Abstract
Objectives
To determine whether objectively measured physical activity levels are associated with physical function and mobility in older men.
Design
Cross-sectional.
Setting
Academic research center.
Participants
Eighty-two community-dwelling men ≥ 65 years of age with self-reported mobility limitations were divided into a low activity and a high activity group based on the median average daily physical activity counts of the whole sample.
Measurements
Physical activity by triaxial accelerometers; physical function and mobility by the Short Physical Performance Battery (SPPB), gait speed, stair climb time, and a lift and lower task; aerobic capacity by maximum oxygen consumption (VO2max); and leg press and chest press maximal strength and peak power.
Results
Older men with higher compared to lower physical activity levels demonstrated a > 1.4 point higher mean SPPB score and a 0.35 m/s faster walking speed. They also climbed a standard flight of stairs 1.85 sec faster and completed 60% more shelves in a lift and lower task (all p < 0.01). Muscle strength and power measures, however, were not significantly different between the low and high activity group. Correlation analyses and multiple linear regression models showed that physical activity is positively associated with all physical function and mobility measures, leg press strength, and VO2max.
Conclusion
Older men with higher physical activity levels demonstrate better physical function and mobility than less active peers. Moreover, in older men physical activity levels are predictive of performance in measures of physical function and mobility. Future work is needed to determine whether modifications in physical activity levels can improve or preserve physical performance in later-life.
Keywords: aging, sarcopenia, muscle strength, disability, exercise
INTRODUCTION
Beyond the sixth decade of life there is a progressive increase in the perceived and measured difficulty in performing activities such as walking, climbing stairs and lifting objects 1. In older individuals, limitations in these and other metrics of physical function and mobility are strongly predictive of falls 2, disability 3, hospitalization 4, quality of life 5 and even mortality 6. Thus, there is substantial merit in identifying strategies that attenuate and/or reverse age-related declines in physical performance.
Physical activity levels decline significantly with age 7 and nearly 60% of older persons without disabilities are either insufficiently active or overtly inactive 8. A number of reports from large epidemiological studies have shown that self-reported physical activity levels in older adults are associated with performance in mobility tasks 9, independence in activities of daily living 10, and number of disability-free years 11. While these studies have drawn attention to physical inactivity as a potential contributor to limitations in physical function and mobility, there are substantive concerns regarding their use of self-report methods to quantify physical activity. Limitations to self-report methods include social desirability bias (over-reporting good behavior), poor sensitivity, subjectivity, and dependence on recall. Of note, analysis of self-reported physical activity data from the 2003–2004 National Health and Nutrition Examination Survey revealed subjects significantly overestimated physical activity count, duration and adherence 12.
Thus, in the present study we used tri-axial accelerometers to objectively quantify physical activity and examine its relationship with measures of physical performance. Accelerometers have demonstrated excellent reliability, validity and utility in older cohorts 13–15 and the ability to distinguish physical activity levels between individuals 16. We tested the hypothesis that older men with higher compared to lower habitual physical activity levels would exhibit better performance in objective measures of physical function and mobility, aerobic capacity and muscle strength and power. For measures that differed between groups, we determined the contribution of physical activity to the variance in measures of physical performance amongst all subjects.
METHODS
Subjects
Baseline data of older men with low testosterone levels (< 350 ng/dl by liquid chromatography tandem mass spectrometry) participating in a clinical trial at Boston Medical Center were used in the present study. Subjects were recruited from the greater Boston area through community-based organizations, direct mailings and a media campaign (detailed in 17). Inclusion criteria included age of ≥ 65 years, community-dwelling, and self-reported difficulty in walking two blocks on a level surface or in climbing a flight of stairs. Subjects completed a supervised graded exercise test prior to enrollment. Those who demonstrated signs and/or symptoms of ischemia or had a cardiac history that precluded participation in the more strenuous tasks participated in a subset of outcome measures. Exclusion criteria included myocardial infarction or fracture within the past 6 months and other orthopedic, cardiac (e.g., symptomatic coronary artery disease or uncontrolled hypertension), cognitive or neurological impairments that would prohibit participation. The study protocol was approved by the Institutional Review Board for Human Subjects research At Boston University Medical Center. All subjects provided written informed consent.
