Abstract
Background
HSP90 is a chaperone protein regulating several client proteins involved in thyroid cancer development. The purpose of this study is to mechanisticially evaluate a novel natural-product HSP90 inhibitor in thyroid cancer cell lines for future translational applications.
Methods
285 plant-extracts/compounds were evaluated for anti-cancer activity by MTS assay. Apoptosis and cell-cycle-arrest were characterized by annexinV-PI flow cytometry. HSP90 and client-protein inhibition along with apoptosis confirmation was demonstrated by Western blot analysis.
Results
45 of 285extracts/compounds demonstrated anti-proliferative activity in thyroid cancers by MTS assay. BTIMNP_D004 demonstrated the highest inhibition [IC50(NPA)=0.19±0.02mM, IC50(DRO)=0.26±0.03mM]vs. 17-AAG [IC50(NPA)=0.51±0.02mM; IC50(DRO)=0.75±0.04mM;p<0.001]. D004 induced cell-cycle-arrest after 18hours (G1/G0→S and G2/M) with 26%DRO cells shifted and 23%NPA cells shifted vs. controls (p<0.001 and <0.01 respectively). 1mM D004 induced significant apoptosis with 76%DRO cells gated after 18hrs. (Annexin V/PI staining) vs <2% in controls;p<0.001 and 80%NPA cells vs. 4%controls(p<0.001). Western analysis demonstrated inhibition of HSP90, HSF-1, AKT, and cleavage of procaspase3 and PARP in both NPA and DRO cells.
Conclusion
BTIMNP_D004 is a potent, novel HSP90inhibitor with selective activity against papillary and anaplastic thyroid cancers through modulation of client proteins, induction of apoptosis and cell cycle arrest. These data support future pre-clinical studies for translational applications.
INTRODUCTION
Thyroid cancer is the most prevalent endocrine malignancy diagnosed each year and represents 1% of all malignancies worldwide with papillary cancers representing the majority of cases. In the United States, thyroid cancers represent the majority of all endocrine malignancies and endocrine cancer deaths with 37,000 new cases diagnosed in 2008 and 1600 cancer-related deaths.1 While papillary cancers account for up to 85% of all thyroid malignancies, over 80% of these carry an excellent prognosis with 20-year cause-specific mortality < 1% following thyroidectomy and frequently radioiodine ablative therapy.2Poorly differentiated and recurrent thyroid cancers, medullary cancers and anaplastic cancers on the other hand carry a much lower 5 and 10 year disease-specific survival mainly due to lack of effective systemic therapies.3
Recent discoveries have improved our understanding of the genetic and molecular basis of thyroid cancer with new therapies and targeted approaches being tested in Phase I and II human trials worldwide. Papillary thyroid cancers (PTC) have been characterized by alterations of one of several kinases including rearrangements of the RET (RET/PTC) receptor tyrosine kinase (13-43% of cases), point mutations in the BRAF serine/threonine kinase (29-69% of cases), rarely rearrangements of the NRTK1 receptor tyrosine kinase (5-13% of cases) or amplification of the catalytic subunit of phosphatidylinositol-3-kinase (up to 12% of cases).4-9 Follicular thyroid cancers (FTC), which make up approximately 10-15% of all thyroid cancers, are often associated with RAS oncogene mutations in 40-53% of cases or rearrangements between the PAX8 transcription factor and the peroxisome proliferator-activated receptor (PPAR)in 25-63% of cases.9,10 Medullary thyroid cancers (MTC) (5-9% of all thyroid malignancies) are familial in 25% of cases as part of the MEN 2 syndromes or sporadic in 75% of cases.11 Almost all familial and over 50% of sporadic MTCs are due to mutations of the transmembrane tyrosine kinase receptor RET proto-oncogene. Recent evidence also points to a high prevalence (up to 50%) of TP53 mutations in MTC.9 Anaplastic thyroid cancers (ATC; 1-5% of all thyroid cancers) carry the worst clinical prognosis with most patients dying of the disease within months of diagnosis. ATCs also have mutations in BRAF (10-35% of cases) and RAS proto-oncogenes (20-60% of cases), but uniquely have a high prevalence of TP53 mutations (67-88% of cases).12
Heat-shock protein 90 (HSP90) is a cellular chaperone protein required for the activation of several eukaryotic protein kinases, including the cyclin-dependent kinase CDK4. Because multiple oncogenic proteins are substrates for the Hsp90-mediated protein folding process, Hsp90 has emerged as an exciting target for the development of cancer chemotherapeutics. Examples of client proteins dependent upon the Hsp90 protein folding machinery include the steroid hormone receptors, AKT, Her2, c-Raf, Bcr-Abl kinase, MEK, mutant p53, and telomerase.13 Many of these same proteins are part of oncogenic pathways responsible for several different thyroid cancers. Therefore, inhibition of Hsp90 results in the simultaneous disruption of multiple signaling nodes and leads to induction of apoptosis. Currently, there are more than 20 clinical trials in progress based on Hsp90-targeted drugs, and several reports have attempted to explain the high level of differential selectivity observed for Hsp90 inhibitors.14 This combination of attributes makes Hsp90 a novel target for the development of new drugs. Most translational research to date on HSP90 inhibitors has been focused on N-terminal inhibitors such as 17-allylamino-17-demethoxygeldanamycin (17-AAG).14
Medicinal plants and their derivatives have become increasingly important in drug discovery for the treatment of human diseases including cancer. Solanaceas are a species of plants that produce withanolides, of which the most important and well-described is Withaferin A.15These compounds exert a number of different effects including anti-stress, immunomodulatory, cardiac, and cytotoxic activities.16Their role as anticancer agents in thyroid cancer is undefined. While recent years have generated new targeted therapies in thyroid cancer, a gap remains in the treatment of anaplastic tumors and recurrent papillary cancers not amenable to surgical resection. The search for novel small molecule inhibitors with good efficacy against anaplastic and recurrent papillary thyroid cancer with a low toxicity profile is critical to advancing the treatment of these malignancies. The purpose of this study is to evaluate the mechanism of action of a novel natural-product HSP90 inhibitor in papillary and anaplastic thyroid cancer cell lines for future translational applications.
