Abstract
Purpose
The purpose of this study is to examine the use of daikenchuto (DKT), a traditional Japanese medicine, as a potential treatment for opiate-induced slowing of intestinal transit in an isolated guinea-pig colon model of motility.
Methods
Isolated segments of distal guinea-pig colon were mounted in a perfusion chamber and imaged with a digital video camera interfaced with a computer. Fecal pellets were inserted into the oral end of the colonic segment and the rates of propulsive motility over a 3-4 cm segment of colon were determined in the presence and absence of test compounds. In addition, intracellular recordings were obtained from intact circular muscle, and the responsiveness of inhibitory and excitatory junction potentials to DKT was evaluated.
Results
The addition of DAMGO (D-Ala2, N-Me-Phe4, Gly-ol5), a selective mu-receptor agonist, caused a concentration dependent decrease in colon motility. Naloxone did not affect basal activity, but partially restored motility in the DAMGO treated preparations. DKT (1×10-4 – 3×10-4 g/ml) also reversed the inhibitory effect of DAMGO treated colon in a concentration dependent manner. At higher concentrations (1×10-3 – 3×10-3 g/ml), however, this effect was lost. Motility slowed even further when naloxone and DKT were combined with noticeable disruptions in spatiotemporal patterns. Interestingly, when added alone, DKT resulted in reverse peristalsis of the pellet. In electrophysiological studies DKT inhibited both excitatory and inhibitory junction potentials.
Conclusions
DKT appears to be as effective as naloxone in restoring motility in DAMGO treated colon. These two agents, however, do not appear to have an additive effect. When used on untreated colon segments, DKT appears to cause disruptions in the intrinsic reflex circuit of the gut resulting in a disruption of neuromuscular communication.
Introduction
Opiates are alkaloid derivatives of opium, which is an extract from the seed pods of the opium poppy plant (Papaver somniferum.). These products have long been known to relieve pain, produce euphoria, and treat the symptoms of diarrhea. Opioid peptides act presynaptically to suppress the release of the excitatory musculomotor neurotransmitters, acetylcholine and substance P, resulting in an inhibition of neurogenic contractile responses in guinea-pig myenteric plexus-longitudinal muscle preparations.1,2 A similar mechanism of action has been proposed to explain the clinically observed entity of opiate-induced paralytic ileus in patients receiving narcotic pain control. In general, morphine increases resting contractile tone of human large intestine with associated nonpropulsive, phasic contractions and changes in smooth muscle electrical activity when subjects were administered morphine postoperatively.3
Opiates such as morphine achieve their action through the activation of opioid receptors. One receptor subtype, the mu receptor, has recently received considerable attention as a potential pharmacotherapeutic target. Activation of this peripherally located receptor, by both exogenous and endogenous opioid peptides, impairs gastric emptying and transit through both small and large intestine.4 Therefore, antagonists of this receptor that do not cross the blood-brain barrier have been investigated as potential prokinetic agents for individuals taking opiate analgesics and for the treatment of post-operative ileus. One such compound is alvimopan, a peripherally acting, selective mu opioid receptor antagonist with minimal systemic absorption, and limited oral bioavailability.4,5 When administered in a rat model of post-surgical ileus, alvimopan significantly reverses delayed GI transit. This effect is even more dramatic when the rats are administered morphine post-operatively.6 The therapeutic potential of this mu antagonist was demonstrated in a recent Phase III clinical trial demonstrating that post-surgical patients receiving alvimopan had earlier return of bowel function and earlier discharge than those receiving placebo.7
Another agent that may be useful in the treatment of POI and opiate-induced constipation is daikenchuto (DKT). DKT is an herbal medicine that has been used to treat adhesive bowel disease in Japan.8,9,10 DKT improves delayed GI transit induced by intestinal manipulation with and without concomitant morphine administration.6 In addition, the stimulatory effect of DKT on delayed GI transit after surgery was abolished by the pretreatment with 5-HT3 and 5-HT4 antagonists.6 DKT also has a stimulatory effect on canine GI motility that is thought to involve cholinergic and 5-HT3 receptor activation.11 This reversal of morphine-induced slowing of transit by DKT is thought to involve both moderate contraction of morphine-treated longitudinal muscle and relaxation of morphine-induced tonic contraction of circular muscle.12
The purpose of the current study was to examine the use of DKT as a potential treatment for opiate-induced slowing of intestinal transit in an isolated guinea-pig colon model of motility. In addition, we investigated whether DKT could act synergistically with the non-selective opiate receptor antagonist, naloxone, to promote propulsive motility in opiate treated colon segments. Lastly, we attempted to determine the effects of DKT on neuromuscular transmission in the colon.