Body weight (kg) and height (m) were measured and used to calculate body mass index (BMI). Number of medications and existing medical conditions were captured through self-report using a standardized questionnaire.
Physical Activity
Triaxial accelerometers (Actigraph, Pensacola, FL) were used to quantify habitual physical activity. The sum of acceleration changes in the anterior-posterior, medial-lateral and vertical axes were measured 30 times per second and a microprocessor in the accelerometer calculated and stored the number of activity counts recorded over one-minute sampling intervals (epochs). Subjects were instructed to wear the accelerometer on an elastic belt over their left or right hip for 7 days while awake and were provided a log to record their compliance. For inclusion in the present study, subjects were required to have worn the accelerometer on at least five complete days to determine their average daily physical activity counts. The median average daily physical activity counts was calculated for the sample. Older men who had average daily physical activity counts below the median value for the sample were assigned to the low activity group and older men above the median value were assigned to the high activity group.
Physical Function and Mobility
The Short Physical Performance Battery (SPPB) was employed as a composite measure of lower extremity function 18. Standing balance, 4-meter walk time and repeated chair rise time (5 sit-to-stand sequences) were each scored on 0–4 point categorical scale. The summary performance score (0–12) was derived from the 3 individual measures and used for analyses.
To capture gait speed, subjects were instructed to walk as fast as possible over 50 meters as previously described 19. Assistive devices (e.g., canes and walkers) were allowed. Time was measured to the nearest 0.001 second using a switch mat and infrared timing system (Lafayette Instrument Company, Lafayette, IN) and used to calculate gait speed (m/sec). Two trials were performed and the best performance was used for analyses. The ICC of the 50m gait measure in this cohort is 0.98819.
Stair climb time was determined on a single flight of stairs consisting of 12 steps (step height = 16 cm) as previously described using a switch mat timing system (Lafayette Instrument Company, Lafayette, IN) 19. Subjects were instructed to climb the stairs as fast as possible while touching every step and allowed to use the handrail only if needed. Two trials were performed (ICC = 0.99219) and the best performance was used for analyses.
As a measure of upper body function, a lift and lower task was performed in which subjects were instructed to lift a weighted basket (equivalent to 15 percent of body weight) from a shelf positioned at standard desk height (78.5 cm) and place it on a shelf positioned at their respective shoulder height, then to a shelf positioned at their respective head height and then to lower it back down in the reverse sequence 19. Subjects repeated this sequence as many times as possible in 1 minute and the number of shelves completed was recorded. Two trials were performed (ICC = 0.94719) and the best performance was used for analyses.
Aerobic Capacity
Maximum oxygen consumption (VO2max) was measured during a graded maximal cardiopulmonary exercise test in subjects using an electrically-braked cycle ergometer (Ergoline, Bitz, Germany). In brief, subjects pedaled at 60 revolutions per minute as the work rate progressively increased by a predetermined (per patient health and predicted VO2max) increment (e.g., 5, 10 or 15 watts/min). Breath-by-breath analysis for metabolic parameters was performed by a SensorMedics Vmax Encore System (Yorba Linda, CA) that was integrated with a 12-lead cardiac monitoring system (Cardiosoft, Cardinal Health, Columbus, OH). Metabolic data collected from subjects whose tests were terminated due to volitional fatigue, and not due to abnormalities in metabolic, hemodynamic or cardiac parameters, were included in the analyses.
Muscle Strength and Power
Maximal voluntary strength of the lower extremities and the upper body was quantified by measuring the one repetition maximum (1RM) for the leg press and chest press, respectively, with Keiser A420 pneumatic resistance machines and integrated software (Keiser Sport, Fresno, CA). A standardized protocol was executed as previously described and two trials were performed separated by at least 2 but not more than 7 days19. The intra-class correlation coefficient of the 1RM protocol in this cohort is ≥ 0.98319. Handgrip strength was also assessed using a dynamometer (Jamar Technologies, Inc., Hatfield, PA). Three trials were completed for each hand and the highest value (kg) was used for analyses.
Peak muscle power for the chest press and leg press were also quantified using the Keiser A420 platform as previously described 20. Briefly, power was measured at 50, 60 and 70 % and 40, 50 and 60 % of the 1RM for the leg press and chest press, respectively. Five repetitions were performed at a selected percentage (in a randomized order) and the highest power generated was recorded as peak power.