MATERIALS AND METHODS
Cell Culture Conditions
Established human thyroid cancer cell lines were grown in selective media. Papillary cancer (NPA) cells and anaplastic (DRO) cells (obtained from Dr. Fiemu Nwariarku, University of Texas Southwestern Medical Center) were cultured in T-75 flasks using RPMI 1640 media (Sigma Chemicals, St. Louis, MO, USA) with glutamine (0.584g/L), sodium bicarbonate (3.7 g/L), 10% (w/v) fetal bovine serum (FBS; Sigma Chemicals, St. Louis, MO, USA), sodium pyruvate (0.11 g/L), MEM non-essential amino acids (1X)(Sigma Chemicals, St. Louis, MO, USA), and a standard 1X antibiotic/antimycotic (Invitrogen, Carlsbad, CA, USA). Follicular cancer cell lines (FTC 236, 238) were obtained from Dr. Peter Goretzki (Dusseldorf, Germany) and established from a single patient. FTC 236 from a lymph node metastasis, and FTC 238 from a lung metastasis. These cells were also cultured in T-75 flasks using DMEM:F-12 media (1:1) with glutamine (0.365 g/L) and 10% (w/v) FBS, supplemented with 1X penicillin/streptomycin solution (Sigma Chemicals, St. Louis, MO, USA), insulin (Sigma Chemicals, St. Louis, MO, USA), and human TSH (Sigma Chemicals, St. Louis, MO, USA). Medullary thyroid cancer (DRO81-1) cells (were gifts of Dr. G. Juillard; University of California, Los Angeles, CA) were cultured in T-75 flasks using DMEM media with glutamine, 10% (w/v) FBS, 1X penicillin/streptomycin solution and amphotericin B (0.25 μg/mL). All cell lines were incubated at 37 °C in humidified atmosphere containing 5% CO2. D004 stock solution (10 mM) was prepared in DMSO.
Cell Proliferation Assay (MTS)
Once 75% confluent, cells were trypsinized (0.25% trypsin), counted and plated in 96-well microtiter plates (Costar, Cambridge, MA, USA) (2×103 cells/well) in 100 mL of growth media. After an overnight attachment period, cells were treated with varying concentration of D004 for 3 days. All studies were performed in triplicate and repeated at least three times independently. After the 3-d treatment period, the number of viable cells was determined using a colorimetric Cell Proliferation assay (CellTiter96 Aqueous Nonradioactive Cell Proliferation assay; Promega, Madison WI, USA), which measures the bioreduction of the MTS (3-[4,5-dimethylthiazol-2yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium) by dehydrogenase enzymes of metabolically active cells into soluble formazon product, in the presence of the electron coupling reagent PMS (phenazine methosulfate). To perform the assay, 20 mL of combined MTS/PMS solution containing 2 mg/mL MTS and 150 mmole/L PMS in buffer (0.2 g/L KCl, 8.0 g/L NaCl, 0.2 g/L KH2PO4, 1.15 g/L, 133 mg/mL CaCl2-2H2O, 100 mg/mL, MgCl2.6H2O, pH 7.35) was added to each well and then after 3 hr of incubation at 37°C in a humidified 5% CO2 atmosphere, absorbance was measured at 490 nm in microplate reader. Triplicate wells with predetermined cell numbers were subjected to the above assay in parallel with the test samples to normalize the absorbance readings; this also provided internal confirmation that the assay was linear over the range of absorbance and cell numbers measured. Data was plotted as a function of % viability from controls (cell viability) vs. drug concentration (x-axis). The concentration of drug at which 50% of cells were inhibited from growth (IC50 level) was determined as the point of inflection on a standard absorbance-concentration curve.
