Abstract
A diverse group of neurodegenerative diseases — including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and Alzheimer's disease (AD) among others, collectively referred to as tauopathies — are characterized by progressive, age-dependent intracellular formations of misfolded protein aggregates that play key roles in the initiation and progression of neuropathogenesis. Recent studies from our laboratory reveal that grape seed-derived polyphenolic extracts (GSPE) potently prevent tau fibrillization into neurotoxic aggregates and therapeutically promote the dissociation of preformed tau aggregates (Ho et al., 2009). Based on our extensive bioavailability, bioactivity and functional pre-clinical studies, combined with the safety of GSPE in laboratory animals and in humans, we initiated a series of studies exploring the role of GSPE (Meganatural-Az® GSPE) as a potential novel botanical drug for the treatment of certain forms of tauopathies including PSP, a neurodegenerative disorder involving the accumulation and deposition of misfolded tau proteins in the brain characterized, in part, by abnormal intracellular tau inclusions in specific anatomical areas involving astrocytes, oligodendrocytes and neurons (Takahashi et al., 2002). In this mini-review article, we discuss the biochemical characterization of GSPE in our laboratory and its potential preventative and therapeutic role in model systems of abnormal tau processing pertinent to PSP and related tauopathies.
Keywords: neurodegeneration, progressive supranuclear palsy, tau, grape seed polyphenolic extract, botanical medicine
Meganatural-Az® GSPE: a potential novel therapeutic agent in neurodegenerative disorders
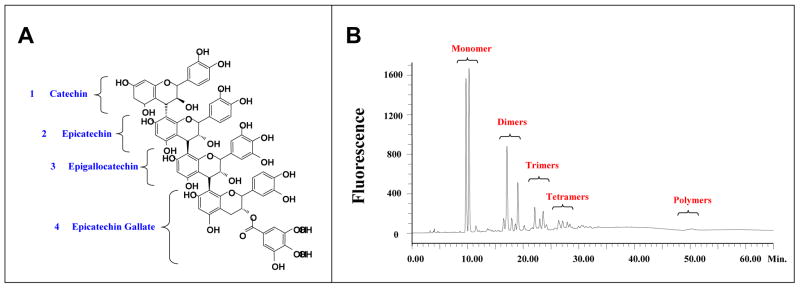
Meganatural-Az® GSPE is a highly purified, 100% water-soluble experimental polyphenolic extract from Vitis vinifera grape seeds (Polyphenolics, Inc.). The polyphenolic component in this GSPE is comprised entirely of catechin and epicatechin, derivatives of catechin and epicatechin (e.g., eipcatechin gallate in which epicatechin is modified with the addition of a gallic acid), and proanthocyanidins which are polymeric compounds derived from catechin, epicatechin and their derivatives (Fig. 1). Meganatural-Az® GSPE is generated from the seeds of grapes grown in California and harvested typically between August to October. The GSPE extraction procedure is based on standard procedures at Polyphenolics, Inc (Madera, CA). GSPE is stable in factory-sealed containers at room temperature for at least 3 years. HPLC analysis shows that Meganatural-Az® GSPE is comprised primarily of catechin and epicatechin in the form of monomeric, oligomeric and polymeric forms (see Fig.1). Typically, Meganatural-Az® GSPE contains about 8% monomers, 75% oligomers and about 17% polymers (Fig. 2A-C). Finally, no detectable resveratrol is found in Meganatural-Az® GSPE (data not shown).
Fig. 1. Component analysis of Meganatural-Az® GSPE.
(A) Molecular structure of a catechin and an epicatechin. (B) Molecular structure of a typical proanthocyanidin comprised of catechin / epicatechin base units and their derivatives. (C) Normal phase HPLC analysis of GSPE demonstrates the presence of monomeric and polymeric units. (Published in Wang et al., 2008)
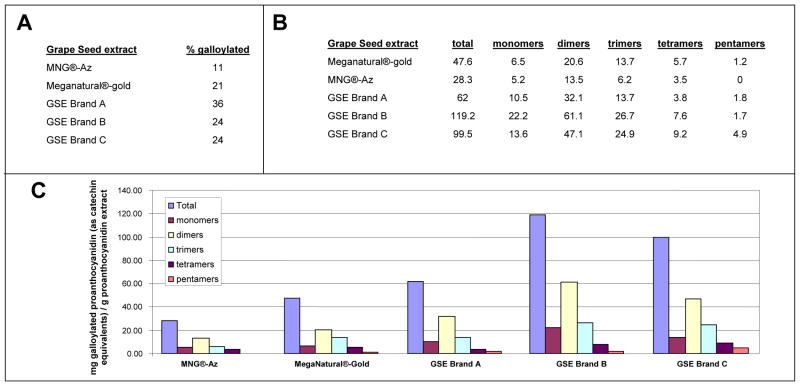
Fig. 2. Meganatural-Az® GSPE contains low contents of gallate side chain.
(A) The percentage of galloylated proanthocyanidins (out of total proanthocyanidins) in Meganatural-Az® GSPE compared to four other commercially available GSPE preparations: Meganatural®-Gold, GSPE Brand A, GSPE Brand B and GSPE Brand C (Brand A is “Activin”, a GSPE obtained from San Joaquin Valley Concentrates; Brand B is “Masquelier® OPC”, a GSPE from France; Brand C is a GSPE from Indena S.p.A., Italy). (B,C) The levels of galloylated proanthocyanidin in Meganatural-Az® GSPE and some other commercially available GSPEs. Abbreviation: MNG®-Az, Meganatural-Az® GSPE
Meganatural-Az® GSPE has unique features which allow it to be readily absorbed through the intestinal mucosa due to removal of the gallate moiety from the constituent polyphenols (Siva et al., 2006). In the manufacturing process for Meganatural-Az®, the crude polyphenolic extract is subjected to mixed culture yeast fermentation to hydrolyze the gallic acid from gallated monomers and proanthocyanidin oligomers. The extract is further processed to a powder containing greater than 90% polyphenols by weight, and greater than 3% gallic acid (Patent # US65444581). The resulting Meganatural-Az® GSPE is characterized by having less than 12% of galloylated proanthocyanidins by weight of the total proanthocyanidins (Fig. 2A). The low contents of galloylated proanthocyanidins is a key distinction between Meganatural-Az® GSPE and other GPSE preparations that are currently available commercially (Fig. 3A-C). The consistency among Meganatural-Az® GSPE lots is very high based, on quality control assessments by Mass Spectrometric chemical analysis (data not shown).
