Abstract
Background
Head Computerized Tomography (CT) has significant risks, especially in children. To reduce this burden, we sought to develop a biomarker panel that predicts the absence of traumatic brain injury (TBI) on head CT.
Methods
We conducted a prospective cohort observational study followed by validation in a retrospective cohort at a regional pediatric trauma center. The prospective cohort included 57 consecutive children evaluated for TBI in the emergency department between September 2007 and March 2008. At the time of initial evaluation, blood was obtained to measure electrolytes, coagulation markers, complete blood count, and plasma levels of s100β, D-dimer, and matrix metalloproteinase-9. We conducted routine statistical analysis to determine which predicted TBI on head CT. The independent retrospective cohort included 57 consecutive patients evaluated for the same indication.
Results
All patients generally met common clinical criteria (such as the CHALICE criteria 4) for head CT after trauma. Plasma levels of D-dimer were associated with TBI on head CT by univariate analysis (p < 0.001). Other markers including prothrombin time, partial thromboplastin time, and s100β were not. D-Dimer also had the strongest association in multivariate analysis (p = 0.02). This association was independent of and stronger than the baseline Glascow Coma Scale (p = 0.08). A D-dimer level cut-off of 500 pg/μl had 94% negative predictive value (p < 0.001) for brain injury on head CT. The discriminatory capacity of this D-dimer level was confirmed in the independent retrospective cohort.
Conclusions
In children who meet clinical criteria for a head CT scan after trauma, low plasma D-dimer suggests the absence of significant brain injury.
Keywords: Head trauma, Biomarker, D-dimer, CT
Traumatic brain injury (TBI) is the leading cause of death and morbidity in children.1 Because of the unpredictable and potentially severe outcome of seemingly mild TBI, it is recommended to evaluate such patients with head computerized tomography (CT).2 A rise in CT use has raised concerns regarding resource utilization and, more recently, the clinical consequences of radiation.3 These concerns are heightened in children who often need anesthesia and are more susceptible to adverse effects of radiation,3 including brain cancer. Despite many clinical guidelines that define the indications for CT in mild head trauma,4–10 none are well validated in children. Given the heightened concerns about use of head CT and the limitations of clinical scoring systems in children, an objective laboratory-based predictor of significant TBI is needed. Because the consequences of missed injury are potentially severe, a useful test requires a nearly perfect negative predictive value.
We describe a prospective cohort study investigating a number of biomarkers to devise a laboratory-based predictive test for the absence of traumatic brain injury. D-dimer levels score <500 pg/μL predicted the absence of head trauma on head CT with high accuracy, and this negative predictive value was confirmed in an independent cohort.
MATERIALS AND METHODS
Patient Selection
In the first cohort, 57 patients were enrolled from September 2007 to March 2008 at Rady Children’s Hospital San Diego, a Level I pediatric trauma center. Only patients admitted via the county-based trauma system were included. These patients were admitted directly to the receiving trauma team for immediate evaluation and care. Patients with suspected traumatic brain injury were considered for enrollment if the attending trauma surgeon ordered a head CT and a “head trauma” panel. The patients enrolled generally met published clinical criteria for a head CT scan, such as the children’s head injury algorithm for the prediction of important clinical events (CHALICE) decision rule,4 although, this was not a defined entry requirement. The “head trauma” panel included a complete blood count, sodium, potassium, chloride, bicarbonate, prothrombin time (PT), partial thromboplastin time, fibrinogen, and qualitative D-dimer. Phlebotomy was conducted in the trauma receiving room before head CT. Patients with antecedent brain injury, such as brain surgery or cerebral palsy were excluded. Patient data included sex, age, co-morbid injuries, time of injury, time of phlebotomy, Glasgow coma score (GCS), and loss of consciousness or amnesia. Descriptions of the head CT results were obtained from the pediatric radiologist’s dictated report. All the CT scans were performed with 5 mm slices on a 64 channel General Electric LightSpeed VCT (GE Healthcare, United Kingdom).
The validation cohort consisted of 57 consecutive patients evaluated from March 2008 to June 2008 using the same inclusion and exclusion criteria. The protocol was approved with a waiver of consent by the Institutional Review Board of the University of California San Diego.