Methods
All methods used in this study were approved by The University of Vermont Animal Care and Use Committee. Experiments were performed on Hartley guinea pigs (Charles River, Montreal, QC, Canada) of either sex housed in cages with soft bedding. The animals had access to food and water ad libitum and were maintained at 23–24 °C on a 12:12 h light cycle. At the time of tissue collection, animals anaesthetized with isoflurane, and exsanguinated.
DKT preparation
The DKT for these experiments was generously provided by the Tsumura & Co. (Tokyo, Japan). DKT extract powder is an aqueous extract containing processed ginger, ginseng, and zanthoxylum fruit in the ratio of 5:3:2. The DKT used in these experiments was prepared by mixing DKT extract powder and maltose syrup powder at a ratio of 1:8. Final concentrations shown in figures were achieved by mixing this mixture with a specified volume of Kreb’s solution in the organ bath.
Gastrointestinal motility assay
The distal colon of the guinea-pig, identified as the part of the colon between the hypogastric flexure and the pelvic brim, was removed and placed in iced cold Kreb’s (mmol L−1: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose 8; aerated with 95%O2/5%CO2; Sigma, St Louis, MO, USA). A 7 cm segment of distal colon was pinned on either end in a 50 mL organ bath lined with Sylgard and continuously perfused with prewarmed oxygenated Kreb’s at 10 mL min−1. The final temperature of the bath was 37 °C. Gastrointestinal motility was monitored using the Gastrointestinal Motility Monitoring system (GIMM; Med-Associates Inc., Saint Albans, VT, USA). Briefly, the distal colon was illuminated from beneath and a digital video camera was used to film the fecal pellet being propelled in the anal direction. The digital movies were stored on a PC and analyzed at a later time using software designed for the GIMM. Control values for rate of propulsion were obtained for each preparation by obtaining an average rate of propulsion from 5 trials prior to the addition of any drugs. Five individual runs were performed for each new drug or concentration change. Also, each experiment was performed on at least 5 different colons from at least 5 different animals. Approximately 5 min was allowed to elapse between runs. The rate of motility of fecal pellets was analyzed by monitoring the time it took for a fecal pellet to traverse 5 cm of the colon. The rates of propulsion were compared between all groups.
Spatiotemporal maps were constructed from the digital videos that were acquired from individual runs. Briefly, changes in colonic diameter were plotted over time (vertical-axis). The image of the colon in each video frame was converted to a silhouette and the diameter along the entire length was calculated and converted into a grey-scale. The smallest diameter, or fully contracted, is white, and the largest diameter, or dilated, is black. Each frame of video produced a single row of pixels corresponding to the diameter of each segment along the entire length of imaged colon. Each computed image has a calibration scale that indicates the change in diameter in grey-scale (mm), time (s) and length (mm) for each spatio-temporal map produced for each experiment (GIMM software, Med-Associates Inc.)
Electrophysiological recordings
Experiments were performed on the distal colons removed from animals as described above. Colons were placed in iced Kreb’s solution for ½ to 3 hours prior to performing experiments. The tissue was then placed in a Sylgard coated dissecting dish with ice cold Kreb’s containing Nifedipine (5 μmol L–1) and atropine (200 nmol L–1) (Sigma) to eliminate smooth muscle contraction. The tissue was cut open along the mesenteric border and the mucosa and submucosa were subsequently removed with forceps exposing the smooth muscle. The preparation was then moved to a 2.5 mL recording chamber.
