Abstract
Purpose
To examine the association between longitudinal changes in visual acuity (VA) and Health Related Quality of Life (HRQOL) in a population-based sample of adult Latinos.
Design
A population-based cohort study of eye disease in Latinos.
Participants
3,169 adult Latino participants who live in the city of La Puente, California.
Methods
Data for these analyses were collected for the Los Angeles Latino Eye Study (LALES). Distance visual acuity (VA) was measured during a detailed ophthalmologic examination using the standard Early Treatment Diabetic Retinopathy Study protocol at baseline and a 4 year follow-up examination. HRQOL was assessed by the National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) and the Medical Outcomes Study 12-Item Short-Form Health Survey version 1 (SF-12 v.1).
Main Outcome Measures
Mean differences in HRQOL composite and subscale scores between baseline and follow-up were calculated for 3,169 participants with complete clinical examination and HRQOL data at both time points. Mean differences and effect sizes (ES) for NEI-VFQ and SF-12 v.1 scores were calculated for 3 categories of VA change over the 4 year follow-up period (VA improved ≥ 2 lines, no change in VA or −2<VA<2, VA loss ≥ 2 lines).
Results
For participants with a 2 line loss in VA, we noted an approximate 5 point loss in the NEI-VFQ-25 composite score; with the largest score changes found for the driving difficulties, vision related mental health, and vision related dependency subscales (−12.7, −11.5, and −11.3 respectively). For participants with a 2 line improvement in VA we also noted an approximate 5 point gain in the NEI-VFQ-25 composite score. The largest change (ES = 0.80) was observed for the driving difficulties subscale. No measurable differences in HRQOL were observed for individuals without change in VA from baseline to follow-up.
Conclusions
Clinically important, longitudinal changes in visual acuity (2 line changes or greater) were associated with significant changes in self-reported visual function and well-being. Both the size and direction of visual acuity change influenced change in HRQOL scores.
INTRODUCTION
Vision plays an important role in the ability of people to process information from their environment and to participate in many everyday activities such as reading, working at home or in the office, walking, driving, and interacting with others. People with visual impairment may face challenges completing these activities, which in some cases may lead to depression, social isolation, and difficulties at home, in school, or at work. We previously reported on significant associations between visual impairment, including visual acuity and visual field loss, and health related quality of Life (HRQOL) in the Los Angeles Latino Eye Study (LALES), a population-based study of adults.1
The National Institute Visual Function Questionnaire (NEI-VFQ) was designed to measure areas of vision-targeted functioning and well-being that were identified as important by persons with eye disease.2 Findings from previous studies,3–8 indicate that individuals with visual impairment have worse HRQOL than those without visual impairment. Using LALES cross-sectional data, a 2-line difference in presenting, binocular visual acuity was associated with a 5-point difference in the composite NEI-VFQ score.9 In total, the findings from the literature suggest that early detection and appropriate treatment of visual impairment is important for maintenance of HRQOL. The analyses also indicate that the NEI-VFQ is sensitive enough to detect differences in HRQOL associated with clinically important differences in visual acuity.
In the current analysis, we used longitudinal data to examine the association between changes in visual acuity and changes in HRQOL over a 4 year follow-up period. We hypothesize based on our earlier cross-sectional data that clinically meaningful improvements or losses in visual acuity during the follow-up period would result in significant changes in HRQOL. Specifically, we wanted to determine if the association previously reported for cross sectional data (a 2-line change in visual acuity was associated with a 5 point change in HRQOL) was a reasonable indicator of the magnitude of the association for longitudinal data. Finally, we wanted to determine which NEI-VFQ sub-scale scores were most likely to be impacted by either worsening or improvement in VA and whether the magnitude of the changes mirrored each other (is a 2 line loss or improvement in VA associated with a similar change in HRQOL).
METHODS
Data for these analyses were collected as part of a population-based study of eye disease in adults living in California. Details of the study design and data collected have been described previously.1 We completed a census of all residential households in 6 census tracts in La Puente to identify individuals eligible to be included in the study. The definition of eligibility included men and women who were 40 years of age and older, self-described as Latino and who lived in one of the 6 census tracts. Participants were given a verbal and written description of the study and invited to participate in both a home interview and a clinic examination between February 2000 and May 2003. A follow-up interview and clinical examination was completed approximately 4 years later (January 2004 through May 2008). Informed consent was completed with all participants before they participated in the clinical examination or questionnaire. All study procedures adhered to the principles outlined in the Declaration of Helsinki for research involving human subjects. Institutional review board (IRB) ethics committee approval was obtained from the Los Angeles County/University of Southern California Medical Center Institutional Review Board (approval #969004 and 041004).
Sociodemographic and Clinical Data
An interview was completed at each participant’s home that included information on demographics, history of medical conditions and eye diseases, access to health care, health insurance, vision insurance, and degree of acculturation.10 Definitions were based on variables described in the Hispanic Health and Nutrition Examination Survey.11, 12 History of 12 medical conditions was asked and summarized using a co-morbidity summation score that included diabetes mellitus, arthritis, stroke or brain hemorrhage, high blood pressure, angina, heart attack, heart failure, asthma, skin cancer, other cancers, back problems, and deafness or hearing problems.13–15 Acculturation was measured using the short-form Cuellar Acculturation Scale, with scale scores ranging from 1 to 5 (5 representing the highest level of acculturation).12
Visual Acuity Testing
Measurement of distance visual acuity in LALES has been described previously.16–18 Presenting distance visual acuity for each LALES participant was measured with the presenting correction (if any) at 4 meters using modified Early Treatment Diabetic Retinopathy Study distance charts trans-illuminated with the chart illuminator (Precision Vision, La Salle, Ill). Presenting visual acuity was scored as the total number of lines read correctly and converted to a logarithm of the minimum angle of resolution (logMAR) score. If a participant read fewer than 55 letters at 4 meters in either eye, an automated refraction (Humphrey Autorefractor model 509, Carl Zeiss Meditec, Dublin, CA) was performed. Those who subsequently read fewer than 55 letters while viewing through the prescription determined from the autorefractor underwent a subjective refraction using a standard protocol followed by a measurement of best-corrected visual acuity. Testing began at the top of the chart and progressed to sequentially smaller lines (logMAR levels) provided 3 of 5 letters were identified correctly. When 2 or fewer letters were identified correctly, testing was discontinued. Best-corrected visual acuity was defined as best visual acuity measured at distance after subjective refraction was determined for each eye and was determined based on the person’s better-seeing eye. If a participant was unable to read 20 letters (20/100 Snellen) at 4 meters, visual acuity measurement was performed at 1 meter.