Sample Processing
In the first cohort, 0.8 mL of whole blood was collected in an ethylenediamine tetra-acetic acid tube at the time of initial patient evaluation, and the plasma separated and stored at −80° Celsius. D-Dimer, matrix metalloproteinase-9, and s100β were measured using an antibody-based capillary detection system (Biosite Triage system, Biosite, San Diego, CA). C-reactive protein was also measured in the hospital laboratory. In the validation cohort, D-dimer levels were measured by the hospital clinical laboratory using the same methodology.
Statistical Analysis
The primary outcome was the presence of brain injury (subdural bleed, epidural bleed, subarachnoid hemorrhage, contusion, or edema) on head CT. This was used as a dichotomous outcome in all related statistical analyses. Statistical analysis was performed using STATA 9 for Windows. Univariate analysis was performed with the two-tailed student’s t test and Wilcoxon’s rank-sum method as appropriate. Pair-wise correlations were conducted with Spearman’s technique. Multivariate analysis was conducted by logistic regression using continuous variables when possible. The D-dimer levels were analyzed using a receiver operator characteristic curve, and the cut off chosen to be the point furthest from the line of insignificance with the highest sensitivity for the presence of brain injury on head CT and adjusted for clinical utility. Analysis of proportions was performed with the Fisher’s Exact Test.
RESULTS
In the first cohort, 19 out of 57 head CT scans (34%) were interpreted as abnormal. Injuries ranged from small bleeds to diffuse parenchymal injury. Loss of consciousness (n = 18) and a GCS less than 15 (n = 22) were the most common primary indications for head CT. Other indications included emesis (n = 4), amnesia (n = 3), evidence of other head injury (n = 7), neurologic abnormality (n = 2), and severity of head trauma mechanism (n = 1). These patients generally met the CHALICE criteria,4 although, the duration of loss of consciousness was often unclear. About 75% (43 of 57) of the patients were admitted to the hospital. Of these patients, almost 60% (25 of 43) were admitted to the pediatric intensive care unit. There were no significant differences in the demographic and clinical characteristics of the two cohorts (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Study Population by Cohort
| Cohort 1 (n = 7) | Cohort 2 (n = 57) | |
|---|---|---|
| Male (%) | 35 (61%) | 41 (72%) |
| Mean age (yrs) ± 1 standard deviation | 7.2 ± 5.3 | 7.3 ± 5.2 |
| Glasgow Coma Score | ||
| <8 | 5 | 3 |
| 8–10 | 1 | 1 |
| 10–12 | 5 | 2 |
| 13–14 | 11 | 11 |
| 15 | 35 | 40 |
| Head CT | ||
| Normal | 38 (67%) | 37 (65%) |
| Abnormal* | 19 (33%) | 20 (35%) |
| EDH | 5 | 13 |
| SDH | 9 | 5 |
| ICH/edema† | 6 | 6 |
| SAH | 8 | 2 |
| ICP‡ | 2 | 1 |
| Interval between injury and phlebotomy | ||
| Median (range) | 93 min (10–1020 min) | |
| Main secondary injuries or condition | ||
| None | 35 | 31 |
| ETOH intoxication | 2 | 0 |
| Large organ injury§ | 2 | 0 |
| Skull Fx | 7 | 16 |
| Face Fx | 6 | 6 |
| Other Fx | 4 | 4 |
| Cardiac arrest | 1 | 0 |
EDH, epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; ICP, intracranial pressure; Fx, fracture; ETOH, ethanol.
Sum of individual injury types is greater than the total abnormal because some studies showed more than one injury type.
Includes small areas of intracranial hemorrhage, edema, or contusion.
Includes evidence of increased ICP such as midline shift or ventricular or cisternal effacement.
Large organ injury includes one liver laceration and one pulmonary contusion.
D-dimer and GCS strongly predicted brain injury on head CT (Table 2). S100β was a poor predictor of brain injury. Several potential confounding variables were not correlated with the D-dimer level (time interval to phlebotomy [p = 0.54], sex [p = 0.69], and age [p = 0.77]). All the associations presented in Table 2 were similar even among the subsets of patients with time interval to phlebotomy less than 3 (n = 38) or 2 (n = 28) hours (data not shown). None of the 49 patients who had platelet count, fibrinogen, and PT values in addition to D-dimer met criteria for disseminated intravascular coagulation.11 The level of C-reactive protein was not associated with the presence of brain injury (p = 0.92) or correlated with the D-dimer level (p = 0.49).
TABLE 2.