Data Analysis
All variables were examined visually and statistically for normality of distribution and a logarithmic transformation was applied for non-normally distributed variables. The subjects were evenly divided into low (n = 41) and high activity groups (n = 41) based on the median average daily physical activity counts for the whole sample. Specifically, older men who had average daily physical activity counts below the median value for the sample were assigned to the low activity group and older men above the median value were assigned to the high activity group. Student’s t-test for independent samples was used to identify significant differences in subject characteristics and physical performance measures between the low and high activity groups. Pearson product-moment correlation coefficients were calculated to measure and interpret the relationships between average daily physical activity, subject characteristics and the physical performance variables. Outcome measures that were significantly different between low and high activity groups were further analyzed using multiple linear regression models to determine whether average daily physical activity could predict performance. The strength of association between physical activity (presented as average daily physical activity counts × 10−5) and the corresponding dependent variable was assessed using partial correlations (partial r), after covarying for age, BMI and number of medications. Regression model assumptions were examined both graphically and analytically. All analyses were performed using SPSS software (Version 16.0 for Windows, Chicago, IL) and GraphPad Prism software (Version 4.03 for Windows, La Jolla, CA). P values ≤ 0.05 were considered statistically significant.
RESULTS
Subjects
Of the 125 older men who met the inclusion criteria for the parent study, 82 subjects had worn the accelerometer for ≥ 5 days (mean ± SE = 6.6 ± 0.09 days) and were included in the analysis. Based on medical history and the cardiopulmonary exercise test, 76 % (n = 62) of the subjects were permitted to complete all outcome measures, and the remaining 20 subjects participated in only a subset of outcome measures that included the SPPB and 50 meter walk to optimize participant safety.
Subjects were evenly divided to high (n = 41) and low (n = 41) activity groups based on the median average daily physical activity counts of the sample (115166 counts). Comparisons of high and low activity groups revealed no difference in age or BMI. The number of medications and number of existing medical conditions, however, were significantly greater in the low compared to the high activity group (both p < 0.03). Subject characteristics are reported in Table 1.
Table 1.
Characteristics of older male subjects (mean ± standard error) and analysis of differences between subjects with low compared to high physical activity levels.
| All Subjects (n = 82) | Low Activity Group (n = 41) | High Activity Group (n = 41) | p value | |
|---|---|---|---|---|
| Age (years) | 74.1 ± 5.3 | 74.5 ± 5.6 | 73.7 ± 5.0 | 0.471 |
| Race | ||||
| • Black/African American (%) | 10.0 | 7.9 | 11.9 | |
| • Caucasian (%) | 87.5 | 89.5 | 85.7 | |
| • Asian/Pacific Islander (%) | 0.0 | 0.0 | 0.0 | |
| • Other (%) | 2.5 | 2.6 | 2.4 | |
| BMI (kg/m2) | 30.4 ± 4.6 | 30.6 ± 4.6 | 30.3 ± 4.6 | 0.758 |
| Medications (#) | 5.8 ± 3.5 | 6.6 ± 3.5 | 4.9 ± 3.4* | 0.027 |
| Conditions (#)* | 3.0 ± 1.7 | 3.4 ± 1.7 | 2.6 ± 1.6* | 0.023 |
| • Cardiovascular (%) | 51.9 | 67.5 | 36.6 | |
| • Respiratory/pulmonary (%) | 21.0 | 25.0 | 17.1 | |
| • Diabetes (%) | 30.5 | 42.5 | 19.0 | |
| • Depression (%) | 19.8 | 20.0 | 19.5 | |
| • Musculoskeletal (%) | 58.2 | 61.5 | 55.5 | |
| Tobacco Use (packs/week) | 1.04 ± 0.13 | 1.24 ± 0.22 | 0.88 ± 0.14 | 0.131 |
