Abstract
Long term consumption of a high fat diet (HFD) contributes to increased morbidity and mortality. Yet the specific effects of HFD consumption on brain aging are poorly understood. In the present study 20-month old male C57Bl/6 mice were fed either “Western Diet” (WD, 41% fat), very high fat lard diet (HFL, 60% fat), or corresponding control diets for 16 weeks and then assessed for changes in metabolism and brain homeostasis. Although both HFDs increased adiposity and fasting blood glucose, only the HFL diet increased age-related oxidative damage (protein carbonyls) and impaired retention in the behavioral test. This selective increase in oxidative damage and cognitive decline was also associated with a decline in Nrf2 levels and Nrf2 activity, suggesting a potential role for decreased antioxidant response. Taken together, these data suggest that while adiposity and insulin resistance following HFD consumption are linked to increased morbidity, the relationship between these factors and brain homeostasis during aging is not a linear relationship. More specifically, these data implicate impaired Nrf2 signaling and increased cerebral oxidative stress as mechanisms underlying HFD-induced declines in cognitive performance in the aged brain.
Keywords: High fat, oxidative stress, aging, cognition
Introduction
Metabolism is a composite of multiple cellular processes which regulate both lipid storage and substrate utilization to ensure that sufficient energy is available for vital functions in the face of continually changing energy demands and food availability (Figlewicz 2003, Gao & Horvath 2007, Lam et al. 2009). Consumption of high fat diets (HFDs) negatively impacts this system, resulting in excess energy intake, increased adiposity and impaired regulation of multiple metabolic processes (Woods et al. 2004, Zhang et al. 2009). Epidemiological and experimental studies implicate glucose dysregulation and obesity as factors which increase morbidity and mortality (Cornier et al. 2008, Zimmet et al. 2001), yet the impact of HFD on the brain remains poorly understood, particularly the potential synergies between aging and HFD consumption in regards to the neurochemistry and function of the brain.
Aging is associated with a decline in multiple aspects of cognitive performance, including reductions in mental speed, attention, and multiple aspects of learning and memory (Park & Reuter-Lorenz 2009). Several studies implicate diet and metabolism as modulators of brain aging, with reduced caloric intake preserving cognitive performance (Mattson 2005) but diabetes and obesity in contrast impairing cognitive performance (Bruce-Keller et al. 2009, Farr et al. 2008, Zhang et al. 2009). While overt diabetes and obesity are implicated as mediators of brain dysfunction, they represent relative extremes of metabolic dysfunction, and little is known regarding the effects of subtle metabolic disturbances on brain health within the elderly. Specifically, the possibility that HFD might potentiate age-related cognitive decline in the absence of overt diabetes has not been investigated systematically, nor have studies identified the relative contribution of subclinical glucose dysregulation or adiposity to a decline in cognitive performance in the aged brain.
Both aging and HFD consumption lead to increased brain oxidative damage (Bruce-Keller et al. 2009, Cecarini et al. 2007, Zhang et al. 2009). Considering the well documented relationship between oxidative stress and cognitive decline (Moreira et al. 2008, Su et al. 2008, Swerdlow 2007, Smith et al. 1991, Forster et al. 1996, Head 2009, Perry et al. 2002), these data are consistent with the possibility that increased oxidative stress mediates the effects of HFD consumption on brain pathogenesis and cognitive disturbances in the elderly. While there are multiple forms of oxidative damage, protein oxidation (particularly protein carbonyls) is highly associated with cognitive decline and cellular dysfunction (Sohal 2002, Dalle-Donne et al. 2003, Smith et al. 1991, Forster et al. 1996). Although the mechanisms that lead to these increases in protein oxidation are varied, a number of studies suggest an important role for NF-E2-related factor 2 (Nrf2)-mediated signaling in the protection against brain oxidative damage (Johnson et al. 2008). Via its interaction with the antioxidant response element (ARE), Nrf2 is a major pathway regulating phase II antioxidant responses; however, the role of Nrf2 as a regulator of HFD effects on brain has not been elucidated previously. In the current study, we demonstrate that HFD consumption impairs cognitive function in aged mice, and that this cognitive impairment is specifically associated with increased protein oxidation and impaired Nrf2 signaling.
Materials and Methods
Animals and dietary treatment
All animal experiments were approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Twenty-month old male C57Bl/6 mice were obtained from the contract colony of the National Institute on Aging maintained at Charles River Laboratories and housed in standard caging with 12:12 light:dark cycle and food and water provided ad libitum unless otherwise noted. At the start of the experiment, mice were randomly assigned to one of two high fat diets or their respective control diets (12/group): Western diet (WD; D12079B) consisting of 40% butterfat and 29% sucrose, a High Fat Lard diet (HFL; D12492) composed of 60% animal fat, or their corresponding low fat control diets (WD-C or HFL-C; 98052602, D12450B). All diets were purchased from Research Diets (New Brunswick, NJ). Mice were maintained on diet for 16 weeks, with body weight recorded every 2 weeks.