Plant Library Screens
A screening analysis for anti-cancer activity was performed on a subset of the medicinal plant library at the University of Kansas (Laboratory of B. Timmermann, Lawrence, KS)demonstrating antioxidant properties. This subset included 285 extracts or purely-derived compounds. Activity was measured using MTS assay (run in triplicate) and positive activity was defined for compounds/extracts that demonstrated IC50 values less than 20 μM in at least two different thyroid cancer cell-lines.
Western Blot Analysis
Thyroid cancer cell lines described above were grown (80% confluence) in 100 mm plates. At the end of treatment cells were washed twice in cold phosphate-buffered saline (PBS), treated with 0.25% trypsin to remove adherent cells from the culture plate and cells were collected by centrifugation (750 × g for 5 min) and washed once with cold PBS. The cells were lysed in lysis buffer containing lysis buffer (50 mM Tris-HCl, pH 6.8, 150 mM NaCl, 1% (v/v) Nonidet NP-40, 0.5% (w/v) sodium deoxycholate, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mMPMSF, 10 mM sodium pyrophosphate, 0.1% (w/v, SDS) supplemented with 1X antiprotease solution (Calbiochem, San Diego, CA, USA). After lysis on ice for 20 min, protein samples were collected from the supernatant after centrifugation of the samples at 12,000 × g for 20 min, and protein levels in whole cell lysates were determined using Protein Assay Reagent (Pierce Rockford, IL, USA). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransfered onto a Hybond nitrocellulose membrane (Amersham, Piscataway, NJ, USA). Membranes were blocked with PBS (phosphate buffered saline) supplemented with 3% (w/v) non-fat dry milk (Bio-Rad Laboratories, Hercules, CA, USA), and probed with the appropriate dilution of primary antibody overnight at 4°C. The blots were washed three times in PBS-Tween 20 for 10 min, and then incubated with 1:1000 dilution of horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in PBS-Tween 20 at room temperature for 1 hr. After washing three times in PBS-Tween 20 for 10 min, the proteins were visualized by enhanced chemiluminescence (Amersham, Piscataway, NJ, USA) was captured on Kodak XAR-5 film (Eastman Kodak, Rochester, NY). Where indicated, the blots were reprobed with antibody against beta-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to ensure equal loading and transfer of proteins.
The primary antibodies to phospho-extracellular signal-related kinase1/2 (thr180/Tyr204) (ERK1/2),Akt, HSP27, cleaved caspase 3, and cleaved poly (adenosine diphosphate-ribose)polymerase (PARP) were obtained from Cell Signaling Technologies (Beverly, MA, USA). Antibodies against HSF-1, HSP90, HSP70, Grp94 and Trap1 were purchased from Stressgen (Ann Arbor, MI);B-Raffrom Upstate Biotechnologies (Lake Placid, NY); and ERK2 and secondary antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell Cycle Arrest Analysis
NPA and DRO cells at 80% confluence are grown overnight and treated with 1 μM D004. After trypsinization, cells (1×106 cells) were washed with 0.9% NaCl and fixed with 70% cold ethanol for 30 min at room temperature. Cells were collected by centrifugation (700 × g for 5 min) and stained with propidium iodide (50 mg/mL in PBS for 30 min. Cells then were treated with DNAse free RNAse (1mg/mL) for 30 min and analyzed by flow cytometry.
Apoptosis Analysis
Preliminary studies to evaluate for induction of apoptosis were also performed including annexinV-propidium iodide (PI) co-staining with flow cytometry. An analysis of phosphatidylserine (PS) on the outer leaflet of apoptotic cell membranes was performed using annexin V-FITC and PI to distinguish between apoptotic and necrotic cells. Cells were cultured to monolayer confluence in 100 mm Petri dishes for 24 hr then rinsed twice with PBS and trypsinzed and cultured in 6-well plates (1×105 cells/ml) for 24 hr. After treatment with D004, media was removed and cells were washed with PBS. Cells were trypsinized, washed with PBS and suspended in 1X Annexin binding buffer at a concentration of 1×106 cells/ml. Cells were transferred to a culture tube and annexin-V and PI (BD Pharmingen (San Diego, CA) were added and after gentle vortex, the cells were incubated for 20 minutes at room temperature in the dark. After adding 400 μL of 1X annexin binding buffer to each tube, cells were analyzed by flow cytometry (BD LSRII; Becton Dickerson, San Diego, CA).
Statistics
All result data points were run in triplicate and expressed as a mean ± SEM. Raw data was analyzed by a student’s unpaired t-test and Fisher exact tests using Graphpad Prism 5.0 statistical software (GraphPad Software, La Jolla, CA). Significance was defined as a p value < 0.05.
