Abstract
Substantial evidence suggests that glutamatergic neurotransmission is a critical mediator of the experience-dependent synaptic plasticity that may underlie alcohol dependence. Substance abuse typically begins in adolescence; therefore, the impact of alcohol on glutamatergic systems during this critical time in brain development is of particular importance. The N-methyl-D-aspartate receptor (NMDAR) is involved in developmental mechanisms underlying neuronal differentiation and synaptogenesis and as such may be a target system for alcohol effects during adolescence. In the present study quantitative biochemical determinations were made of the relative abundance of different protein expressions of NMDAR subunits in adolescents and adults after 2 wks of ethanol vapor exposure, and 24 hrs and 2 wks following withdrawal. After 2 wks of ethanol vapor exposure NR1, NR2A, and NR2B subunit expression was found to be increased in hippocampus of the adults. In contrast, 2 wks of ethanol exposure resulted in no significant changes in NR1 and NR2B subunits and a reduction NR2A subunit expression in hippocampus in adolescents. Twenty-four hours and 2 wks following withdrawal from ethanol vapor NR1 and NR2A subunit expression in hippocampus was decreased in adolescents, whereas in adults it had returned to control levels. In frontal cortex, 2 wks of chronic ethanol exposure produced decreases in NR1 subunit expression in both adults and adolescents but also produced decreases in NR2A and NR2B subunit expression in adults that returned or exceeded control levels by 2 wks following withdrawal from ethanol vapor. These results demonstrate that NMDAR subunit composition can be modulated differentially between adolescents and adults by chronic ethanol exposure and withdrawal. These developmental differences in NMDAR subunits composition may also be associated with the enhanced vulnerability of the adolescent brain to ethanol dependence.
Keywords: NR1, NR2A, NR2B, Alcohol Withdrawal, Hippocampus, Frontal Cortex
Adolescence is a critical stage of brain development when humans are initially exposed to potentially toxic external stimuli such as ethanol and other drugs of abuse (Johnston, 1995). Ethanol is one of the most abused drugs during adolescence and can produce detrimental effects on brain development (Spear, 2000; Crews et al., 2007). Given that the brain continues to develop throughout the adolescent period (Markus and Petit, 1987; Sowell et al., 1999), early ethanol exposure may have unique deleterious consequences.
Studies characterizing the cellular and molecular mechanisms underlying the enhanced vulnerability to ethanol exposure during adolescence have frequently focused on studying glutamatergic neurotransmission (see Fadda and Rossetti, 1998; Crews et al., 2002), and in particular the N-methyl-D-aspartate (NMDA) type of glutamate receptor. Substantial loss of synapses, especially the excitatory glutamatergic inputs to the forebrain, occurs during adolescence (Huttenlocher, 1984; Zecevic et al., 1989) and may be vulnerable to ethanol exposure. In the hippocampus, the exuberant outgrowth of excitatory axon collaterals and synapses that occur earlier during young ages are morphologically remodeled and branches within dendritic arbors are pruned during adolescent maturation with most synaptic pruning involving glutamatergic receptors (Swann et al., 1999).
Evidence has shown that NMDA receptor subunit expression is developmentally regulated during brain maturation (Watanabe et al., 1992, 1993; Jin et al., 1997; Wenzel et al., 1997; Barria and Malinow, 2002; Magnusson et al., 2002; Law et al., 2003; Chang et al., 2009). This regulation, which has been demonstrated both in vivo and in vitro, leads to changes in the functional and pharmacological properties of NMDAR. Several studies suggest that NMDA receptors influence synaptogenesis, neuronal growth and plasticity, as well as the fine-tuning of neuronal connections (see Kloda et al., 2007). One of the fundamental changes that occur in NMDA receptor subunit composition during development is a gradual reduction in NR2B levels, with a concomitant increase in NR2A levels (Monyer et al., 1994; Jin et al., 1997).
The NMDAR has also been shown to be an important site for ethanol actions (see Lovinger et al., 1989; Peoples and Weight, 1999; Schummers and Browning, 2001; Hoffman, 2003) and to play a role mediating ethanol dependence, tolerance, and withdrawal (Eckardt et al., 1998; Kalluri et al, 1998; Krystal et al., 1998; Darstein et al., 2000). Studies have shown that acute ethanol exposure inhibits the expression of NMDAR subunits. There is considerable evidence to suggest that the NR2A- and NR2B subunits are the most sensitive NMDAR subunits to ethanol actions (Masood et al., 1994; Trevisan et al., 1994; Grant and Lovinger, 1995; Allgaier, 2002; Nixon et al., 2004; Hendricson et al., 2007). However, the consequences of adolescent ethanol exposure on the expression of NMDAR subunits in the cortex and hippocampus in vivo are just beginning to be understood (Sircar and Sircar, 2006; Pascual et al., 2009). Characterizing the effects of adolescent versus adult ethanol exposure on the expression of NMDAR subunits could provide important information on the unique mechanisms that may mediate the neurophysiological consequences of adolescent ethanol exposure previously shown in our laboratory (Slawecki et al., 2004; Criado et al., 2008a, 2008b). The objective of the present study was to determine whether expression of the NR1, NR2A and NR2B subunits in the hippocampus and frontal cortex is influenced by chronic ethanol exposure and/or withdrawal in adolescent versus adult rats. Adolescent and adult rats were exposed to intermittent chronic ethanol vapor for a period of 2 wks. Expression protein levels of the three different NMDA receptor subunits (NR1, NR2A, and NR2B) were quantified in the frontal cortex and hippocampus at three different time points following chronic ethanol treatment: no withdrawal period (chronic ethanol group), 24-hr withdrawal (WD) period (24-hr WD group) and 2-wk withdrawal period (2-wk WD group).