After 13 weeks on their respective diets, total body adiposity was measured via nuclear magnetic resonance (NMR) spectroscopy (Minispec, Brucker Optics, Billerica MA). Additionally, during week 13 the mice were fasted overnight and blood glucose levels measured on tail-blood samples using a commercially available glucometer (AccuCheck Advantage, Roche). On weeks 14-15 of diet treatment, all mice were measured for cognitive performance using a newly designed 14-Unit T-Maze ((Pistell & Ingram 2009) described below). At week 16 of diet treatment, mice were fasted overnight and euthanized by isoflurane anesthesia followed by rapid decapitation. Trunk-blood was collected and fat pads (epididymal, retroperitoneal, visceral) manually dissected and weighed (White et al. 2009). Brains were rapidly removed following decapitation, and the hippocampus manually dissected and frozen on dry ice for measures of protein oxidation (see below).
Serum hormone analysis
Trunk blood was collected at sacrifice, allowed to clot overnight at 4°C, spun at 3000xg, and serum stored a −80°C. Serum levels of leptin and insulin were measured via a multiplex hormone analysis system (Milliplex Panel; Millipore Corporation, Billerica, MA), using a Luminex 100 IS.
Analysis of protein oxidation
Oxidative modifications to proteins were evaluated by quantifying protein carbonylation in brain tissue homogenates by spectrophotometric analyses using modifications of previously described procedures (Dalle-Donne et al. 2003, Ding et al. 2006, Forster et al. 1996). Hippocampal tissue samples were homogenized in PBS (pH 7.0) containing protease inhibitor cocktail (Sigma Aldrich, Inc.), and homogenates (10 μg of total protein) were incubated with an excess of 2,4-dinitrophenylhydrazone (DNPH) for 20 minutes, followed by the addition of 12% sodium dodecylsulfate (SDS). Each experiment included samples that underwent the protein carbonyl detection procedure without the derivatization step as negative controls. Protein carbonyls were quantified by monitoring the absorbance 370 nm in a quartz 96 well plate using a spectrophotometer, and calculated using extinction coefficient of 22.0 M-1 × cm −1 for aliphatic hydrazones to calculate the amount of protein carbonyls in terms of nmol/mg protein.
Analysis of Nrf2 levels, Nrf2 activity and Nrf2 responsive pathways
Levels of Nrf2 protein were measured via Western blot analysis, using beta-actin to ensure equal loading of the samples. It should be noted that while the levels of Nrf2 protein could be observed to be decreased in HFL fed mice, we did notice considerable variability in the degree to which Nrf2 levels were changed, which is likely based on the fact that beta-actin levels also change with aging and the fact nuclear localization of Nrf2 may interfere with our ability to detect total Nrf2 in pre-cleared lysates. Therefore, we also quantified Nrf2 DNA binding activity using a TransAM™ Kit (Active Motif, Carlsbad, CA) which consisted of an immobilized double-stranded oligonucleotide specific for Nrf2 binding motifs. In this assay increased levels of Nrf2 binding are detected as increased optical density. For these studies a total of 2.5 μg of nuclear extract was used in accordance with manufactures instructions, with data reported as relative Nrf2 activity. Levels of heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO-1), which are established Nrf2-responsive pathways, were measured via Western Blot analysis as an additional measure of Nrf2 activity.
Behavioral analysis of cognitive performance: 14-Unit T-maze
Apparatus and Training
A straight runway (60 cm), constructed of acrylic with opaque sides and clear top was used to pre-train the mice to run for escape in the goal box. The mice were pre-trained in the straight runway to meet a criterion of 13/15 successful escapes in under 15 sec. Following this training, the 14-unit T-maze (Supplementary Figure 1; constructed of the same material as the straight runway), was used for behavioral analysis. Both the straight run and maze were placed in a tray of water (21-23°C) filled to a level (1.5 cm) that allowed the mice to maintain their head out of the water while maintaining contact with the floor, but the height of the ceiling prevented rearing. In all training and testing procedures, successful task performance was rewarded by escape into a dark and dry goal box. Specifics of the maze and its applicability to aging research is described by Pistell and Ingram (Pistell & Ingram).
