Abstract
Skeletal myoblast differentiation involves acquisition of the muscle-specific transcriptional program and morphological changes, including fusion into multinucleated myofibers. Differentiation is regulated by extracellular signaling cues, including cell-cell contact and adhesion. Cadherin and Ig adhesion receptors have been implicated in distinct but overlapping stages of myogenesis. N-cadherin signals through the Ig receptor Cdo to activate p38 MAP kinase, while the Ig receptor neogenin signals to activate FAK; both processes promote muscle-specific gene expression and myoblast fusion. M-cadherin activates Rac1 to enhance fusion. Specific Ig receptors (Kirre, Sns) are essential for myoblast fusion in Drosophila, also signaling through Rac, and vertebrate orthologs of Kirre and Sns have partially conserved function. Mice lacking specific cytoplasmic signaling factors activated by multiple receptors (e.g., Rac1) have strong muscle phenotypes in vivo. In contrast, mice lacking individual adhesion receptors that lie upstream of these factors have modest phenotypes. Redundancy among receptors may account for this. Many of the mammalian Ig receptors and cadherins associate with each other, and multivalent interactions within these complexes may require removal of multiple components to reveal dramatic defects in vivo. Nevertheless, it is possible that the murine adhesion receptors rate-limiting in vivo have not yet been identified or fully assessed.
Keywords: cell adhesion, signal transduction, myogenesis, differentiation, cadherin, Ig superfamily, receptor
Introduction
Skeletal muscle, the most abundant tissue in the vertebrate body, develops through multiple stages that occur in a sequential yet overlapping manner [1]. The trunk and limb musculature arise from muscle progenitors in the dorsal somite, whereas progenitors of the head musculature arise variously from cranial paraxial, prechordal and splanchnic mesoderm [2]. These distinct progenitor populations are characterized by distinct sets of transcriptional regulators, but all ultimately rely on members of the MyoD family of myogenic regulatory factors (MRFs) for specification to lineage-restricted myoblasts and differentiation into myofibers [3]. Myf5, MyoD and Mrf4 are involved with lineage determination, whereas MyoD, myogenin and Mrf4 play roles in differentiation [3]. Additionally, adult muscle has impressive regenerative properties. The primary source of this capability is the satellite cell, a quiescent muscle-resident precursor cell that, following injury, undergoes activation, proliferation and differentiation to accomplish muscle repair [4].
Myoblast differentiation is itself a multistage process and includes withdrawal from the cell cycle, expression of the muscle-specific transcriptional program, cellular elongation, and fusion into syncytial myofibers. Myoblasts therefore undergo dramatic changes in morphology and gene expression, i.e., in structure and function, during this process. Myoblast differentiation is influenced by extracellular signaling cues, including secreted, soluble factors; extracellular matrix and its associated factors; and factors involved with cell-cell contact and adhesion. While the birth of muscle progenitors and their myogenic determination are most often studied in vivo, primary myoblasts and myoblast cell lines are easily grown in culture, and differentiation is therefore accessible to mechanistic analyses that are often difficult to perform in vivo. Many investigators have observed that high cell density promotes myoblast differentiation, suggesting that cell-cell contact and adhesion regulate signaling pathways that influence both biochemical (expression of muscle-specific proteins) and morphological (elongation and fusion) aspects of cell differentiation. Ig superfamily (IgSF) members and cadherins mediate many cell-cell contact-based morphogenetic events [5, 6], and most of the known cell surface proteins implicated in promyogenic signaling initiated by cell-cell contact belong to these families. Recent studies have begun to identify the signaling pathways that lie directly downstream of specific cell surface adhesion receptors, revealing both specific and common factors and mechanisms. Here I review progress in this area, with particular reference to mammalian systems.