RESULTS
Inhibition of thyroid cancer proliferation in vitro
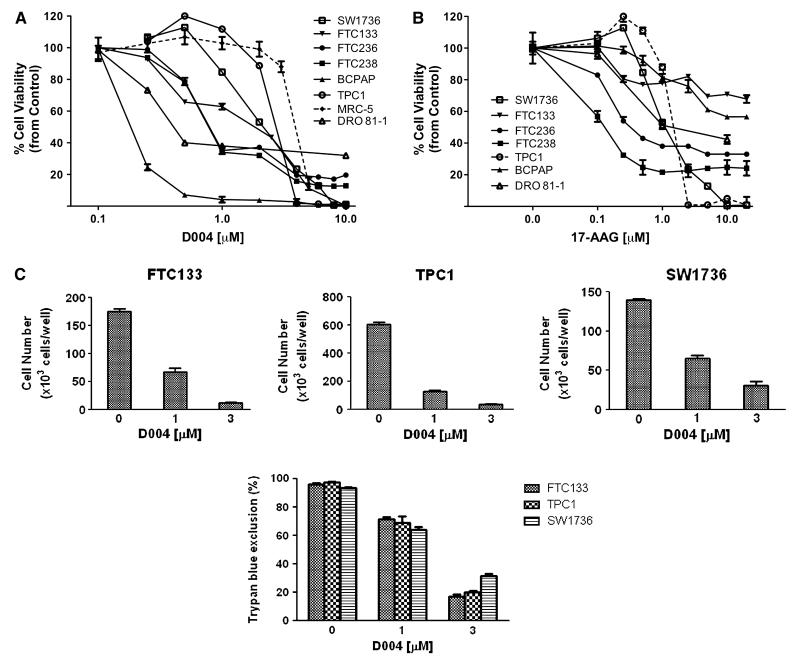
Of the 285 natural product compounds and extracts tested, 45 (16%) met criteria for activity. From this group of compounds/extracts with activity, only one compound, BTIMNP_D004 (D004), demonstrated IC50 levels in the nanomolar concentration range. We next evaluated,using MTS assays, the in vitro activity of D004 against a diverse panel of six thyroid cancer cell lines(Figure 1). In these cell-lines, we observed that D004 exhibited potent in vitro anti-thyroid cancer activity with IC50 levels well below 1 μM. The majority of thyroid cancer cells treated with D004 at 300 nM exhibited ≤30% viability compared with their respective controls (Fig 1). IC50 levels for each thyroid cancer cell line are listed in Table 1 with a range of 190.7 ± 19.8 nM for papillary cancer to 904.1 ± 48.2 nM for one of the anaplastic (KAT-4B) cell lines. For comparison we used 17-allylamino-17-demethoxygeldanamycin (17-AAG) a well-known HSP90 inhibitor used in clinical trials. Treatment of NPA and DRO cells with 17-AAG induced decrease in cell viability. However, these cells were significantly more resistant to 17-AAG compared to D004, by three to five-fold.
Figure 1. Antiproliferative Activity of Compounds by MTS assay.
Antiproliferation effects of D004and 17-AAG on thyroid cancer cell lines by MTS assay in vitro. The viability of D004-treated or 17-AAG treated thyroid cancer cells (expressed for each cell line as the percent of its respective control) after 72 hr treatment in NPA, DRO, KAT4B, FTC236, FTC238 and DRO81.1 cells. 17-AAG treated cells are graphed as dotted lines. IC50 values for each cell-line treated are listed in Table 1.
Table.
IC50 levels in thyroid cancer cell lines
| Cell type | IC50* D004 (nM) | IC50 17-AAG (nM) | IC70 D004 (nM) | IC70 17-AAG (nM) |
|---|---|---|---|---|
| BCPAP (papillary thyroid cancer) | 155 ± 15 | >20,000 | 205 ± 10 | >20,000 |
| TPC1 (papillary thyroid cancer) | 2,890 ± 35 | 1,750 ± 25 | 3,180 ± 35 | 1,840 ± 30 |
| SW1736 (anaplastic thyroid cancer) | 2,540 ± 20 | 1,470 ± 35 | 3,210 ± 40 | 2,050 ± 35 |
| FTC133 (follicular thyroid cancer) | 2,150 ± 25 | >20,000 | 2,825 ± 40 | >20,000 |
| FTC236 (follicular thyroid cancer) | 745 ± 30 | 315 ± 20 | 2,450 ± 48 | >20,000 |
| FTC238 (follicular thyroid cancer) | 770 ± 25 | 180 ± 15 | 2,080 ± 35 | 340 ± 15 |
| DRO81-1 (medullary thyroid cancer) | 689 ± 30 | 2,410 ± 30 | 1,005 ± 15 | >20,000 |
| MRC-5 (normal lung fibroblast cells) | 5,750 ± 35 | 7,450 ± 50 |
The cell-proliferation of D004-treated and 17-AAG treated cells were measured by MTS assay.