Maze Performance
Following successful performance in the straight runway, mice were given 15 trials in a single day within the T-maze. The mice were run through sequentially in squads of 8-10, such that all mice were given their first trial before the 1st mouse received trial 2 (protocol similar to traditional protocols used for water maze training). This approach insures that each mouse had sufficient rest between trials to reduce the potential impact of fatigue. Both the latency to reach the goal box and the number of errors committed were recorded, with errors committed as the primary measure of learning because it is unbiased by potential confounds resulting from differences in motor function. Each trial was also recorded using video tracking software (Viewpoint Lifesciences, Inc) to allow re-analysis following the session.
Following the 15-trial training (acquisition) session, which was conducted in a single day, mice were returned to their homecage. Seven days later, mice were administered 5 more trials in the maze to evaluate retention, with both latency and errors recorded as above. Because completion of the first retention trial essentially serves as an additional acquisition trial, the most accurate representation of retention is provided by errors committed on the first retention trial.
Statistical Analysis
Analytical calculations were performed using SAS V9, SAS Institute, Cary, NC. Changes in body weight and other outcomes were summarized as means ± SE. Analysis of variance was conducted via the general linear statistical model to test the experiment-wide significance of differences among dietary outcome. Means and least significant difference tests were employed for specific preplanned post hoc comparisons. Statistical associations were assessed in terms of Pearson product-moment correlation coefficients (r).Statistical significance was determined as P≤ 0.05.
Results
Changes in body weight, adiposity and leptin
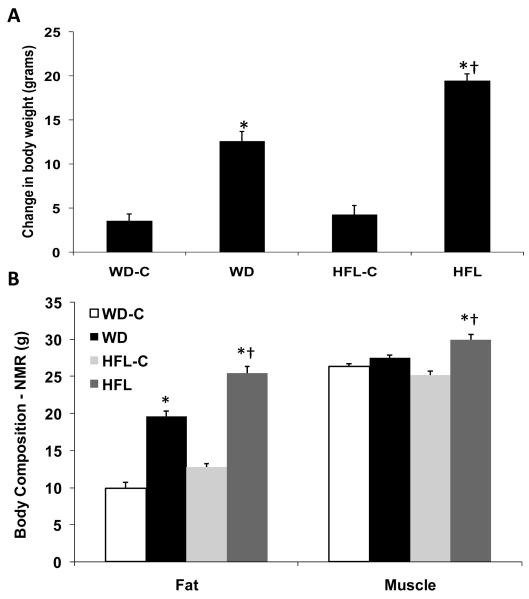
Following 16 weeks on the individual diets, both groups exposed to high fat exhibited significant increases in body weight gain relative to their low fat controls (P < 0.01; Fig 1A), but animals on HFL exhibited a significantly greater weight gain on average compared to all other groups (P = 0.001 vs. WD; Fig 1A). No significant differences in body weight gain were observed for mice on the two control diets. Each high fat diet also increased total body fat relative to its respective control diet (P < 0.001; Figure 1B), with the HFL diet again producing the largest increase in body adiposity (P = 0.001 vs WD; Fig 1B). The high fat diets produced much smaller increases in muscle mass, with WD having a non-significant effect (P = 0.10 vs WD-C) but HFL significantly in increasing muscle mass (P = 0.01; Fig 1B).
Figure 1. Changes in body weight and total adiposity.
Twenty-month old male C57Bl/6 mice were placed on Western diet (WD), High Fat Lard diet (HFL), or their respective control diets (WD-C,HFL-C) for 16 weeks and analyzed for changes in body weight (A) or total body fat using NMR (B). Data are presented as the mean and S.E.M. from 12 animals per group. *P < 0.01 for each high fat diet vs. its control; †P < 0.01 for HFL vs. WD.
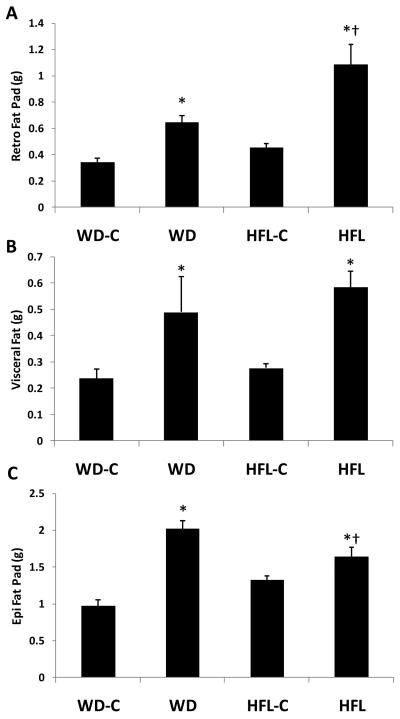
Both WD and HFD also increased the size of individual fat depots relative to their control (P < 0.01), with WD having the largest effect on epididymal fat (P = 0.008 vs HFL; Fig 2) but HFL having the largest effect on retroperitoneal fat (P = 0.001 vs WD; Fig 2). Visceral fat was increased to a similar degree in both diet groups (Fig 2).