Preparations were continuously perfused at 10 mL min–1 with Kreb’s solution containing nifedipine and atropine maintained at 37 °C. Glass microelectrodes used for recording were filled to the shoulder with 1.0 mol L–1 KCl, and the remainder filled with 2.0 mol L–1 KCl and had resistances in the range of 50–150 MΩ. Smooth muscle cells a were visualized at ×200 with Hoffman modulation contrast optics through an inverted microscope (Nikon Diaphot, Melville, NY, USA) and individual smooth muscle cells were randomly impaled. Transmembrane potential was measured with an Axoclamp-2A amplifier (Axon Instruments, Union City, CA, USA) and electrical signals were acquired and analysed using PowerLab Chart (version 5.01; AD Instruments, Castle Hills, Australia). Input resistance and resting membrane potential were determined for each cell. Junction potentials were elicited with a depolarizing current pulse from either an oral or aborally placed stimulating monopolar extracellular electrode made from Teflon-insulated platinum wire (a single pulse of 5 ms).
Statistical analyses
Statistical analyses were performed using GraphPad Prism software (version 4.0a for Macintosh, GraphPad Software, San Diego, CA, USA). Continuous data differences between groups were determined by performing a Student’s unpaired t-test or a one-way anova with a Newman–Keuls multiple comparison post-test. Proportional data were analyzed by Fisher’s exact test. P-values < 0.05 were determined to be significant. n values represent individual colons for each experiment and are indicated where appropriate.
Results
Effects of opioid receptor agonists and antagonists on propulsive motility
DAMGO (D-Ala2, N-Me-Phe4, Gly-ol5), a selective mu-receptor agonist, caused a concentration-dependent decrease in the rate of fecal pellet propulsion (fig. 1). It was determined that a DAMGO concentration of 100nM caused a 49% +/- 4 (n=6) decrease in propulsion compared to a control run performed in the absence of DAMGO (p=<0.001). Spontaneous neurogenic motility, evident in the spatiotemporal maps, was also decreased in the presence of DAMGO (not shown.) Based on these findings, we used a DAMGO concentration of 100 nM in subsequent experiments.
Figure 1.
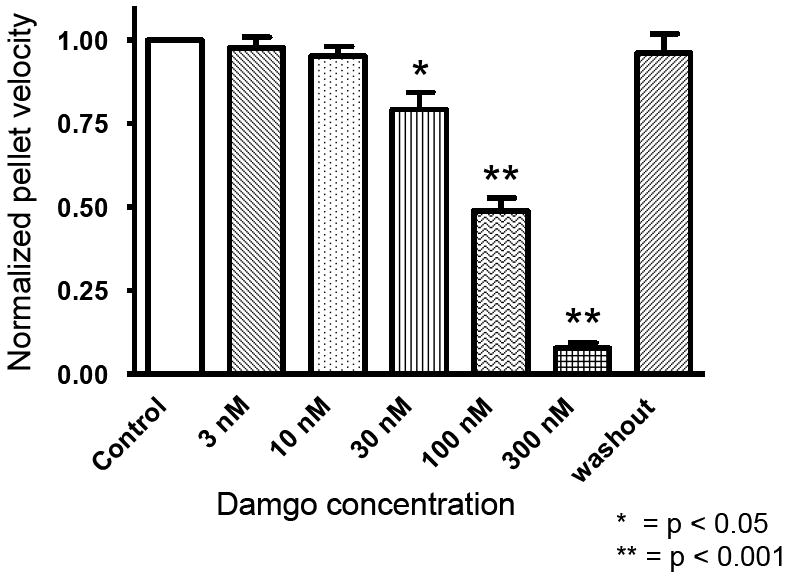
Addition of the selective μ-receptor agonist, DAMGO (D-Ala2, N-Me-Phe4, Gly-ol5), reduced spontaneous neurogenic motility (not shown) and the rate of fecal pellet propulsion in isolated segments of the guinea pig distal colon. (n=5 colons at each concentration)
Having determined that an opioid-receptor agonist suppresses propulsive motility in our model, we next examined whether or not propulsive motility could be restored with the application of an opioid-receptor antagonist. Both low (1 μM) and high (10 μM) concentrations of naloxone (a non-selective opioid receptor antagonist) were added to a bath containing an isolated segment of colon in the presence of 100 nM of DAMGO (fig 2). Naloxone was observed to restore propulsive motility to 83%+/-3 and 86%+/-3 (n=6) of the control rate respectively (p=<0.05 for both concentrations). To verify that there was no intrinsic effect from naloxone and/or the presence of endogenous opioids, the same concentrations of naloxone were added to a bath containing the isolated colon segment in the absence of DAMGO. Naloxone alone had no effect on propulsive motility as compared to control (Fig 3).