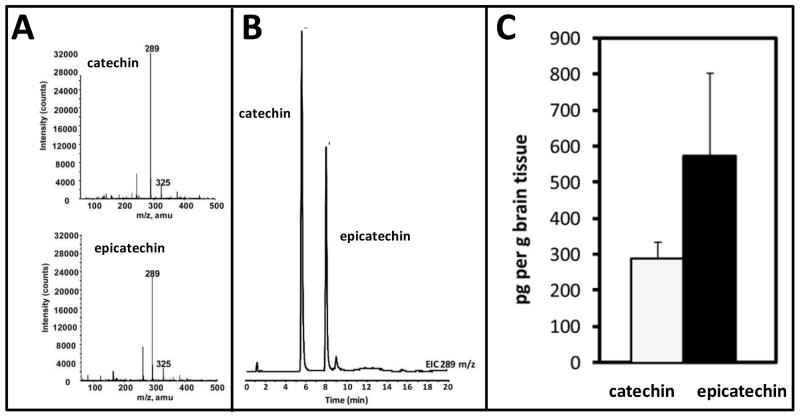
Fig. 3. Identification of catechin and epicatechin in the brain following oral administration of Meganatural-Az® GSPE.
In this study rats were treated repeatedly with GSPE (10 mg/kg BW per day, p.o.) for 120 days. Thereafter, brain specimens were harvested. Polyphenolic contents were extracted from brain specimens followed by quantitative assessments of catechin and epicatechin by LC-MS. (A) Extracted ion chromatograms at 289 m/z for catechin and epicatechin. (B) Collected in-line MS spectrum of catechin and epicatechin. (C) Concentration (pg/g) of catechin and epicatechin in brain tissue. Data represents mean ± SEM n=8 rats per group. Please refer to Ferruzzi et al., 2009 for details on experimental methodologies. (Adapted from Ferruzzi et al., 2009)
Meganatural-Az® GSPE safety data
Meganatural-Az® GSPE is highly tolerable and safe when examined in experimental animal models. For example, we found that chronic 5-month treatment of Tg2576 mice, which model AD β-amyloid (Aβ) neuropathology, with 200 mg/kg/day of Meganatural-Az® GSPE was highly safe and characterized by no detectable sign of adverse events (Wang et al., 2008; Bentivegna and Whitney, 2002). Translating Meganatural-Az® GSPE treatments from animal models to human dosage, we used the UDSA FDA-recommended formulation for converting equivalent drug dosage between species (U.S. Food and Drug Administration: http://www.fda.gov/cber/gdlns/dose.htm) and calculated that a dosage of 200 mg/kg/day in mice is equivalent to a human dosage of 1000mg/day. Sivaprakasapillaia and colleagues (2009) recently found that treatment with Meganatural-Az® GSPE at 300mg/day for 8 weeks is highly safe and tolerable in a clinical study exploring the role of Meganatural-Az® GSPE in human subjects with pre-hypertensive conditions meeting the JNC7 criteria (systolic blood pressures between 120 and 139 mmHg and diastolic blood pressures between 80 and 89 mmHg) (Sivaprakasapillaia et al., 2009). This study found that treatment with 300 mg/day of Meganatural-Az® GSPE significantly reduced both systolic and diastolic blood pressure by an average of 8 mmHg and 5 mmHg, respectively; no adverse events were observed in response to the Meganatural-Az® GSPE treatment (Sivaprakasapillaia et al., 2009). Collectively, this evidence suggests that Meganatural-Az® GSPE is likely to be highly safe and tolerable in preclinical and translational human applications. Based on observations from the pre-hypertension study that treatment with Meganatural-Az® GSPE at 300 mg/day led to a moderate reduction in systolic and diastolic blood pressure by an average of 5-8 mmHg (Sivaprakasapillaia et al., 2009), we do not anticipate Meganatural-Az® GSPE, when given in doses ranging from 300 mg/day to 1000 mg/day, to lower blood pressure below acceptable levels (i.e., systolic pressure 90 mmHg and diastolic pressure 60 mmHg) in normotensive subjects. Consistent with our expectation, Dr. Tissa C. Kappagoda has anecdotal evidence from three normotensive cases that Meganatural-Az® GSPE, when dosed at 300 mg/day, led to only very small reductions (<2 mmHg) in systolic and diastolic blood pressure (T. Kappagoda, unpublished observation). However, it is critical to conduct detailed safety and human efficacy studies in PSP patients. Tolerability and dose escalation studies in PSP subjects are presently underway in our Institution, and will provide fundamental information for the characterization of appropriate doses of Meganatural-Az® GSPE in the treatment of PSP.
Bioavailabilty of GSPE polyphenolic components in the brain
In ongoing studies in our laboratories, we detected monomeric GSPE components (catechin, epicatechin and gallic acid) in the blood following a single acute per orem dosage of the GSPE (150 mg/kg BW) in rats, but very little of the GSPE multimeric components (Ferruzzi et al., 2009). No GSPE polyphenolic components were detected in the brain following acute GSPE administration (Ferruzzi et al., 2009). In contrast, repeated GSPE exposure that simulates long-term dietary GSPE supplementation in humans led to significant accumulations of GSPE polyphenolic components (e.g., 290.7+45.9 pg catechin and 576.7+227.2 pg epicatechin per g brain tissues) in the brain (Fig. 3A-C). Multimeric GSPE components were not detected in the brain following repeated GSPE administration (data not shown). Similar to our observation in rats, we detected monomeric GSPE polyphenolic components, but not multimeric GSPE components, in human blood following repeated exposure of Meganatural-Az® GSPE (Pasinetti, unpublished observation).
GSPE as a disease-modifying agent in neurodegenerative disorders characterized by misfolded proteins in the brain: Implications for PSP
Tau plays a regulatory role in microtubule assembly and stability in neuronal and glial cells (see below). The expression of tau is developmentally regulated. The normal adult brain contains six tau isoforms, with the longest isoform containing 441 amino acid residues. Tau isoforms are derived from alternate splicing of exons 2 and 3 in the N-terminus and exon 10 in the microtubule-binding domain, which may contain either three or four imperfect repeats (D'Sousa and Schellenberg, 2005). The relevance of disease-specific distribution, composition, and abnormal phosphorylation of tau paired helical filaments (PHFs) in AD and other neurodegenerative disorders, such as PSP, CBD, Fronto Temporal Dementia and Parkinsonism (FTDP-17), Argyrophilic-Grain-Disease (AgD) and Parkinson's disease (PD), etc., are not yet fully understood (Lee et al., 2001). Phosphorylation is not required for tau to assemble into PHF-like filaments (Buee et al., 2000; Drewes, 2004). Nonetheless, tau mutations associated with tauopathies are characterized by hyperphosphorylation and accelerated aggregation into filament formations in vitro (Goedert and Jakes, 2005). The molecular mechanisms leading to aggregation of tau remain unclear, and models of PHFs based on studies of native and assembled filaments are limited (Barghorn and Mandelkow, 2002; Berriman et al., 2003; Margittai and Langen, 2004).