Figure 2. Changes in individual fat pad weight.
Twenty month old male C57Bl/6 mice were placed on Western diet (WD), High Fat Lard diet (HFL), or the respective control diets (WD-C,HFL-C) for 16 weeks and analyzed for changes in fat pad weight, which were manually dissected and weighed. Animals were analyzed for total fad pad weight (A), retroperitoneal fat pad (B), epididymal fat pad (C), and visceral fat pad weight (D). Data are presented as the mean and S.E.M. from 12 animals per group. *P < 0.01 for each high fat diet vs. its control; †P < 0.01 for HFL vs. WD.
Changes in circulating levels of insulin, leptin and glucose
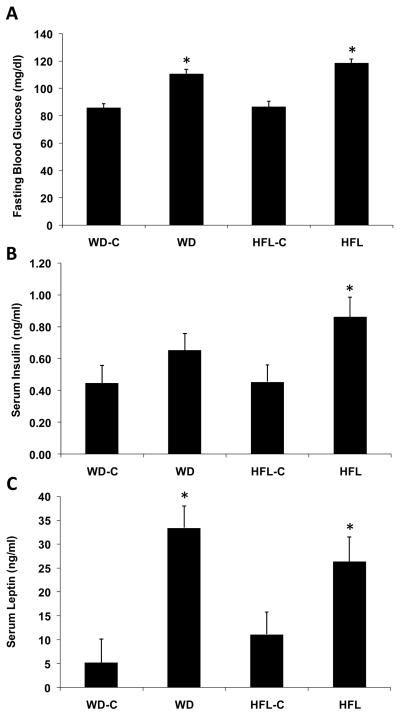
Fasting glucose levels were elevated in the mice on WD and HFL relative to their low fat controls (P < 0.001; Fig 3), but there was no significant difference in glucose levels between the two HFDs (P = 0.15; Fig 3). Insulin levels were significantly higher in mice on HFL relative to control diet (P = 0.006: Fig 3), but the effect of WD did not reach statistical significance (P = 0.13). Circulating leptin levels were significantly higher in both WD and HFL groups relative to their respective control groups (P < 0.04; Fig 3), but leptin levels were similar between the two HFDs.
Figure 3. Changes in fasting insulin, glucose, and leptin levels.
Twenty month old male C57Bl/6 mice were placed on Western diet (WD), High Fat Lard diet (HFL), or the respective control diets (WD-C,HFL-C) for 16 weeks and analyzed for changes in fasting glucose (measured at 13 weeks; A), fasting insulin (B), and fasting leptin levels (C). Data are presented as the mean and S.E.M. from 12 animals per group. *P < 0.01 for each high fat diet vs. its control; †P < 0.01 for HFL vs. WD.
Changes in cerebral oxidative stress
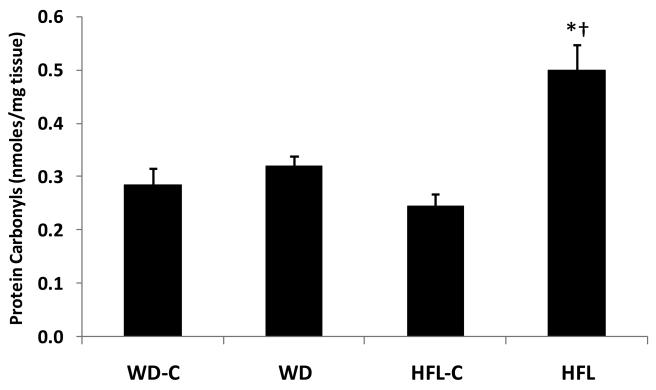
WD did not significantly increase protein carbonyls compared to its control (P = 0.44; Fig 4), but mice on the HFL diet exhibited nearly double the amount of protein carbonyls within their hippocampus compared to animals on HFL-C (P = 0.001; Fig 4). No significant differences in protein carbonyls were observed for the control groups. Significant positive correlations were observed between protein carbonyls and body weight change (r = 0.69, P = 0.001), total body fat (r = 0.67, P = 0.001), glucose (r = 0.63, P = 0.001), retroperitoneal fat pads (r = 0.56, P = 0.001), insulin (r = 0.49, P = 0.005) and visceral fat (r = 0.40, P = 0.03; Table 1).
Figure 4. High fat lard, but not Western diet, consumption promotes increased oxidative stress.