Figure 2.
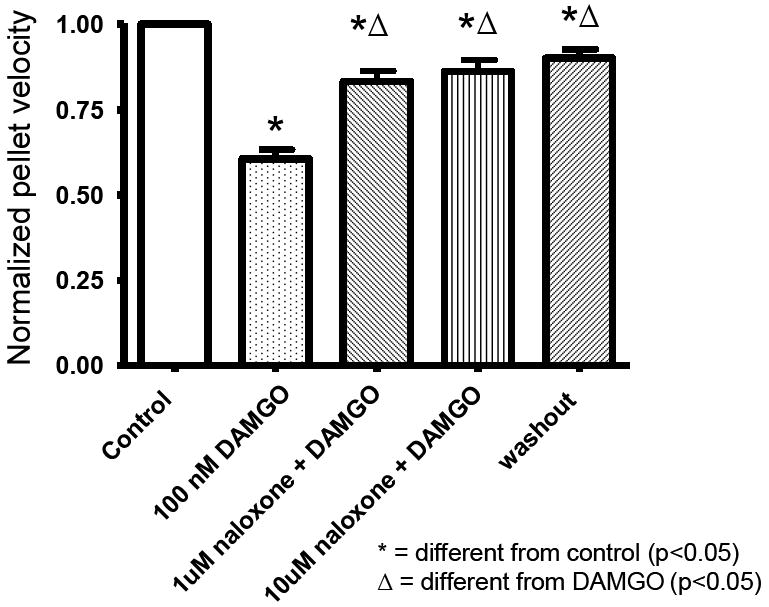
The non-selective opioid receptor antagonist, naloxone partially restored intestinal motility in the DAMGO-treated isolated guinea-pig distal colon.
Figure 3.
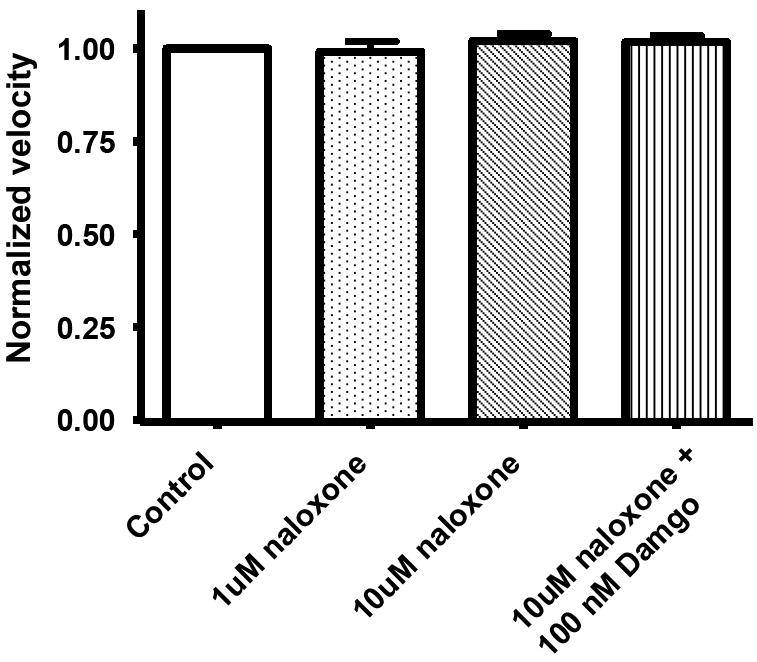
Naloxone by itself did not affect the rate of pellet propulsion in the isolated guinea-pig distal colon. This indicates that endogenous opioids do not typically contribute to peristalsis in the guinea pig distal colon.