PSP is one of the rare neurodegenerative disorders clinically characterized by supranuclear ocular palsy, pseudobulbar palsy, parkinsonism with axial dystonia and postural instability, and progressive subcortical dementia (Steele et al., 1964; Golbe et al., 1988; Goedert et al., 1998). The abnormal tau inclusions are composed of aggregated and highly phosphorylated tau proteins, a microtubule-associated protein playing a role in microtubule assembly and stability. Some of the neurodegenerative disorders designated as tauopathies such as PSP are linked to specific abnormalities in the tau gene affecting the splicing ratio of 3Rtau vs 4Rtau and binding of tau to microtubules (Murrell et al., 1999). Normal brain tau contains 6 isoforms, with the 1:1 ratio between isoforms expressing 3 repeats (3Rtau) and 4 repeats (4Rtau) in the microtubule binding domain (Ksiezak-Reding et al., 1995). The isoforms are generated by the alternative splicing of a single tau gene on chromosome 17.
While AD is characterized by both Aβ and tau neuropathology, other tauopathies, including PSP are characterized by the accumulation of intracellular tau inclusions in the absence of Aβ pathology. In selected brain regions of PSP, 4Rtau isoforms appear to predominate as reported at mRNA (Chambers et al., 1999) and protein (Litvan et al., 2000; Sergeant et al., 1999) levels. Tau gene mutations favoring the 4Rtau splicing, however, have been only rarely associated with familial PSP (Stanford et al., 2000). Instead, the tau A0 allele may represent a genetic risk factor for PSP (Conrad et al., 1997). Analysis of tau levels, phosphorylated epitopes, and tau isoform content clarified some of the morphological and biochemical differences among tauopathies (Feany et al., 1995; Feany et al., 1996, Hoffmann et al., 1997). Tau may therefore serve as a potential biomarker identifying PSP and other tauopathies.
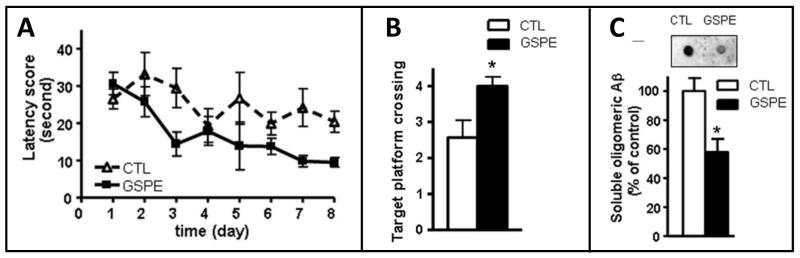
We recently demonstrated that GSPE exerts anti-oligomeric bioactivity and is capable of interfering not only with the abnormal assembly of tau, but also Aβ (Wang et al., 2008, Ono et al., 2008, Ho et., 2009). More importantly, preclinical feasibility studies in our laboratories demonstrate that GSPE may exert anti-oligomeric bioactivity in vivo and attenuate misfolded protein-mediated phenotypes coincidental with neuroprotection in vitro (Ono et al., 2008). For example, we demonstrated that dietary supplementation with the GSPE significantly attenuates the development of AD-type Aβ neuropathology and cognitive deterioration (Wang et al., 2008). In particular, in in vitro studies, we demonstrated that the GSPE exerts anti-oligomeric bioactivity and potently interferes with aggregation of Aβ1-40 and Aβ1-42 peptides into misfolded aggregates and destabilized preformed misfolded Aβ peptide aggregates (Wang et al., 2008; Ono et al., 2008). Importantly, we also demonstrated that GSPE is bioactive in vivo and that long-term dietary supplementation with GSPE in the Tg2576 mouse AD model (200 mg/kg BW/day equivalent to 1 g/day in humans) significantly reduced Aβ neuropatholoy (Fig. 4A-B) and mitigated Aβ-mediated cognitive deterioration (Fig. 4C). Collectively, our evidence suggests that the GSPE exerts anti-oligomeric bioactivity in vivo and mitigates misfolded Aβ-mediated neurological mechanism by interfering with either the generation and/or stability of misfolded Aβ aggregates.
Fig. 4. Dietary GSPE supplementation significantly mitigates Aβ-mediated neuropathology and cognitive deteriorating in the Tg2576 mouse model of AD.
The influence of GSPE on Aβ-related spatial memory in Tg2576 mice versus the untreated control Tg2576 mice were assessed by the Morris water maze test. (A) Hidden escape platform acquisition. Latency score represents time taken to escape to the platform from the water. (B) Probe trial to assess memory retention when tested in the absence of the hidden escape platform, 24h after the last acquisition trial. Platform crossing reflects the capability to correctly remember the location of the hidden platform during acquisition trials and is calculated as the number of crosses over the exact location of the hidden platform. In control studies, we confirmed GSPE exposure did no modulate the capability of the animals to swim in the water maze (not shown). (C) Assessments of soluble, extracellular misfolded, soluble high-molecular weight (HMW) Aβ aggregated oligmieric contents in the brain of Tg2576 mice using an antibody specific for soluble HMW oligomeric Aβ peptides in a dot blot analysis. (C, Inset) Representative dot blot analysis of misfolded HMW soluble Aβ contents. Values represent group mean ± SEM. n = 6–7 mice per group. (A-C) *p < 0.05 by two-tailed Student's t test analysis. CTL, Control non-treated Tg2576 mice. (Adapted from Wang et al., 2008)
Aside from modulating the generation and stability of misfolded Aβ peptides (Wang et al., 2008), our recent evidence, as discussed below, demonstrated that dietary GSPE supplementation may also mitigate misfolded tau-mediated neuropathologic and clinical phenotypes in animal models of tauopathies.
Meganatural-Az® GSPE as a potential novel therapeutic reagent for other forms of tauopathies
Besides PSP, a diverse group of neurodegenerative diseases are also characterized by progressive intracellular formations of misfolded tau aggregates (Dimakopoulos, 2005; Li et al., 1008; Outeiro et al., 2007). While these diseases are distinguished by important clinical manifestations, they share common features: these clinical disorders appear late in life and are accompanied by extensive neuronal loss and synaptic abnormalities and the presence of misfolded protein aggregates in the brain (Dimakopoulos, 2005). Misfolded – hyperphosphorylated – tau protein aggregates are also deposited in other tauopathies (e.g., FTDP-17, AgD and PD, etc.), although characteristics of misfolded tau deposits - such as the compositions of tau isoforms, hyperphosphorylation sites, ultrastructures of tau fibrils, as well as regional distributions of misfolded tau fibrils in the brain — differ among tau-mediated neurodegenerative disorders (Guillozet-Bongaart et al., 2007; Yoshida, 2006).
For example, in PD, misfolded α-synuclein proteins are deposited as Lewy bodies in substantia nigra neurons (Roodveldt et al., 2008). Patients with Huntington's disease show intranuclear deposits of polyglutaminated huntingtin proteins in the brain (Rose et al., 2008). A common feature of these disorders is that misfolded protein polymers are enriched in β-sheet conformations, whereas the native monomeric protein is mainly composed of α-helical and unordered structures (Dimakopoulos, 2005). A current hypothesis suggests that aggregations of misfolded proteins in diverse neurodegenerative disorders follow a nucleation model (Dimakopoulos, 2005).