Health Related Quality of Life (HRQOL)
SF-12
The Medical Outcomes Study 12-Item Short-Form Health Survey version 1 (SF-12, v. 1)19 was used to calculate the standard U.S. norm-based SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores.20 Larger PCS and MCS scores represent better HRQOL.20 The PCS and MCS are scored on a T-score metric with the mean equal to 50 and SD equal to 10 in the U.S. general population.
NEI-VFQ-25
Vision-targeted HRQOL was assessed by the NEI-VFQ-25.2, 21 The survey measures the influence of visual impairment and symptoms on generic health domains such as emotional well-being and social functioning, in addition to task-oriented domains related to daily visual functioning.2, 21 The survey is composed of 12 vision-targeted scales: general health (similar to one of the SF-12 items), general vision, near and distance vision activities, ocular pain, vision-related social function, vision-related role function, vision-related mental health, vision-related dependency, driving difficulties, color vision, and peripheral vision. Each scale consisted of a minimum of 1 and a maximum of 4 items. The standard recommended algorithm was used to calculate the scale scores, which have a possible range from 0 to 100. Higher score represents better visual functioning and well-being. Eleven of the 12 scale scores (excluding the general health rating question) were averaged together to yield a composite score.21
Interviewers administered the questionnaires (before the clinical examination) in either English or Spanish at the LALES Local Eye Examination Center.
Statistical Analyses
Mean differences in HRQOL scores between baseline and follow-up examinations were calculated and tested for significance using a paired t-tests (p<0.05). The proportion of participants who had five or more points differences in NEI-VFQ scores between the baseline and follow-up examinations were calculated.
Analysis of variance was used to compare the mean differences in HRQOL scores by 3 categories of visual acuity change (VA improved ≥ 2 lines, no change in VA, VA loss ≥ 2 lines) for best-corrected, better seeing eye and presenting, better seeing eye or both eyes. Effect sizes (ES) for NEI-VFQ scores were calculated for 3 categories of VA change for both presenting and the best-corrected better-seeing eye. ES is an index used to measure the magnitude of impact of one variable on an outcome variable. To measure the impact of the magnitude of visual acuity on HRQOL, ES was calculated as the difference in the mean scores between the baseline and follow-up examinations divided by the standard deviation (SD) of the scores for the baseline group.22 Based on Cohen’s suggestion, an ES of 0.20–0.49 is considered small, 0.50–0.79 moderate, and 0.80 or greater is large.23, 24
To examine the possible non-linear relationship between changes in VA and changes in HRQOL, predicted QOL values for NEI-VFQ scores were obtained through a regression model conditioned on an individual’s change in VA (i.e., 2 line loss or worse, less than a 2 line change, 2 line improvement or greater); models were adjusted for age, gender, education, employment status, income, acculturation, health insurance, vision insurance, number of co-morbidities, visual-field loss, and baseline visual acuity. Predicted mean changes in NEI-VFQ-25 scores were plotted against change in visual acuity for the best-corrected, better-seeing eye. An iterative, locally weighted, least squares method was used to generate lines of best fit (LOWESS fit line).25
The reliable change index (RCI) was calculated as a measure of the statistical significance of individual change in HRQOL. RCI is a z test of change between baseline and follow-up, divided by the standard error of the difference.26 An RCI beyond ±1.96 is indicative of reliable or statistically significant individual change at the p<.05 level.
Analyses were conducted using SAS software 9.1 (SAS Institute, Inc., Cary, NC) at the 0.05 significance level. LOWESS plots were created with STATA.
RESULTS
Description of HRQOL Study Cohort
The HRQOL study cohort includes 3,169 LALES participants with complete clinical examination and HRQOL data collected at baseline and at 4-years of follow-up. A total 7,789 participants were identified as eligible for LALES at baseline; 6,142 (79%) had complete ophthalmic examination data and of these 4,650 (76%) also had complete examination data at the 4-year follow-up examination. A total of 1,192 participants were excluded from the analyses because they did not answer the NEI-VFQ-25 survey at one of the two time points and an additional 67 participants were excluded because they did not answer the SF-12 v.1 on the baseline (N=58) or follow-up (N=9) questionnaires.
The majority of the LALES HRQOL study cohort is female (61%) with a mean age of 55 years at baseline and 1 to 2 chronic health conditions reported on average per person. Half the population reported they were employed at baseline (49.2%) with approximately 53% reporting they had vision insurance. Sixty-six percent of the population reported less than a high school education.