EXPERIMENTAL PROCEDURES
Subjects
Male Wistar rats at postnatal day (PND) 23 (n = 42; Charles River, USA) and at PND 60 (n = 42; Charles River, USA) were used in this study. Adolescent (PND 23) and adult (PND 60) rats were housed four and two per cage respectively in standard cages for the duration of the experiment. Animals were kept in a light/dark (12h light/12h dark, lights on at 06:00 a.m.) and temperature-controlled environment except for during the vapor exposure (see below). Food and water were available ad libitum throughout the experiment, except where noted. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Scripps Research Institute and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
Ethanol vapor exposure
Ethanol vapor exposure has been shown to reliably allow for the titration of blood alcohol levels (BALs) that are sufficient for inducing ethanol physical dependence as indicated by signs of withdrawal (Roberts et al., 1996, 2000; O’Dell et al, 2004). The ethanol vapor inhalation procedure and the chambers used in this study were previously described (Rogers et al., 1979; Slawecki et al., 2001; Slawecki, 2002). Ethanol vapor chambers were calibrated to produce moderate BALs between 175–225 mg/dL. In brief, adolescent (n = 42) and adult (n = 42) rats were divided into two groups each (ethanol-exposed group, n = 21; control group, n = 21). Ethanol-exposed rats were housed in sealed chambers, which were infused with vaporized 95% ethanol from 8 PM to 10 AM. For the remaining of the 10 hours of the day, ethanol vapor was not infused into the chamber. This ethanol exposure regimen continued for 2 wks. At the start of the ethanol exposure, adolescent rats were 23 days old and the exposure continued until they were 37 days old. Adult rats were 60 days old and exposure continued until they were 74 days old. Age-matched controls were handled identically to ethanol-exposed rats. Food and water were always available. Blood samples were collected from the tip of the tail three times per week to assess blood alcohol levels (BALs) (target: 150 to 200 mg/dl). BALs were determined using the Analox micro-statGM7 (Analox Instr. Ltd., Lunenberg, MA).
Adolescent (n = 21) and adult (n = 21) ethanol-exposed rats were randomly subdivided into three groups each: the chronic ethanol treatment group (CET) (n = 7), the 24-hr ethanol withdrawal (WD) group (n = 7), and the 2-wk ethanol WD group (n = 7). In the CET group, rats were sacrificed and brains dissected immediately after the 2-wks of ethanol exposure. In the 24-hr WD group, brains were dissected 24 hrs after ethanol exposure. In the 2-wk WD group, brains were dissected 2 wks after ethanol exposure. Each ethanol group was compared to its respective control group. When ethanol exposure ended, rats from groups assigned to the withdrawal periods (24-hr WD and 2-wk WD) were maintained in the Scripps vivarium.
Tissue dissection and preparation
Figure 1 shows graphical representation of the experimental protocol used to harvest adolescent (PND 37–52) and adult (PND 74–88) rat brains. Frontal cortex and hippocampus were dissected and frozen until solubilization and homogenization. Brain tissue was solubilized and homogenized in ice-cold buffer containing 50 mM Tris-HCl (pH 7.5), 1% deoxycholate and Complete Mini Protease Cocktail Inhibitor (Roche, Switzerland) using a tissue sonicator. The homogenate was then centrifuged for 35 minutes at 4 °C. The resulting supernatant was used as the membrane fraction. Protein concentrations were determined using a BCA assay (Pierce, Rockford, IL).
Figure 1.
Ethanol Exposure Protocol.
Western blotting and analysis
Samples of frontal cortical and hippocampal homogenates (5–15 ug of total protein per lane) were heated with NuPAGE 4×LDF sample buffer (Invitrogen), and then separated on 4%-8% gradient Tris-acetate minigels (Invitrogen) and electrotransferred to nitrocellulose membranes. Non-specific binding of antibodies to membranes was prevented by blocking with a solution containing 3% (w/v) nonfat dry milk, 0.05% (v/v) Tween-20 (Fisher Scientific), in Tris Buffered Saline (Fisher Scientific). Membranes were incubated with diluted primary antibodies in buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20): rabbit anti-NMDAR1 affinity-purified polyclonal antibody (1:6,000; Chemicon, Temecula, CA), rabbit anti-NMDAR2A affinity-purified polyclonal antibody (1:4,000; Chemicon), rabbit anti-NMDAR2B affinity-purified polyclonal antibody (1:3,000; Chemicon), or mouse polyclonal antibody to β-actin (1:1,000; Chemicon) overnight at 4 °C, and the –HRP conjugated secondary goat anti-rabbit IgG (1:5,000) and goat anti-mouse IgG (1:1,000) antibodies (Southern Biotechnology Associates, Inc., Birmingham, AL) incubated for one hour at room temperature. Immuno-reactive bands were visualized with the ECL Plus Western Blotting Detection System (Pierce, Rockford, IL), and band intensity quantified using Quantity One software (Bio-Rad, Hercules, CA). Protein levels are expressed as optical units obtained by normalization of NMDAR subunits against β-actin.
Data Analysis
Statistical analyses were performed by using SPSS (SPSS, Inc., Chicago, IL). ANOVAs were used to determine the effects of chronic ethanol exposure on body weight and BALs. Ethanol and control groups were compared after termination of the chronic ethanol treatment and 2-wks after withdrawal from chronic ethanol (P < 0.05 for significance). BALs in adolescent and adult rats exposed to ethanol were compared using one-way ANOVA (P < 0.05 for significance).
Brain regions (frontal cortex and hippocampus) and NMDAR subunits (NR1, NR2A and NR2B) were assessed independently. Independent analysis of variance (ANOVA) was used to assess the expression of the NR1, NR2A and NR2B NMDAR subunits in the frontal cortex and hippocampus in adolescent (PND 37–52) and adult (PND 74–88) rats. Independent ANOVAs were also used to assess the effects of CET on the expression of the NMDAR subunits in adolescent and adult rats at three different withdrawal periods following chronic ethanol exposure: 0-hr, 24-hr and 2-wk. These groups were designated as the CET, the 24-hr WD and the 2-wk WD. When appropriate, post hoc analysis of the ANOVA utilized the Fisher’s Least Significance Difference test to assess group differences. ANOVAs were also used to assess developmental differences in the expression of NMDAR subunits and the NR2A/NR2B ratio in animals from control groups. To correct for multiple comparisons in the expression of NMDAR subunits, P-values for all ANOVAs were set at P < 0.01 to determine the levels of statistical significance. P-values for post hoc analyses were set at P < 0.05.