Meganatural-Az® GSPE a potential novel botanical drug in the “prevention” of tauopathies: Implications for PSP
As discussed below, in a series of biochemistry studies, we found that the Meganatural-Az® GSPE may significantly promote abnormal oligomerization of Aβ peptides into soluble neurotoxic high molecular weight species that play major role in the onset and progression of AD (Wang et al., 2008; Ono et al., 2008). These studies were extremely exciting and demonstrated that GSPE may exert bioactivity in vivo and attenuate AD-type cognitive deterioration in a transgenic AD mouse model by significantly reducing the accumulation of Aβ aggregates in the brain (Wang et al., 2008). Based on this observation, we decided to explore whether Meganatural-Az® GSPE may also exert similar bioactivity to beneficially influence other neurodegenerative disorders involving accumulations of misfolded protein aggregates in the brain such as tau.
Tau proteins, particularly hyperphosphorylated tau, tend to aggregate in multimeric, soluble, oligomeric species; continual progressive aggregation leads to the deposition of insoluble tau into cellular PHFs. We used the spontaneous aggregation of a synthetic Ac-306VQIVYK311 tau peptide (Goux et al., 2004) as a model system to explore whether Meganatural-Az® might modulate tau protein aggregation. In this model system, aggregation of the tau peptide is reflected by intercalation of thioflavin S (ThS) into tau peptides that are aggregated into β-sheet conformers, and is monitored as fluorescence emission of the intercalated ThS (Goux et al., 2004). Consistent with published observations (Goux et al., 2004), we found that the synthetic Ac-306VQIVYK311 tau peptide readily forms into aggregates over time in the presence of salt, as reflected by increasing ThS fluorescence as a function of reaction time (Ho et al., 2009). However and most importantly, we previously reported that that the addition of increasing concentrations of GSPE significantly interfered with aggregations of the tau peptide in a dose-dependent manner. Collectively, the studies reported by Ho and colleagues (2009) tentatively suggested that Meganatural-Az® GSPE may significantly interfere with the initial oligomerization of the tau peptide (as schematized in Figure 5), and that the GSPE may inhibit tau peptide aggregation, in part, by interfering with the initial stages of tau peptide self-association.
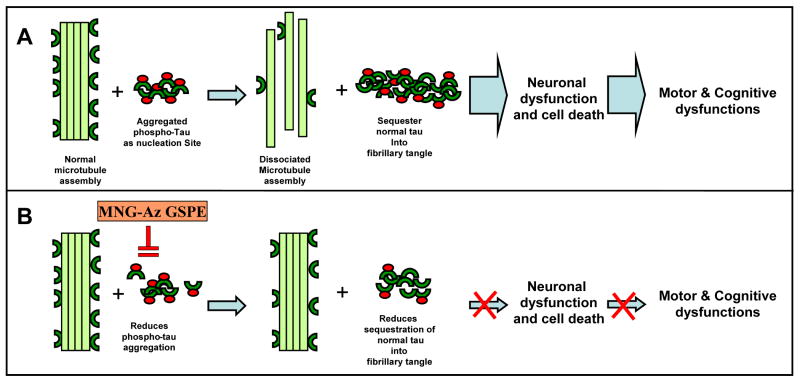
Fig. 5. Hypothetical mechanism by which Meganatural-Az® GSPE may mitigate tau-related neurodegeneration and resultant motor and cognitive dysfunction in PSP and other tauopathies.
(A) Aggregation of abnormally hyperphosphorylated tau in neurons and glial cells provides nucleation sites that recruit aggregation of normal, non-phosphorylated tau into intracellular deposits of neurofibrillary tangles. Microtubules are disabled by the loss of tau, leading to neuronal dysfunction, which ultimately culminates in neuronal cell death and cognitive impairment. (B) Meganatural-Az® may attenuate tau-mediated neurodegeneration and cognitive deterioration in PSP and other tauopathies by inhibiting aggregation of hyperphosphorylated tau and/or aggregation of normal tau into neurofibrillary tangles. Abbreviation: GSPE, grape seed polyphenol extract; MNG-Az GSPE, Meganatural-Az grape seed polyphenol extract. (Published in Ho et al., 2009)
An important consideration for therapeutic intervention in patients with existing tau neuropathology is whether the GSPE may beneficially modulate oligomeric tau fibrils, or PHFs, that are deposited in the brain. In follow-up biophysical chemical studies using tau PHFs isolated from PSP brain specimens, as well as brain specimens from other neurodegenerative disorders, we demonstrated that the GSPE is capable of interfering with the accumulation of misfolded tau aggregates by simultaneously interfering with their assembly and/or disrupting their ultra structures, possibly leading to their increased sensitivity to protease degradation. These studies discussed below have a profound implication in the development of GSPE as a novel agent not only for treating, but also for preventing PSP and other tauopathies.
Meganatural-Az® GSPE attenuates mutant-tau-mediated clinical phenotypes in phylogenetically diverse Drosophila and murine models
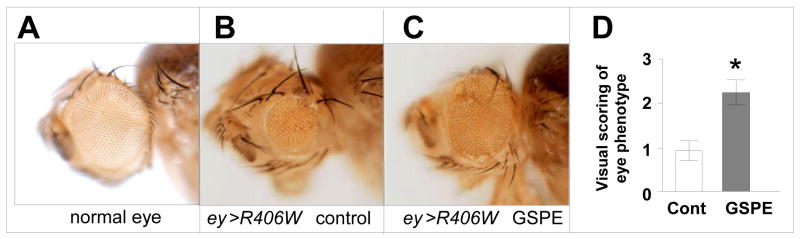
Based on the exciting evidence that GSPE may mechanistically attenuate tauopathy through mechanisms involving anti-oligomerization activities which may lead to the promotion of proteolytic degradation of PHF protofibrils (see below), we explored whether GSPE may exert tau-modifying bioactivity in a transgenic ey-gal4/SM6-TM6B Drosophila model of tauopathy. This fly model is engineered to selectively express the R406W mutant tau in developing eyes, resulting in abnormal eye development. Eyes from adult R406W mutant tau flies are characterized by reduced size and abnormal morphologies (Fig. 6B) compared to WT adult flies (Fig. 6A). We treated R406W mutant tau flies with GSPE, starting at the larval stage and continuing until maturity. We found that GSPE treatment significantly attenuated abnormal eye phenotypes in adult R406W mutant flies (Fig. 6C compared to Fig. 6B,). In a quantitative analysis of adult eye morphology, using a four-point scoring system where 0 = no eye and 4 = normal eye, we confirmed that GSPE treatment significantly improved eye phenotypes in adult R406W mutant tau flies (Fig. 6D) (Pasinetti, unpublished observation).
Fig. 6. Meganatural-Az® GSPE treatment suppressed abnormal eye phenotypes in R406W mutant tau flies.