N-cadherin ligation signals via Cdo to activate Cdc42 and p38 MAP kinase
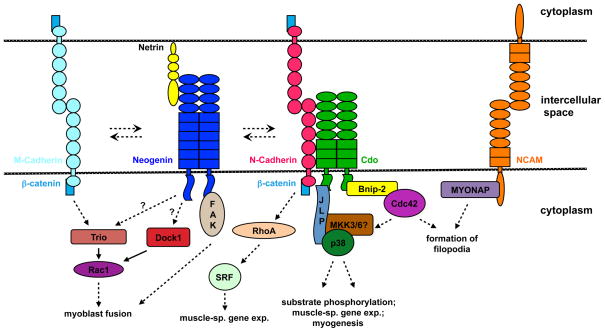
Classical cadherins mediate cell-cell adhesion through calcium-dependent, homophilic binding of their ectodomains on the surfaces of neighboring cells [5]. Intracellularly, they bind β-catenin and, indirectly via β-catenin, α-catenin, which functions to dynamically tether the complex to the actin cytoskeleton [5]. N-cadherin (encoded by Cdh2) is expressed throughout myogenesis, from progenitors in the dorsal somite through formation of myofibers and mature neuromuscular junctions; furthermore, Cdh2 expression is induced during adult regenerative myogenesis (for review, see [7]). Studies in which wild-type or interfering mutant forms of N-cadherin were expressed in the dorsal somite of the chick embryo argue that N-cadherin-mediated adhesion is important for the integrity of the dermomyotome and for myogenic determination and differentiation of specific somatic muscle progenitors [8]. N-cadherin ligation in myoblasts in vitro, either naturally through cell-cell contact or via recombinant N-cadherin ectodomains affixed to solid substrata or beads, promotes differentiation, including cell cycle withdrawal and expression of muscle-specific proteins [7]. Cdh2−/− mice die before E10 with disorganized somites, preventing analysis of skeletal muscle development [9]. Explanted, cultured Cdh2−/− somites express skeletal muscle-specific myosin heavy chain (MHC) but also display normal β-catenin staining at cell-cell contacts [9]. Similarly, primary Cdh2−/− myoblasts differentiate and fuse normally but express additional classical cadherins (e.g., M-cadherin and cadherin 11) [10]. Together, these results indicate that N-cadherin is not essential for myogenesis but that other cadherins can function in its absence, perhaps in a redundant or compensatory manner.
Cdo is a cell surface protein with Ig and FnIII repeats in its ectodomain and a long intracellular region, and it serves as a multifunctional co-receptor for a limited number of signaling pathways [11, 12]. Cdo binds in cis (in the plane of the same membrane) to N-cadherin in myoblasts, independent of cadherin ligation status (i.e., N-cadherin and Cdo interact in cells with or without cell-cell adhesive contact) [12]. Upon N-cadherin ligation, the Cdo intracellular region associates directly with Bnip-2, a scaffold protein for the small GTPase, Cdc42, and with JLP, a scaffold protein for the p38α/β MAP kinase [13, 14]. This results in activation of Cdc42 and Cdc42-dependent activation of JLP-bound p38α/β (Fig. 1). p38α/β in turn promotes myogenesis through phosphorylation of substrates that stimulate MyoD-dependent, muscle-specific gene expression [15]. N-cadherin-initiated, Cdo-dependent signaling is likely to be a major initiator of p38α/β activity during myogenic differentiation in vitro, but additional pathways clearly exist and contribute, particularly at later stages [12, 13]. Mice lacking Cdo display a mild delay in skeletal muscle development and are born with small but normally patterned muscles [16]. Primary Cdo−/− myoblasts have a more severe differentiation defect: they fail to activate p38α/β fully in differentiation medium, express low levels of MyoD target genes and form myotubes inefficiently [14, 16]. Furthermore, restoration of p38α/β activity in Cdo−/− myoblasts by expression of a constitutively active form of the immediate upstream p38α/β activating kinase, MKK6, rescues differentiation of these cells, arguing that p38α/β is functionally downstream of Cdo [14].
Fig. 1.
Signaling pathways activated by engagement of cadherin and IgSF receptors. Factors touching each other (e.g., N-cadherin and β-catenin; Cdo and JLP) or that are connected by a solid arrow have been shown to bind directly to one another. Dashed arrows indicate that the interactions have not been shown to be direct or relate to complex processes (e.g., gene expression, myoblast fusion). Dashed arrows with a question mark represent signaling pathways observed in other cell types and that may occur in myoblasts but have not been tested. The double, anti-parallel dashed arrows between cell surface proteins indicate that these factors have been shown to interact (e.g., Cdo associates with neogenin and M-cadherin; neogenin associates with cadherins) but the how these interactions influence the signaling pathways shown is not clear. Activated MKK6 rescues the defective differentiation program caused by loss of Cdo or Bnip-2, but the role of endogenous MKK3/6 has not been established, so a question mark accompanies their position.
While Cdc42-dependent p38α/β activity is obviously a critical aspect of Cdo function in myoblasts, Cdc42 is also known for its ability to promote formation of filopodia, and C2C12 myoblasts plated on N-cadherin substrate form filopodia in a Cdo- and Cdc42-dependent, p38α/β activity-independent manner (M. Lu and RSK, unpublished). Filopodia form dynamically during myoblast differentiation and may be important for myoblast fusion, a process that requires Cdc42 in vivo [17, 18].