RESULTS
Body weight and BALs
Prior to the initiation of the experiment, there were no differences in body weight between the ethanol (52 ± 2 g) and control (53 ± 1 g) groups in adolescent rats. The body weight of the ethanol (355 ± 4 g) and control (362 ± 3 g) groups was also not significantly different in adult rats. Ethanol vapor exposure produced BALs averaging 193 ± 10 mg/dl in adolescent rats and 197 ± 6 mg/dl in adult rats. Following chronic ethanol exposure, no differences in body weight were observed between ethanol exposed and control adolescent rats (Ethanol exposed: 132 ± 3 g; Control: 142 ± 3 g). In contrast, differences in body weight were found between ethanol exposed and control adult rats following chronic ethanol exposure (Ethanol exposed: 387 ± 6 g; Control: 420 ± 4 g; F(1,41)=23.0, P < 0.01). 2-wks after ethanol WD, differences in body weight were observed between ethanol exposed and control adolescent rats (Ethanol exposed: 251 ± 4 g; Control: 272 ± 5 g; F(1,12)=10.8, P < 0.01). No differences in body weight were observed between ethanol exposed and control adult rats after 2-wks of ethanol WD (Ethanol exposed: 477 ± 9 g; Control: 464 ± 8 g).
Developmental regulation of NMDAR subunit expression
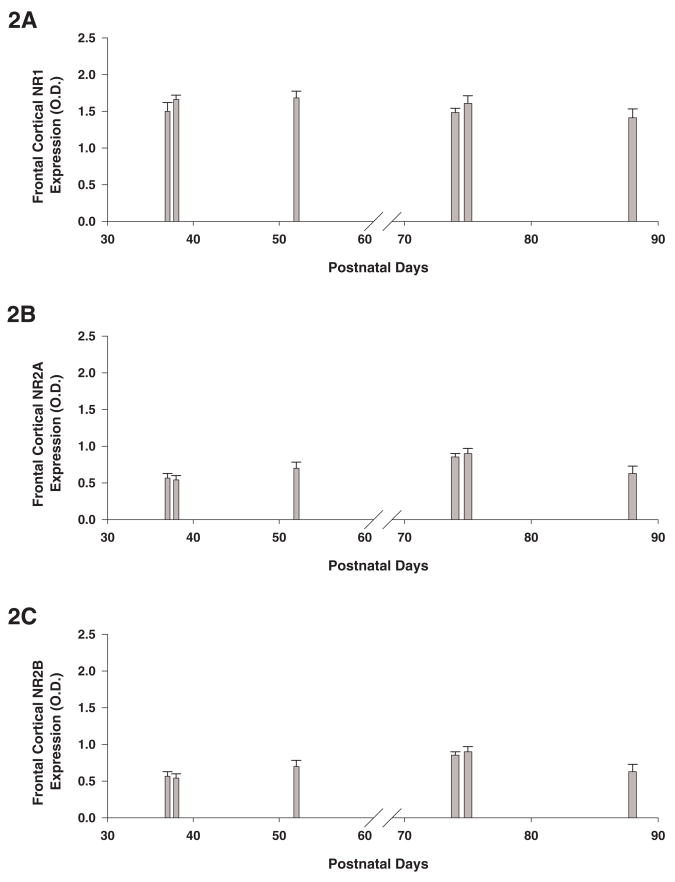
We studied the expression of the NR1, NR2A and NR2B NMDAR subunits in the frontal cortex and hippocampus in adolescent (PND 37–52) and adult (PND 74–88) rats (n = 15/group). The expression of the NR1 subunit in the frontal cortex was not different between adolescent and adult rats. In contrast, the expression of the NR2A and NR2B subunits in the frontal cortex was greater in adult than in adolescent rats (F’s(1,29)<77.6, P’s < 0.01). The ratio of NR2A to NR2B subunit protein expression was significantly increased in frontal cortex in adult versus adolescent control animals (adolescent: 0.92 ± 0.1; adult: 1.75 ± 0.1; F(1,29) = 31.2, P < 0.001). Figures 2A-C illustrate the expression of the NR1, NR2A and NR2B NMDAR subunits in the frontal cortex in rats at PND 37, 38, 52, 74, 75 and 88.
Figure 2. Age-related developmental changes in the protein expression of the NMDAR NR1, NR2A and NR2B subunits.
Figure 2A-F represents the protein expression levels of the NMDAR subunits in the frontal cortex (Fig. 2A-C) and hippocampus (Fig. 2D-F) from naive rats at postnatal day 37, 38, 52, 74, 75 and 88 (n=5/group). Protein levels are expressed as optical units obtained by normalization of NMDAR subunits against β-actin. Error bars= S.E.M.
The expression of the NR1, NR2A and NR2B subunits in the hippocampus was greater in adult than in adolescent rats (F’s(1,29)<87.5, P’s < 0.001). The ratio of NR2A to NR2B subunit protein expression was significantly increased in hippocampus in adult versus adolescent control animals (adolescent: 0.40 ± 0.03 adult: 0.64 ± 0.03; F(1,29) = 25.5, P < 0.001). Figures 2D-2F illustrate the expression of the NR1, NR2A and NR2B NMDAR subunits in hippocampus in rats at PND 37, 38, 52, 74, 75 and 88.