Twenty month old male C57Bl/6 mice were placed on Western diet (WD), High Fat Lard diet (HFL), or the respective control diets (WD-C,HFL-C) for 16 weeks and analyzed for changes in protein oxidation (protein carbonyls) within the hippocampus. Data are presented as the mean and S.E.M. from 8 animals per group. *P < 0.01 for HFL vs. its control diet.
Table 1.
Correlation of metabolic indices, protein oxidation, and cognition
| Correlation to Maze Retention | Correlation to Protein Carbonyls | ||||
|---|---|---|---|---|---|
| Variable | r | P | Variable | r | P |
| BW Change | 0.38 | 0.010 | BW Change | 0.69 | 0.0001 |
| Total Body Fat | 0.44 | 0.002 | Total Body Fat | 0.67 | 0.0001 |
| Glucose | 0.41 | 0.005 | Glucose | 0.63 | 0.0001 |
| Insulin | 0.02 | 0.860 | Insulin | 0.49 | 0.0045 |
| Leptin | 0.27 | 0.060 | Leptin | 0.18 | 0.3171 |
| Retro | 0.22 | 0.140 | Retro | 0.56 | 0.0008 |
| Epi | 0.35 | 0.019 | Epi | 0.29 | 0.1015 |
| Visceral | 0.26 | 0.087 | Visceral | 0.40 | 0.0241 |
| Retention | 0.37 | 0.0363 | |||
Changes in Nrf2 levels and activity
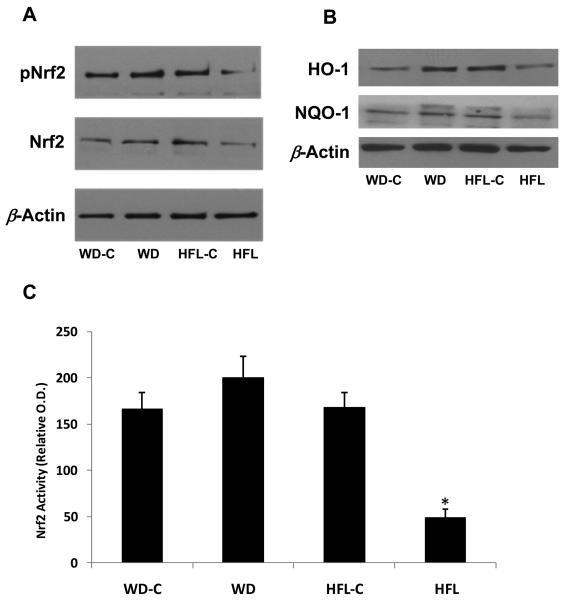
While total levels of Nrf2 protein as well as phosphorylated Nrf2 tended to decrease in mice fed the HFL diet (Fig 5A), significant variability was detected when measuring these endpoints via Western Blot. Contrastingly, a quantitative ELISA system revealed a clear and reproducible decrease in Nrf2 DNA binding activity in HFL-fed mice as compared to their controls (P < 0.001; Fig 5C). Nrf2 activity was not altered following consumption of the WD (P = 0.18 vs WD-C). Similarly, Western Blot analysis of the Nrf2 the responsive proteins HO-1 and NQO-1 demonstrated that these proteins were increased in WD but decreased in HFL animals relative to controls (Fig 5B). Taken together, these data indicate that Nrf2 activity was selectively impaired within animals exhibiting increased protein oxidation.
Figure 5. High fat lard, but not Western diet, consumption promotes decreases in Nrf2 levels and Nrf2 DNA binding activity.
Twenty month old male C57Bl/6 mice were placed on Western diet (WD), High Fat Lard diet (HFL), or the respective control diets (WD-C,HFL-C) for 16 weeks and analyzed for changes in Nrf2 protein levels (A), in the NRF2 responsive proteins HO-1 and NQO-1 (B), or changes in Nrf2 DNA binding activity (C). *P < 0.01 for HFL vs. control.