N-cadherin ligation also activates the small GTPase, RhoA, although the direct signaling mechanism involved is not known [19] (Fig. 1). RhoA plays complex roles in myogenesis. It signals to activate the serum response factor (SRF), which in turn stimulates expression of MyoD and other muscle-specific genes in vivo and in vitro [20, 21]. However, RhoA activity decreases at the onset of cell fusion in differentiating cultures of C2C12 cells, and sustained RhoA activity (via expression of a constitutively active RhoA mutant) results in internalization and degradation of M-cadherin, which positively regulates fusion of these cells ([22] and see below).
M-cadherin signals via Trio to activate Rac1
M-cadherin (a classical cadherin encoded by Cdh15) is expressed mainly, though not exclusively, in the skeletal muscle lineage, including myotomes and developing muscle masses; it is also expressed in quiescent satellite cells and induced during adult myogenesis [7]. Studies in cell lines with blocking peptides, neutralizing antibodies and RNAi suggest that M-cadherin functions to promote myoblast fusion and, in some studies, expression of muscle-specific proteins [7, 23]. Cdh15−/− mice do not, however, display obvious defects in skeletal muscle development or adult muscle regeneration, and primary myoblasts from such mice differentiate normally into myotubes in vitro [24]. Other cadherins, including N-cadherin, are expressed in the absence of M-cadherin and may serve in a redundant or compensatory manner [24]. Like N-cadherin, M-cadherin mediates catenin-dependent cell-cell adhesion, and M-cadherin ligation stimulates Cdo-dependent p38α/β activity in C2C12 cells, offering two potentially redundant functions held in common between these two cadherins [7, 12]. Nevertheless, it is not clear why M-cadherin is of greater importance to fusion in cell lines than in primary cultures derived from satellite cells.
The small GTPase, Rac1, is required for myoblast fusion in organisms as diverse as fruit flies, zebrafish and mice ([25] and see below). Additionally, two distinct guanine nucleotide exchange factors (GEFs) for Rac1, Trio and Dock1 (also called Dock180), play roles in myoblast fusion in vivo. Mice lacking Trio display a failure of myoblasts to fuse with nascent myofibers during secondary myogenesis, after E14.5 [26]. (The role of Dock1 is discussed below). M-cadherin ligation in C2C12 cells activates Rac1 in a Trio-dependent manner [23] (Fig. 1). Furthermore, RNAi-mediated depletion of M-cadherin or Trio, or treatment with a small molecule inhibitor of Rac1, all result in inhibition of C2C12 cell fusion without affecting expression of muscle-specific genes [23]. M-cadherin and Trio co-immunoprecipitate from lysates of differentiating C2C12 cells, but it is not known whether this interaction is direct or mediated through additional proteins. Therefore, at least some of M-cadherin’s ability to promote fusion in myoblast cell lines is linked to activation of the essential fusion regulator, Rac1.
Neogenin signals to FAK
Neogenin (encoded by Neo1), like Cdo, contains an ectodomain with Ig and FnIII repeats and a long intracellular region devoid of intrinsic catalytic activity [27]. It serves as a receptor for two families of secreted ligands, netrins and repulsive guidance molecules (RGMs) [27]. Furthermore, neogenin and Cdo bind to each other in cis, and Cdo is a likely context-specific co-receptor for neogenin [11]. Netrin- and RGM-initiated signaling through neogenin is best studied in axon guidance, a form of directed cell motility [27]. However, netrins are usually associated with cell membranes and extracellular matrix, rather than freely soluble, and netrin-neogenin interaction also promotes cell-cell adhesion in specific contexts [27]. Neogenin is expressed in dorsal somites and at high levels in developing skeletal muscle ([11] and references therein). Mice homozygous for a strongly hypomorphic gene-trap mutation in the Neo1 locus (Neo1Gt/Gt mice) develop myotomes normally but express low levels of myogenin at E15.5 and produce myofibers of very small diameter at E18.5 and P21 [11]. Muscle patterning overall appears roughly normal, including in limbs. Furthermore, netrins, which are likely to be the most relevant ligands for neogenin in myogenesis, are expressed with neogenin in developing musculature (for discussion, see [11]). These results suggest that netrin-neogenin signaling may play a role in myoblast-myoblast interactions, rather than long-range motility as in axon guidance. Consistent with this notion, cultured primary myoblasts from Neo1Gt/Gt mice also express low levels of myogenin and form small myotubes with fewer nuclei than control cells.