Effects of ethanol on NMDAR subunits expression in the frontal cortex
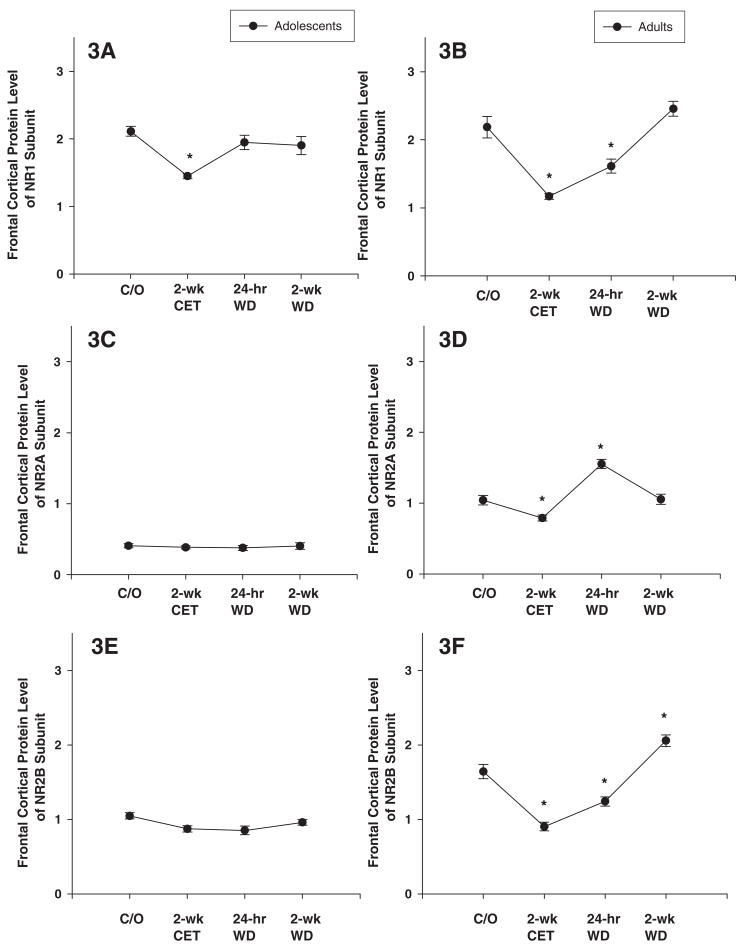
Use of ANOVA provided statistically significant differences in the expression of the NR1 subunit in the frontal cortex in adolescent and adult rats (Figs. 3A-B; F’s(3, 41) < 8.9; P’s < 0.001). Post hoc assessment revealed that the expression of the NR1 subunit in the frontal cortex was decreased in the CET group in adolescent rats, and in the CET and 24-hr WD groups in adult rats, compared to their respective control groups (Figs. 3A-B).
Figure 3. Chronic ethanol treatment (CET) and ethanol withdrawal (WD) alters the protein expression of NMDAR subunits in adolescent and adult frontal cortical tissue.
(A) In frontal cortex of CET adolescent rats, the NR1 subunit protein level is significantly decreased compared with that from naïve adolescent rats. NR1 subunit at 24-hr and 2-wk WD is returned to the protein levels of naïve adolescent rats. (B) In frontal cortex of CET adult rats, the NR1 subunit protein level is significantly decreased compared with naïve adult rats. NR1 subunit at 24-hr WD is remained decreased compared with naïve adult rats but at 2-wk WD the level of NR1 subunit returned to the level of naïve rats. (C) CET, 24-hr and 2-wk WD had no effect on protein expression of NR2A subunit in adolescent rats. (D) CET significantly decreased NR2A subunit levels as compared to naïve adult rats. At 24-hr WD, the NR2A subunit is significantly increased compared to naïve adult rats. At 2-wk WD, NR2A subunit returned to the protein levels of naïve adult rats. (E) CET, 24-hr and 2-wk WD had no effect on NR2B subunit protein expression in adolescent rats. (F) CET and 24-hr WD decreased the NR2B subunit compared to naïve adult rats. Adult NR2B subunit at at 2-wk WD was increased compared to naïve rats. Protein levels are expressed as optical units obtained by normalization of NMDAR subunits against β-actin. * indicates P< 0.05 for significant difference from control rats. Error bars= S.E.M.
Ethanol exposure had no significant effect on the expression of the NR2A subunit in the frontal cortex of adolescent rats (Fig. 3C). In contrast, ethanol had a significant effect on the expression of the NR2A subunit in adult rats (F(3,41) = 12.1; P < 0.001). Post hoc assessment showed that CET significantly reduced NR2A expression, whereas the 24-hr WD group showed a significant increase (Fig. 3D). Chronic ethanol exposure had no significant effect on the expression of the NR2B subunit in the frontal cortex of adolescent rats (Fig. 3E; F’s(3,41) = 3.5; P > 0.01). However, chronic ethanol exposure had a significant effect on the expression of the NR2B subunit in the frontal cortex of adult rats (Fig. 3F; F’s(3,41) = 15.8; P < 0.01). Post hoc assessment revealed that the expression of the NR2B subunit in the frontal cortex was decreased in the CET and 24-hr WD groups in adult rats, compared to the control group (Figs. 3F). However, the adult 2-wk WD group showed an increase in the expression of the NR2B subunit (Fig. 3F).
Effects of ethanol on NMDAR subunits expression in the hippocampus
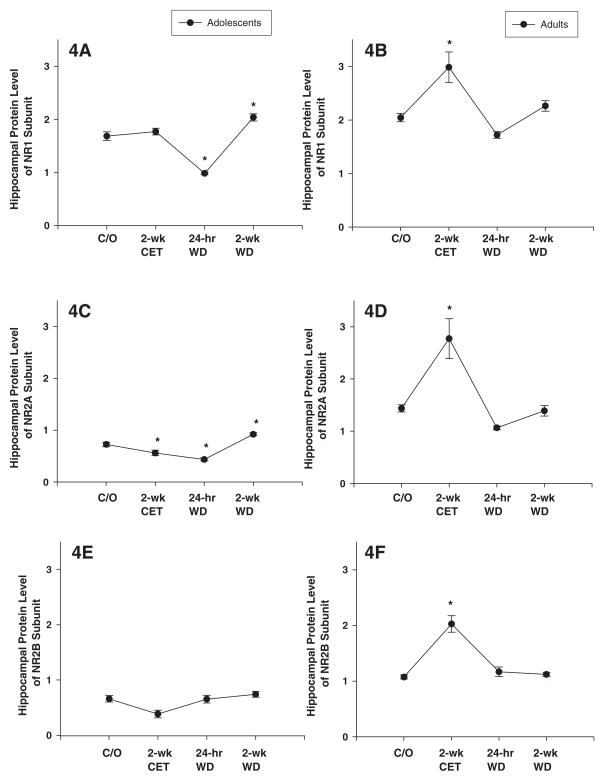
ANOVA revealed that ethanol had a significant effect on the expression of the NR1 subunit in the hippocampus in adolescent and adult rats (Figs. 4A-B; F’s(3, 41) < 17.1; P’s < 0.001). Post hoc assessment revealed that the expression of the NR1 subunit in the adolescent hippocampus was decreased in the 24-hr WD group and increased in the 2-wk WD group, compared to the control group (Fig. 4A). In contrast, the expression of the NR1 subunit in the adult hippocampus was increased in the CET group, compared to the control group (Fig. 4B).