Effects of DKT on propulsive motility
Next we examined whether the application of DKT could similarly improve propulsive motility in DAMGO-treated colon segments. Figure 4 shows the concentration-dependent effects of DKT on intestinal transit in colon segments treated with 100nM DAMGO. Colonic propulsive motility increased up to a concentration of 3×10-4 g/ml. At higher concentrations of DKT, the rate of propulsive motility decreased.
Figure 4.
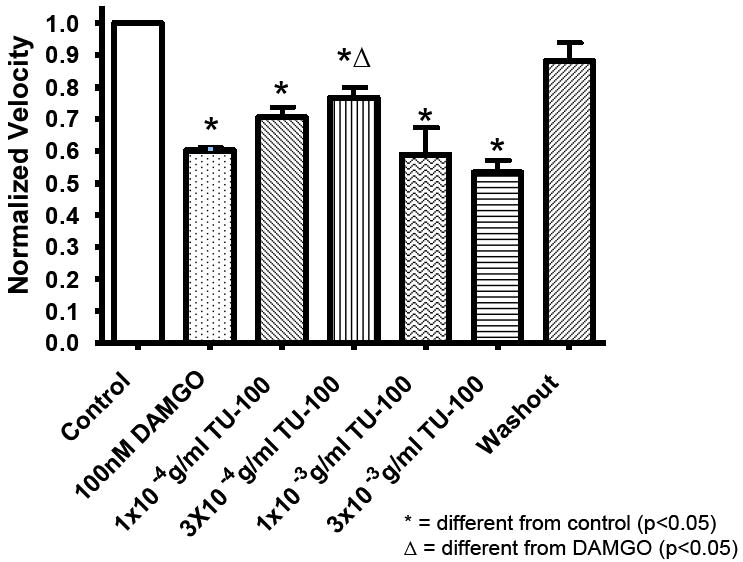
TU-100 caused a concentration-dependent reversal of the inhibitory effect of DAMGO on the rate of fecal pellet propulsion in the guinea pig distal colon.
Given these results, we next examined whether the combined application of naloxone and DKT could work synergistically to fully restore the propulsive motility of DAMGO treated colon. Figure 5 shows spatiotemporal maps from before and after application of naloxone to colon segments bathed in DAMGO and DKT. The combined application resulted in disrupted motor patterns of motility that included halted motility (5a) or episodic periods of reverse peristalsis (5b).
Figure 5.

Spatiotemporal maps showing pellet motility in a segment of guinea pig colon after the addition of naloxone to a bath containing DAMGO plus DKT. Combined application resulted in disrupted motor patterns of motility that included halted motility 5a or episodic periods of reverse peristalsis.5b.
As documented earlier, 3×10-4 g/ml of DKT attenuated the inhibitory effect of DAMGO on propulsive motility. However, when added alone to an isolated segment of colon, DKT caused disruptive changes in the pattern of motility (Fig 6.) The pellet was observed to slowly advance to a point and then reverse direction until it exited from the oral end. These results suggest that DKT affects activity in the neuromuscular circuitry of the colon.
Figure 6.
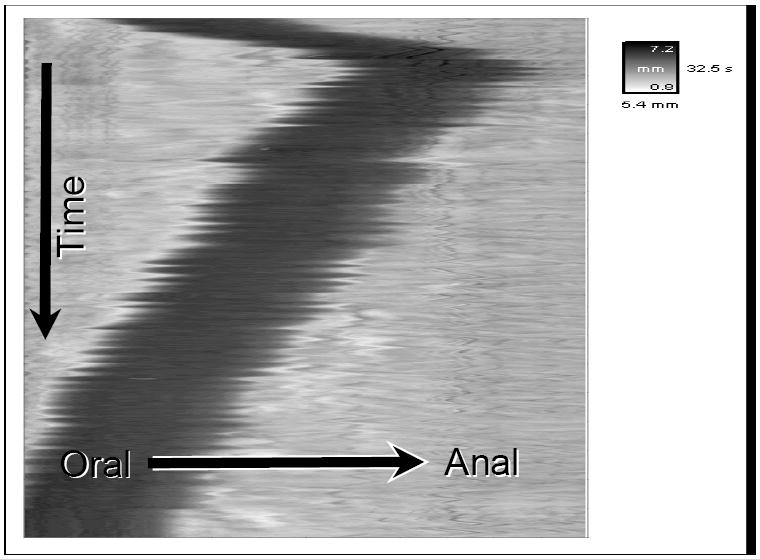
Spatiotemporal map showing pellet motility in a segment of guinea pig colon in the presence on DKT alone.