IC50 level is expressed for each cell line in nanomolar concentration (average of 3 experiments for each condition ± standard deviation) and is defined as the concentration of D004 required to reduce cell proliferation/viability by 50% compared to controls.
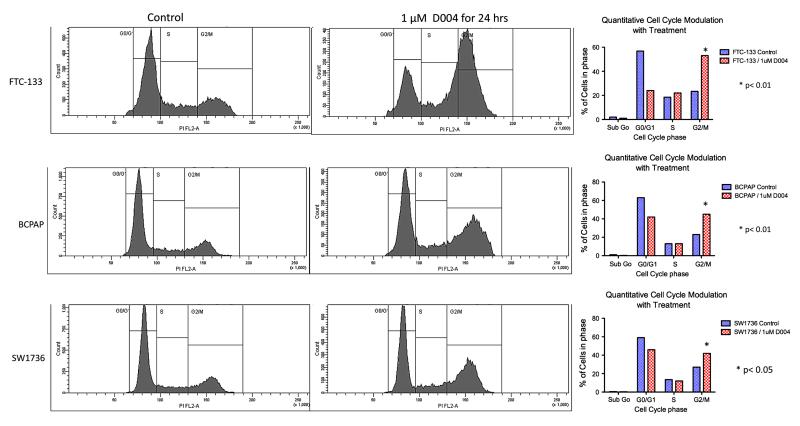
Induction of cell cycle arrest and apoptosis
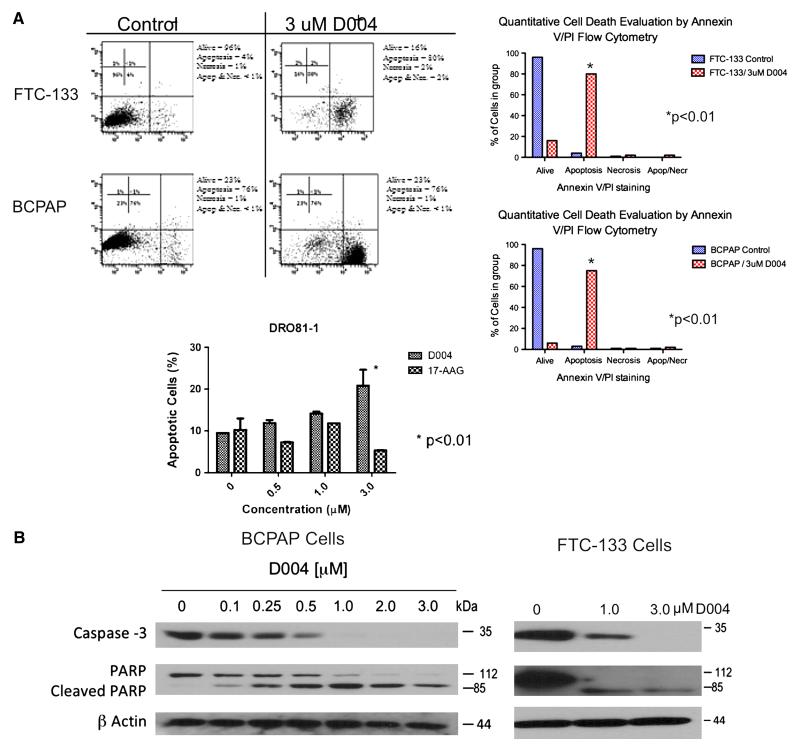
Treatment of both anaplastic (DRO) and papillary (NPA) thyroid cancer cells with 1uM of D004 induced a shift in cell cycle arrest (normally arrested in G0/G1)with an increase in the percentage of cells in the sub G0, G2-M, and S phases (26% cells shifted out of G0/G1 in DRO cells (p<0.001 compared to controls) and 23% shifted in NPA cells (p<0.01 compared to controls; Figure 2). We next examined D004-induced apoptosis using annexin V/PI assay (Figure 3A). D004 greatly increased the population of NPA (76% vs. 2% controls; p<0.001) and DRO (80% vs. 4% controls; p<0.001) cells stained with annexin V after 18 hr of treatment. Confirmation of apoptosis was performed by Western analysis for cleavage of caspase 3 and PARP (Figure 3B). D004 concentration dependently induced cleavage of procaspase-3 to an active form (p19/p17), which is maximally cleaved at 3μM concentration of D004. Cleaved caspase 3 appeared after treatment with 1 μM treatment with D004 for 24 hr. PARP cleavage also occurred in both cell-lines (1μM D004 in NPA cells and at 3μM D004 in DRO cells).
Figure 2. D004 treatment alters cell cycle arrest mechanisms.