Figure 4. Chronic ethanol treatment (CET) and ethanol withdrawal (WD) alters the protein expression of NMDAR subunits in adolescent and adult hippocampal tissue.
(A) At 24-hr WD, the hippocampal NR1 subunit protein level of adolescent rats is significantly decreased compared to naïve adolescent rats. At 2-wk WD, the NR1 subunit expression is increased compared to naïve adolescent rats. (B) In hippocampus of CET adult rats, the NR1 subunit protein level is significantly increased compared to naïve adult rats. At 24-hr and 2-wk WD, the protein expression of NR1 subunit is returned to the levels of the same-age naïve adult rats. (C) CET and 24-hr WD decreased the NR2A subunit compared to naïve adolescent rats. At 2-wk WD, NR2A subunit is increased as compared to naïve adolescent rats. (D) CET increased the NR2A subunit compared to naïve adult rats. At 24-hr and 2-wk WD, the expression of the NR2A subunit is returned to naïve adult control levels. (E) CET, 24-hr and 2-wk WD had no effect on NR2B subunit protein expression in adolescent rats. (F) CET increased the NR2B subunit compared to naïve adult rats. At 24-hr and 2-wk WD, the NR2B subunit is returned to the naïve control levels. Protein levels are expressed as optical units obtained by normalization of NMDAR subunits against β-actin. * indicates P< 0.05 for significant difference from control rats. Error bars= S.E.M.
Ethanol exposure had a significant effect on the expression of the NR2A subunit in the hippocampus of adolescent and adult rats (Figs. 4C-D; F’s(3, 41) < 18.6; P’s < 0.001). Post hoc assessment revealed that the adolescent hippocampus showed a significant reduction in NR2A expression in the CET and 24-hr WD groups, whereas the 2-wk WD group showed a significant increase, compared to their respective controls (Fig. 4C). In contrast, the adult hippocampus showed a significant increase in the expression of the NR2A subunit in the CET group (Fig. 4D). Chronic ethanol exposure had no effect on the expression of the NR2B subunit in the hippocampus of adolescent rats (Fig. 4E; F(3,41)=3.5; P > 0.01). However, chronic ethanol exposure had a significant effect on the expression of the NR2B subunit in the hippocampus of adult rats (Fig. 4F; F(3,41) = 34.5; P < 0.01). Post hoc assessment revealed that the expression of the NR2B subunit in the adult hippocampus showed an increase in the expression of the NR2B subunit in the CET group (Fig. 4F). Figure 5 shows representative Western blots of hippocampal frontal cortex NMDAR NR1, NR2A and NR2B expression in adolescent and adult rats from the CET 24-h WD and 2-weeks WD groups.
Figure 5. Effect of chronic ethanol treatment (CET), 24-hr and 2-wk ethanol withdrawal (WD) on the protein expression of NMDAR subunits in hippocampus and frontal cortex of adolescent and adult rats.
Representative Western blot analysis of NMDAR NR1, NR2A and NR2B subunits expression in hippocampus and frontal cortex of adolescent (top) and adult (bottom) rats
DISCUSSION
In the present study quantitative biochemical determinations were made of the relative abundance of different protein expressions of NMDAR subunits in adolescents and adults after 2 wks of ethanol vapor exposure, and 24 hrs and 2 wks following withdrawal. Quantitative Western blot analysis revealed that NR1, NR2A and NR2B subunits were increased in the hippocampus of adult rats, whereas the NR2A subunit was reduced in the hippocampus of adolescent rats after 2 wks of ethanol vapor exposure. Our data also showed that while the NR1, NR2A and NR2B subunits were reduced in the frontal cortex of adult rats, only the NR1 subunit was reduced in adolescent rats after 2 wks of ethanol exposure. These findings suggest that the effects of 2 wks of ethanol exposure on the expression of NMDAR subunits were age- and region-dependent.
Western blot analysis showed no change in the expression of the NMDAR subunits in the hippocampus of adult rats during the ethanol withdrawal period. While NR1 and NR2A subunits in the hippocampus of adolescent rats were reduced after 24 hr withdrawal, expression of these subunits increased after 2 wks of ethanol exposure. These changes in the expression of NR1 and NR2A subunits in the hippocampus suggest enhanced vulnerability to ethanol withdrawal in adolescent rats. In contrast, our data indicated that in adolescents, NMDAR subunits in frontal cortex were not changed following ethanol withdrawal. Western blot analysis showed that in adult rats, NR1 and NR2B subunits were reduced after 24 hr withdrawal, while NR2A subunits were increased. NR2B subunits in frontal cortex in adult rats were also increased after 2 wks of withdrawal from ethanol exposure. These findings suggest that the effects of ethanol withdrawal on the expression of NMDAR subunits were age- and region-dependent. These results demonstrate that NMDAR subunit composition can be modulated differentially between adolescents and adults by chronic ethanol exposure and withdrawal. These developmental differences in NMDAR subunits composition may be associated with the enhanced vulnerability of the adolescent brain to ethanol dependence. Our data also revealed that the ratio of NR2A to NR2B was significantly increased in both hippocampus and frontal cortex in adult versus adolescent control animals. Further research is needed to determine whether developmental differences in the NR2A to NR2B ratio play a role in the effects of chronic ethanol exposure and withdrawal in vivo on the expression of NMDAR subunits.