Neogenin binds directly to the non-receptor tyrosine kinase FAK [27], and netrin signaling activates FAK in primary myoblasts in a neogenin- and Cdo-dependent manner (Fig. 1); in contrast, RGM has little ability to do so [11]. Furthermore, E15.5 muscle from Neo1Gt/Gt mice has lower levels of tyrosine phosphorylated (active) FAK than control muscle [11]. FAK is activated transiently during myoblast differentiation, and inhibition of FAK in primary myoblasts by RNAi-mediated depletion or expression of a dominant-negative form of FAK impairs myoblast fusion [28]. Therefore, neogenin → FAK signaling appears to be important in myogenic differentiation and production of myofibers in vivo and in vitro. It should be noted, however, that neogenin activates additional pathways that may play roles in myogenesis. For example, ERK → p90RSK signaling is reduced in Neo1Gt/Gt mice and cells [11]. Furthermore, neogenin binds Dock1 in neurons, and neogenin orthologs and paralogs bind Trio to activate Rac1, suggesting neogenin may also activate Rac1 during myogenesis [29, 30] (Fig. 1). Similarly, FAK is activated in many systems by integrin signaling, and β1 integrin is required for myoblast fusion in vivo and in vitro [31]. Neogenin signaling is therefore likely to occur within a broader network of signaling events that branch from and converge with other pathways.
NCAM may signal via MYONAP/C6orf32 to promote formation of filopodia
Neural cell adhesion molecule (NCAM) is an IgSF member that mediates homophilic cell-cell adhesion [32]. NCAM is expressed in developing and regenerating skeletal muscle and in myoblasts in vitro [7]. Careful analysis of primary satellite cells cultured for a limited time and then switched to differentiation medium (DM) revealed that NCAM is expressed in only ~10% of such cells prior to induction of differentiation but in >95% of these cells 48 hr afterwards; the kinetics of NCAM expression are similar to that of myogenin, an MRF critical for differentiation [33]. NCAM is alternatively spliced into multiple isoforms, some of which include a muscle-specific domain (MSD) in the extracellular region that is subject to O-linked glycosylation. Overexpression of MSD-containing NCAM isoforms in C2C12 cells enhances differentiation, including myoblast fusion, with O-linked glycosylation of MSD a positive regulator of these effects. Furthermore, mice that express an NCAM transgene specifically in skeletal muscle display enhancement of secondary myoblast fusion (for review, see [7]). In contrast, NCAM-null mice and myoblasts do not display obvious differences in muscle development or fusion in vitro, respectively [34, 35]. Therefore, it has been suggested that, while NCAM may participate in cell adhesion events that promote myogenesis, other adhesive systems compensate for its loss and that the promyogenic effects of NCAM overexpression may be a consequence of enhanced adhesion [7, 34].
The intracellular regions of various NCAM isoforms interact, directly or indirectly, with signaling and cytoskeletal proteins, including some implicated in myoblast differentiation and fusion (e.g., FAK) [32]. However, there is little known about the signaling mechanisms that underlie NCAM’s ability to promote myogenesis when overexpressed, or why such mechanisms are not rate-limiting in the absence of NCAM. It was recently reported that the intracellular region of NCAM binds directly to a protein known as MYONAP or C6orf32 [36, 37] (Fig. 1). Expression of MYONAP/C6orf32 mRNA is induced during differentiation of primary human myoblasts and a quail myoblast cell line but is not muscle-specific [36, 37]. MYONAP/C6orf32 does not contain recognizable structural motifs but is partially localized at the cell membrane, cytoskeleton and filopodia [37]. Overexpression of MYONAP/C6orf32 in myoblast cell lines induces filopodia-like protrusions, and RNAi-mediated depletion reduces fusion into myotubes; in one study this was accompanied by a failure to induce muscle-specific gene products, in another it was more specific for fusion [36, 37]. This difference may be due to the degree of protein depletion or use of different cell systems. Deletion of a 34-amino acid region of MYONAP/C6orf32 required for association with NCAM resulted in loss of ability to produce filopodia when overexpressed [36]. MYONAP/C6orf32 may therefore transduce signals initiated by NCAM-mediated adhesion to factors that regulate the cytoskeleton, in turn modulating differentiation and/or fusion. This may offer an alternative explanation for why NCAM can promote myogenesis when overexpressed but is dispensable for this process: several myoblast signaling systems may link to similar cytoskeletal regulatory events and, even if NCAM normally does so at specific times and locations, if production of filopodia is the key outcome, the required events may occur sufficiently well through other pathways in the absence of NCAM, for example via N-cadherin/Cdo/Cdc42 signaling (see above).
IgSF receptors required for myoblast fusion in Drosophila and their vertebrate counterparts
The process of myoblast fusion (including fusion between two myoblasts and between myoblasts and nascent myofibers) obviously requires cell-cell contact. Myoblast fusion is best understood for embryonic myogenesis in Drosophila, where genetic screens have identified signaling pathways that are critical and specific for cell-cell fusion. The cell surface IgSF adhesion receptors Kirre and Rst bind in trans to the distinct IgSF receptors Sns and Hibris to initiate signals that regulate myoblast fusion in flies. Recent results indicate that the myoblast fusion process is at least partially conserved in vertebrates. Furthermore, vertebrate orthologs of Kirre/Rst and Sns/Hibris, called Neph1–3 and Nephrin, respectively, have been identified, and their roles in myogenesis are being explored; this topic will therefore be covered here from the point of view of adhesion-mediated signaling. For comprehensive overviews of myoblast fusion in Drosophila and vertebrate organisms, please refer to the following reviews [25, 38, 39].