Effects of DKT on neuromuscular transmission
To test whether DKT affects neuromuscular transmission in the colon, intracellular recordings were obtained from smooth muscle cells. Both spontaneous and evoked junction potentials were evaluated. Figure 7A depicts a typical tracing of spontaneous activity neuromuscular in an isolated segment of colon placed in a control bath. Spontaneous junction potential activity decreased when 3×10-4 g/ml of DKT was added (Fig 7B.) When the colon segment in the control bath solution was stimulated from the oral end with a depolarizing current, inhibitory junction potentials were detected (Fig 7C), but they were blocked in the presence of DKT (Fig 7D). Furthermore, when stimulated from the aboral end with a depolarizing current pulse, excitatory junction potentials were detected in the untreated colon (Fig 7E) but were eliminated in the presence of DKT (Fig 7F).
Figure 7.
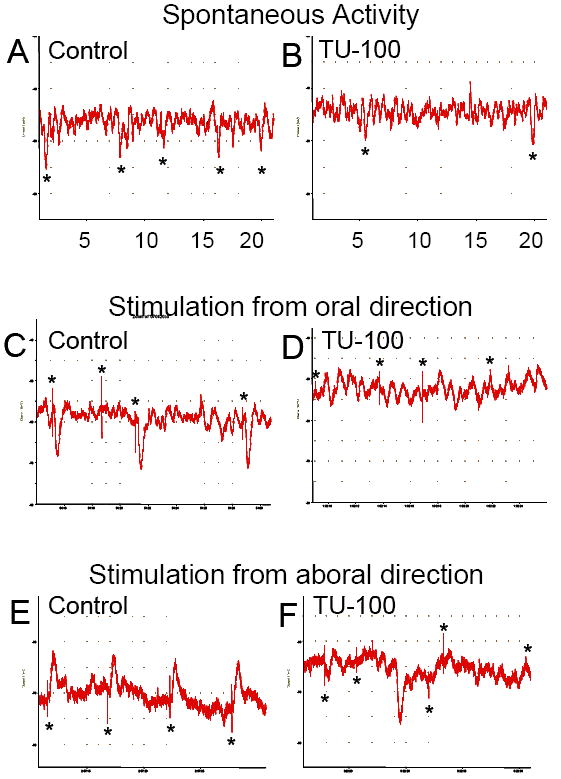
Intracellular recordings obtained from smooth muscle cells. A) A typical tracing of spontaneous activity neuromuscular in an isolated segment of colon placed in a control bath. B) Spontaneous junction potential activity decreased when 3×10-4 g/ml of DKT was added to bath. C) Inhibitory junction potentials elicited by a depolarizing current to the oral end of an untreated isolated colon segment. D) Same inhibitory junction potentials blocked in the presence of DKT. E) Excitatory junction potentials elicited by a depolarizing current to the anal end of an untreated isolated colon segment. F) Excitatory junction potentials eliminated in the presence of DKT.
Discussion
In the studies reported here, we have demonstrated that DKT partially reverses opiate-induced slowing of propulsive motility in the guinea pig distal colon. The extent of this effect is comparable in magnitude to that of the opioid receptor antagonist, naloxone. The inhibitory effects of opiates, such as morphine, and opiate analogs, such as DAMGO, are largely attributed to the suppression of neuronal excitability resulting in subsequent inhibition of neurotransmitter release from enteric excitatory and inhibitory musculomotor neurons.13 Experiments performed in guinea-pig myenteric plexus-longitudinal muscle preparations have demonstrated that exposure to opioids results in suppression of acetylcholine and substance P release. Since the primary input to longitudinal muscle is excitatory, the absence of these excitatory neurotransmitters results in inhibition of longitudinal muscle contraction.14,15 Unlike longitudinal muscle, the predominant neuromuscular input to the circular muscle layer is inhibitory. In a quiescent state, there is ongoing firing of inhibitory motor neurons resulting in relaxation of the circular muscle layer.16 This allows the bowel to relax and receive advancing intraluminal contents. In the presence of opioids and opioid analogs, however, the firing of these inhibitory motorneurons is suppressed thereby resulting in elevated contractile activity of this muscle layer. Taken together, the lack of longitudinal muscle contraction and the absence of circular muscle relaxation result in decreased propulsive motility.