A number of studies have shown that NMDAR subunit expression is differentially regulated during development (Watanabe et al., 1992, 1993; Monyer et al., 1994; Zhong et al., 1995; Jin et al., 1997; Wenzel et al., 1997; Hsieh et al., 2002; Magnusson et al., 2002; Law et al., 2003; Chang et al., 2009). Most of these studies have demonstrated in the rat that NR2A expression is generally low at birth but then increases up to about P21–P28, whereas NR2B is high at birth and then peaks around P10–21. Few studies have specifically compared subunit expression between the adolescent and young adult developmental periods. In the present study, the NMDAR subunits, NR2A and NR2B, were found to be expressed at significantly lower levels in hippocampus and frontal cortex in the adolescents as compared to the adults. In one study, investigating human hippocampus, NR1, NR2A and NR2B levels did not appear to be significantly different between adolescents (n=9) and young adults (n=6) (Law et al., 2003). In another study, in Sprague-Dawley rats, little differences in expression of these subunits were seen between P25 and “adult” rats (Hsieh et al., 2002). Additionally, it has been reported that no effects of age were seen in NR1 and NR2A subunit mRNA between 3, 10 and 30 month old mice (Magnusson et al., 2006). However, highly consistent with the literature (see Monyer et al., 1994; Jin et al., 1997), we found that the ratio of NR2A to NR2B subunit protein expression levels to be significantly increased in both hippocampus and frontal cortex in adult versus adolescent control animals. Discrepancies observed between studies in subunit expression as a function of developmental trajectory may be a result of the use of different species or strains of animals and remains to be replicated in additional studies.
An abundance of data suggests that chronic ethanol exposure induces changes in the NMDA receptor (see Hoffman, 2003, for review). Early reports demonstrated that an “up-regulation” occurs in NMDA receptors in the brains of animals that have been treated chronically with ethanol (see Tabakoff and Hoffman, 1996, Kumari and Ticku, 2000). Increases in the number of NMDA receptors has been shown by ligand binding and autoradiographic technique in mice chronically fed ethanol in a liquid diet (Grant et al., 1990; Gulya et al., 1991; Snell et al., 1993). Increases in NR1 and NR2A (Snell et al., 1996) and NR2B (Narita et al., 2000) receptor subunit proteins have also been reported from studies examining brains from mice fed ethanol in a liquid diet. In rats, increases in ligand binding of NMDA receptors (Sanna et al., 1993; Rudolph et al., 1997) and increases in mRNA and/or protein levels for NR1, NR2A, and NR2B have been reported in numerous studies in vivo (Trevisan et al., 1994; Ortiz et al., 1995; Hardy et al., 1999) and in vitro (Follesa and Ticku, 1996; Hoffman et al., 1996; Hu et al., 1996; Chandler et al., 1999; Nagy et al., 2003; Hendricson et al., 2007). However, some studies have found no changes in NMDA receptor proteins in chronically ethanol treated rats (Ferreira et al., 2001).
Consistent with previously published studies we found that protein expression of the hippocampal NMDAR subunits: NR1, NR2A and NR2B, were significantly increased after chronic ethanol exposure, in adult male Wistar rats. However, we found chronic ethanol exposure decreased protein expressions of NMDAR subunits NR1, NR2B, and NR2A in frontal cortex. Thus, our study suggests that there may be regional specificity in the effects of ethanol on NMDARs in adult rats. Different findings were observed in adolescent rats where NR1 but not NR2A or NR2B subunits were found to be reduced in cortex and NR2A but not NR2B or NR1 subunits were reduced in hippocampus by chronic ethanol exposure. Findings from the present study also showed that chronic ethanol exposure had no effect on body weight in adolescent rats, but significantly reduced the body weight of adult rats. In contrast, adolescent rats, but not adult rats, showed a significant reduction in body weight following 2-wk ethanol withdrawal. These results showed age-dependent differences in the effects of chronic ethanol and withdrawal on body weight. However, these age-dependent effects of chronic ethanol exposure on body weight were not consistent with the age-dependent effects of chronic ethanol on adolescent and adult NMDAR expression. Chronic ethanol produced changes on cortical and hippocampal NMDAR expression in adolescent rats in the CET as well as in the 2-wk WD group. While hippocampal NMDAR expression in adult rats was only altered in the CET group, cortical NMDAR expression was altered at all time points. Thus, body weight changes between the groups do not appear to be correlated with the neurochemical findings.
Only a few previous studies have investigated the effects of ethanol exposure specifically on adolescent animals. One study found down-regulation of the expression of NMDAR2B phosphorylation in adolescent but not adult rats 24 hours following a regime of intermittent I.P. (3 g/kg) ethanol treatment that occurred over a period of 14 days during adolescence in Wistar rats (Pascual et al., 2009). However in another study in Sprague Dawley rats, daily I.P. ethanol injections of 2 g/kg for five days followed by 7 days of withdrawal during adolescence was found to produce an increased in [3H] MK-801 binding and an increase in immunohistochemical measures of NR2B subunit protein in frontal cortex (Sircar and Sircar, 2006). Differences between studies may be due to length of ethanol treatment as both our study and that of Pascual et al. (2009) treated animals over a 2-wk period. Additionally, both our study and the study of Pascual et al. (2009) used Wistar rats as subjects. Another potential source of differences between studies may be related to the methods used to isolate and quantitate tissue samples, as it has been suggested that ethanol exposure may significantly affect the assembly and transport of NMDA receptors (Hughes et al., 2001).
Molecular and cellular adaptations to drug exposure are believed to lead to persistent changes in transcription, translation, synaptic morphology and function that are extremely long-lived and are analogous to the plastic processes that underlie learning and memory (Nestler, 2001; Ron and Jurd, 2005). Long-lasting adaptive changes after chronic ethanol exposure during adolescent development in NMDAR subunit expression in different brain regions may contribute to permanent changes within neural circuitry when chronic ethanol exposure is withdrawn. This has been shown in studies investigating chronic prenatal ethanol exposure where long-lasting decreases in NR2B subunit protein expression have been found in the forebrain and hippocampus of the guinea pig and rat (Hughes et al., 1998; Dettmer et al., 2003; Samudio-Ruiz et al., 2010), and reductions in NR2A and [3H] MK-801 binding density in rat (Honse et al., 2003a,b). Such changes may potentially contribute to fetal ethanol syndrome (FAS) and also to deficits observed following adolescent ethanol exposure. Although our results demonstrate that chronic ethanol exposure had no effect on the expression of the NR2B subunit in the hippocampus of adolescent rats, the NR1 and NR2A subunits were still affected after 2-wks of ethanol withdrawal. These long-lasting changes in the normal composition of NMDARs may result in long-lasting changes in the functional activity of NMDARs.