| Alcohol Use (drinks/week) | 1.94 ± 0.15 | 1.74 ± 0.18 | 2.15 ± 0.25 | 0.229 |
| Activity (counts)† | 12.25 ± 6.98 | 7.29 ± 2.63 | 17.20 ± 6.44 | < 0.001 |
Conditions: Percentage of subjects (%) with indicated conditions. Cardiovascular includes myocardial infarction, coronary artery bypass graft, cerebrovascular accident, hypertension, and peripheral vascular disease; respiratory/pulmonary includes asthma, emphysema, and chronic obstructive pulmonary disease; diabetes (type 2 diabetes mellitus); musculoskeletal includes osteoarthritis, joint surgery, and joint pain
Activity: average daily physical activity counts reported × 10−5
Older men with higher compared to lower physical activity levels demonstrate better physical function and mobility
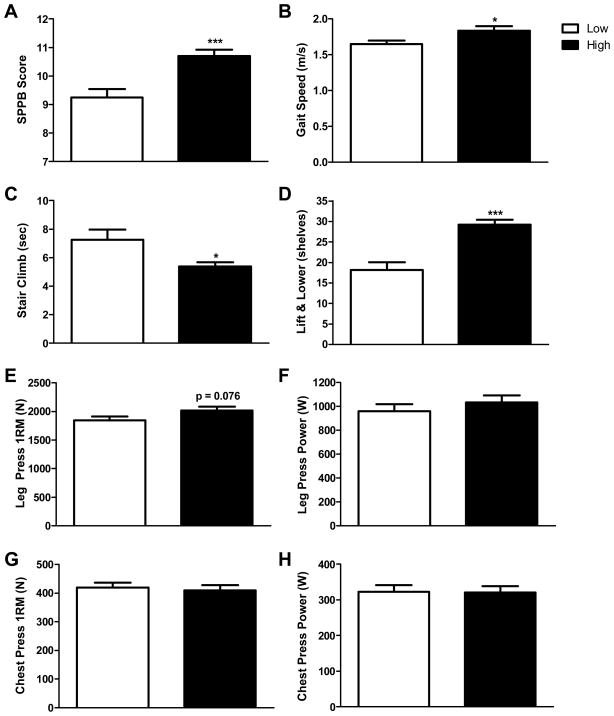
Comparison of lower extremity mobility by the SPPB demonstrated a significant difference of 1.46 points (95% confidence interval = 0.72 to 2.21) between the high and low activity groups (p < 0.001) (Figure 1A). In addition, subjects in the high activity group exhibited gait speeds that were 0.35 m/s faster than the low activity group in the 50m walk measure (p< 0.001) (Figure 1B). Similarly, older men in the high compared to low activity group ascended a standard flight of stairs 1.87 seconds (25.7%, p = 0.01) faster (Figures 1C). In respect to upper extremity function, the mean number of shelves completed in the lift and lower task was 11 (60.4%) more in the high activity group than the low (p < 0.001) (Figure 1D).
Figure 1.
Older men with high compared to low habitual physical activity demonstrate better performance in measures of physical function and mobility but not strength or power. Accelerometers were used to determine the average daily physical activity counts in 82 older men and the median average daily physical activity counts was calculated for the sample. Older men who had average daily physical activity counts below the median value for the sample were assigned to the low activity group and older men above the median value were assigned to the high activity group. Evaluation of lower extremity mobility and function using the SPPB demonstrated significant differences between men with low compared to high physical activity levels (A). Likewise, measures of gait speed over a 50 meter distance (B) and the time to climb a standard flight of stairs (C) revealed significant differences between group means. Examination of upper extremity function using a lift and lower measure demonstrated that individuals with relatively high compared to low physical activity levels could complete significantly more shelves (D). Determination of maximum voluntary strength by the 1RM measure (E) and peak muscle power (F) for the leg press demonstrated a modest but insignificant difference (p = 0.076) and no difference (p = 0.359) between men with low compared to high levels of habitual physical activity, respectively. Upper extremity strength and power quantified by measuring the chest press 1RM (G) and peak power (H) were also not different between groups (p = 0.710 and 0.945, respectively). * p < 0.05 and *** p < 0.001.
In examining the relationship between habitual physical activity and measures of physical function and mobility across all subjects, correlation analysis revealed significant associations between physical activity level and SPPB score (r= 0.48, p < 0.001) as well as gait speed (r= 0.40, p < 0.001) (Table 2). Stair climb time was also significantly, but less strongly, associated with physical activity levels (r= 0.27, p = 0.03). In addition to measures of lower extremity function and mobility, a significant and robust correlation between physical activity and the lift and lower measure was observed (r= 0.48, p < 0.001).