Effects on Cognitive Performance
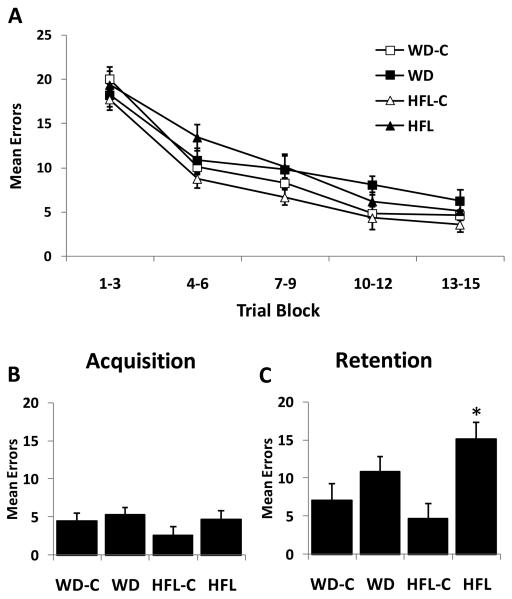
All groups exhibited a distinct learning curve (decrease in errors made) across the acquisition trials in the 14-Unit Stone Maze (Pistell & Ingram), with no difference in errors made among groups at any point during this learning stage (P > 0.10; Fig 6). However, the ability to retain this information 7 days later was selectively impaired in the HFL group (Fig 6C), as indicated by an increase in the number of errors made in the retention trial in the HFL group (P=0.002 relative to HFL-C), but not in the WD group (P = 0.20 relative to WD-C). A significant correlation was observed between retention errors and total body fat (r = 0.44; P = 0.002), glucose (r = 0.41; P = 0.005), body weight change (r = 0.38, P = 0.01), and epididymal fat pad weights (r = 0.35, P = 0.019), with a trend for leptin (r = 0.27, P = 0.06). Contrastingly, no significant relationship was detected between retention and visceral fat, retroperitoneal fat or insulin. In addition, a significant correlation was also detected between retention errors made and levels of protein carbonyls within the hippocampus (r = 0.37; P = 0.036).
Figure 6. High fat lard, but not Western diet, consumption promotes increased cognitive decline.
Twenty month old male C57Bl/6 mice were placed on Western diet (WD), High Fat Lard diet (HFL), or the respective control diets (WD-C,HFL-C) and cognitive performance was assessed in a 14-Unit T-maze. A. Mean errors during the acquisition training across 15 trials (collapsed into 3 trial blocks). B. Mean errors during the final acquisition trial. C. Mean errors during the retention trial, 7 days following acquisition. *P < 0.05 vs control.
Discussion
The present study demonstrates for the first time the ability of a relatively moderate (16 week) exposure to a HFL diet to increase brain oxidative stress, alter Nrf2 signaling, and impair cognitive function in aged animals. However, these effects seemly do not extend to all high-fat diets, as mice consuming the WD exhibited significant alterations in glucose homeostasis and body adiposity, but failed to exhibit age-related brain impairments (oxidative damage and cognitive dysfunction). Taken together, these data suggest that subtle differences in either the magnitude or pattern of diet-induced metabolic perturbations underlie the basis for HFD mediating brain pathogenesis, and that the use of multiple diets or metabolic indices can lead to unique interpretations as compared to studies in which a single diet or metabolic endpoint is evaluated. Lastly, these data demonstrate that the observed increases in oxidative damage were associated with a selective impairment of Nrf2 activity within the HFL animals, suggesting that an impaired antioxidant response may contribute to the observed effects of HFL consumption. In particular, these data implicate an important role for Nrf2-mediated gene expression in the amelioration of oxidative stress and cognitive decline following HFD consumption.
In the current study both HFDs significantly increased body weight gain and total body adiposity, with the HFL diet producing a larger effect. Although we did not directly assess caloric intake in these animals, our experience in other groups of animals suggests that both HFDs lead to an increase in caloric intake which likely contributes to their increased weight and adiposity. Whether subtle differences in food intake exist between these diets remains undetermined. The larger effect on weight and adiposity was mirrored by larger changes in brain oxidative damage and impaired cognition in the HFL as compared to WD group. Thus, while weight gain and total body adiposity are not sufficient to predict brain pathogenesis, they are highly associated with negative outcomes for the brain. Indeed, correlation analysis indicated that body weight gain and total body adiposity exhibited relatively strong relationships to both oxidative damage and cognitive decline. In addition, whole body adiposity exhibited a stronger relationship to age-related brain pathogenesis than any specific fat depot. Considering recent data indicating that visceral or abdominal fat is more ’pathogenic’ when it comes to glucose homeostasis and cardiovascular disease (Gabriely et al. 2002, Muzumdar et al. 2008), these data suggest an additional role for fat deposited outside of the abdominal cavity in promoting cerebral oxidative stress and cognitive decline in the aged brain (Kuk et al. 2009).