Previous studies have suggested that DKT is able to overcome morphine-induced slowing of transit by causing moderate contraction of morphine-treated longitudinal muscle and relaxation of morphine-induced tonic contraction of circular muscle.12 The exact neuronal mechanism by which this occurs is unknown, but appears to be partly associated with stimulation of serotonin receptors and vanilloid receptors.12,17
The various constituents of DKT were not examined independently in these experiments, but previous experiments indicate that the active ingredient in DKT is the Sichuan pepper. Experiments performed on guinea pig colon revealed similar contractile effects between DKT and Sichuan pepper alone. Meanwhile, the Asian ginseng and ginger had no contractile effect.18
Clearly, clinical trials utilizing DKT are needed, but the results of these experiments suggest that DKT could potentially offer a therapeutic role in the management of opiate induced ileus. Unlike naloxone which blocks the opiate receptor and thereby negates any positive analgesic effect, DKT appears to have no alteration of the anti-nociceptive affect in mice.12 This would be of particular use in a post-operative setting where systemic opiates would be needed for analgesia.
When used in the absence of opiates, DKT was observed to cause disruptions in the intrinsic reflex circuit of the gut by interfering with neuromuscular transmission. This was first apparent by visualizing spontaneous contractile activity of the colonic segments and with our spatial-temporal maps. The isolated colon segment was observed to move erratically in the presence of DKT rather than contract rhythmically as it did in the absence of DKT. This interesting observation may also help explain why DKT has been useful for the prevention of adhesive bowel obstructions. One proposed mechanism by which post-operative adhesions form has to due with areas of bowel stasis. The theory suggests that areas of hypokinesis allow more time for scar tissue to form and adhere, thereby causing an obstruction. Such a theory is supported when one encounters a single adhesive band during laparotomy. Since DKT apparently causes hyperkinesis, it would seem reasonable that this increased motion would not allow sufficient time or stasis for adhesions to form.
From these experiments, it is clear that DKT is effective in overcoming the slowing of motility produced by the presence of opiates such as DAMGO. Furthermore, this amelioration seems to work by a mechanism that is different from that of opiate-receptor antagonists. When used in the absence of opiates, however, a very different result becomes apparent. Rather than accelerate motility or even produce no effect, DKT seemed to cause drastic disruptions in propulsive motor activity. It is unknown what effect, if any, these disruptions would have in human subjects. It would seem that further work needs to be done in order to understand the exact mechanism by which these disruptions occur. This could include examining how the individual ingredients of DKT influence both neuronal and muscle signal transmission. This, unfortunately is beyond the scope of this present study.
In summary, the results of this investigation suggest that DKT is as effective as naloxone in restoring motility in DAMGO treated guinea pig colon. The actions of these two agents, however, were not additive. These findings suggest that DKT might provide some benefit in the restoration of colonic motility in post-operative patients receiving opiate analgesic therapy and/or in patients requiring chronic opiate use.
Acknowledgments
We gratefully acknowledge the technical and intellectual contributions of Drs. Onesmo Balemba and Eric Krauter. This publication was made possible by NIH grant DK62267 to GMM and NIH Grant Number P20 RR16435 from the COBRE Program of the National Center for Research Resources.