Functional physiological studies in animal models have shown that some of the consequences of chronic ethanol exposure can differ between adolescents and adults. For instance, long-term impairments in neurophysiological function, including reductions in the P3 component of the event-related potential (ERP) are observed after 10 days of ethanol exposure in adolescent (Slawecki et al., 2001), but not in adult rats (Ehlers and Chaplin, 1991). Suggesting that ethanol exposure during adolescence could result in more severe consequences relative to adult ethanol exposure. We have previously shown that administration of the NMDA antagonist MK-801 reduces the amplitude of the hippocampal P3 ERP in rats (Ehlers et al., 1992). These data suggest an important role of the glutamatergic NMDA system in the generation of the P3 ERP (Ehlers et al., 1992). More recently, we provided evidence of protracted changes in NMDA systems in cortical and hippocampal neurophysiological activity following adolescent ethanol exposure in rats (Criado et al., 2008a). In that study, we showed that MK-801 significantly reduced P3 ERP amplitude and increased P3 ERP latency in control, but not in adolescent rats exposed to ethanol. Whether the lack of NMDA-mediated regulation of P3 amplitude is due to glutamate neurotoxicity following adolescent ethanol exposure is presently unknown, however, these results provide evidence to suggest that adolescent ethanol exposure produces long-lasting effects on the efficacy of NMDA systems regulating cortical and hippocampal ERPs. Further studies will be necessary to determine whether NMDAR subunits composition is associated with to the enhanced vulnerability of the adolescent brain to alcohol dependence.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH) National Institute on Alcoholism and Alcohol Abuse (NIAAA) grants awarded to CLE, AA006059 and AA014339. Jerry P. Pian was supported by NIAAA training grant 5T32 AA007456. RM was supported by a Harry Weaver Neuroscience Scholar Award (JF 2125A1/1) from the National Multiple Sclerosis Society. The authors thank Greta Berg and Derek Wills for their assistance in data collection.
Abbreviations
- ANOVA
analysis of variance
- BALs
blood alcohol levels
- BCA assay
bicinchoninic acid assay
- C
degree Celsius
- CET
chronic ethanol treatment
- ERP
event-related potential
- FAS
fetal alcohol syndrome
- g
grams
- g/kg
grams per kilogram
- IgG
immunoglobulin G
- I.P
intraperitoneal
- mg/dl
miligrams per deciliter
- MK-801
Dizocilpine maleate
- mM
millimolar
- NIH
National Institutes of Health
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- NR1
N-methyl-D-aspartate receptor NR1 subunit
- NR2A
N-methyl-D-aspartate receptor NR2A subunit
- NR2B
N-methyl-D-aspartate receptor NR2B subunit
- PND
postnatal day
- WD
withdrawal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Chang LR, Liu JP, Zhang N, Wang YJ, Gao XL, Wu Y. Different expression of NR2B and PSD-95 in rat hippocampal subregions during postnatal development. Microsc Res Tech. 2009;72:517–524. doi: 10.1002/jemt.20708. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Rudolph JG, Chandler LJ. Glutamate and alcohol-induced neurotoxicity. In: Herman BH, Frankenheim J, Litten RZ, Sheridan PH, Weight FF, Zukin SR, editors. Glutamate and addiction. Totowa, N.J: Humana Press; 2002. pp. 357–374. [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of Dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008a;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008b;42:631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darstein MB, Landwehrmeyer GB, Feuerstein TJ. Changes in NMDA receptor subunit gene expression in the rat brain following withdrawal from forced long-term ethanol intake. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:206–213. doi: 10.1007/s002109900180. [DOI] [PubMed] [Google Scholar]
- Dettmer TS, Barnes A, Iqbal U, Bailey CD, Reynolds JN, Brien JF, Valenzuela CF. Chronic prenatal ethanol exposure alters ionotropic glutamate receptor subunit protein levels in the adult guinea pig cerebral cortex. Alcohol Clin Exp Res. 2003;27:677–681. doi: 10.1097/01.ALC.0000060521.32215.E9. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacology (Berl) 1991;104:67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Wall TL, Chaplin RI. Effects of dizocilpine (MK-801) and ethanol on the EEG and event-related potentials (ERPS in rats. Neuropharmacology. 1992;31:369–378. doi: 10.1016/0028-3908(92)90069-2. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Ferreira VM, Frausto S, Browning MD, Savage DD, Morato GS, Valenzuela CF. Ionotropic glutamate receptor subunit expression in the rat hippocampus: lack of an effect of a long-term ethanol exposure paradigm. Alcohol Clin Exp Res. 2001;25:1536–1541. [PubMed] [Google Scholar]
- Follesa P, Ticku MK. Chronic ethanol-mediated up-regulation of the N-methyl-D-aspartate receptor polypeptide subunits in mouse cortical neurons in culture. J Biol Chem. 1996;271:13297–13299. doi: 10.1074/jbc.271.23.13297. [DOI] [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res. 1991;547:129–134. [PubMed] [Google Scholar]
- Hardy PA, Chen W, Wilce PA. Chronic ethanol exposure and withdrawal influence NMDA receptor subunit and splice variant mRNA expression in the rat cerebral cortex. Brain Res. 1999;819:33–39. doi: 10.1016/s0006-8993(98)01340-7. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of N-methyl-D-aspartate receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Bhave SV, Kumar KN, Iorio KR, Snell LD, Tabakoff B, Michaelis EK. The 71 kDa glutamate-binding protein is increased in cerebellar granule cells after chronic ethanol treatment. Brain Res Mol Brain Res. 1996;39:167–176. doi: 10.1016/0169-328x(96)00021-6. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. NMDA receptors in alcoholism. Int Rev Neurobiol. 2003;56:35–82. doi: 10.1016/s0074-7742(03)56002-0. [DOI] [PubMed] [Google Scholar]
- Honse Y, Randall PK, Leslie SW. Prenatal ethanol exposure modifies [3H]MK-801 binding to NMDA receptors: spermidine and ifenprodil. Alcohol Clin Exp Res. 2003a;27:1993–2001. doi: 10.1097/01.ALC.0000099029.55026.C6. [DOI] [PubMed] [Google Scholar]