Univariate and Multivariate Analysis of Laboratory Parameters to Predict Presence of TBI on Head CT From Patients in Cohort 1
| No Brain Injury (n = 38)* | Brain Injury (n = 19) ICP | Univariate† | Multivariate‡ | |
|---|---|---|---|---|
| Na (mmol/l) | 140 ± 2.2 | 141 ± 3.3 | p = 0.40 | |
| K (mmol/l) | 3.74 ± 0.45 | 3.35 ± 0.7 | p = 0.02 | p = 0.8 |
| PT (s) | 13 (11.5–15.8) | 13 (11.4–18.7) | p = 0.86 | |
| PTT (s) | 25 (18–52) | 28 (19–45) | p = 0.15 | |
| Fibrinogen (mg/dl) | 263 (157–520) | 230 (132–320) | p = 0.04 | p = 0.26 |
| Platelets (103/μl) | 295 (183–519) | 283 (71–514) | p = 0.50 | |
| D-dimer (pg/μ) | 688 (150–5000) | 5000 (154–5000) | p < 0.001 | p = 0.02 |
| MMP9 (pg/ml) | 183 (25–686) | 170 (25–1300) | p = 0.94 | |
| S100β (pg/ml) | 100 (100–1250) | 100 (100–1260) | p = 0.65 | |
| GCS | 15 (6–15) | 13 (3–15) | p < 0.001 | p = 0.08 |
Na, sodium; K, potassium; PTT, partial thromboplastin time; MMP-9, major metalloproteinase-9; GCS, Glascow Coma Scale.
Mean ± SD or median (range).
Statistical tests: Na and K, student’s t test; all other variables, Wilcoxon rank sum.
Standard logistic regression using variables with P ≤ 0.05 from univariate analysis.
Variables with a p ≤ 0.05 in the univariate analysis were analyzed in a logistic regression model. D-Dimer was identified as an independent predictor of brain injury on head CT and was a stronger predictor than initial GCS (Table 2).
Receiver operator characteristic curve analysis suggested that a cut off of 500 pg/μL predicts brain injury (area under curve = 0.77, data not shown). Using this cut off, one patient with a D-dimer score <500 pg/μL was misclassified (Table 3). To test the suggested cut off, we conducted a follow-up validation study on a second cohort. Again, only one patient with a positive head CT had a D-dimer score < 500 pg/μL, whereas, almost half of those with a higher D-dimer had a positive head CT. Using the combined data, a D-dimer cut off of 500 pg/μL had a negative predictive value of 94%. In patients with an initial GCS of 13 to 15 or 15, the cut off generated a negative predictive value of 97% and 96%, respectively, with one false negative (Table 4).
TABLE 3.
Analysis of Proposed D-Dimer Cut-Off to Predict TBI
| D-Dimer (pg/μl) |
|||
|---|---|---|---|
| <500 | ≥ 500 | ||
| Cohort 1 | p = 0.002 | ||
| No TBI | 17 | 21 | |
| TBI | 1 | 18 | |
| Cohort 2 | p = 0.002 | ||
| No TBI | 16 | 21 | |
| TBI | 1* | 19 | |
| Combined cohorts | p < 0.001 | ||
| No TBI | 33 | 42 | |
| TBI | 2 | 37 | |
Patient had a small single slice intracerebral hemorrhage versus antecedent calcification on head CT.
TABLE 4.
Analysis of Proposed D-Dimer Cut-Off to Predict TBI in Patient Subsets
| D-Dimer (pg/μl) |
|||
|---|---|---|---|
| <500 | ≥500 | ||
| Patients with GCS 13–15 | p < 0.001 | ||
| No TBI | 30 | 39 | |
| TBI | 1* | 27 | |
| Patients with GCS 15 | p = 0.004 | ||
| No TBI | 23 | 32 | |
| TBI | 1* | 18 | |
Patient had a small single-slice intracerebral hemorrhage versus antecedent calcification on head CT.
Two patients with a D-dimer level score <500 pg/μL were misclassified. The first patient was 15 years old with an initial GCS of 12 and no other injury; the injury to phlebotomy time was 60 minutes, the D-dimer level 154 pg/μL, and the patient had a 48-hour period of disorientation. The head CT scan showed several, small intracerebral hemorrhages and a tiny subarachnoid hemorrhage. This patient received minimal medical therapy, no surgical therapy, and had slightly disorganized speech at discharge. The second patient was 8 months old and had no neurologic deficit with an initial GCS of 15 and no other injury; the injury to phlebotomy time was 280 minutes, the D-dimer level 198 pg/μL, and the patient’s head CT had a small single-slice intracerebral hemorrhage, which may have been an artifact or an antecedent calcification. The patient was observed in the hospital for a brief period because of the suspected intracerebral hemorrhage and received no medical or surgical therapy.