Changes in Visual Acuity and HRQOL Scores from Baseline to Follow-up
Table 1 summarizes 4-year changes in visual acuity and HRQOL scores from baseline to the 4-year follow-up examination. LogMar visual acuity score differences for presenting (binocular vision or better eye) and best corrected visual acuity (better eye) indicate that mean visual acuity became worse over the 4 year follow-up period. The mean difference was small, but statistically significant. With respect to HRQOL scores, significant increases (p<0.0001) were found for 8 of 12 NEI-VFQ-25 scales and for the composite score (p<0.0001). A significant decrease in the NEI-VFQ-25 General Health score was found during the same time interval (p<0.0001). Consistent with this finding, the SF-12 v.1 PCS was significantly lower at follow-up than at baseline (p=0.0015), however the actual change was small. The SF-12 v.1 MCS was significantly higher (p<0.0001).
Table 1.
Difference between mean Health Related Quality of Life Scores and Visual Acuity LogMar Scores in the Los Angeles Latino Eye Study at the baseline and 4-year follow-up examination (N=3,169)
| LALES I | LALES II | DIFF | Proportion of persons with 5 or more Points Change |
|||
|---|---|---|---|---|---|---|
| P-value* | ||||||
| Mean (SD) | Mean (SD) | Mean (SD) | ↓ | ↑ | ||
| NEI-VFQ 25 | ||||||
| Color Vision | 94.3 (14.8) | 95.4 (13.6) | 1.1 (16.4) | 0.0002 | 9 | 12 |
| Vision Related Dependency | 90.2 (19.2) | 93.1 (19.2) | 2.9 (20.1) | <.0001 | 12 | 27 |
| Driving Difficulty† | 88.4 (16.9) | 88.2 (17.4) | −0.3 (16.3) | 0.31 | 25 | 23 |
| Distance Vision | 86.4 (18.0) | 86.2 (18.3) | −0.1 (18.4) | 0.65 | 26 | 29 |
| General Health | 46.6 (22.9) | 44.2 (22.9) | −2.3 (22.8) | <.0001 | 27 | 19 |
| General Vision | 68.9 (16.3) | 69.5 (16.7) | 0.6 (18.6) | 0.09 | 25 | 27 |
| Vision Related Mental Health | 76.8 (21.2) | 80.4 (20.9) | 3.5 (21.8) | <.0001 | 32 | 47 |
| Near Vision | 80.1 (19.4) | 82.3 (18.3) | 2.2 (20.4) | <.0001 | 34 | 40 |
| Ocular Pain | 78.1 (20.2) | 81.1 (18.9) | 3.1 (20.7) | <.0001 | 27 | 40 |
| Peripheral Vision | 87.6 (20.1) | 89.4 (18.8) | 1.8 (21.3) | <.0001 | 16 | 20 |
| Vision Related Role Function | 88.6 (20.7) | 90.6 (19.3) | 2.0 (21.9) | <.0001 | 17 | 23 |
| Vision Related Social Function | 93.2 (13.8) | 94.6 (12.7) | 1.3 (15.0) | <.0001 | 14 | 20 |
| Composite Score | 84.2 (14.3) | 86.1 (13.8) | 1.9 (12.5) | <.0001 | 20 | 32 |
| SF12 | ||||||
| Physical Composite Score | 46.4 ( 9.6) | 45.9 ( 9.6) | −0.5 (9.3) | 0.0015 | 25 | 22 |
| Mental Composite Score | 50.1 (10.7) | 51.7 (10.6) | 1.6 (11.3) | <.0001 | 23 | 32 |
| Visual Acuity logMar | ||||||
| Presenting VA binocular | 0.002 (0.148) | 0.012 (0.168) | 0.014 (0.141) | <.0001 | --- | --- |
| Presenting VA better eye | 0.035 (0.154) | 0.049 (0.171) | 0.010 (0.134) | <.0001 | --- | --- |
| Best corrected better eye | −0.019 (0.107) | −0.013 (0.131) | 0.006 (0.093) | 0.0006 | --- | --- |
P-value is from paired T-test.
Score could be generated for only 2266 of the participants in the whole sample.
DIFF=score difference between LALES II (4-year follow-up examination) and LALES I (baseline examination). SD=standard deviation. LALES = Los Angeles Latino Eye Study. NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire. SF12 = Medical Outcomes Study 12-Item Short-Form Health Survey version 1.
Change in Health Related Quality of Life Stratified by Change in Visual Acuity
Table 2 presents mean changes in HRQOL and resulting effect sizes (ES) by best corrected visual acuity change in the better-seeing eye (visual acuity improvement of ≥ 2 lines, no visual acuity change, visual acuity loss ≥ 2 lines) between LALES baseline and follow-up examinations. Although we also examined HRQOL differences for presenting visual acuity of the better-seeing eye, and for presenting visual acuity for both eyes, the strongest associations were found for best corrected visual acuity in the better seeing eye.
Table 2.