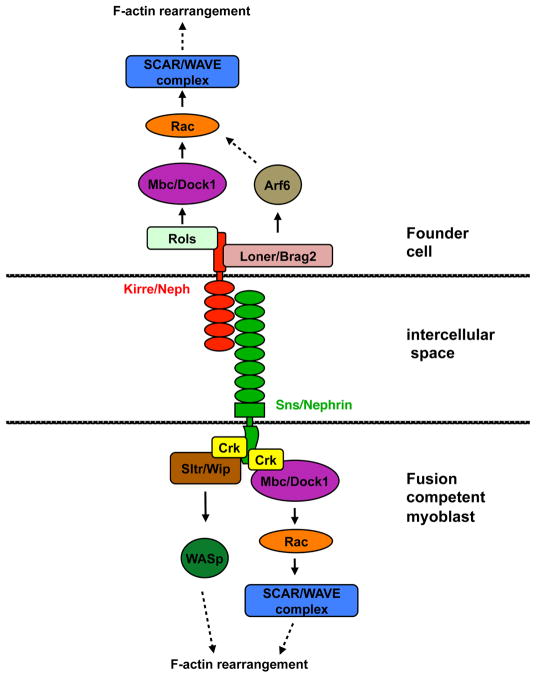
Two types of myoblasts contribute to Drosophila embryonic muscle: 1) muscle founder cells that seed formation of specific muscles; and 2) fusion-competent cells that fuse with founder cells and with nascent myofibers produced by earlier rounds of fusion. Fusion involves a set of mechanistically interconnected events, including cell attraction and migration, cell-cell adhesion, membrane alignment, and membrane merger via formation and resolution of membrane pores [25]. Founder cells express the related receptors Kirre (also called Duf) and Rst (also called IrreC), which function to attract fusion-competent cells and initiate fusion. Fusion-competent cells are attracted to and adhere with founder cells via expression of Sns and Hibris, which bind directly in trans to Kirre and Rst. Upon formation of an adhesive complex called a fusion-restricted myogenic-adhesive structure (FuRMAS), these receptors signal through pathways that regulate actin nucleation to produce F-actin foci at future sites of cell-cell fusion [25, 39]. Genetic and biochemical evidence, plus analogy with related signaling molecules in other systems, has led to the following scenario (Fig. 2). Upon adhesion, the intracellular region of founder cell-associated Kirre/Rst binds to the adaptor protein Rols (also called Ants), which associates with Mbc, a GEF for Rac GTPases; Rols, Mbc and Rac are all required for fusion [25, 39]. Rac in turn activates the SCAR/WAVE actin-nucleating complex, which works via Arp2/3 to regulate the dynamics of the F-actin foci. Kirre/Rst also signals via Loner, a GEF for the small GTPase Arf6, which regulates subcellular localization of Rac [25, 39]. The IgSF receptor-based adhesive interaction also promotes Sns-dependent Mbc/Rac/SCAR signaling in fusion competent myoblasts. Sns binds to Crk, an adaptor protein that interacts directly with Mbc, though structure-function analyses suggest direct Crk-Mbc interaction is dispensable for fusion [25, 39]. Crk also binds to Sltr, which in turn associates with WASp, a second actin-nucleating factor [25, 39]. These pathways regulate formation and dynamics of the F-actin foci that are important for fusion.
Fig. 2.
Signaling pathways activated by engagement of Kirre and Sns during myoblast fusion in Drosophila. Most factors shown have been assessed for a role in vertebrate myoblast fusion also, as described in the text. Factors touching each other or that are connected by a solid arrow have been shown to bind directly to one another. Dashed arrows indicate that the interactions have not been shown to be direct or relate to complex processes, as described in the legend to Fig. 1.
Several of the factors implicated in Drosophila myoblast fusion have been assessed for a similar role in zebrafish, mice and/or mammalian cell culture. Similar to Drosophila, Rac1 is critical for myoblast fusion during primary myogenesis in both zebrafish and mice [18, 40]. Interestingly, the related GTPase Cdc42 is required for fusion in the mouse but not the fly, revealing a degree of evolutionary divergence in the requirement for such factors [18]. F-actin and the cytoskeletal regulators ena, vinculin and Arp2/3 accumulate at sites of cadherin-based myoblast adhesion (marked by β- and α-catenin) [18]. In the absence of Rac1 or Cdc42, β- and α-catenin are present at adhesive contacts, but F-actin, ena and vinculin are not; Arp2/3 accumulates in Cdc42-null cells but not Rac1-null cells [18].
