Abstract
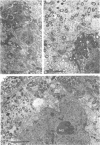
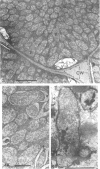
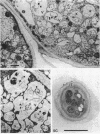
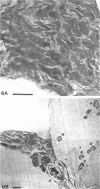
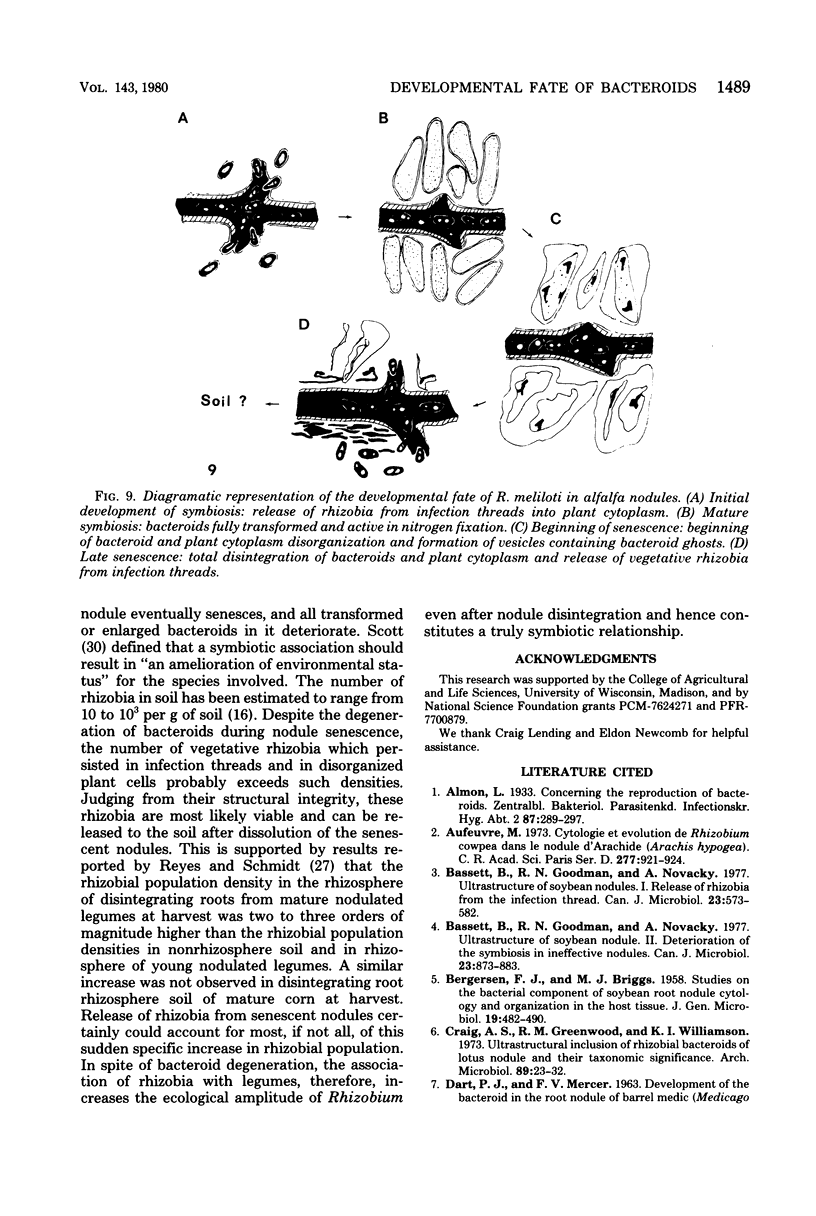
Nitrogen-fixing bacteroids are degraded during nodule senescence. This is in contrast to recent implications that viable bacteroids can be released into soil from legume nodules. Rhizobia originating from persistent infection threads in senescing nodule plant cells seem to be the source of viable cells required for perpetuation of the Rhizobium spp. population in the soil. Our conclusions were derived from electron microscopic examination of stages of development and senescence of alfalfa root nodules.