References
- 1.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004 Oct;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishiwaki H, Saitoh N, Nishio H, et al. Possible role of potassium channels in mu-receptor mediated inhibition and muscarinic autoinhibition in acetylcholine release from the myenteric plexus of guinea pig ileum. Jpn J Pharmacol. 2000;82:343–349. doi: 10.1254/jjp.82.343. [DOI] [PubMed] [Google Scholar]
- 3.Frantzides CT, Cowles V, Salaymeh B, et al. Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg. 1992;163:144–148. doi: 10.1016/0002-9610(92)90267-u. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt WK. Alvimopan (ADL 8-2698) is a novel peripheral opioid antagonist. Am J Surg. 2001;182:27S–38S. doi: 10.1016/s0002-9610(01)00784-x. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M. Alvimopan, a selective peripherally acting μ-opioid antagonist. Neurogastroenterol Motil. 2005;7:157–165. doi: 10.1111/j.1365-2982.2005.00640.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda H, Chen C, Mantyh C, Ludwig K, Pappas TN, Takahashi T. The Herbal Medicine, Dai-Kenchu-To, Accelerates Delayed Gastrointestinal Transit after the Operation in Rats. J Surg Res. 2006;131:290–295. doi: 10.1016/j.jss.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Delaney CP, Weese JL, Hyman NH, Bauer J, Techner L, Gabriel K, Du W, Schmidt WK, Wallin BA Alvimopan Postoperative Ileus Study Group. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. 2005 Jun;48(6):1114–25. doi: 10.1007/s10350-005-0035-7. [DOI] [PubMed] [Google Scholar]
- 8.Moriwaki Y, Yamamoto T, Katamura H, Sugiyama M. Clinical research to the effect of dai-kenchuto for simple intestinal obstruction. Nihon Touyou Igaku Zassi. 1992;43:303. [Google Scholar]
- 9.Furukawa Y, Shiga Y, Hanyu N, Hashimoto Y, Mukai H. Effect of Chinese herbal medicine on gastrointestinal motility and bowel obstruction. Jpn J Gastroenterol Surg. 1995;28:956. [Google Scholar]
- 10.Itoh T, Yamakawa J, Mai M, Yamaguchi N, Kanda T. The effect of the herbal medicine dai-kenchu-to on postoperative ileus. J Int Med Res. 2002;30:428. doi: 10.1177/147323000203000410. [DOI] [PubMed] [Google Scholar]
- 11.Shibata C, Sasaki I, Naito H, Ueno T, Matsuno S. The herbal medicine Dai-Kenchu-Tou stimulates upper gut motility through cholinergic and 5-hydroxytryptamine 3 receptors in conscious dogs. Surgery. 1999;126:918. [PubMed] [Google Scholar]
- 12.Nakamura T, Sakai A, Isogami I, Noda K, Ueno K, Yano S. Abatement of Morphine-Induced Slowing in Gastrointestinal Transit by Dai-kenchu-to, a Traditional Japanese Herbal Medicine. Jpn J Pharmacol. 2002;88:217–221. doi: 10.1254/jjp.88.217. [DOI] [PubMed] [Google Scholar]
- 13.Wood JD. Opioids, the enteric nervous system, and postoperative ileus. Semin Colon Rectal Surg. 2005;16:188–196. [Google Scholar]
- 14.Grider JR, Makhlouf GM. Role of opioid neurons in the regulation of intestinal peristalsis. Am J Physiol. 1987;253:G226–231. doi: 10.1152/ajpgi.1987.253.2.G226. [DOI] [PubMed] [Google Scholar]
- 15.Nishiwaki H, Saitoh N, Nisho H, et al. Possible role of potassium channels in mu-receptor-mediated inhibition and muscarinic autoinhibition in acetycholine release from myenteric plexus of guinea pig ileum. Jpn J Pharmacol. 2000;82:343–349. doi: 10.1254/jjp.82.343. [DOI] [PubMed] [Google Scholar]
- 16.Manaka H, Manaka Y, Kostolanska F, et al. Release of VIP and substance P from isolated perfused canine ileum. Am J Physiol. 1989;257:G182–G190. doi: 10.1152/ajpgi.1989.257.2.G182. [DOI] [PubMed] [Google Scholar]
- 17.Prins NH, Akkermans LM, Lefebvre RA, Schuurkes JA. 5HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol. 2000;131:927–932. doi: 10.1038/sj.bjp.0703615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurosawa S, Nishikawa S, Kaneko M, Ogiwara S, Ro S, Nakamura T. The Herbal Medicine Dai-Kenchu-To (DKT) contracts guinea pig distal colon muscle through acetylcholine release. Gastroenterology. 1998;114:A782. [Google Scholar]