Effect size and mean change in Health Related Quality of Life by change in best corrected visual acuity of the better seeing eye (N=3,169)
| Mean and standard deviation of the difference in HRQOL scores Between LALES baseline and follow-up examinations |
||||||
|---|---|---|---|---|---|---|
| VA Improved ≥ 2 Lines N=31 |
No VA Change N=3,055 |
VA Loss ≥ 2 Lines N=83 |
||||
| Mean (SD) | ES†† | Mean (SD) | ES†† | Mean (SD) | ES†† | |
| NEI-VFQ-25 | ||||||
| Color Vision* | 8.6 (24.3) | 0.39 | 1.2 (16.2) | 0.08 | 3.6 (21.1) | −0.19 |
| Vision Related Dependency* | 1.3 (38.4) | 0.04 | 3.3 (19.0) | 0.18 | −11.3 (37.5) | −0.41 |
| Driving Difficulty*† | 25.0 (31.9) | 0.80 | − 0.2 (15.7) | −0.01 | −12.7 (29.6) | −0.42 |
| Distance Vision* | 5.9 (30.5) | 0.24 | −0.05 (18.0) | −0.003 | −4.9 (24.1) | −0.23 |
| General Health | −1.6 (26.9) | −0.07 | −2.4 (22.6) | −0.11 | −2.1 (25.6) | −0.09 |
| General Vision* | 1.9 (18.6) | 0.11 | 0.7 (18.5) | 0.04 | −5.6 (21.5) | −0.33 |
| Vision Related Mental Health* | 6.3 (30.4) | 0.22 | 3.8 (21.4) | 0.18 | −11.5 (30.9) | −0.43 |
| Near Vision* | 11.5 (20.1) | 0.46 | 2.3 (20.3) | 0.11 | 0.6 (24.8) | 0.02 |
| Ocular Pain | 8.6 (24.1) | 0.30 | 3.1 (20.6) | 0.16 | 1.6 (22.6) | 0.07 |
| Peripheral Vision | −1.6 (32.3) | −0.07 | 1.9 (21.0) | 0.10 | −2.7 (26.6) | −0.11 |
| Vision Related Role Function | −0.4 (31.2) | −0.01 | 2.1 (21.6) | 0.10 | −3.4 (28.9) | −0.11 |
| Vision Related Social Function* | 5.9 (24.0) | 0.27 | 1.5 (14.5) | 0.11 | −6.3 (23.4) | −0.32 |
| Composite Score* | 5.8 (18.3) | 0.28 | 2.1 (12.2) | 0.15 | −5.9 (18.5) | −0.31 |
| SF12 | ||||||
| Physical Composite Score | 0.1 (10.0) | 0.008 | −0.6 ( 9.3) | −0.06 | −0.6 (10.8) | −0.06 |
| Mental Composite Score | 3.1 (11.0) | 0.22 | 1.6 (11.2) | 0.15 | 0.6 (15.1) | 0.05 |
One way analysis of variance P<0.05.
Score could be generated for only 2266 of the participants in the whole sample.
Effect size (ES) is defined as mean difference between LALES baseline and follow-up scores divided by standard deviation of baseline scores.
ES: Small effect 0.2–0.49; Moderate effect 0.50–0.79; Large effect 0.80+.
SD = standard deviation. VA = visual acuity. HRQOL = health related quality of life. NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire. SF12 = Medical Outcomes Study 12-Item Short-Form Health Survey version 1.
For participants with a 2 lines loss in visual acuity over the 4 year period based on best corrected visual acuity in the better seeing eye, we found an average 5.8 point loss in the NEI-VFQ-25 composite score (Table 2). Losses were greatest for driving difficulty (−12.7), vision-related mental health (−11.5), and vision related dependency (−11.3). Small effects (ES = −0.20 to −0.49) were observed for 6 NEI-VFQ-25 subscales and the composite score. No measurable effect was found for the NEI-VFQ-25 general health scale or the SF-12 v.1 MCS or PCS.
For participants with a 2 line improvement in VA over the 4 year period based on best corrected visual acuity in the better seeing eye, we found an approximate 5 point gain in the NEI-VFQ-25 composite score. Small effects (ES = 0.20 to 0.49) were observed for 6 NEI-VFQ-25 subscales and the composite score. A large score change (25.0) and effect (ES=0.80) was found for the driving subscale, suggesting that a 2 line improvement or greater in VA had a strong, beneficial impact on participants’ perception of their ability to drive independently. No measurable effect was found for the NEI-VFQ-25 general health scale or the SF-12 v.1 MCS or PCS. No measurable differences in HRQOL scores were observed for individuals without change in VA from LALES baseline to follow-up examinations.
For presenting VA in the better seeing eye or presenting VA based on both eyes, a 2 line or greater loss in VA was associated with small effects for the NEI-VFQ-25 driving difficulty subscale. We observed a small effect for driving difficulty (ES= −0.28) when considering presenting visual acuity in the better seeing eye and a small effect (ES= −0.43) when considering presenting visual acuity in both eyes (data not shown). No effects were found among participants with minimal or no change in VA. For a 2 line improvement in presenting VA in the better seeing eye, small effects were found for 3 NEI-VFQ-25 subscales (vision-related mental health, near vision, ocular pain) and the composite score. For presenting VA based on both eyes, small effects were found for 9 of 12 subscales (color vision, vision related dependency, distance vision, general vision, vision related mental health, near vision, ocular pain, vision related role function and social function) and the composite score (data not shown). No effects were found for the SF-12 v. 1 MCS or PCS for presenting visual acuity in both eyes; a small effect was found for the MCS for participants with a 2 line loss or greater for presenting VA in the better-seeing eye (data not shown).
The RCI was used to evaluate the proportion of people with significant changes in NEI-VFQ-25 scores by visual acuity change using the best corrected visual acuity of the better-seeing eye (Table 3, available at http://aaojournal.org). For participants with a 2 line improvement in visual acuity, the largest proportions of people with significant improvements in HRQOL were found for ocular pain, driving difficulties, general vision, color vision, distance vision, peripheral vision, and near vision (bolded in Table 3, available at http://aaojournal.org). The scale with the smallest proportional improvement was vision-related social function. Cataract surgery was the reason for the 2 line improvement in visual acuity for more than half of the participants with visual acuity improvement. For participants with a 2 line loss or greater, the largest proportion of people with significant loss in HRQOL were found for driving difficulty, general vision, peripheral vision, vision related dependency, and vision related mental health (bolded in Table 3, available at http://aaojournal.org). The smallest proportional loss in HRQOL was found for the ocular pain scale.
Table 3.