Full text
PDF

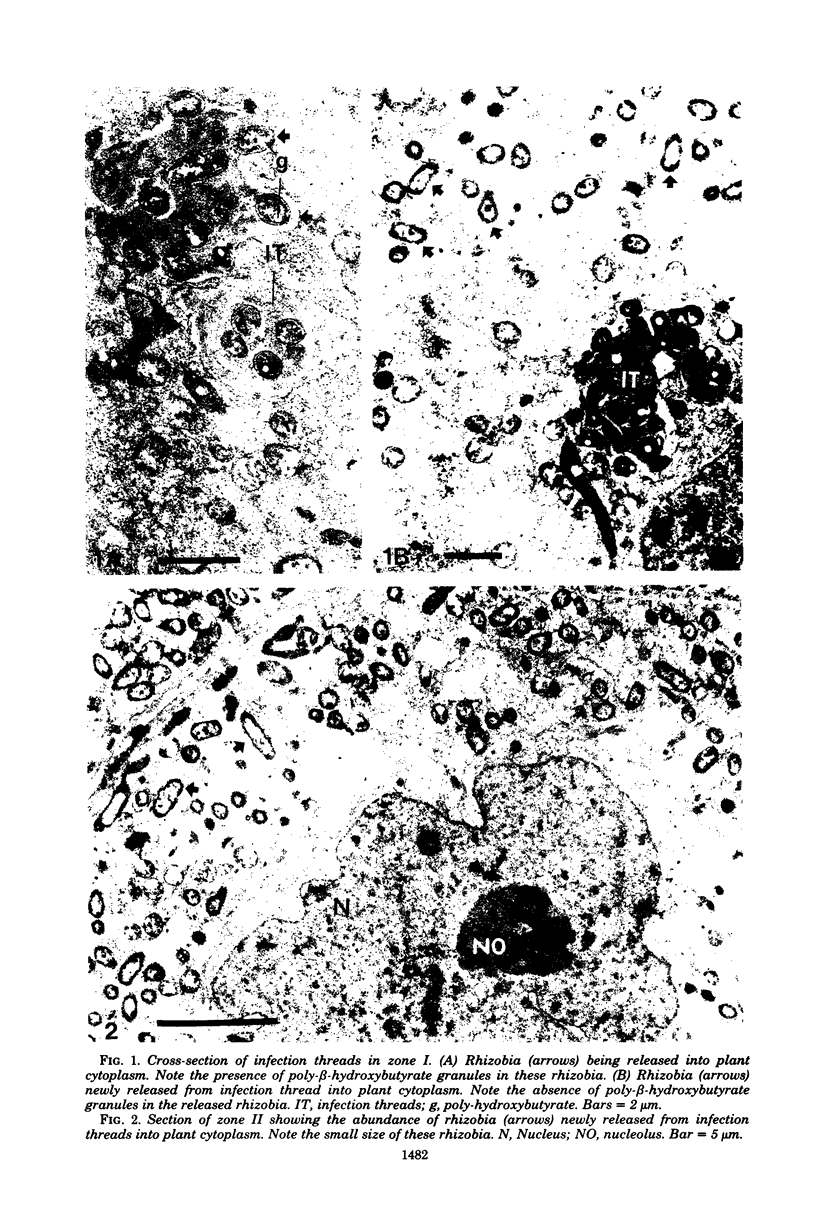

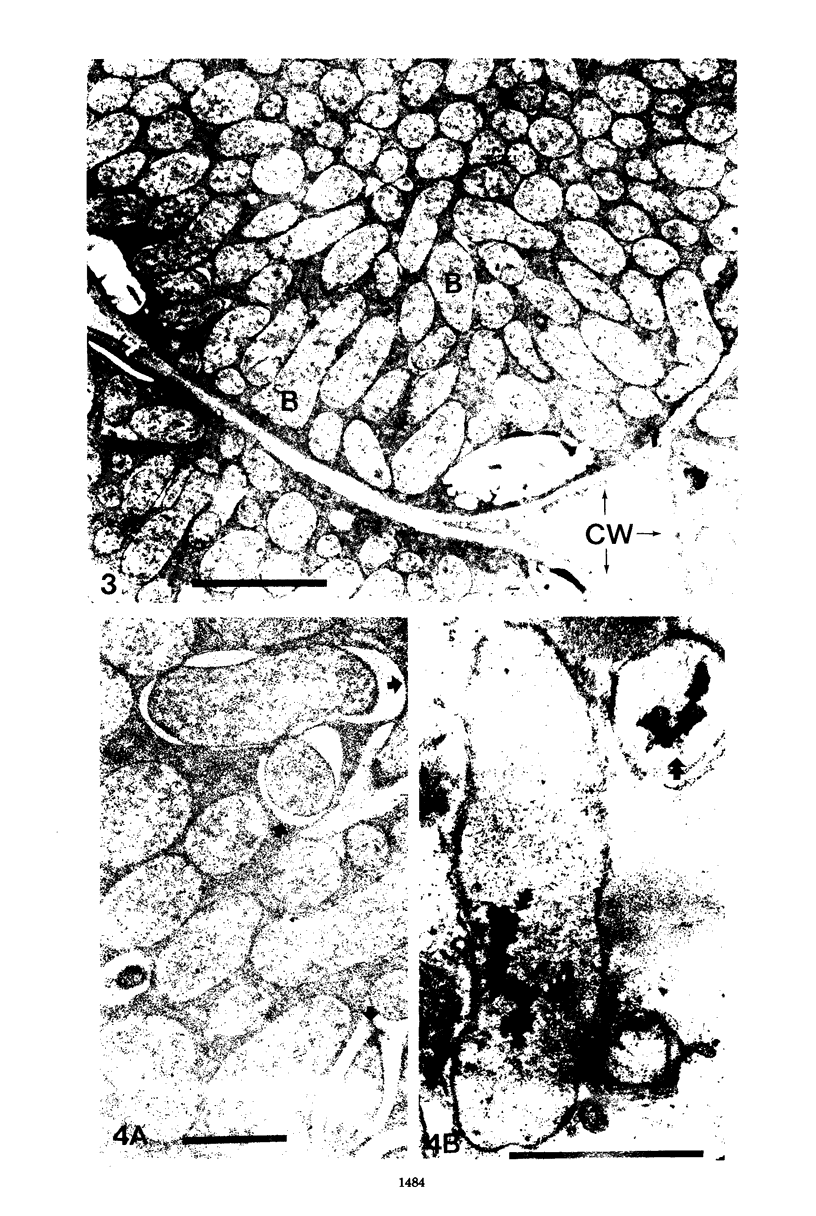
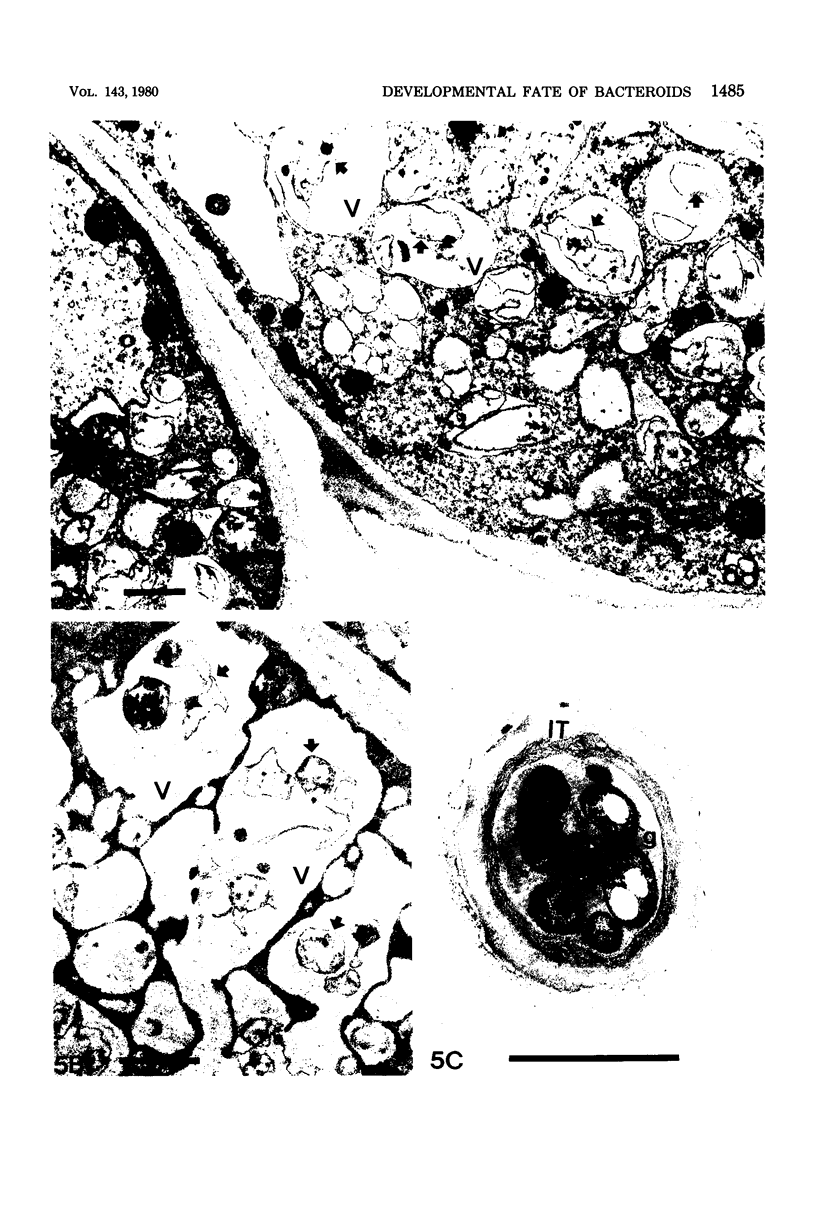

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGERSEN F. J., BRIGGS M. J. Studies on the bacterial component of soybean root nodules: cytology and organization in the host tissue. J Gen Microbiol. 1958 Dec;19(3):482–490. doi: 10.1099/00221287-19-3-482. [DOI] [PubMed] [Google Scholar]
- Bassett B., Goodman R. N., Novacky A. Ultrastructure of soybean nodules. I: release of rhizobia from the infection thread. Can J Microbiol. 1977 May;23(5):573–582. doi: 10.1139/m77-083. [DOI] [PubMed] [Google Scholar]
- Bassett B., Goodman R. N., Novacky A. Ultrastructure of soybean nodules. II: deterioration of the symbiosis in ineffective nodules. Can J Microbiol. 1977 Jul;23(7):873–883. doi: 10.1139/m77-129. [DOI] [PubMed] [Google Scholar]
- Bretherton K. N., Day A. J., Skinner S. L. Incorporation in vitro of 14C-labelled acetate and 32P-labelled phosphate into lipid in thoracic aortae from hypertensive and nomotensive rabbits. Atherosclerosis. 1976 Sep;24(3):431–440. doi: 10.1016/0021-9150(76)90135-0. [DOI] [PubMed] [Google Scholar]
- Dart P. J., Mercer F. V. Fine structure of bacteroids in root nodules of Vigna sinensis, Acacia longifolia, Viminaria juncea, and Lupinus angustifolius. J Bacteriol. 1966 Mar;91(3):1314–1319. doi: 10.1128/jb.91.3.1314-1319.