NPA and DRO cells at 80% confluence are grown overnight and treated with 1 μM D004. After trypsinization, cells were washed with saline and fixed with 70% cold ethanol for 30 min at room temperature. Cells were collected by centrifugation and stained with propidium iodide (50 mg/mL in PBS for 30 min. Cells then were treated with DNAse free RNAse (1mg/mL) for 30 min and analyzed by flow cytometry. D004 treatment resulted in a decrease in G0/G1 arrested cells from 61% (DRO) and 55% (NPA) in controls to 35% and 32% respectively. Additionally, treatment resulted in increases in sub G0 (6% controls to 13% treated), G2/M (19% controls to 31% treated) and S phase (13% controls to 20% treated) populations in DRO cells as well as in NPA cells (sub G0: 1% controls to 15% treated), G2/M (24% controls to 27% treated) and S phase (19% controls to 25% treated). Of note an increase in sub G0 population in indicative of DNA fragmentation which likely represents apoptosis.
Figure 3. D004 Induces Apoptotic Cell-Death in NPA and DRO cells in vitro.
Figure 3A. Annexin V–FITC/PI assay. Anaplastic (DRO) and Papillary (NPA) thyroid cancer cells were incubated with either 1% DMSO or D004 (1 mM) for 18 hours. Cells were then incubated with annexin V–FITC and PI and analyzed by flow cytometry. The number of events analyzed for each condition was 10,000. Three independent experiments were performed and percentage of annexin positive cells in each treatment protocol was determined. NPA and DRO cells demonstrate a shift to the lower right quadrant with treatment indicating gating of cells toward apoptotic cell death. In NPA cells this gating occurred in 76% of cells compared to 2% in controls (p<0.001) while in DRO cells this was 80% compared to 4% in controls (p<0.001). Apotosis was confirmed by Western Blot analysis (Figure 3B). D004 induced cleavage of both caspase 3 and its substrate PARP after 24 hours exposure to drug at 1 and 3 μM concentrations indicating apotosis was occurring with treatment.
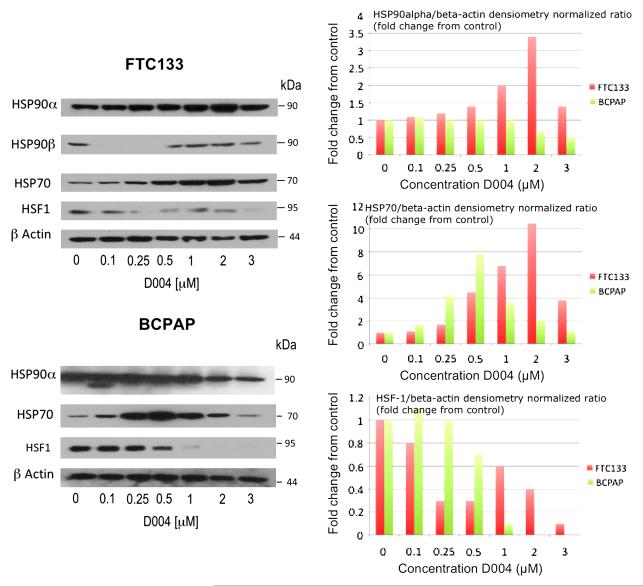
Modulation ofHSP90 and Client Proteins
To determine if D004 was indeed a HSP90 inhibitor, it was evaluated by Western analysis for effects on heat-shock chaperone proteins in DRO and NPA cells, specifically HSP90 isoforms (of which there are 4 in humans: HSP90α, HSP90β, GRP94, and TRAP-1) as well as HSF-1, HSP70 and HSP27. HSP90α and HSP27 levels increased slightly at 3μM D004 in both cell lines. HSP90β levels decreased with increasing D004 concentration. Both GRP94 and HSP70 levels increased at 1μM and 3μM D004 in both cell-lines. TRAP-1 levels increased in DRO cells more than NPA cells with increasing D004 concentration. Finally, HSF-1 levels decreased in both cell lines in a dose-dependant manner (Figure 4).
Figure 4. Modulation of Heat Shock Protein Expression by D004.
D004 modulates HSP expression in thyroid cancer cells. Anaplastic (DRO) and papillary (NPA) thyroid cancer cells were treated with 1% DMSO (as control) or D004 at concentrations from 0.25 to 3μM for 24 hr. Lysates were prepared and subjected to Western blotting with the indicated antibodies. HSP90α and HSP27 levels increased slightly at 3μM D004 in both cell lines. HSP90β levels decreased with increasing D004 concentration. Both GRP94 and HSP70 levels increased at 1μM and 3μM D004 in both cell-lines. TRAP-1 levels increased in DRO cells more than NPA cells with increasing D004 concentration. Finally, HSF-1 levels decreased in both cell lines in a dose-dependant manner.