The orthologs of the Rac GEF Mbc (Dock1 and Dock5) are also essential for myoblast fusion during muscle development in zebrafish and mice; Dock1 and Dock5 are equally important in zebrafish, whereas Dock1 is the primary factor in the mouse, with Dock5 playing a non-essential, subsidiary role [41, 42]. It is noteworthy that loss of two different Rac1 GEFs, Dock1 and Trio, lead to two distinct fusion defects in mice, with Dock1−/− mice having the earlier, more severe phenotype and more closely resembling conditional removal of Rac from early myogenic precursor cells and Trio−/− mice displaying a defect in secondary myogenesis [26, 41]. It seems likely that Rac activity is controlled by different GEFs at distinct times during developmental myogenesis and that signaling through these individual GEFs may be regulated by distinct adhesion receptors.
The mammalian orthologs of other Drosophila factors involved in myoblast fusion have also been studied in mammalian cell culture with RNAi and dominant-negative approaches. Individual inhibition of Dock1, Brag2 (the mouse ortholog of Loner), Arf6, Wip (the mouse ortholog of Sltr), N-Wasp, and Kette (a component of the SCAR/WAVE complex) function reduces fusion in C2C12 cells [17, 43–45]. Although the reduction in multinucleated myotube formation in these cases appears mainly to be due to specific defects in cell-cell fusion, it is interesting that depletion of Dock1 and Brag2 also leads to delayed cell cycle withdrawal and impaired expression of myogenin and MHC early in the differentiation process [45]. It is possible that the mechanisms of muscle-specific gene expression and myoblast fusion are more tightly overlapping (and potentially coupled) in mammalian cells than in Drosophila.
The role of the IgSF receptors is less clear. Zebrafish Kirrel morphants (Kirrel is an ortholog of Drosophila Kirre/Rst) have a phenotype similar to that of flies lacking both Kirre and Rst, i.e., morphants display a specific reduction of myoblast fusion during somitic myogenesis [40]. There are three Kirre/Rst orthologs in the mouse, Neph1–3 (Neph1, Neph2 and Neph3 are also called Kirrel1, Kirrel3 and Kirrel2, respectively); Neph1−/− mice do not display a muscle phenotype [46], but mice carrying mutations in Neph2 and Neph3 have not been assessed and redundancy is possible. Mice lacking nephrin (the vertebrate ortholog of Sns) appear normal at birth and can breathe and suckle, indicating that there are no severe defects in fetal myogenesis [47]. These mice die within 2 days with severe nephrotic dysfunction, but myoblasts derived from neonatal nephrin mutants display inefficient fusion in vitro, particularly myoblasts with nascent myotubes [48]. Zebrafish nephrin morphants had shorter, less organized myosepta, and some myofibers showed clustered nuclei but had no obvious defects in myoblast fusion [48]. Taken together, the emerging picture is one in which the signaling pathways that promote myoblast fusion are evolutionarily conserved, but the mechanisms whereby these pathways are initiated by cell-cell adhesion are not well understood in vertebrates.
Perspectives
During myogenesis cell-cell contact and adhesion promotes both expression of muscle-specific genes and myoblast fusion. Several myoblast cell surface proteins that mediate cell-cell adhesion are known, and substantial progress has been made in identification of the signaling pathways they activate. While a degree of evolutionary conservation exists, the precise roles these factors and pathways play in mammalian skeletal muscle development are still not well understood. In loss-of-function analyses, several in vivo/in vitro discrepancies exist, with in vitro effects stronger than in vivo effects. This may not be surprising as a greater level of redundancy and/or compensation may be present in vivo, a not uncommon phenomenon. Furthermore, primary myoblasts sometimes behave differently than myoblast cell lines. For example, M-cadherin depletion in C2C12 cells blocks fusion but primary myoblasts from M-cadherin-null mice are reported to fuse normally [23, 24]. While most of what we have learned from cell lines extrapolates well to more physiological settings, it is clear that C2C12 cells, the best studied line, are different from primary myoblasts in several ways [49]. It is noteworthy that mice lacking specific cytoplasmic signaling factors activated (or likely to be activated) by multiple cell surface proteins have strong phenotypes in vivo (e.g., the GTPases Rac1 and Cdc42; the Rac GEFs Dock1 and Trio). In contrast, mice lacking cell surface proteins that lie upstream of these cytoplasmic signaling factors generally have more modest, if any, muscle phenotypes. Redundancy among family members may account for some of this; for example, N- and M- (and possibly additional) cadherins can easily be envisaged to possess compensatory functions, though this needs to be tested by construction of double- or multiple-mutants. Likewise, IgSF receptors of the same or even divergent subfamilies, may compensate for each