Table 2.
Pearson correlation coefficients among covariates, the independent variable, and dependent variables.
| BMI | Medications | Conditions | Activity | SPPB | Gait Speed | Stair Climb | Lift & Lower | VO2 | LPa 1RM (N) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | −0.18 | 0.04 | 0.16 | 0.01 | −0.01 | 0.28* | 0.06 | −0.17 | 0.02 | −0.18 |
| BMI (kg/m2) | −0.07 | −0.06 | −0.02 | −0.06 | 0.05 | 0.10 | −0.33* | −0.02 | 0.24* | |
| Medications(#) | 0.47* | −0.29** | −0.29** | 0.31* | 0.11 | −0.19 | −0.20 | −0.13 | ||
| Conditions (#) | −0.22* | −0.11 | −0.33* | 0.07 | −0.09 | −0.06 | −0.07 | |||
| Activity (counts) | 0.48** | 0.40** | −0.27* | 0.48** | 0.23* | 0.29* | ||||
| SPPB Score | 0.78** | −0.60** | 0.52** | 0.23* | 0.46** | |||||
| Gait Speed (m/s) | 0.85** | 0.47** | −0.18 | −0.39* | ||||||
| Stair Climb (sec) | −0.38** | −0.22 | −0.39** | |||||||
| Lift & Lower (shelves) | 0.37** | 0.43** | ||||||||
| VO2max (ml/kg·min) | 0.16 |
p < 0.05
p < 0.01
Leg Press (LP)
The results of the multiple regression analyses demonstrated that hysical activity was a significant independent predictor of SPPB, explaining 19% of the variance in SPPB score after adjustment for covariates (β = 1.13, partial r2 = 0.19, p < 0.001). Similarly, for upper extremity function, physical activity was also found to be a significantly predictor of the number of shelves completed in the lift and lower task (β = 5.93, partial r2 = 0.22, p < 0.001).
Older men with higher compared to lower physical activity levels demonstrate increased aerobic capacity
Aerobic capacity, determined by measuring VO2max, was 12 % higher in subjects exhibiting high (20.13 ± 0.72 ml/kg·min) compared to low physical activity levels (17.90 ± 0.73 ml/kg·min) (p = 0.04). A small but significant relationship was found between physical activity and VO2max across all subjects (r = 0.23, p < 0.05) (Table 2), however, multiple regression analysis demonstrated that physical activity was no longer a significant predictor of VO2max (model r2 = 0.076, p = 0.28) (data not shown).
Older men with low compared to high physical activity levels are not differentiated among measures of muscle strength or power
In contrast to measures of physical function and mobility, overall, comparisons of upper body and lower extremity muscle strength and power between low and high activity groups showed no significant differences. Leg press strength showed a positive trend (p=0.08) as the high activity group exerted 171 N (9 %) more force than the low activity group (Figure 1E); however, comparisons of mean chest press (Figure 1G) and handgrip strength (29.7 ± 1.0 and 29.8 ± 1.1 kg in low and high activity groups, respectively) and leg press and chest press power (Figures 1F and 1G, respectively) revealed no significant differences between groups (all p ≥ 0.36).
Leg press strength was included in the correlation matrix given the trend for a difference between the low and high activity groups and a significant correlation with physical activity level was observed (r = 0.29, p = 0.02) (Table 2). The multiple regression model revealed a small but significant predictive relationship with leg press strength across all subjects (β = 200.0, partial r2 = 0.09, p < 0.01).
DISCUSSION
The data presented here show remarkable distinctions between older men with differing levels of objectively measured habitual physical activity. In regards to general health, participants of similar age and BMI, but exhibiting low relative physical activity levels, had more medical conditions (particularly of cardiovascular and metabolic origin) and were prescribed more medications. Consistent with our hypothesis, subjects in the high activity group demonstrated better performance on objective measures of physical function and mobility. Physical activity explained a significant portion of the variance in performance of these measures amongst participants even when adjusted for age, BMI and medications. Surprisingly, physical activity demonstrated a less robust relationship with measures of muscle strength and aerobic capacity, and no association with peak muscle power.