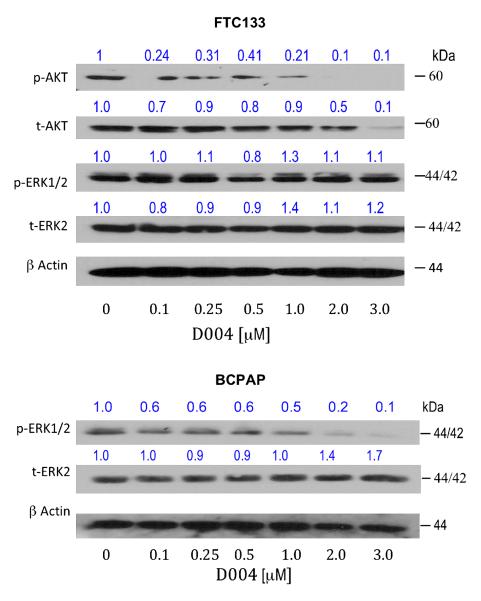
With evidence of modulation of heat shock chaperones on Western analysis, the next experiment evaluated the effect of D004 on client protein levels, specifically AKT, B-Raf (overexpressed in papillary cancers and in NPA cells) and ERK1/2 (Figure 5). D004 down-regulates AKT in both cell lines in a dose-dependant manner, while both B-Raf and phospho-ERK levels increased (total ERK levels remained unchanged). Similarly, 17-AAG down-regulates AKT in both cell lines although not as robustly as D004. The N-terminal HSP90 inhibitor, 17-AAG, also reduced phosho-ERK levels in both cell lines without altering total ERK levels (data not shown).
Figure 5. Modulation of HSP90 Client Protein Expression.
D004 modulates HSP90 client proteins in thyroid cancer cells. Anaplastic (DRO) and papillary (NPA) thyroid cancer cells were treated with 1% DMSO (as control), D004 or 17-AAG at concentrations from 0.1 to 3μM for 24 hr. Lysates were prepared and subjected to Western blotting with the indicated antibodies. D004 and 17-AAG depleted total AKT levels in DRO and NPA cells in a dose-dependant manner, although more potently with D004. Phosphorylated ERK and B-raf levels were increased in both DRO and NPA cell lines after 24 hr treatment in a concentration-dependent manner with D004 treatment. Total ERK protein levels did not changed significantly in DRO and NPA cells.
DISCUSSION
As a molecular chaperone, Hsp90 is responsible for proper folding many of the proteins directly associated with malignant progression, and hence inhibition of the Hsp90 protein folding machinery results in a combinatorial attack on numerous pathways 17. Recent studies have revealed that Hsp90 inhibitors accumulate in tumor cells more efficiently than in normal tissue leading to high differential selectivities.18,19 Currently, there are 26 Hsp90-targeted clinical trials in progress and these studies have demonstrated that Hsp90 can be inhibited at doses that are well tolerated by patients.20-23 Consequently, Hsp90 has emerged as a promising therapeutic target for the treatment of cancer because inhibition of Hsp90 results in the simultaneous degradation of multiple anti-cancer targets.11-14,23-24
BTIMNP_D004 is a natural product withanolide which demonstrates potent anti-cancer activity against thyroid cancers with specific activity against papillary (NPA cells) and anaplastic (DRO) cancers that is more robust than 17-AAG (which has been used clinically). Regarding the mechanism of action of D004, cell-cycle arrest studies indicate that there is a shift with drug-treatment out of G0/G1 arrest toward increased sub G0 phase as well as increases in G2/M and S phase. By allowing cells to come out of arrest and move through the cell cycle may be one mechanism in how this drug leads to cancer cell death. Additionally, an increase in the sub G0population in both cell lines when treated with D004 suggests that the cells may be undergoing DNA fragmentation, which is a biochemical hallmark of apoptosis. Apoptotic mechanisms were then evaluated to confirm this effect. Annexin V/PI flow cytometry analyses demonstrate that there is a significant shift of cells gated toward programmed cell death, as high as 76-80% with drug-treatment. This effect is confirmed by observation of cleavage of caspase 3 and PARP on Western analysis following treatment with D004.
Since pro-apoptotic proteins such as AKT are clients of HSP90, it is important mechanistically to determine the role of this drug on heat shock chaperone protein and client protein inhibition. Western analysis data support that the beta-isoform of HSP90 is inhibited by D004 as is AKT expression. Cancer cells when stressed will often induce or overexpress pro-survival pathways as a compensatory mechanism and it is likely that this balance of apoptosis and pro-survival is occurring in these cells with drug treatment. While AKT, HSF-1 and HSP90β levels are down-regulated, we also observed increases in B-Raf, HSP70, GRP94, and phosphoERK levels with drug treatment. Several papillary and anaplastic thyroid cancers (including NPA and DRO cells) utilize B-Raf overexpression as a pro-survival pathway. An increase in B-raf expression with treatment may therefore represent the cell’s attempt to survive and proliferate in response to activation of pro-apoptotic pathways. Although, ERK1/2 is not a HSP90 client protein, upstream signaling proteins such as Raf-1and MEK are in fact HSP90 client proteins, and HSP90 inhibitors may therefore indirectly alter activation of ERK1/2 via upstream MAP-kinase modulation.18 Additionally, other withanolide compounds have demonstrated a multiplicity of mechanistic actions including activation of ERK and modulation of NF-κB pathways. 25
Overall these data support that D004 is a novel heat shock protein inhibitor which acts specifically on papillary and anaplastic thyroid cancers to inhibit cell growth and proliferation through induction of apoptosis, modulation of client signaling proteins, and altering cell-cycle arrest mechanisms. This natural product is a more potent inhibitor than 17-AAG, which has been used in clinical trials with thyroid cancers. Future studies are warranted to evaluate the efficacy and toxicity of this novel drug in animal models of thyroid cancer to support its role for further translational applications in patients.