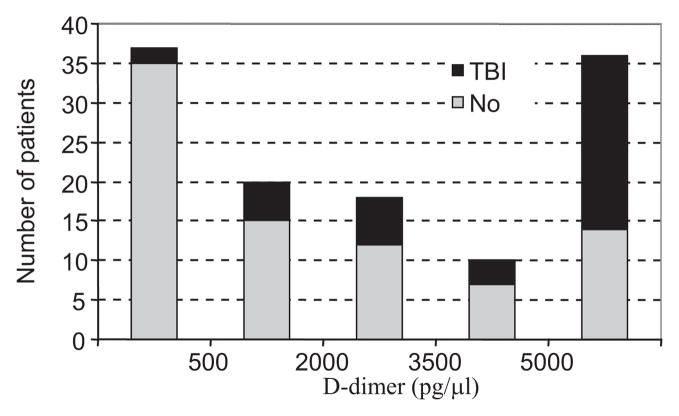
The distribution of D-dimer levels from the combined data (Fig. 1) shows that the majority of patients were above or below the range of the measurement device. Six percent (2 of 35) of the patients with D-dimer score < 500 pg/μL had brain injury on head CT. Sixty-one percent (22 of 36) of the patients with a D-dimer score >5000 pg/μL had brain injury. The patients with more severe findings tended to have a higher D-dimer level (Table 5). Twenty-one patients had an abnormal GCS for greater than 12 hours, and of these, only two had D-dimer score less than 1500 pg/μL (data not shown). One was the 15 year old mentioned previously; the other had had a seizure with an initial GCS of 9, a negative head CT, and an uneventful recovery. Six patients had abnormal GCS greater than 48 hours, and all had a D-dimer level greater than 5000 pg/μL. Only one patient required neurosurgical intervention (besides monitoring or drainage devices). This patient had an epidural hematoma evacuated with quick recovery and a D-dimer level of 4840 pg/μL (data not shown).
Figure 1.
Distribution of D-dimer levels. The histogram combines data from the prospective and retrospective cohorts. The black bars represent the portion of patients with evidence of brain injury on Head CT.
TABLE 5.
Head CT Findings Categorized by D-Dimer Level
| D-Dimer (pg/μl) | <500 | 500–1999 | 2000–3499 | 3500–4999 | ≥5000 |
|---|---|---|---|---|---|
| Total | 35 | 15 | 18 | 10 | 36 |
| TBI-any | 2 | 6 | 6 | 3 | 22 |
| EDH | 0 | 3 | 4 | 2 | 9 |
| SDH | 0 | 2 | 3 | 1 | 8 |
| ICH* | 1 | 0 | 0 | 1 | 10 |
| SAH | 1 | 3 | 1 | 0 | 5 |
| ICP† | 0 | 0 | 0 | 1‡ | 2 |
EDH, epidural hematoma; SDH, subdural hematoma; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; ICP, intracranial pressure.
Includes small areas of intracranial hemorrhage, edema, or contusion.
Includes evidence of increased ICP such as midline shift or ventricular or cisternal effacement.
Patient had an EDH that was evacuated with complete and rapid recovery, D-dimer 4840 pg/μl.
In the combined cohorts, 33% (13 of 39) of patients with a brain injury also had a skull fracture, and 57% (13 of 23) of patients with a skull fracture also had a brain injury. To evaluate the specificity of D-dimer for brain injury versus skull fracture, we conducted subset analyses (data not shown). The D-dimer level was not associated with a skull fracture in the subsets of patients with (p = 0.36) or without (p = 0.29) brain injury. Conversely, D-dimer level was associated with brain injury in subsets of patients with (p = 0.006) or without (p < 0.001) skull fracture. We also conducted logistic regression to determine which factors (brain injury, face fracture, skull fracture, or other fracture) predict a D-dimer level ≥500 pg/μL. These results suggest that brain injury (odds ratio [OR] = 11.2, p = 0.002) is a stronger predictor of D-dimer level >500 pg/μL than face fracture (OR = 2.8, p = 0.24), skull fracture (OR = 7.8, p = 0.02), or other fracture (OR = 5.5, p = 0.06) (data not shown).