Eggs from R406W mutant tau flies and WT flies were laid in and reared on Formula 4-24 instant fly medium supplemented with 2.8 μg/ml GSPE (“GSPE food”) or control food supplemented with an equivalent volume of water (GSPE solvent, vehicle control). GSPE treatment continued into adulthood. (A-C) Representative eye phenotypes in WT (A) and R406W mutant tau flies in the absence (B) or presence (C) of GSPE treatment. (D) Quantitative analysis of adult eye morphology, using a four-point scoring system where 0 = no eye and 4 = normal eye, in male and female flies across three independent trials. The number of flies scored per trial is indicated. Bar graphs represent mean ± SEM. (*P <0.05, comparing GSPE-treated vs. non-treated R406W mutant tau flies in individual trials).
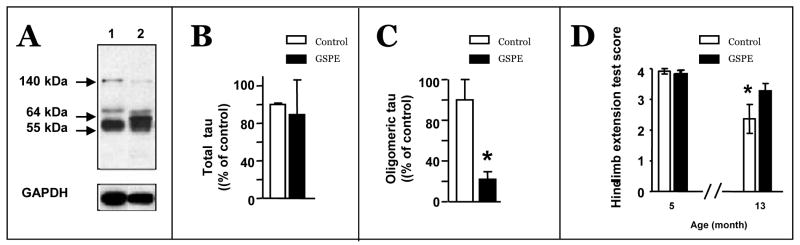
In parallel studies, we explored the preclinical efficacy of the GSPE to modulate tau-mediated neuropathological phenotypes in the transgenic JNPL3 mouse model of tauopathy. The JNPL3 mouse model is engineered to express the human familial P301L mutant tau that leads to age-related neurodegeneration, which is reflected by motor dysfunction (Lewis et al., 2000). In ongoing studies, we assessed the impacts of GSPE treatment on the development of tau neuropathology and motor impairment in JNPL3 mice. We treated JNPL3 mice with 150 mg/kg BW/day of the GSPE, starting at approximately seven months of age, prior to the initiation of mutant tau-mediated motor impairment that typically begins to develop by approximately 12 months of age. Consistent with our evidence from the Tg2576 AD mouse model (Wang et al., 2008), GSPE treatment did not result in detectable adverse effects, including changes in body weight or water consumption (not shown). We found that GSPE treatment significantly reduced the accumulation of oligomeric tau species in the brain (Fig. 7 A,B,C), coincidental with attenuating the severity of motor impairment which normally occurs with aging in this mouse model (Fig. 7D).
Fig. 7. GSPE treatment attenuated clinical tauopathy phenotypes JNPL3 mutant mice.
(A-C) GSPE treatment was accomplished by incorporating the GSPE (0.9 g/L) into the drinking water, corresponding to 150 mg/kg BW/day. Western blot assessments of tau protein species in the brain using PHF1 antibody. (A) Representative analysis of brain specimens from non-treated control (Lane 1) and GSPE-treated (Lane 2) JNPL3 mice. (B, C) Quantitative analysis of total tau (55 kDa) (B) and oligomeric tau (140 kDa) (C) species. (D) Assessments of motor functions impairment using a Hind limb extension assay for evaluating motor function impairment in JNPL3 mice. A four-point rating system reflecting motor functions by evaluating the animals' natural tendency to extend their hind limbs laterally when they are hung inverted by their tails. 4 = normal function, 3 = mild impairment, 2 = moderate impairment, 1 = severe impairment. Hind limb extension test shows that GSPE treatment significantly reduced motor impairments in GSPE-treated mice. (B, C, D) Bar graphs: Means ± SEM; * p<0.05, student t-test.
Collectively, our observations from distinct drosophila and mouse tauopathy models suggest, for the first time, that GSPE may exert anti-oligomerization bioactivity in vivo and beneficially attenuate mutant-tau-mediated pathological responses.
Meganatural-Az® GSPE induces ultrastructure changes in native, isolated PHFs which may promote clearance of the PHFs by proteolysis
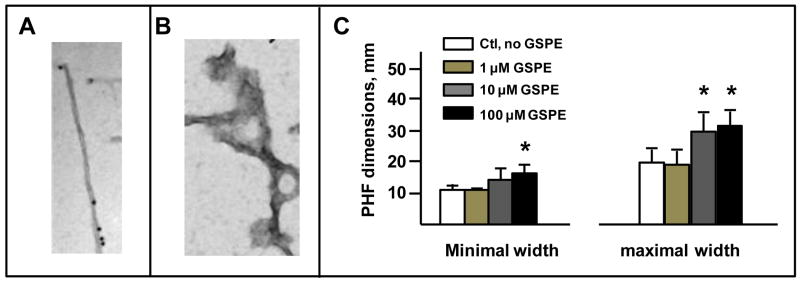
Based on in vitro evidence from our lab that the GSPE may interfere with the stability of tau aggregates (Ho et al., 2009), we continued to explore the impact of the GSPE on the ultra structure of native tau fibrils isolated from the brains of PSP, but also CBD, and AD cases. For example, we recently found that incubation of PHFs isolated from AD brains with GSPE in vitro results in a significant disruption of PHF ultra-structure. In particular, we found that GSPE can elicit a stable PHF change in dimension. For example, we found that incubation of PHFs with increasing concentrations of GSPE (1-100 μM) elicited a dose-dependent alteration in maximal or minimal width of PHF dimension (Fig. 8 A-C). This evidence is of extreme interest and suggests that GSPE may disrupt the normally tight conformations of PHFs.
Fig. 8. Treatment with GSPE widens PHFs from AD brain.
PHFs were isolated from patients with AD using our standard procedure (Ksiezak-Reding and Wall, 1994). In brief, autopsy brain specimens were homogenized in an aqueous buffer solution and PHFs were isolated using step-wise centrifugation that lead to sedimentation of highly purified PHFs for our studies. Details on the isolation of PHFs are available in Ksiezak-Reding and Wall (1994). Isolated PHFs were either treated by incubation with GSPE or vehicle for 1 h, followed by electron microscopy (EM) assessments using a Hitachi H7000 instrument (Japan) operated at 75 kV. Dimensions and twisting intervals of PHFs were measured in random images of electron micrographs using Scale Lupe 10X (Peak, Japan). Filaments from at least 5-10 micrographs were used for measurements. Results were corrected for magnification and expressed in nm. (A,B) Representative electron microscopy image of an native PHF (A) and a PHF following GSPE treatment. (C) Dose response changes in PHF minimal width (left panel) and maximal width (right panel). Bar graphs represent mean ± SEM; * p<0.001, Student t-test comparing control, non-treated vs. GSPE treated PHFs.