ACKNOWLEDGEMENT
The authors are grateful to Dr. Joyce Slusser, PhD (University of Kansas Medical Center, Flow Cytometry Core Director) for assistance in flow cytometry studies as well as Dr. John Robertson (Department of Pharmacology, University of Kansas Medical Center) for caspase-3 antibody. This work was partially funded through support from NIH P20 RR016443 (PI: B. Timmermann, Program Award: M.S. Cohen) through the University of Kansas CCET COBRE Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Cancer Society: Facts & Figures 2008. 2008.
- 2.Randolph GW, Thompson GB, Branovan DI, Tuttle RM. Treatment of thyroid cancer: 2007--a basic review. Int J Radiat Oncol Biol Phys. 2007;69:S92–97. doi: 10.1016/j.ijrobp.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Sanders EM, Jr., LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidence-based review of poorly differentiated thyroid cancer. World J Surg. 2007;31:934–945. doi: 10.1007/s00268-007-9033-3. [DOI] [PubMed] [Google Scholar]
- 4.Santoro M, Carlomagno F. Drug insight: Small-molecule inhibitors of protein kinases in the treatment of thyroid cancer. Nat Clin Pract Endocrinol Metab. 2006;2:42–52. doi: 10.1038/ncpendmet0073. [DOI] [PubMed] [Google Scholar]
- 5.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 6.Groussin L, Fagin JA. Significance of BRAF mutations in papillary thyroid carcinoma: prognostic and therapeutic implications. Nat Clin Pract Endocrinol Metab. 2006;2:180–181. doi: 10.1038/ncpendmet0161. [DOI] [PubMed] [Google Scholar]
- 7.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 8.Pierotti MA, Greco A. Oncogenic rearrangements of the NTRK1/NGF receptor. Cancer Lett. 2006;232:90–98. doi: 10.1016/j.canlet.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 9.Santoro M, Fusco A. New drugs in thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:857–861. doi: 10.1590/s0004-27302007000500025. [DOI] [PubMed] [Google Scholar]
- 10.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 11.Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M. New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4:22–32. doi: 10.1038/ncpendmet0717. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rostan G, Costa AM, Pereira-Castro I, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 13.Blagg BS, Kerr TD. Hsp90 inhibitors: small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med Res Rev. 2006;26:310–338. doi: 10.1002/med.20052. [DOI] [PubMed] [Google Scholar]
- 14.Messaoudi S, Peyrat JF, Brion JD, Alami M. Recent advances in Hsp90 inhibitors as antitumor agents. Anticancer Agents Med Chem. 2008;8:761–782. doi: 10.2174/187152008785914824. [DOI] [PubMed] [Google Scholar]
- 15.Yokota Y, Bargagna-Mohan P, Ravindranath PP, Kim KB, Mohan R. Development of withaferin A analogs as probes of angiogenesis. Bioorg Med Chem Lett. 2006;16:2603–2607. doi: 10.1016/j.bmcl.2006.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 17.Yu XM, Shen G, Neckers L, et al. Hsp90 inhibitors identified from a library of novobiocin analogues. J Am Chem Soc. 2005;127:12778–12779. doi: 10.1021/ja0535864. [DOI] [PubMed] [Google Scholar]
- 18.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 19.Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem Biol. 2007;14:1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhury S, Welch TR, Blagg BS. Hsp90 as a target for drug development. ChemMedChem. 2006;1:1331–1340. doi: 10.1002/cmdc.200600112. [DOI] [PubMed] [Google Scholar]
- 21.Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007;98:1536–1539. doi: 10.1111/j.1349-7006.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiosis G, Huezo H, Rosen N, Mimnaugh E, Whitesell L, Neckers L. 17AAG: low target binding affinity and potent cell activity--finding an explanation. Mol Cancer Ther. 2003;2:123–129. [PubMed] [Google Scholar]
- 23.Banerji U, Judson I, Workman P. The clinical applications of heat shock protein inhibitors in cancer - present and future. Curr Cancer Drug Targets. 2003;3:385–390. doi: 10.2174/1568009033481813. [DOI] [PubMed] [Google Scholar]
- 24.Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets. 2003;3:377–383. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- 25.Kaileh M, Berghe W Vanden, Heyerick A, et al. Withaferin a strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J Biol Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]