DISCUSSION
These results suggest that quantitative D-dimer level may be an important adjunct in the evaluation of children with head trauma, especially, in light of the concerns regarding the expanding use and adverse effects of head CT. In fact, it was recently estimated that current CT use accounts for 2% of all cancers in the United States, and it is predicted that this number will increase as the large number of children exposed to even a single CT scan age.3,12
Several laboratory-based tests have been proposed to predict brain injury. Two recent studies13,14 suggest a s100β level less than 100 pg/mL predicts a negative CT in patients with initial GCS 13 to 15. One of our initial goals was to build a biomarker panel including s100β to overcome the concerns about its specificity in trauma15 and usefulness in pediatrics.16 But s100β was a poor predictor of brain injury in our study. There are several possible explanations for this unexpected result: (a) the higher low-detection limit of our study device (100 vs. 5 and 13 pg/mL), (b) differences in normative ranges between devices, (c) differences in sample preparation, (d) the higher rate of positive CT scans (26 vs. 7 and 9%) in our population, (e) the previous studies were restricted to patients with isolated head trauma and/or included the presence of skull fracture in the analysis, and (f) sample degradation.
Other laboratory-based studies have evaluated the association between brain injury and coagulopathy, thought to be triggered by the release of thromboplastin17 or other factors.18 A study published by the International Mission on Prognosis and Clinical Trail Design in TBI study group19 suggested that elevated PT is an independent predictor of poor outcome in patients with TBI. Other studies have suggested that elevated D-dimer levels predict poor outcome in TBI20 and after acute intracerebral hemorrhage.21 We found no studies that have rigorously evaluated D-dimer levels in comparison to head CT results.
Clinical scales designed to limit head CT use, such as The New Orleans Criteria7 and the Canadian CT Rule,5 were recently compared in prospective studies.10,22 In general, these scales are very sensitive but lack specificity (0–41%), and their application to the pediatric population has been limited. One study examined the New Orleans Criteria in children older than 5 years and suggested that CT use could be safely reduced to 23% using the criteria.23 The large, prospective CHALICE study4 in children proposed that if any of the 14 criteria is met, a head CT is justified, and the algorithm had 99% sensitivity and 87% specificity for significant injury. Similarly, a large meta-analysis9 suggested that focal neurologic deficit, abnormal GCS, and loss of consciousness correlate with brain injury. But despite the promise of these clinical indicators and scales, their utility has not been broadly accepted. This is perhaps because of the often limited history that is available during trauma screening and the confusing clinical picture often encountered in children.
An appreciation of injury-induced coagulopathy and familiarity with these clinical scales is important to understanding the potential applications of our finding. D-Dimer levels are used routinely in the evaluation of deep venous thrombosis and pulmonary embolism. In these scenarios, the D-dimer level is used as a negative predictor as proposed here for head trauma. A common feature of these pathologic states is activated fibrinolysis. Stoichiometrically, a relatively small loss of fibrinogen produces a relatively large amount of D-dimer. Therefore, D-dimer is a far more sensitive measure of activated coagulation than does parameters based on the disappearance of factors, such as PT, and might be more useful in the context of mild injury.
The biggest concern in limiting head CT scans is that clinicians would miss potentially severe injuries. Consideration of the misclassified patients in our study is encouraging, but the number is limited. The first patient had a low initial GCS with clear traumatic mechanism and would require head CT by any reasonable criteria and had a relatively long period of disorientation. The second had a normal GCS and a small finding on head CT. Neither patient required medical therapy beyond intravenous fluids and observation.
Based on these results, we suggest that the D-dimer level is a potential negative predictive tool in patients with suspected TBI who meet current indications for head CT (as defined by the practitioner, institution, or published criteria) with an initial GCS of 13 to 15 or a confounding condition, such as seizure or alcohol intoxication. Such patients might include those with loss of consciousness but appear well, recurrent emesis, abnormal GCS that is stable or improving, or the likely intoxicated or postictal. Injudicious use of the D-dimer test outside of this clinical context might, inadvertently, increase the use of head CT. This is akin to the widespread use of D-dimer to rule out deep vein thrombosis with high-negative predictive value (96–99%) and low-positive predictive value (14–37%).24
There are several points of caution regarding the use of D-dimer to evaluate TBI. Most importantly, although it is tempting to use the D-dimer level as a positive predictor of brain injury, our data do not support this use. Nevertheless, the observation that patients with higher D-dimer tended to have more severe injuries suggests that a value beyond the range of the device used herein might provide positive-predictive power.