These data lend support to previous epidemiological studies that have reported associations between self-reported physical activity levels and various metrics of physical function and mobility. Even in this generally inactive cohort of older men, the differences in physical function and mobility measures between the low and high activity groups were striking. The SPPB score of the low activity group suggests a mild to moderate risk for mobility-related disability and dependency in activities of daily living compared the high activity group 3. The difference in SPPB score between low and high activity groups of 1.47 points far exceeds the recent estimation of a small (0.54 points) as well as a substantial (1.34 points) clinically meaningful difference in this measure in community dwelling older individuals 21. The significant relationship between physical activity and SPPB and the difference between groups revealed by the SPPB are corroborated by other measures of lower extremity function (gait speed and stair climb time) as well as upper extremity function (lift and lower measure). For example, the calculated difference in gait speed of 0.35 m/s suggests that the low activity group would require an additional 2 minutes and 20 seconds to walk 400 meters; a clinically important and substantial difference 22 in a favored metric for capturing mobility disability in older populations 22–24.
In contrast to previous epidemiological studies (e.g., 9, 25), differences in objective measures of muscle strength between low and high activity groups were negligible with the exception of the leg press. This may reflect inherent differences in the characterization of activity by self-report compared to accelerometry. Alternatively, the modest differences in strength, as well as aerobic capacity, between groups, and their weak associations with physical activity, may simply reflect the smaller and more homogenous cohort studied here. Whether interventions designed to promote physical activity in older men and women, independent of improving muscle strength or power or aerobic capacity, can improve or prevent limitations in physical function and mobility remains to be determined.
Questionnaire-based methods that qualitatively assess physical activity, such as the Physical Activity Scale for the Elderly (PASE), remain useful in large epidemiological studies because of their speed and ease of administration and scoring. With respect to accuracy, however, the widely used PASE has demonstrated only modest correlations (r = 0.28–0.49) with objective measures of physical activity including doubly labeled water and accelerometers in older individuals 26, 27. Moreover, these instruments lack sensitivity to change. For example, 3 of 10 questions on the PASE ask if a subject has participated in certain household activities in the previous 7 days, but does not capture either the frequency or duration of these activities. Collectively, the previously mentioned concerns (bias, subjectivity and dependency on recall), limitations in accuracy, and lack of sensitivity to change limit the functionality of questionnaire-based metrics of physical activity. Accelerometers overcome these limitations and provide a means to accurately and objectively measure physical activity, evaluate the effectiveness of strategies to promote physical activity, identify minimal detectable changes, and determine the amount and/or intensity of physical activity required to maintain or enhance physical function and mobility in older persons.
It should be noted that the findings of the present study may be limited to older men; and having used the baseline data of subjects participating in a clinical trial, the generalizability may be further limited to men with low testosterone levels (< 350 ng/dl). In regards to the related nature of physical activity and physical function and mobility, we chose to examine physical activity as the independent variable given the opportunity to modify activity levels in older individuals for the purpose of attenuating, preventing and/or reversing the loss of physical function and mobility in later life. Recent data from the Lifestyle Interventions and Independence for Elders (LIFE) pilot study suggests this may in fact be a viable strategy 28. Lastly, we failed to include measures of depression or cognition in the current study and determine their potential relationships and contributions to physical activity levels and physical performance. While several reports have concluded physical activity positively affects cognition (e.g., 29) and depression (e.g., 30) in older adults, we cannot exclude reverse causation; e.g., depression accounts for the reduced physical activity levels and subsequently the poorer performance in measures of physical function and mobility in the low activity group.
In summary, the data advance the concept that physical activity level is a key determinant of physical function and mobility in older men. Our data also suggest older individuals with relatively low physical activity levels may be at an elevated risk for disability based on poor performance on objective outcome measures of physical function and mobility. In the face of an aging epidemic and the preponderance of sedentary lifestyles, studies are warranted to determine whether strategies to promote physical activity may counter the onset and consequences of limitations in physical function and mobility.