We also found that co-incubation of PHF filaments isolated from CBD and PSP as well as AD brain specimens with Meganatural-Az® GSPE (100 μM, 1 hr) resulted in a reduction of the quantity of PHF filaments (Fig. 9), relative to untreated PHF. This evidence is of high interest and tentatively suggests that GSPE may promote the unwinding/de-aggregation of the PHFs, which may lead to the sensitivity of PHFs to proteolytic digestion, as schematized in the Scheme I (see below), which may ultimately promote clearance of PHFs.
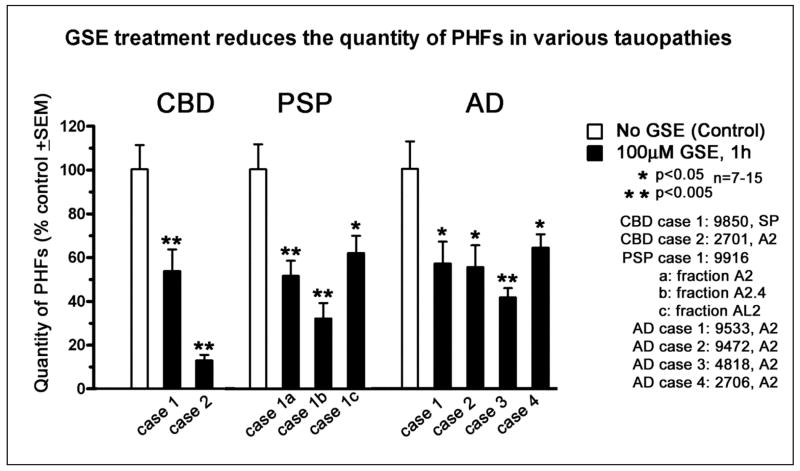
Fig. 9. Meganatural-Az® GSPE treatment attenuates the PHFs in CBD, PSP and AD brain specimens in vitro.
In this study, various fractions of PHFs were isolated from patients with CBD, PSP and AD and subjected to 100 μM GSE treatments for 1h. The isolation and assessment of PHFs are essentially as described for Fig. 8. Quantity of PHFs was determined as total length of filaments in 7 to 15 electron micrographs. In all tauopathies, GSE reduced the total length of filaments in 7 to 15 electron micrographs. In all tauopathies, GSE reduced the quantity of PHF's approximately by 50%
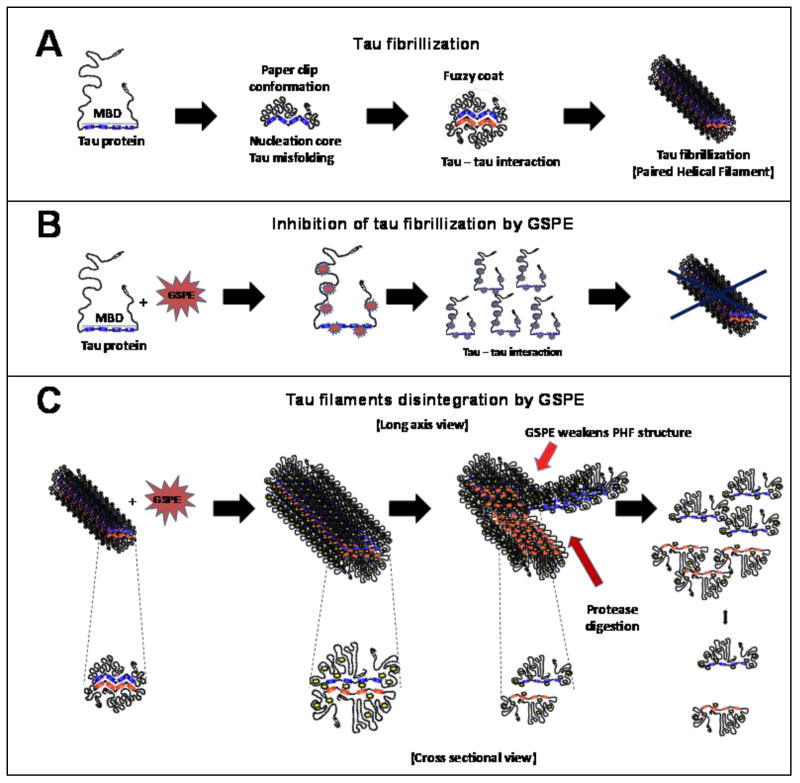
Scheme I. Scheme of the working hypothesis: Effect of Meganatural-Az® GSPE in tau fibrillization and filament disintergration.
(A) Model of tau fibrillization process in which tau protein due to several factors such as mutations or postranslational modifications (i.e. phosphorylation, glycation) becomes aberrantly misfolded and is prone to form fibrillar polymers. (B) Model of a mechanism by which GSPE inhibits tau filament formation. Interaction of GSPE with tau interferes with tau-to-tau interactions, therby inhibiting the formation of tau filaments. (C) Long axis and cross sectional view of tau filament disintegration provoked by addition of GSPE. GSPE alters aberrantly misfolded state of tau in polymers leading to ultrastructural changes that weakens the normally tight fibrillar structure that may also promote protease digestion. Abbrev. MBD, microtubule binding domain
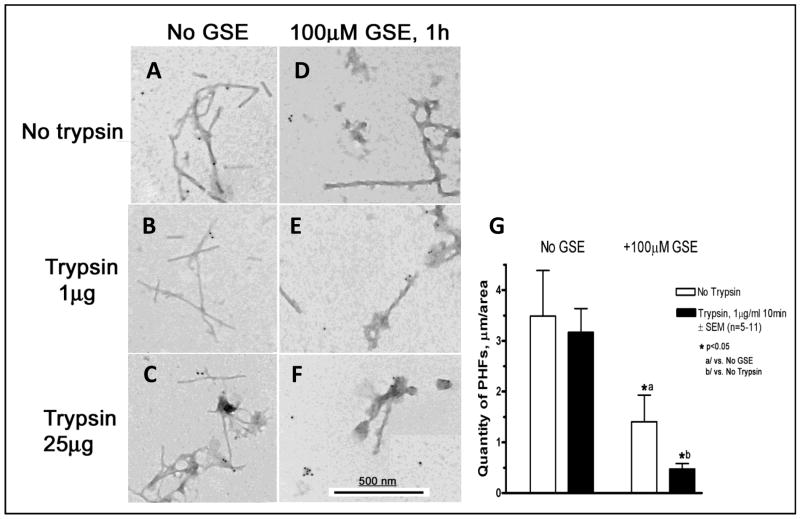
We hypothesize that the unfolding of PHFs in response to Meganatural-Az® GSPE treatment might promote accessibility of tau fibrils to cellular proteases and thereby facilitate clearance of tau fibrils by proteolytic degradation. Consistent with our hypothesis, we found that the treatment of isolated native tau filaments with GSPE (100 μM) promoted trypsin digestion of the tau filaments (Fig. 10A-F). The quantification of GSPE-mediated promotion of trypsin-dependent PHF disintegration is shown in Fig.10G. Trypsin is an endopeptidase that selectively digests peptide bonds adjacent to lysine and arginine. Our observation that GSPE treatment promotes trypsin digestion of PHFs suggests that GSPE may induce unfolding of the typically tight fibril structure, thereby promoting accessibility of lysine and arginine residues for proteolytic digestion. Thus, our biochemical evidence suggests that Meganatural-Az® GSPE might reduce tau-mediated pathology, in part, by interfering with the stability of abnormal tau aggregates and/or by promoting cellular protease-dependent disintegration. Ongoing studies in our laboratory will clarify whether GSPE may similarly destabilize the ultrastructure of native PHFs isolated from brains of individual with other tauopathies. Outcomes of these studies will provide insight into the mechanism by which dietary supplementation with the GSPE may exert beneficial activities in PSP and other tauopathies.