Our patient population had a relatively high-incidence of positive head CT. This is likely because of the strict inclusion criteria (admission via trauma system activation), our tendency to image patients who meet the CHALICE criteria,4 and the use of high-resolution −64 channel VCT technology. The higher incidence effectively amplifies the difference between pre- and post-test odds of a negative result, because the pretest odds of a negative head CT are lower. But if D-dimer were applied to populations with a lower pretest probability (but still meet head CT criteria determined by clinician, institution, or guideline-based criteria), the negative predictive value would increase. These statistical observations reinforce the important point that this test be applied to individual patients after clinical assessment and determination of the need for head CT to rule out injury and not as a positive predictor.
Furthermore, the time interval between injury and phlebotomy might confuse interpretation of D-dimer. In this study, the interval was not associated with D-dimer level, and adjustments to include only patients with a shorter time interval had no effect on the results. We could not find a detailed description of the kinetics of D-dimer degradation. Because patients enrolled in our study were admitted through the county trauma system protocol, there was little variation in triage time between patients, although there was large variation in transport time. It is possible in other settings where triage might be altered based on clinical condition, and the time interval to phlebotomy may be important. Nevertheless, a low D-dimer level, in the context of rapid D-dimer degradation, would likely imply that there is no ongoing release of thromboplastin. Conversely, a high D-dimer level, in the context of slow degradation, would not alter the negative-predictive power of the D-dimer level.
The coagulation and fibrinolytic systems undergo developmental changes throughout childhood,25–27 and D-dimer level seems to be elevated in children.25 It is possible age-related normative D-dimer values might alter our conclusions. We found no correlation between D-dimer and age. Also, there was no such correlation in patients with or without brain injury (data not shown). A larger study would be required to determine age-related effects. Because younger children presumably have higher normative D-dimer levels, the age effect would not alter the negative-predictive power of D-dimer, and D-dimer might be more useful in adults who seem to have a lower and a more narrow normative range.
Our data are not sufficient to delineate the specificity of D-dimer for brain injury in the context of polytrauma. The data do suggest that brain injury is a potent stimulator of fibrinolysis, but many traumatic injuries trigger this state. Furthermore, as another study has suggested,28 these results question the use of D-dimer to evaluate for deep vein thrombosis in patients with TBI.
Finally, other limitations of our study include the relatively small number of patients and its retrospective design, especially, considering the importance of missing severe brain injury. In addition, reading of head CT scans was not standardized, and there may have been variation in interpretation among radiologists. A larger clinical study will be required to validate these findings and address these concerns. We note that D-dimer is a commonly used marker; confirmation of these results in adult and other centers should be relatively straightforward.
CONCLUSIONS
In children who meet clinical criteria for a head CT scan after trauma, low-plasma D-dimer strongly suggests the absence of significant brain injury. This common test may prove especially valuable to help assess such patients with GCS from 13 to 15 or clinical comorbidity and reduce the overall burden of head CT, but further study is required to fully ascertain its limitations.
References
- 1.White JR, Farukhi Z, Bull C, et al. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29:534–540. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Stein SC, Burnett MG, Glick HA. Indications for CT scanning in mild traumatic brain injury: a cost-effectiveness study. J Trauma. 2006;61:558–566. doi: 10.1097/01.ta.0000233766.60315.5e. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 4.Dunning J, Daly JP, Lomas JP, Lecky F, Batchelor J, Mackway-Jones K Children’s Head Injury Algorithm for the Prediction of Important Clinical Events Study Group. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child. 2006;91:885–891. doi: 10.1136/adc.2005.083980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391–1396. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- 6.Smits M, Dippel DW, de Haan GG, et al. Minor head injury: guidelines for the use of CT—a multicenter validation study. Radiology. 2007;245:831–838. doi: 10.1148/radiol.2452061509. [DOI] [PubMed] [Google Scholar]