Acknowledgments
The authors would like to thank the research coordinators and staff of the BUMC GCRC. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Funding Sources: This work was supported by the Boston Claude D. Pepper Older Americans Independence Center Grant (P30-AG031679) and grant U01-AG01436906 to SB. NKL was supported by NIH grant K01-AG031154. This work is based upon work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Sponsor’s Role
The sponsor’s had no role in the design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions
Marina Morie: Data acquisition, analysis and interpretation, manuscript preparation
Kieran F. Reid: Data analysis and interpretation, manuscript preparation
Renee Miciek: Study concept and design, data acquisition, analysis and interpretation, manuscript preparation
Newsha Lajevardi: Data acquisition, manuscript preparation
Karen Choong: Data acquisition, interpretation
Joanne B. Krasnoff: Data acquisition, analysis and interpretation, manuscript preparation
Thomas W. Storer: Study design, data interpretation
Roger A. Fielding: Study concept and design, data analysis and interpretation, manuscript preparation
Shalender Bhasin: Study concept and design, data interpretation, manuscript preparation
Nathan K. LeBrasseur: Study concept and design, data acquisition, analysis and interpretation, manuscript preparation.
References
- 1.Jette AM, Pinsky JL, Branch LG, et al. The Framingham Disability Study: Physical disability among community-dwelling survivors of stroke. J Clin Epidemiol. 1988;41:719–726. doi: 10.1016/0895-4356(88)90157-6. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 6.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 7.Westerterp KR, Meijer EP. Physical activity and parameters of aging: A physiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 2):7–12. doi: 10.1093/gerona/56.suppl_2.7. [DOI] [PubMed] [Google Scholar]
- 8.Brown DR, Yore MM, Ham SA, et al. Physical activity among adults >or=50 yr with and without disabilities, BRFSS 2001. Med Sci Sports Exerc. 2005;37:620–629. doi: 10.1249/01.mss.0000158189.17546.ed. [DOI] [PubMed] [Google Scholar]
- 9.Rantanen T, Guralnik JM, Sakari-Rantala R, et al. Disability, physical activity, and muscle strength in older women: The Women’s Health and Aging Study. Arch Phys Med Rehabil. 1999;80:130–135. doi: 10.1016/s0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- 10.LaCroix AZ, Guralnik JM, Berkman LF, et al. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–869. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 11.Nusselder WJ, Looman CW, Franco OH, et al. The relation between non-occupational physical activity and years lived with and without disability. J Epidemiol Community Health. 2008;62:823–828. doi: 10.1136/jech.2007.067165. [DOI] [PubMed] [Google Scholar]
- 12.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 13.Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol. 2007;100:581–589. doi: 10.1007/s00421-006-0320-8. [DOI] [PubMed] [Google Scholar]
- 14.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Activity. 2009;17:17–30. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Kochersberger G, McConnell E, Kuchibhatla MN, et al. The reliability, validity, and stability of a measure of physical activity in the elderly. Arch Phys Med Rehabil. 1996;77:793–795. doi: 10.1016/s0003-9993(96)90258-0. [DOI] [PubMed] [Google Scholar]
- 16.Westerterp KR. Assessment of physical activity level in relation to obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S522–525. doi: 10.1097/00005768-199911001-00006. [DOI] [PubMed] [Google Scholar]
- 17.LeBrasseur NK, Lajevardi N, Miciek R, et al. Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (The TOM Trial): design and methods. Contemp Clin Trials. 2009;30:133–140. doi: 10.1016/j.cct.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.LeBrasseur NK, Bhasin S, Miciek R, et al. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56:2118–2123. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielding RA, LeBrasseur NK, Cuoco A, et al. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50:655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 21.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 22.Kwon S, Perera S, Pahor M, et al. What Is a Meaningful Change in Physical Performance? Findings from a Clinical Trial in Older Adults (The LIFE-P Study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolland YM, Cesari M, Miller ME, et al. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang M, Cohen-Mansfield J, Ferrucci L, et al. Incidence of loss of ability to walk 400 meters in a functionally limited older population. J Am Geriatr Soc. 2004;52:2094–2098. doi: 10.1111/j.1532-5415.2004.52570.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuh D, Bassey EJ, Butterworth S, et al. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: Associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005;60:224–231. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Bonnefoy M, Normand S, Pachiaudi C, et al. Simultaneous validation of ten physical activity questionnaires in older men: A doubly labeled water study. J Am Geriatr Soc. 2001;49:28–35. doi: 10.1046/j.1532-5415.2001.49006.x. [DOI] [PubMed] [Google Scholar]
- 27.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 28.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 29.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 30.Strawbridge WJ, Deleger S, Roberts RE, et al. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156:328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]