Proportion of people with significant changes in National Eye Institute Visual Function Questionnaire-25 scores for individuals with 2 or more line changes in best corrected visual acuity of the better seeing eye as determined by the reliable change index.
| Change in visual acuity between Los Angeles Latino Eye Study baseline and follow-up examinations |
||||
|---|---|---|---|---|
| Increase 2 lines VA (N=31) |
Loss 2 lines VA (N=83) |
|||
| Loss | Improvement | Loss | Improvement | |
| RCI<=−1.96 | RCI†>=1.96 | RCI†<=−1.96 | RCI>=1.96 | |
| NEI-VFQ-25 | N (%) | N (%) | N (%) | N (%) |
| Color Vision | 4 (13) | 10 (32) | 21 (25) | 13 (16) |
| Vision Related Dependency | 7 (23) | 8 (26) | 26 (31) | 16 (19) |
| Driving Difficulty | 0 ( 0) | 3 (38) | 16 (42) | 3 ( 8) |
| Distance Vision | 5 (16) | 10 (32) | 24 (29) | 14 (17) |
| General Health | 11 (35) | 8 (26) | 22 (26) | 14 (17) |
| General Vision | 7 (23) | 11 (35) | 32 (39) | 17 (20) |
| Vision Related Mental Health | 7 (23) | 9 (29) | 26 (31) | 10 (12) |
| Near Vision | 2 ( 6) | 10 (32) | 14 (17) | 16 (19) |
| Ocular Pain | 5 (16) | 12 (39) | 13 (16) | 16 (19) |
| Peripheral Vision | 9 (29) | 10 (32) | 27 (33) | 22 (27) |
| Vision Related Role Function | 6 (19) | 8 (26) | 21 (25) | 14 (17) |
| Vision Related Social Function | 3 (10) | 7 (23) | 20 (24) | 10 (12) |
| Composite Score | 6 (19) | 11 (36) | 26 (31) | 12 (15) |
RCI= Reliable change index. VA=visual acuity.
Bolded number and percent indicate those NEI-VFQ scores with a large (30% or greater) percent of individuals with statistically significant change in score in the same direction as the direction of VA change.
Note: Percentage is for each line change group. For example, 31 participants increased 2 lines best corrected better seeing eye visual acuity, 11 (11/31=35.48%) participants had RCI between less than or equal to −1.96 subscale general health.
LALES = Los Angeles Latino Eye Study. NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire.
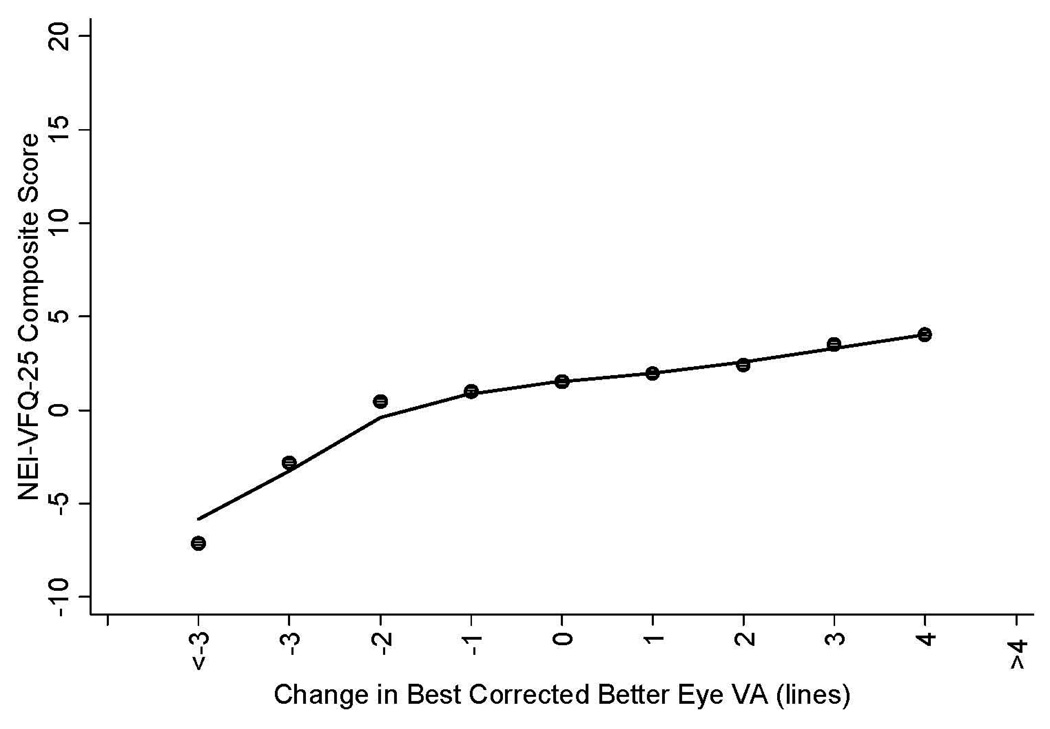
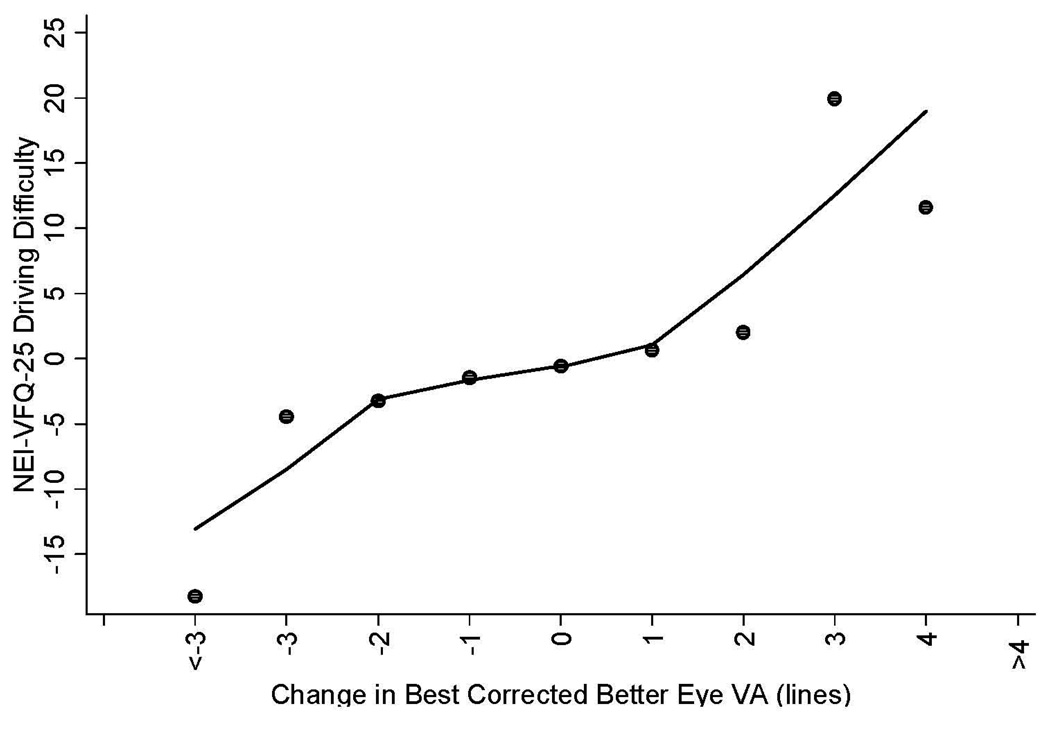
Figure 1 and Figure 2 show selected plots of predicted change in mean NEI-VFQ scores (composite scores, driving scores) by change in best corrected visual acuity of the better seeing eye. Plots of predicted change in mean NEI-VFQ composite scores show an approximate linear relationship with change in the best corrected visual acuity of the better-seeing eye when considering data for a 2 line loss in VA through an approximate 4 line gain in VA (Figure 1); a more dramatic loss in composite scores was found for VA loss of 3 lines or greater, however these points were based on small numbers of participants. The same plot for the NEI-VFQ driving difficulty scale also shows an approximate linear relationship between change in driving scores and change in best corrected VA for a 3 line loss in VA to a 2 line gain in VA; a larger change in NEI-VFQ driving scores was found for a VA gain of 3 lines or more or for a VA loss of 4 lines or more (Figure 2).
Figure 1.
Linear regression plot of the relationship between change in NEI-VFQ-25 composite scores (adjusted for co-variates including age, gender, education, employment status, income, acculturation, co-morbidities, health insurance, vision insurance, and baseline visual acuity (VA)) by change in best corrected visual acuity in the better seeing eye of participants in the Los Angeles Latino Eye Study. The adjusted NEI-VFQ-25 composite scores were obtained using the regression model conditioned on VA status (vision loss, no change, vision improvement). The mean change in NEI-VFQ composite score of all participants by each unit of mean change in VA were plotted.
NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire. VA = visual acuity.
Figure 2.
Linear regression plot of the relationship between change in NEI-VFQ-25 driving difficulty scores (adjusted for co-variates including age, gender, education, employment status, income, acculturation, co-morbidities, health insurance, vision insurance, and baseline VA) by change in best corrected visual acuity in the better seeing eye of participants in the Los Angeles Latino Eye Study. The adjusted NEI-VFQ-25 composite scores were obtained using the regression model conditioned on VA status (vision loss, no change, vision improvement). The mean change in NEI-VFQ composite score of all participants by each unit of mean change in VA were plotted.
NEI-VFQ-25 = National Eye Institute Visual Function Questionnaire. VA0020= visual acuity.
DISCUSSION
In this population-based study of adults, we found statistically significant changes in mean HRQOL scores over the 4 year follow-up period for participants who had clinically meaningful changes in visual acuity, while mean changes in HRQOL scores were small for participants with little or no change in visual acuity.
Several studies of clinic based populations also have examined the relationships between longitudinal changes in visual acuity and HRQOL. Mangione and colleagues found that improvements in visual acuity following cataract surgery were associated with improvements in HRQOL as measured by the Activities of Daily Vision Scale (ADVS). 5 The participants included 419 cataract surgery patients age 65 years and older with ADVS data available to assess changes in HRQOL following surgery. The ADVS includes 20 questions on visual activities related to 5 subscales: night driving, daytime driving, distance-vision activities, near-vision activities, and glare.27 Twelve months after cataract extraction, improvement in the best corrected visual acuity of the operated eye was associated with improvement in all 5 ADVS subscales after adjusting for visual acuity of the non-operated eye.
The Submacular Surgery Trials Research Group also found NEI-VFQ-25 scores were sensitive to changes in visual acuity. The study included 828 patients from 3 clinical trials of submacular surgery for subfoveal choroidal neovascularization. Data on visual acuity and NEI-VFQ-25 scores were collected at baseline (pre-randomization) and 2 years post-randomization. The median age of the population was 75 years, approximately 20 years older than the LALES population. Best-corrected visual acuity of each eye was measured. Investigators concluded that a 3 point difference in the NEI-VFQ-25 composite score and a 5.4 difference in the driving difficulty score represented small, but clinically meaningful changes when evaluating the visual acuity data.28, 29 When restricting to patients with a 2 line or more improvement in visual acuity of the better-seeing eye, investigators found a mean NEI-VFQ-25 composite score change of 8.3 (SD=12.8) and driving difficulty score change of 6.6 (SD=24.4) over the 2 year interval. This improvement was greater than found in the LALES population, however their finding of a 5.9 point loss (SD=17.4) in the NEI-VFQ-25 composite score for patients with a 2 line loss or greater was the same as found in LALES. In general, mean changes in HRQOL scores for 2 or more line changes in visual acuity for the clinical populations were larger than found for LALES.