- 7.Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100–105. doi: 10.1056/NEJM200007133430204. [DOI] [PubMed] [Google Scholar]
- 8.Dunning J, Stratford-Smith P, Lecky F, et al. Emergency Medicine Research Group. A meta-analysis of clinical correlates that predict significant intracranial injury in adults with minor head trauma. J Neurotrauma. 2004;21:877–885. doi: 10.1089/0897715041526122. [DOI] [PubMed] [Google Scholar]
- 9.Dunning J, Batchelor J, Stratford-Smith P, et al. A meta-analysis of variables that predict significant intracranial injury in minor head trauma. Arch Dis Child. 2004;89:653–659. doi: 10.1136/adc.2003.027722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiell IG, Clement CM, Rowe BH, et al. Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA. 2005;294:1511–1518. doi: 10.1001/jama.294.12.1511. [DOI] [PubMed] [Google Scholar]
- 11.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective evaluation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2416–2421. doi: 10.1097/01.ccm.0000147769.07699.e3. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell CD, Gorelick M, Holmes JF, Bandyopadhyay S, Kuppermann N. Pediatric head trauma: changes in use of computed tomography in emergency departments in the United States over time. Ann Emerg Med. 2007;49:320–324. doi: 10.1016/j.annemergmed.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Muller K, Townend W, Biasca N, et al. S100B serum level predicts computed tomography findings after minor head injury. J Trauma. 2007;62:1452–1456. doi: 10.1097/TA.0b013e318047bfaa. [DOI] [PubMed] [Google Scholar]
- 14.Biberthaler P, Linsenmeier U, Pfeifer KJ, et al. Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: a prospective multi-center study. Shock. 2006;25:446–453. doi: 10.1097/01.shk.0000209534.61058.35. [DOI] [PubMed] [Google Scholar]
- 15.Pickering A, Carter J, Hanning I, Townend W. Emergency department measurement of urinary S100B in children following head injury: can extracranial injury confound findings? Emerg Med J. 2008;25:88–89. doi: 10.1136/emj.2007.046631. [DOI] [PubMed] [Google Scholar]
- 16.Piazza O, Storti MP, Cotena S, et al. S100B is not a reliable prognostic index in paediatric TBI. Pediatr Neurosurg. 2007;43:258–264. doi: 10.1159/000103304. [DOI] [PubMed] [Google Scholar]
- 17.Harhangi BS, Kompanje EJ, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien) 2008;150:165–175. doi: 10.1007/s00701-007-1475-8. discussion 175. [DOI] [PubMed] [Google Scholar]
- 18.Morel N, Morel O, Petit L, et al. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J Trauma. 2008;64:698–704. doi: 10.1097/TA.0b013e31816493ad. [DOI] [PubMed] [Google Scholar]
- 19.Murray GD, Butcher I, McHugh GS, et al. Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24:329–337. doi: 10.1089/neu.2006.0035. [DOI] [PubMed] [Google Scholar]
- 20.Bayir A, Kalkan E, Kocak S, Ak A, Cander B, Bodur S. Fibrinolytic markers and neurologic outcome in traumatic brain injury. Neurol India. 2006;54:363–365. doi: 10.4103/0028-3886.28106. [DOI] [PubMed] [Google Scholar]
- 21.Delgado P, Alvarez-Sabin J, Abilleira S, et al. Plasma D-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology. 2006;67:94–98. doi: 10.1212/01.wnl.0000223349.97278.e0. [DOI] [PubMed] [Google Scholar]
- 22.Smits M, Dippel DW, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519–1525. doi: 10.1001/jama.294.12.1519. [DOI] [PubMed] [Google Scholar]
- 23.Haydel MJ, Shembekar AD. Prediction of intracranial injury in children aged five years and older with loss of consciousness after minor head injury due to nontrivial mechanisms. Ann Emerg Med. 2003;42:507–514. doi: 10.1067/s0196-0644(03)00512-2. [DOI] [PubMed] [Google Scholar]
- 24.Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 25.Sosothikul D, Seksarn P, Lusher JM. Pediatric reference values for molecular markers in hemostasis. J Pediatr Hematol Oncol. 2007;29:19–22. doi: 10.1097/MPH.0b013e3180308749. [DOI] [PubMed] [Google Scholar]
- 26.Andrew M, Paes B, Johnston M. Development of the hemostatic system in the neonate and young infant. Am J Pediatr Hematol Oncol. 1990;12:95–104. doi: 10.1097/00043426-199021000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80:1998–2005. [PubMed] [Google Scholar]
- 28.Meythaler JM, Fisher WS, Rue LW, Johnson A, Davis L, Brunner RC. Screening for venous thromboembolism in traumatic brain injury: limitations of D-dimer assay. Arch Phys Med Rehabil. 2003;84:285–290. doi: 10.1053/apmr.2003.50116. [DOI] [PubMed] [Google Scholar]