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOTHERGILL P. G., CHILD J. H. COMPARATIVE STUDIES OF THE MINERAL NUTRITION OF THREE SPECIES OF PHYTOPHTHORA. J Gen Microbiol. 1964 Jul;36:49–66. doi: 10.1099/00221287-36-1-49. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Bergersen F. J. Electron microscopy of the infection and subsequent development of soybean nodule cells. J Bacteriol. 1966 Jul;92(1):204–213. doi: 10.1128/jb.92.1.204-213.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourret J. P., Fernandez-Arias H. Etude ultrastructurale et cytochimique de la différenciation des bactéroïdes de Rhizobium trifolii Dangeard dans les nodules de Trifolium repens L. Can J Microbiol. 1974 Aug;20(8):1169–1181. [PubMed] [Google Scholar]
- Gunning B. E. Lateral fusion of membranes in bacteroid-containing cells of leguminous root nodules. J Cell Sci. 1970 Jul;7(1):307–317. doi: 10.1242/jcs.7.1.307. [DOI] [PubMed] [Google Scholar]
- JORDAN D. C., GRINYER I., COULTER W. H. ELECTRON MICROSCOPY OF INFECTION THREADS AND BACTERIA IN YOUNG ROOT NODULES OF MEDICAGO SATIVA. J Bacteriol. 1963 Jul;86:125–137. doi: 10.1128/jb.86.1.125-137.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. C., Grinyer I. Electron microscopy of the bacteroids and root nodules of Lupinus luteus. Can J Microbiol. 1965 Aug;11(4):721–725. doi: 10.1139/m65-095. [DOI] [PubMed] [Google Scholar]