The Age-Related Eye Disease Study Research Group (AREDS) also examined change in HRQOL with change in visual acuity and found a 3 line loss in visual acuity or greater was associated with a 10 point change in the NEI-VFQ composite score. The sample for the VA analyses included 3,624 people, aged 55–80 years at enrollment, identified from 11 clinical centers. Progression to visual acuity loss was defined as a decrease of 3 lines or more between 2 NEI-VFQ-25 administrations at least 1 year and up to 4 years apart. The median age of the population was 72 years. AREDS investigators found effects (ES) were moderate for 7 of 12 NEI-VFQ subscales and the composite score.
A 5 point change in NEI-VFQ measures has been suggested as a guideline for interpreting score differences as minimally important or as representing clinically meaningful change with respect to visual acuity.29 Several studies have examined the change in HRQOL scores for a 2 or 3 line change in visual acuity. As mentioned above, the Submacular Surgery Trials Research Group used the SD of change in NEI-VFQ scores from baseline to the 2 year follow-up examination to calculate a clinically meaningful change of the NEI-VFQ; score changes of 3.8 were considered small, 9.4 were considered medium, and 15 were considered large. In LALES, we chose to examine the change in HRQOL scores associated with a 2 line or greater change in visual acuity, because 2 lines or greater is recognized as having clinical significance and is a level of change observed in the population. Clinical trial patients often achieve larger improvements in visual acuity with treatment and therefore may achieve greater changes in HRQOL scores than can be expected when studying a general, population based sample. Other differences that may influence the size of change in HRQOL scores between studies may include the baseline QOL score and the expectation of vision improvement following treatment by the patient.
We examined the proportion of individuals with significant changes in NEI-VFQ scores by change in visual acuity using the RCI. A substantial proportion of individuals with clinically meaningful improvements or loss in VA had significant change in HRQOL scores. For example, 42% of individuals with a 2 line loss or more in VA over the 4 year follow-up period had a significant decline in the NEI-VFQ driving score. The proportion of individuals with significant changes in NEI-VFQ subscale scores was similar by direction of VA change (2 line or more gain versus 2 line or more loss) for many of the HRQOL subscales.
Small, but statistically significant losses in the general health measures (SF-12 v.1 PCS and NEI-VFQ general health scale) were found over the 4 year interval. These changes are likely due to additional health problems in this adult, aging population. The number of self-reported co-morbidities from baseline to follow-up in our sample increased from an average 1.5 to 1.7. It is interesting that despite the worsening of scores for the general health measures, the SF-12 v.1 MCS score improved significantly. After stratifying by change in VA status, we see that the small, positive change in the SF-12 v.1 MCS is attributable to the improvement in scores for the participants with a 2 line or greater change in VA. The data suggest that improvements in vision following cataract surgery, glasses or other clinical interventions are associated with gains in perceived well-being and function.
A limitation of the analyses is the small number of subjects with 2 or more lines of change in visual acuity based on the best corrected, better eye. There were too few participants meeting these criteria to stratify by larger numbers of line changes (3 line changes, 4 line changes) and further explore the difference in HRQOL by line changes in VA. More participants reached a 2 line change based on presenting visual acuity however the perceived impact of vision change in this group (whose vision could be corrected by refraction) was not as strong as in the best corrected vision group. The plots showing change in NEI-VFQ scores by change in visual acuity suggest a linear relationship through 2–3 lines of visual acuity change; additional follow-up data are needed to accurately assess the shape of the relationship for larger changes in visual acuity. While strengths of the study are the collection of visual acuity and visual field data over time, we do not have other longitudinal measures of vision such as contrast sensitivity. Additional strengths of the study include the population-based sample, the availability of both clinical and questionnaire data at 2 time points, and the collection of cataract history and use of glasses to allow for better interpretation of changes in HRQOL scores. The NEI-VFQ was scored using the standard methods based on classical test theory. However, increasing attention to applying item response theory (IRT) methods in the evaluation and scoring of HRQOL measures is occurring30. Methodological research is needed to document the extent to which IRT yields psychometric improvements to the NEI-VFQ and other HRQOL measures in future studies 31.
In summary, clinically important changes in visual acuity were associated with statistically significant, changes in HRQOL based on longitudinal data from a population-based sample of adults. The magnitude of the HRQOL effect was dependent on the size of the visual acuity change and the direction of change (loss or gain in vision). A 2-line or more improvement in visual acuity was associated with an average 5.8 point gain in the NEI-VFQ-25 composite score and a 2 line loss or more in visual acuity was associated with an average 5.9 point loss. Previous studies have defined minimally important changes in HRQOL scores ranging from 3 to 5 points. The largest impact of visual acuity change was found for the NEI-VFQ driving scale; a large effect (ES =0.80) was found for individuals with a 2 line or more loss in visual acuity. These data suggest that changes in a person’s visual acuity have a measurable and clinically important impact on perceived visual function and well-being.
Acknowledgments
Financial Support: National Institutes of Health Grants NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar. Ron D. Hays was supported in part by the UCLA Resource Center for Minority Aging Research/Center for Health Improvement in Minority Elderly (RCMAR/CHIME), NIH/NIA Grant Award Number P30AG021684.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article contains online-only material. The following should appear online-only: Table 3 and Los Angeles Latino Eye Study Group List.