Fig. 10. GSPE treatment promotes the sensitivity of PSP PHFs to proteolytic digestion.
In this study PHFs from PSP cases were pre-treated with 100μM GSE for 1h and then subjected to protease digestion by adding trypsin to PHF samples at 0.01 to 10μg/ml final concentration for 10 min at room temperature. Reaction was terminated by adding phenylmethylsulfonyl fluoride at 1 mM final concentration. PHFs were then immunogold labeled with the AH-1 tau antibody and secondary antibodies conjugated to 10-nm colloidal gold particles. Details on immunogold labeling are essentially as described by Takahashi et al. (2002). Immunogold labeled PHFs are examined by electron microscopy. Quantity of PHFs was determined as total length of filaments in 5 to 11 electron micrographs. Without GSE, trypsin had no effect on the quantity of PHFs. In the presence of GSPE, which alone reduced the quantity of PHFs to 40%, trypsin additionally lowered the quantity of PHFs to less than 14%.
As depicted in Scheme I, our new observation suggests that the GSPE may mitigate tau-mediated phenotypes by interfering with the generation and/or stability of neurotoxic tau protofibrils, suggesting the value of this GSPE in treating tau-mediated neuropathological mechanisms. Based on this and on the roles of calpain and ubiquitin proteosomal system (UPS) in proteolytic degradation of tau in the cell, ongoing studies in the laboratory are exploring whether the GSPE-mediated unfolding of tau fibrils could also increase proteolysis of PHFs, for example by calpain and other proteases.
Meganatural-Az® GSPE as potential novel treatment in PSP
Collectively, our studies tentatively suggest that Meganatural-Az® GSPE exerts anti-oligomerization bioactivity and may mitigate misfolded protein-mediated neuropathologic mechanisms by interfering with the assembly and/or stability of misfolded proteins in the brain. Our novel observation provides, for the first time, the rationale for developing this GSPE for preventing and/or treating a variety of neurodegenerative disorders involving misfolded protein-mediated mechanisms such as in PSP. Based on this and evidence demonstrating the high tolerability and safety of long-term application of the Meganatural-Az® GSPE in both laboratory animals (Wang et al., 2008) and in humans (Sivaprakasapillaia et al., 2009), and from anedoctal evidence showing delayed progression of disease stages in PSP patients five months after treatment at 1200 mg/day in compassionate use phase studies (Pasinetti, personal observation), our studies strongly support the possibility of developing Meganatural-Az® GSPE as a potential novel treatment in PSP and possibly other forms of tauopathy. As schematized in Scheme I, the upper panel shows that tau may undergo through and hypothetical fibrilliarization process (e.g., through formation of Fuzzy coat formation), due to several factors (e.g., mutations, post-translational modifications glycation etc.), creating a permissive environment, ultimately resulting in an environment prone to the formation of fibrillar polymers (e.g., formation of PHF). As schematized in the middle panel (B), we hypothesize that tau oligomerization prevention may be achieved through direct physical intercalation of GSPE with tau, which may ultimately prevent PHF formation by interfering with tau-tau interactions. Finally, in panel C, we hypothesize that GSPE may lead therapeutically to the deconstruction of PHFs (represented longitudinally), possibly through mechanisms involving the weakening of PHF structures by increasing sensitivity to proteolytic digestion, ultimately leading to ultrastructural deconstruction of PHF into simpler clearance of tau units, depicted cross-sectionally.
Naturally occurring polyphenols (Beecher et al 2003) found in GSPE share structural similarity to others tau assembly inhibitors tested in vitro and in cell cultures, such as anthracyclines (Pickhardt et al 2005), N-phenylamines (Khlistunova at al 2006) and phenylthiazolyl-hydrazides (Pickhardt at al 2007). Additionally, recent studies identified several polyphenolic and non polyphenolic inhibitors of fibrillogenesis in vitro using a variety of tau assembly assays (Hasegawa, 2006; Taniguchi et al., 2006; Bulic et al 2010; Chirita et al 2004; Crowe et al 2009; Wischik et al 1996). Similar to GSPE, in vitro experimental evidence revealed some of these compounds, such as anthraquinones and phenylthiazolyl-hydrazides (Pickhardt et al 2005; Pickhardt at al 2007), were able to block fibrillization of tau protein composed of all four microtubule binding repeats of tau or full-length 3-repeats tau protein. Also similar to GSPE (Ho et al., 2009), some of these compounds, such as anthraquinones are capable of promoting disassembly of pre-formed tau fibrils. Unlike GSPE, there is, however, no information regarding the bioavailability of these compounds in the brain, or preclinical evidence supporting the efficacy of these compounds to modulate tau-related neuropathologic or clinical phenotypes in animal models. This information will be critical for evaluating whether, aside from GSPE, bioactive compounds capable of interfering with the generation and stability of tau oligomers should be developed as therapeutics for PSP and other tauopathies.
In conclusion, our studies tentatively support the hypothesis, for the first time, that certain forms of bioavailable polyphenolic compounds in Meganatural-Az® GSPE may promote tau anti-oligomerization activities in the brain, ultimately delaying clinical PSP onset, and possibly therapeutically attenuating the clinical progression of PSP and other tauopathies.
Acknowledgments
Supported by NIH P01AT004511-01 to GMP.
Footnotes
Disclosure: Drs. Pasinetti, Wang and Ho are named inventors of a pending patent application filed by Mount Sinai School of Medicine (MSSM) for grape seed polyphenolic extract. In the event that the pending or issued patent is licensed, Drs. Pasinetti, Wang and Ho would be entitled to a share of any proceeds MSSM receives from the license.