other. Rac1 is a downstream effector of M-cadherin, neph, nephrin, and neogenin in myoblasts of one or more species (or yet to be tested in myoblasts, but downstream in other cell types) (Figs. 1 and 2). If Rac1 serves as a hub for multiple pathways, it is conceivable that cadherins and IgSF receptors have non-orthologous but physiologically overlapping functions. Obviously, these adhesion receptors do not all signal exclusively through Rac1, but this notion is a potential, if not fully satisfactory, way to explain the difference in phenotypes of mice with mutations in adhesion molecules vs. mice with mutations in core signaling factors. It is worth noting that many of the IgSF receptors and cadherins form cis complexes with each other (e.g., Cdo with cadherins; Fig. 1) and that many of these cell surface proteins are found in lipid rafts with additional factors that promote myogenesis [33]. Multivalent interactions among cell surface signaling complexes may require removal of multiple components to reveal a dramatic defect in vivo. An alternative possibility is that the adhesion-related receptors that are rate-limiting in vivo in the mouse have not yet been identified or fully assessed in vivo. The connection between adhesion-directed signaling and the complex process whereby a myoblast differentiates into a myofiber is of intrinsic basic interest and important to understand if therapies for myopathies are to succeed optimally. Work over the past several years with several model organisms and in vitro mechanistic studies have shed much light in this area, and it is anticipated that these approaches will continue to bear fruit and help clarify some of the current discrepancies.
Acknowledgments
I apologize to the many authors whose work has been cited indirectly through other review articles due to limitations in references. Work in the author’s laboratory on this topic is funded by the National Institutes of Health (AR46207).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol. 2007;308:281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Kang JS, Krauss RS. Muscle stem cells in developmental and regenerative myogenesis. Curr Opin Clin Nutr Metab Care. 2010 doi: 10.1097/MCO.0b013e328336ea98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajbakhsh S. Skeletal muscle stem and progenitor cells: reconciling genetics and lineage. Exp Cell Res. 2005;306:364–372. doi: 10.1016/j.yexcr.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Borghi N, Nelson WJ. Intercellular Adhesion in Morphogenesis: Molecular and Biophysical Considerations. Curr Top Dev Biol. 2009;89:1–32. doi: 10.1016/S0070-2153(09)89001-7. [DOI] [PubMed] [Google Scholar]
- 6.Rougon G, Hobert O. New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci. 2003;26:207–238. doi: 10.1146/annurev.neuro.26.041002.131014. [DOI] [PubMed] [Google Scholar]
- 7.Krauss RS, Cole F, Gaio U, Takaesu G, Zhang W, Kang JS. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J Cell Sci. 2005;118:2355–2362. doi: 10.1242/jcs.02397. [DOI] [PubMed] [Google Scholar]
- 8.Cinnamon Y, Ben-Yair R, Kalcheim C. Differential effects of N-cadherin-mediated adhesion on the development of myotomal waves. Development. 2006;133:1102–1112. doi: 10.1242/dev.02291. [DOI] [PubMed] [Google Scholar]
- 9.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 10.Charlton CA, Mohler WA, Radice GL, Hynes RO, Blau HM. Fusion competence of myoblasts rendered genetically null for N-cadherin in culture. J Cell Biol. 1997;138:331–336. doi: 10.1083/jcb.138.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae GU, Yang YJ, Jiang G, Hong M, Lee HJ, Tessier-Lavigne M, Kang JS, Krauss RS. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Mol Biol Cell. 2009;20:4920–4931. doi: 10.1091/mbc.E09-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu M, Krauss RS. N-cadherin ligation, but not Sonic hedgehog binding, initiates Cdo-dependent p38α/β MAPK signaling in skeletal myoblasts. Proc Natl Acad Sci (USA) 2010;107:4212–4217. doi: 10.1073/pnas.0908883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang JS, Bae GU, Yi MJ, Yang YJ, Oh JE, Takaesu G, Zhou YT, Low BC, Krauss RS. A Cdo/Bnip-2/Cdc42 signaling pathway regulates p38α/β MAPK activity and myogenic differentiation. J Cell Biol. 2008;182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM, Reddy EP, Krauss RS. Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guasconi V, Puri PL. Chromatin: the interface between extrinsic cues and the epigenetic regulation of muscle regeneration. Trends Cell Biol. 2009;19:286–294. doi: 10.1016/j.tcb.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole F, Zhang W, Geyra A, Kang JS, Krauss RS. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev Cell. 2004;7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Nowak SJ, Nahirney PC, Hadjantonakis AK, Baylies MK. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J Cell Sci. 2009;122:3282–3293. doi: 10.1242/jcs.047597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc Natl Acad Sci (USA) 2009;106:8935–8940. doi: 10.1073/pnas.0902501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and b-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol Biol Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A, Olson EN. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci (USA) 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouvière C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol Biol Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouvière C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–1743. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollnagel A, Grund C, Franke WW, Arnold HH. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: When it takes more to make one. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien SP, Seipel K, Medley QG, Bronson R, Segal R, Streuli M. Skeletal muscle deformity and neuronal disorder in Trio exchange factor-deficient mouse embryos. Proc Natl Acad Sci (USA) 2000;97:12074–12078. doi: 10.1073/pnas.97.22.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vries M, Cooper HM. Emerging roles for neogenin and its ligands in CNS development. J Neurochem. 2008;106:1483–1492. doi: 10.1111/j.1471-4159.2008.05485.x. [DOI] [PubMed] [Google Scholar]
- 28.Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal Adhesion Kinase Signaling Regulates the Expression of Caveolin 3 and beta1 Integrin, Genes Essential for Normal Myoblast Fusion. Mol Biol Cell. 2009;20:3422–3435. doi: 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briançon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, Piché C, Enslen H, Chebli K, Cloutier JF, Castellani V, Debant A, Lamarche-Vane N. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Gao X, Liu G, Xiong W, Wu J, Rao Y. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. β1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 32.Ditlevsen DK, Povlsen G, Berezin V, Bock E. NCAM-induced intracellular signaling revisited. J Neurosci Res. 2008;86:727–743. doi: 10.1002/jnr.21551. [DOI] [PubMed] [Google Scholar]
- 33.Capkovic KL, Stevenson S, Johnson MC, Thelen JJ, Cornelison DD. Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp Cell Res. 2008;314:1553–1565. doi: 10.1016/j.yexcr.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charlton CA, Mohler WA, Blau HM. Neural cell adhesion molecule (NCAM) and myoblast fusion. Dev Biol. 2000;221:112–119. doi: 10.1006/dbio.2000.9654. [DOI] [PubMed] [Google Scholar]
- 35.Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Wille W. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 36.Hirayama E, Kim J. Identification and characterization of a novel neural cell adhesion molecule (NCAM)-associated protein from quail myoblasts: relationship to myotube formation and induction of neurite-like protrusions. Differentiation. 2008;76:253–266. doi: 10.1111/j.1432-0436.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoon S, Molloy MJ, Wu MP, Cowan DB, Gussoni E. C6ORF32 is upregulated during muscle cell differentiation and induces the formation of cellular filopodia. Dev Biol. 2007;301:70–81. doi: 10.1016/j.ydbio.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansen KM, Pavlath GK. Molecular control of mammalian myoblast fusion. Methods Mol Biol. 2008;475:115–133. doi: 10.1007/978-1-59745-250-2_7. [DOI] [PubMed] [Google Scholar]
- 39.Abmayr SM, Zhuang S, Geisbrecht ER. Myoblast fusion in Drosophila. Methods Mol Biol. 2008;475:75–97. doi: 10.1007/978-1-59745-250-2_5. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas BP, Woo J, Leong WY, Roy S. A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat Genet. 2007;39:781–786. doi: 10.1038/ng2055. [DOI] [PubMed] [Google Scholar]
- 41.Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Côté JF. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci (USA) 2008;105:15446–15451. doi: 10.1073/pnas.0805546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore CA, Parkin CA, Bidet Y, Ingham PW. A role for the Myoblast city homologues Dock1 and Dock5 and the adaptor proteins Crk and Crk-like in zebrafish myoblast fusion. Development. 2007;134:3145–3153. doi: 10.1242/dev.001214. [DOI] [PubMed] [Google Scholar]
- 43.Chen EH, Pryce BA, Tzeng JA, Gonzalez GA, Olson EN. Control of myoblast fusion by a guanine nucleotide exchange factor, Loner, and its effector ARF6. Cell. 2003;114:751–762. doi: 10.1016/s0092-8674(03)00720-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Pajcini KV, Pomerantz JH, Alkan O, Doyonnas R, Blau HM. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J Cell Biol. 2008;180:1005–1019. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Sohn RL, Huang P, Kawahara G, Mitchell M, Guyon J, Kalluri R, Kunkel LM, Gussoni E. A role for nephrin, a renal protein, in vertebrate skeletal muscle cell fusion. Proc Natl Acad Sci (USA) 2009;106:9274–9279. doi: 10.1073/pnas.0904398106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornelison DD. Context matters: in vivo and in vitro influences on muscle satellite cell activity. J Cell Biochem. 2008;105:663–669. doi: 10.1002/jcb.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]