The current studies also assessed circulating levels of glucose, leptin and insulin. Recent work has implicated leptin in the regulation of cognitive function (Morrison 2009), and circulating levels of leptin and brain leptin signaling are altered in settings of HFD consumption. Consistent with the increase in body adiposity, both HFDs significantly increased circulating leptin, while both HFDs also lead to increases in fasting blood glucose. Insulin was also numerically increased in both groups, but this increase was only statistically significant in the HFL group. Based on these outcomes, it is difficult to predict the specific contribution of these circulating signals to the brain pathogenesis, as the both HFDs tended to alter these signals yet only the HFL resulted in brain pathogenesis. Interestingly, correlation analysis reveals glucose as having the strongest relationship to cognitive performance and oxidative damage, again despite the fact that glucose was similarly increased by both diets. Additional circulating signals are also likely to contribute to the negative effects of HFD on the brain. HFD-induced obesity is associated with alterations in circulating fuel substrates (glucose, triglycerides, free fatty acids), as well as many adipose derived hormones and chemokines, collectively known as adipokines, which influence the function of peripheral tissues (Halberg et al. 2008, Rasouli & Kern 2008). Many of these adipokines also impact the brain and thus may contribute to HFD-induced brain pathogenesis. Clearly additional work is required to precisely define whether altered circulating nutritional or adipose-derived signals contribute to increases in protein oxidation and decreases in cognition.
One key observation of the current work is an association between increased protein oxidation within the hippocampus and impaired behavioral performance. While metabolic endpoints were altered by both diets (albeit at times more strongly by the HFL diet), protein oxidation was significantly increased only by the HFL diet, which is also the only diet to produce a significant increase in retention errors during the maze task. The mechanism through which HFL consumption produces this selective increase in hippocampal protein oxidation is currently unclear. Increases in body adiposity and the resulting metabolic dysfunction are likely mediators, and hippocampal oxidative stress was most strongly related to body weight gain and whole body adiposity (NMR). However, aside from blood glucose levels, measures of individual fat pads or circulating hormones (leptin, insulin) were much more weakly correlated with hippocampal protein oxidation. What is more, both HFDs produced significant alterations body adiposity and fasting blood glucose, despite protein oxidation and cognitive decline only being observed in the HFL group. Thus while the current work implicates increased protein oxidation as key contributor to cognitive decline, the physiological or metabolic changes that drive this increased protein oxidation remain undefined.
While the specific changes within the periphery which drive increased hippocampal protein oxidation are unclear, our studies highlight a potential role for decreased Nrf2 signaling as a local mediator of diet-induced oxidative stress within the brain. Nrf2 is a key regulator of the antioxidant response system, being activated in settings of oxidative damage and promoting increased antioxidant enzyme activity, at least in part via its binding to the antioxidant response element (ARE) (Johnson et al. 2008). As such, activation of Nrf2 is believed to be a key mechanism through which cells respond to and protect against oxidation damage, and studies in liver suggest a role for Nrf2 in ameliorating HFD-induced lipid peroxidation (Tanaka et al. 2008). Consistent with this result, the current studies demonstrate that 16 weeks of HFL exposure significantly reduces Nrf2 DNA binding activity; whereas, WD did not produce this decrease in Nrf2 nor the increase in protein oxidation. This selective reduction of Nrf2 activity by HFL was also corroborated by a selective reduction in Nrf2 responsive pathways (HO-1 and NQO-1). Taken together, these data suggest that HFL selectively impairs Nrf2 levels and/or activity, leading to an attenuated antioxidant defense and increased protein oxidation. Currently it is unclear whether this decline in Nrf2 is mediated by a change in neuronal Nrf2 expression or glial Nrf2 expression. Previous studies have demonstrated that selective Nrf2 activation in astrocytes confers neuroprotection in a mouse model of amyotrophic lateral sclerosis (Vargas et al. 2008), presumably via the secretion of astrocyte derived glutathione. Identifying the potential role of such pathways in an aging-HFD mouse model could contribute significantly to understanding the mechanistic basis for the selective induction of oxidative stress and cognitive decline observed in HFL fed mice.
Aging-induced cognitive decline is often associated with insulin resistance and alterations in adipose deposition in the absence of overt diabetes (Barbieri et al. 2008, Barbieri et al. 2001). While the ability of diabetes to promote brain pathogenesis is well documented (Nelson et al. 2009, Stranahan et al. 2008a, Stranahan et al. 2008b), the present study does not examine the effects of overt diabetes on the brain. Instead, the current study demonstrates that moderate term exposure to HFD, in the absence of overt diabetes, is sufficient to induce behavioral and neuropathological changes in aged mice. In future studies it will be important to use these dietary models of metabolic dysfunction in combination with more prolonged periods of HFD exposure and/or periodic HFD consumption as tools to identify the basis by which metabolic disturbances promote brain pathogenesis, and whether these changes are due to increased dietary fat consumption or increased obesity. Similarly, these data are consistent with the established role for reduced energy consumption in promoting beneficial effects on lifespan and brain homeostasis. The current work supports the hypothesis that HFDs promote an excess of energy consumption, resulting in metabolic imbalance and brain pathogenesis, while reduced energy intake protects against deleterious metabolic changes and brain pathogenesis (Ferguson et al. 2008, Mattson 2005, Sohal et al. 2009).
