualised therapy), at a cost of around $2000 per patient.15 The cost of treating 41 patients with the disease would then be $82 000 versus $1148 000. The strength of participants’ claims to treatment might thus increase. However, the need to reduce transmission, the high cost of treatment, and the low cure rate with all available treatment regimens would remain relevant to such assessments. Exactly how we weigh these competing factors remains a difficult challenge.
This second case indicates the tensions that exist between vulnerability, dependency, cost, and scientific considerations. It provides an especially helpful example of the need to prevent extreme vulnerability and dependence from hijacking all other considerations, an unfortunate outcome of the position advocated in the Declaration of Helsinki and by several authors in published work.4,14 Such stances could entail the loss of valuable research findings for communities with important health needs. One great advantage of Richardson and Belsky’s framework is that it explicitly requires that these important moral considerations be assessed in context; one consideration cannot trump all others.
In conclusion, the most important implication of this discussion has to do with the range of investigators’ duties to provide ancillary care. The MOMS project challenges directly Richardson and Belsky’s notion of the investigator-participant relation and the scope of ancillary-care responsibilities. We have suggested that the scope is established by more than what is entrusted to investigators by participants. We have also challenged the notion that the tightness of the connection between a particular ancillary-care need and the research question is an especially important determinant. The range of ancillary-care responsibility is not boundless, and we agree with Richardson and Belsky that it is mainly delimited by the nature of the research relation. However, factors such as the existence of need, the ability to help, and particularly investigators’ level of engagement with participants are all important elements of that association and are thus material to establishing the scope of responsibility in addition to determining the strength of claims to care.
We also hope to have called attention to the many factors that determine the strength of a particular claim to ancillary care by research participants. The DOTS study indicates a serious flaw with the hard-line position that participants must be assured of the best treatment available anywhere. Requiring investigators to provide individualised second-line treatment would have made the study prohibitively expensive and prevented detection of important endpoints. Insistence on such a requirement inflates ancillary-care duties and fails to recognise the important role of research in advancing health in the developing world.
Ancillary-care needs are ubiquitous and warrant interest by the bioethics and research communities. Richardson and Belsky should be commended for recognising these issues as distinct and calling attention to their importance. We hope that discussion of these two cases will advance our understanding of ancillary-care responsibilities and facilitate ethical implementation of important research addressing health issues in developing countries.
Acknowledgments
We thank Rachel Derr, Ezekiel Emanuel, Christine Grady, Franklin Miller, Alfredo Ponce-de-Leon, Jose Sifuentes-Osornio, and Henry Richardson for helpful comments on earlier versions of this report; the audience at the 2004 annual meeting of the American Society for Tropical Medicine and Hygiene, at which these cases were discussed; and the Fogarty International Center for facilitating this collaboration.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
References
- 1.Lie RK, Emanuel E, Grady C, Wendler D. The standard of care debate: the Declaration of Helsinki versus the international consensus opinion. J Med Ethics. 2004;30:190–93. doi: 10.1136/jme.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lurie P, Wolfe SM. Unethical trials of interventions to reduce perinatal transmission of the human immunodeficiency virus in developing countries. N Engl J Med. 1997;337:853–56. doi: 10.1056/NEJM199709183371212. [DOI] [PubMed] [Google Scholar]
- 3.Varmus H, Satcher D. Ethical complexities of conducting research in developing countries. N Engl J Med. 1997;337:1003–05. doi: 10.1056/NEJM199710023371411. [DOI] [PubMed] [Google Scholar]
- 4.WMA. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Ferney-Voltaire: World Medical Association; 2000. [Google Scholar]
- 5.Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries. Moral standards for research in developing countries: from “reasonable availability” to “fair benefits”. Hastings Cent Rep. 2004;34:17–27. [PubMed] [Google Scholar]
- 6.Council for International Organizations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Geneva: Council for International Organizations of Medical Sciences; 2002. [PubMed] [Google Scholar]
- 7.Richardson HS, Belsky L. The ancillary-care responsibilities of medical researchers: an ethical framework for thinking about the clinical care that researchers owe their subjects. Hastings Cent Rep. 2004;34:25–33. [PubMed] [Google Scholar]
- 8.Belsky L, Richardson HS. Medical researchers’ ancillary clinical care responsibilities. BMJ. 2004;328:1494–96. doi: 10.1136/bmj.328.7454.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutabingwa TK, Bolla MC, Li JL, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer P. Famine, affluence, and morality. [accessed Oct 20, 2006];Philosophy and Public Affairs. 1972 1:229–43. Available at: http://www.utilitarian.net/singer/by/1972----.htm.
- 11.DeRiemer K, Garcia-Garcia L, Bobadilla-del-Valle M, et al. Does DOTS work in populations with drug-resistant tuberculosis? Lancet. 2005;365:1239–45. doi: 10.1016/S0140-6736(05)74812-1. [DOI] [PubMed] [Google Scholar]
- 12.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR., Jr Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–32. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 13.Burgos M, Gonzalez LC, Paz EA, et al. Treatment of multidrug-resistant tuberculosis in San Francisco: an outpatient-based approach. Clin Infect Dis. 2005;40:968–75. doi: 10.1086/428582. [DOI] [PubMed] [Google Scholar]
- 14.Schuklenk U. The standard of care debate: against the myth of an “international consensus opinion”. J Med Ethics. 2004;30:194–97. doi: 10.1136/jme.2003.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suarez PG, Floyd K, Portocarrero J, et al. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–89. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]