- Honse Y, Nixon KM, Browning MD, Leslie SW. Cell surface expression of NR1 splice variants and NR2 subunits is modified by prenatal ethanol exposure. Neuroscience. 2003b;122:689–698. doi: 10.1016/s0306-4522(03)00603-1. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Chen Y, Leslie FM, Metherate R. Postnatal development of NR2A and NR2B mRNA expression in rat auditory cortex and thalamus. J Assoc Res Otolaryngol. 2002;3:479–487. doi: 10.1007/s10162-002-2052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36:211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- Hughes PD, Kim YN, Randall PK, Leslie SW. Effect of prenatal ethanol exposure on the developmental profile of the NMDA receptor subunits in rat forebrain and hippocampus. Alcohol Clin Exp Res. 1998;22:1255–1261. [PubMed] [Google Scholar]
- Hughes PD, Wilson WR, Leslie SW. Effect of gestational ethanol exposure on the NMDA receptor complex in rat forebrain: from gene transcription to cell surface. Brain Res Dev Brain Res. 2001;129:135–145. doi: 10.1016/s0165-3806(01)00192-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- Jin DH, Jung YW, Ham SH, Ko BH, Moon IS. Developmental expression, subcellular localization, and tyrosine phosphorylation of NR2A and NR2B in the rat brain. Mol Cells. 1997;7:64–71. [PubMed] [Google Scholar]
- Johnston MV. Neurotransmitters and vulnerability of the developing brain. Brain Dev. 1995;17:301–306. doi: 10.1016/0387-7604(95)00079-q. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of NMDA receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Kloda A, Martinac B, Adams DJ. Polymodal regulation of NMDA receptor channels. Channels (Austin ) 2007;1:334–343. doi: 10.4161/chan.5044. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Kumari M, Ticku MK. Regulation of NMDA receptors by ethanol. Prog Drug Res. 2000;54:152–189. [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur J Neurosci. 2003;18:1197–1205. doi: 10.1046/j.1460-9568.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Kresge D, Supon J. Differential effects of aging on NMDA receptors in the intermediate versus the dorsal hippocampus. Neurobiol Aging. 2006;27:324–333. doi: 10.1016/j.neurobiolaging.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nagy J, Kolok S, Dezso P, Boros A, Szombathelyi Z. Differential alterations in the expression of NMDA receptor subunits following chronic ethanol treatment in primary cultures of rat cortical and hippocampal neurones. Neurochem Int. 2003;42:35–43. doi: 10.1016/s0197-0186(02)00062-1. [DOI] [PubMed] [Google Scholar]
- Narita M, Soma M, Mizoguchi H, Tseng LF, Suzuki T. Implications of the NR2B subunit-containing NMDA receptor localized in mouse limbic forebrain in ethanol dependence. Eur J Pharmacol. 2000;401:191–195. doi: 10.1016/s0014-2999(00)00428-3. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nixon K, Hughes PD, Amsel A, Leslie SW. NMDA receptor subunit expression after combined prenatal and postnatal exposure to ethanol. Alcohol Clin Exp Res. 2004;28:105–112. doi: 10.1097/01.ALC.0000106311.88523.7B. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484:241–247. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Differential alcohol modulation of GABA(A) and NMDA receptors. Neuroreport. 1999;10:97–101. doi: 10.1097/00001756-199901180-00019. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self- administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci STKE. 2005;2005:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Rudolph JG, Walker DW, Iimuro Y, Thurman RG, Crews FT. NMDA receptor binding in adult rat brain after several chronic ethanol treatment protocols. Alcohol Clin Exp Res. 1997;21:1508–1519. [PubMed] [Google Scholar]
- Samudio-Ruiz SL, Allan AM, Sheema S, Caldwell KK. Hippocampal N-methyl-D-aspartate receptor subunit expression profiles in a mouse model of prenatal alcohol exposure. Alcohol Clin Exp Res. 2010;34:342–353. doi: 10.1111/j.1530-0277.2009.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Serra M, Cossu A, Colombo G, Follesa P, Cuccheddu T, Concas A, Biggio G. Chronic ethanol intoxication induces differential effects on GABAA and NMDA receptor function in the rat brain. Alcohol Clin Exp Res. 1993;17:115–123. doi: 10.1111/j.1530-0277.1993.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Schummers J, Browning MD. Evidence for a role for GABA(A) and NMDA receptors in ethanol inhibition of long-term potentiation. Brain Res Mol Brain Res. 2001;94:9–14. doi: 10.1016/s0169-328x(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Repeated ethanol treatment in adolescent rats alters cortical NMDA receptor. Alcohol. 2006;39:51–58. doi: 10.1016/j.alcohol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley EP, Ehlers CL. Neurophysiologic consequences of neonatal ethanol exposure in the rat. Alcohol. 2004;34:187–196. doi: 10.1016/j.alcohol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Snell LD, Tabakoff B, Hoffman PL. Radioligand binding to the N-methyl-D-aspartate receptor/ionophore complex: alterations by ethanol in vitro and by chronic in vivo ethanol ingestion. Brain Res. 1993;602:91–98. doi: 10.1016/0006-8993(93)90246-j. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Swann JW, Pierson MG, Smith KL, Lee CL. Developmental neuroplasticity: roles in early life seizures and chronic epilepsy. Adv Neurol. 1999;79:203–216. [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Alcohol addiction: an enigma among us. Neuron. 1996;16:909–912. doi: 10.1016/s0896-6273(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Fitzgerald LW, Brose N, Gasic GP, Heinemann SF, Duman RS, Nestler EJ. Chronic ingestion of ethanol up-regulates NMDAR1 receptor subunit immunoreactivity in rat hippocampus. J Neurochem. 1994;62:1635–1638. doi: 10.1046/j.1471-4159.1994.62041635.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Distinct distributions of five N-methyl-D-aspartate receptor channel subunit mRNAs in the forebrain. J Comp Neurol. 1993;338:377–390. doi: 10.1002/cne.903380305. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989;50:11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]
- Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB. Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem. 1995;64:531–539. doi: 10.1046/j.1471-4159.1995.64020531.x. [DOI] [PubMed] [Google Scholar]