- MacKenzie C. R., Vail W. J., Jordan D. C. Ultrastructure of free-living and nitrogen-fixing forms of Rhizobium meliloti as revealed by freeze-etching. J Bacteriol. 1973 Jan;113(1):387–393. doi: 10.1128/jb.113.1.387-393.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Cowles J. R. Development of Bacteroids in Alfalfa (Medicago sativa) Nodules. Plant Physiol. 1978 Oct;62(4):526–530. doi: 10.1104/pp.62.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad D. N., De D. N. Ultrastructure of release of Rhizobium and formation of membrane envelope in root nodule. Microbios. 1971 Jul;4(13):13–20. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M., Shaw B. D. Changes in the Number, Viability, and Amino-acid-incorporating Activity of Rhizobium Bacteroids during Lupin Nodule Development. Plant Physiol. 1977 Apr;59(4):741–744. doi: 10.1104/pp.59.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchet G., Coulomb P. Mise en évidence et évolution du système phytolysosomal dans les cellules des différentes zones de nodules radiculaires de pois (Pisum sativum L. J Ultrastruct Res. 1973 Apr;43(1):36–57. [PubMed] [Google Scholar]
- Tsien H. C., Cain P. S., Schmidt E. L. Viability of Rhizobium bacteroids. Appl Environ Microbiol. 1977 Dec;34(6):854–856. doi: 10.1128/aem.34.6.854-856.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Heichel G. H., Barnes D. K., Bryan J. W., Johnson L. E. Nitrogen Fixation, Nodule Development, and Vegetative Regrowth of Alfalfa (Medicago sativa L.) following Harvest. Plant Physiol. 1979 Jul;64(1):1–8. doi: 10.1104/pp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]