References
- Barghorn S, Mandelkow E. Toward a unified scheme for the aggregation of tau into Alzheimer paired helical filaments. Biochemistry. 2002;41:14885–96. doi: 10.1021/bi026469j. [DOI] [PubMed] [Google Scholar]
- Beecher GR. Overview of dietary flavonoids, nomenclature, occurrence and intake. J Nutr. 2003;133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- Berriman J, et al. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc Natl Acad Sci USA. 2003;100:9034–8. doi: 10.1073/pnas.1530287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentivegna SS, Whitney KM. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem Toxicol. 2002;40(12):1731–43. doi: 10.1016/s0278-6915(02)00155-2. [DOI] [PubMed] [Google Scholar]
- Buee L, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Bulic B, et al. Tau protein and tau aggregation inhibitors. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.01.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chambers LB, et al. Overexpression of four-repeat tau mRNA isoforms in progressive supranuclear palsy but not in Alzheimer's disease. Ann Neurol. 1999;46:325–332. doi: 10.1002/1531-8249(199909)46:3<325::aid-ana8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chirita C, et al. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- Conrad C, et al. Genetic evidence for the involvement of tau in progressive supranuclear palsy. Ann Neurol. 1997;41:277–281. doi: 10.1002/ana.410410222. [DOI] [PubMed] [Google Scholar]
- Crowe A, et al. The identification of aminothienopyridazine inhibitors of tau assembly by quantitative high-throughput screening. Biochemistry. 2009;48:7732–7745. doi: 10.1021/bi9006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza L, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta. 2005;1739:140–115. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Dimakopoulos A. Protein aggregation in Alzheimer's disease and other neuropathological disorders. Curr Alz Res. 2005;2:19–28. doi: 10.2174/1567205052772795. [DOI] [PubMed] [Google Scholar]
- Feany MB, et al. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40:139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- Feany MB, et al. Epitope expression and hyperphosphorylaton of tau protein in corticobasal degeneration: differentiation form progressive supranuclear palsy. Acta Neuropathol. 1995;90:37–43. doi: 10.1007/BF00294457. [DOI] [PubMed] [Google Scholar]
- Ferruzzi MG, et al. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is improved by repeated dosing in rats: implications for treatment in Alzheimer's disease. J Alzheimer's Dis. 2009;18(1):113–124. doi: 10.3233/JAD-2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta. 2005;1739(2-3):240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Goedert M, et al. Filamentous nerve cell inclusions in neurodegenerative diseases. Curr Opin Neurobiol. 1998;8:619–632. doi: 10.1016/s0959-4388(98)80090-1. [DOI] [PubMed] [Google Scholar]
- Golbe LI, et al. Prevalence and natural history of progressive supranuclear palsy. Neurology. 1988;38:1031–1034. doi: 10.1212/wnl.38.7.1031. [DOI] [PubMed] [Google Scholar]
- Goux WJ, et al. The formation of straight and twisted filaments from short tau peptides. J Biol Chem. 2004;279(26):26868–26875. doi: 10.1074/jbc.M402379200. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, et al. Phosphorylation and cleavage of tau in non-AD tauopathies. Acta Neuropathol. 2007;113:513–520. doi: 10.1007/s00401-007-0209-6. [DOI] [PubMed] [Google Scholar]
- Hasegawa M. Biochemistry and molecular biology of tauopathies. Neuropathology. 2006;26:484–490. doi: 10.1111/j.1440-1789.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- Ho L, et al. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J Alzheimer's Dis. 2009;16:433–9. doi: 10.3233/JAD-2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann R, et al. Unique Alzheimer's disease paired helical filament specific epitopes involved double phosphorylation at specific sites. Biochemistry. 1997;36:8114–8124. doi: 10.1021/bi970380+. [DOI] [PubMed] [Google Scholar]
- Khlistunova I. Inducible expression of tau repeat domain in cell models of tauopathy—aggregation is toxic to cells but can be reversed by inhibitor drugs. J Biol Chem. 2006;281:1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, et al. Differential expression of exon 10 and 11 in normal tau and tau associated with paired helical filaments. J Neurosci Res. 1995;41:582–593. doi: 10.1002/jnr.490410504. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Wall JS. Mass and physical dimensions differ in two distinct populations of paired helical filaments. Neurobiol Aging. 1994;15:11–19. doi: 10.1016/0197-4580(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Lee VM, et al. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lewis J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;23(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Litvan I, et al. Research goals in progressive supranuclear palsy. Mov Disord. 2000;15:446–458. doi: 10.1002/1531-8257(200005)15:3<446::AID-MDS1005>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mangiraini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Murrel JR, et al. Tau gene mutation G389R causes a tauopathy with abundant Pick body-like inclusions and axonal deposits. Neuropathol Exp Neurol. 1999;58:1207–1226. doi: 10.1097/00005072-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Tetzlaff J. Mechanisms of disease II: cellular protein quality control. Semin Pediatr Neurol. 2007;14(1):15–25. doi: 10.1016/j.spen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Ono K, et al. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J Biol Chem. 2008;283:321–387. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt M. Screening for inhibitors of tau polymerization. Curr Alzheimer Res. 2005;2:219–226. doi: 10.2174/1567205053585891. [DOI] [PubMed] [Google Scholar]
- Pickhardt M. Phenylthiazolyl-hydrazide and its derivatives are potent inhibitors of tau aggregation and toxicity in vitro and in cells. Biochemistry. 2007;46:10016–10023. doi: 10.1021/bi700878g. [DOI] [PubMed] [Google Scholar]
- Roodveldt C, Christodoulou J, Dobson CM. Immunological features of alpha-synuclein in Parkinson's disease. J Cell Mol Med. 2008;12(5B):1820–9. doi: 10.1111/j.1582-4934.2008.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E, et al. Pathophysiology of Huntington's disease: from huntingtin functions to potential treatments. Curr Opin Neurol. 2008;21:497–503. doi: 10.1097/WCO.0b013e328304b692. [DOI] [PubMed] [Google Scholar]
- Sargent N, et al. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusive “exon 10” isoforms. J Neurochem. 1999;72:1243–1249. doi: 10.1046/j.1471-4159.1999.0721243.x. [DOI] [PubMed] [Google Scholar]
- Siva B, et al. Effects of a polyphenoics extracts fo grape seeds (GSE) on blood pressure (BP) in patients with metabolic syndrome (MetS) FASEB J. 2006;20:A305. [Google Scholar]
- Sivaprkasapillai B, Edirisinghe I, Randolph J, et al. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism. 2009;58(12):1743–6. doi: 10.1016/j.metabol.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Stanford PM, Halliday GM, Brooks WS, et al. Progressive supranuclear palsy pathology caused by a novel silent mutation in exon 10 of the tau gene: expansion of the disease phenotype caused by tau gene mutations. Brain. 2000;123:880–893. doi: 10.1093/brain/123.5.880. [DOI] [PubMed] [Google Scholar]
- Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol. 1964;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, et al. Inhibition of heparin-induced tau filament formation by phenothizaines, polyphenols, and porphyrins. J Biol Chem. 2006;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Weidenheim KM, Dickson DW, Ksiezak-Reding H. Morphological and biochemical correlations of abnormal tau filaments in progressive supranuclear palsy. J Neuropath Exp Neurol. 2002;61(1):33–45. doi: 10.1093/jnen/61.1.33. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology. 2006;26:457–470. doi: 10.1111/j.1440-1789.2006.00743.x. [DOI] [PubMed] [Google Scholar]