References
- 1.Varma R, Paz SH, Azen SP, et al. Los Angeles Latino Eye Study Group. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–1131. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Mangione CM, Lee PP, Pitts J, et al. NEI-VFQ Field Test Investigators. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) Arch Ophthalmol. 1998;116:1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 3.Submacular Surgery Trials Research Group. Health- and vision-related quality of life among patients with choroidal neovascularization secondary to age-related macular degeneration at enrollment in randomized trials of submacular surgery: SST report no. 4. Am J Ophthalmol. 2004;138:91–108. doi: 10.1016/j.ajo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Linder M, Chang TS, Scott IU, et al. Validity of the Visual Function Index (VF-14) in patients with retinal disease. Arch Ophthalmol. 1999;117:1611–1616. doi: 10.1001/archopht.117.12.1611. [DOI] [PubMed] [Google Scholar]
- 5.Mangione CM, Phillips RS, Lawrence MG, et al. Improved visual function and attenuation of declines in health-related quality of life after cataract extraction. Arch Ophthalmol. 1994;112:1419–1425. doi: 10.1001/archopht.1994.01090230033017. [DOI] [PubMed] [Google Scholar]
- 6.Parrish RK, II, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997;115:1447–1455. doi: 10.1001/archopht.1997.01100160617016. [DOI] [PubMed] [Google Scholar]
- 7.Scott IU, Smiddy WE, Schiffman J, et al. Quality of life of low-vision patients and the impact of low-vision services. Am J Ophthalmol. 1999;128:54–62. doi: 10.1016/s0002-9394(99)00108-7. [DOI] [PubMed] [Google Scholar]
- 8.Varma R, Wu J, Chong K, et al. Los Angeles Latino Eye Study Group. Impact of severity and bilaterality of visual impairment on health-related quality of life. Ophthalmology. 2006;113:1846–1853. doi: 10.1016/j.ophtha.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Globe DR, Wu J, Azen SP, Varma R Los Angeles Latino Eye Study Group. The impact of visual impairment on self-reported visual functioning in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1141–1149. doi: 10.1016/j.ophtha.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Globe DR, Schoua-Glusberg A, Paz S, et al. Using focus groups to develop a culturally sensitive methodology for epidemiological surveys in a Latino population: findings from the Los Angeles Latino Eye Study (LALES) Ethn Dis. 2002;12:259–266. [PubMed] [Google Scholar]
- 11.Solis JM, Marks G, Garcia M, Shelton D. Acculturation, access to care, and use of preventive services by Hispanics: findings from HHANES 1982-84. Am J Public Health. 1990;80 (suppl):11–19. doi: 10.2105/ajph.80.suppl.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks G, Garcia M, Solis JM. Health risk behaviors of Hispanics in the United States: findings from HHANES, 1982-84. Am J Public Health. 1990;80 (suppl):20–26. doi: 10.2105/ajph.80.suppl.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108:1893–1900. doi: 10.1016/s0161-6420(01)00754-0. discussion 1900-1. [DOI] [PubMed] [Google Scholar]
- 14.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 15.Globe DR, Varma R, Torres M, et al. Los Angeles Latino Eye Study Group. Self-reported comorbidities and visual function in a population-based study: the Los Angeles Latino Eye Study. Arch Ophthalmol. 2005;123:815–821. doi: 10.1001/archopht.123.6.815. [DOI] [PubMed] [Google Scholar]
- 16.Azen SP, Varma R, Preston-Martin S, et al. Los Angeles Latino Eye Study Group. Binocular visual acuity summation and inhibition in an ocular epidemiological study: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2002;43:1742–1748. [PubMed] [Google Scholar]
- 17.Varma R, Fraser-Bell S, Tan S, et al. Los Angeles Latino Eye Study Group. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1288–1297. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Varma R, Torres M, Pena F, et al. Los Angeles Latino Eye Study Group. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1298–1306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. 2nd ed. Boston, MA: The Health Institute, New England Medical Center; 1995. p. 41. [Google Scholar]
- 21.Mangione CM, Lee PP, Gutierrez PR, et al. National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal R. Parametric measures of effect size. In: Cooper HM, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 231–244. [Google Scholar]
- 23.Age-Related Eye Disease Study Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss, and lens opacity: AREDS report no. 14. Arch Ophthalmol. 2005;123:1207–1214. doi: 10.1001/archopht.123.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Earlbaum Associates; 1988. pp. 20–74. [Google Scholar]
- 25.Cleveland WS, Grosse E. Computational methods for local regression. Stat Comput. 1991;1:47–62. [Google Scholar]
- 26.Hays RD, Brodsky M, Johnston MF, et al. Evaluating the statistical significance of health-related quality-of-life change in individual patients. Eval Health Prof. 2005;28:160–171. doi: 10.1177/0163278705275339. [DOI] [PubMed] [Google Scholar]
- 27.Mangione CM, Phillips RS, Seddon JM, et al. Development of the 'Activities of Daily Vision Scale': a measure of visual functional status. Med Care. 1992;30:1111–1126. doi: 10.1097/00005650-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Submacular Surgery Trials Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ): SST report number 19. Ophthalmic Epidemiol. 2007;14:205–215. doi: 10.1080/09286580701502970. [DOI] [PubMed] [Google Scholar]
- 29.Submacular Surgery Trials Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization--SST report no. 1. Arch Ophthalmol. 2003;121:531–539. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hays RD, Morales LS, Reise SP. Item response theory and health outcomes measurement in the 21st century. Med Care. 2000;38 (suppl):II28–II42. doi: 10.1097/00005650-200009002-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeve BB, Hays RD, Bjorner JB PROMIS Cooperative Group. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45 (suppl):S22–S31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]