While the precise molecular events which precipitate aging-induced cognitive decline remain unclear, we envisage two distinct scenarios through which dietary stressors might induce cognitive decline and oxidative damage within the aging brain. In the first scenario, HFD consumption and the resultant metabolic dysfunction act on or through existing pathological processes, resulting in an acceleration or potentiation of normal brain aging. Contrastingly, in the second scenario, HFD consumption and the resultant metabolic dysfunction initiates new pathological processes which occur independently from and in addition to normal brain aging. Identifying which of these mechanisms is responsible for the cognitive decline and oxidative stress following HFD consumption is essential for developing effective therapies. These findings may be particularly important to age-related disorders, such as Alzheimer's disease (AD), considering our previous observation that consumption of HFD accelerates pathogenesis in a mouse model of beta amyloid pathology (Studzinski et al. 2009), and studies suggesting links between metabolic dysfunction and AD (Perry et al. 2003).
In summary, the current data demonstrate that 16 weeks of HFD consumption increases total body adiposity and fasting blood glucose levels in 20-month old mice. Although both the WD and HFL diet produced significant changes in adiposity, blood glucose and circulating leptin, only the HFL diet produced significant cognitive impairment, and this selective effect on cognition was associated with a selective reduction in Nrf2 activity and an increase in oxidative damage within the hippocampus. These data provide the first direct evidence for HFD induced metabolic disturbances promoting increased levels of oxidative stress and cognitive decline in the aged brain, and implicate a loss of Nrf2 as a potential mechanism for this effect.
Supplementary Material
Acknowledgements
This project was supported by National Institutes of Health grants R03-NS051570 and P20-RR021945 (COBRE) to CDM, and by the Metabolic core facilities at PBRC, which are supported in part by a CNRU (NIH 1P30-DK072476) center grant. This work was also supported by grants from NIH/NIA 2PO1 AG005119 (JNK, ABK), the Alzheimer's Association Investigator Initiated Research Grant (JNK), and the Hibernia National Bank/Edward G. Schlieder Chair (JNK).
Footnotes
The authors have no conflicts or financial interest to disclose.
References
- Barbieri M, Gambardella A, Paolisso G, Varricchio M. Metabolic aspects of the extreme longevity. Exp Gerontol. 2008;43:74–78. doi: 10.1016/j.exger.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Rizzo MR, Manzella D, Paolisso G. Age-related insulin resistance: is it an obligatory finding? The lesson from healthy centenarians. Diabetes Metab Res Rev. 2001;17:19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 2009;1792:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim Biophys Acta. 2007;1773:93–104. doi: 10.1016/j.bbamcr.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Ding Q, Martin S, Dimayuga E, Bruce-Keller AJ, Keller JN. LMP2 knock-out mice have reduced proteasome activities and increased levels of oxidatively damaged proteins. Antioxid Redox Signal. 2006;8:130–135. doi: 10.1089/ars.2006.8.130. [DOI] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882–892. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. x-xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem Res. 2009;34:670–678. doi: 10.1007/s11064-008-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Lam CK, Chari M, Lam TK. CNS regulation of glucose homeostasis. Physiology (Bethesda) 2009;24:159–170. doi: 10.1152/physiol.00003.2009. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7:3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta. 2009;1792:401–408. doi: 10.1016/j.bbadis.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Smith CD, Abner EA, et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim Biophys Acta. 2009;1792:454–469. doi: 10.1016/j.bbadis.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Hirai K, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Raina AK, et al. A metabolic basis for Alzheimer disease. Neurochem Res. 2003;28:1549–1552. doi: 10.1023/a:1025678510480. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Ingram DK. Development of a water-escape motivated version of the Stone T-maze for mice. Neuroscience. 2009;166:61–72. doi: 10.1016/j.neuroscience.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ferguson M, Sohal BH, Forster MJ. Life span extension in mice by food restriction depends on an energy imbalance. J Nutr. 2009;139:533–539. doi: 10.3945/jn.108.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, Spangler EL, Ingram DK, Mattson MP. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol Learn Mem. 2008a;90:479–483. doi: 10.1016/j.nlm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008b;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski CM, Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM, Markesbery WR, Murphy MP, Keller JN. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP × PS1 knock-in mice. J Neurochem. 2009;108:860–866. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5:525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a “complex” problem. Antioxid Redox Signal. 2007;9:1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464–1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bruce-Keller AJ, Dasuri K, Nguyen AT, Liu Y, Keller JN. Diet-induced metabolic disturbances as modulators of brain homeostasis. Biochim Biophys Acta. 2009;1792:417–422. doi: 10.1016/j.bbadis.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.