Abstract
Posttraumatic stress disorder (PTSD) accounts for a substantial proportion of casualties among surviving soldiers of the Iraq and Afghanistan wars. Currently, the assessment of PTSD is based exclusively on symptoms, making it difficult to obtain an accurate diagnosis. This study aimed to find potential imaging markers for PTSD using structural, perfusion and diffusion magnetic resonance imaging (MRI) together. Seventeen male veterans with PTSD (45 ± 14 years old) and 15 age-matched male veterans without PTSD had measurements of regional cerebral blood flow (rCBF) using arterial spin labeling (ASL) perfusion MRI. A slightly larger group had also measurements of white matter integrity using diffusion tensor imaging (DTI) with computations of regional fractional anisotropy (FA). The same subjects also had structural MRI of the hippocampal subfields as reported recently (W. Zhen et al. Arch Gen Psych 2010; 67(3):296–303). On ASL-MRI, subjects with PTSD had increased rCBF in primarily right parietal and superior temporal cortices. On DTI, subjects with PTSD had FA reduction in white matter regions of the prefrontal lobe, including areas near the anterior cingulate cortex and prefrontal cortex as well as in the posterior angular gyrus. In conclusion, PTSD is associated with a systematic pattern of physiological and structural abnormalities in predominantly frontal lobe and limbic brain regions. Structural, perfusion and diffusion MRI together may provide a signature for a PTSD marker.
INTRODUCTION
It is estimated that posttraumatic stress disorder (PTSD), a mental condition that can affect individuals who have been exposed to severe emotional or physically life-threatening traumatic events, accounts for as much as 20% of the casualties among surviving soldiers of the Iraq and Afghanistan wars (Tanielian and Jaycox, 2008). The prevalence of PTSD is particularly high - up to 43% (Hoge et al., 2008) - among soldiers who suffer from mild traumatic brain injury (MTBI), a physical injury often caused by a mild blow to the brain without visible damage. Since PTSD and MTBI share many persistent symptoms, such as problems with memory and attention as well as depression, impulsivity, and irritability, a differential diagnosis between these two conditions is extremely difficult. The identification of robust biomarkers for each condition is therefore critical for an accurate diagnosis as well as for the selection of effective treatments, assessment of treatment outcome and disability evaluations. Neuroimaging is among the most promising technologies to address this critical gap. This study primarily aimed to find potential markers for PTSD using magnetic resonance imaging (MRI) in a population that also suffered from various levels of MTBI. However, the study does not include a separate group of MTBI patients without PTSD to conduct a rigorous correspondence analysis between the two conditions, which will be the topic of future work.
We recently studied differences in size of the hippocampal subfields between veterans with and without PTSD using high-resolution structural MRI (Wang et al., 2010). The hippocampus has long been a prime target for studies of PTSD because of its key role for memory processing, which is functionally important for the pathogenesis of the persistent re-experiencing symptoms in the context of trauma as well as for its crucial role in the biological response to stress (Sapolsky, 2000). We found that PTSD was associated with a smaller dentate gyrus, which is a key site for neurogenesis and a smaller CA3 region, which is a major target for glucocorticoids, a class of steroid hormones that are elevated under conditions of stress (McEwen, 2002). Although imaging of the subfields may clarify the role of the hippocampus in PTSD, these measures of brain structure alone may neither yield the most sensitive marker for PTSD nor a marker that is very specific, since other conditions, such as epilepsy and depression, also can impact the hippocampal subfields (Ballmaier et al., 2008; Mueller et al., 2009).
We therefore extended our imaging investigations into PTSD beyond structural brain alterations. Functional imaging studies of regional cerebral blood flow (rCBF) using Positron Emission Tomography (PET) or Single Proton Emission Computed Tomography (SPECT) with radioisotopes have pointed to an extended network of related brain regions that mediate the PTSD symptoms when subjects are exposed to trauma reminders (for a review see (Lanius et al., 2006)). Even without provocative reminders, PTSD subjects show patterns of both increased and decreased rCBF levels in some regions compared to controls. This network include the limbic system, to which the hippocampus belongs as well as the prefrontal cortex, which has a role in the encoding and retrieval of verbal memory (Sachinvala et al., 2000). Similarly to functional studies, recent studies using diffusion tensor imaging (DTI), a new MRI variant that is sensitive to tissue microstructure, reported abnormalities in various subcortical brain regions in PTSD (Abe et al., 2006; Kim et al., 2006). Specifically, DTI studies suggested that PTSD is associated with microstructural degradation of the cingulum bundle, a neural pathway interconnecting many brain regions of the limbic system, such as the hippocampus and the anterior cingulate cortex, which is thought to play a key role in emotion regulation (Vogt et al., 1992). However, previous structural, functional, and DTI studies in PTSD were conducted separately and often tested different aspects of PTSD.
Here we report initial findings of cortical rCBF patterns and subcortical DTI alterations in a group of veterans with PTSD, who also had undergone structural MRI of the hippocampus and its subfields, as previously reported (Wang et al., 2010). Note, we measured rCBF using arterial spin labeling (ASL) perfusion imaging, another relatively new MRI variant and an entirely non-invasive approach to determine blood flow (Detre et al., 1998). ASL imaging also facilitates to obtain rCBF in the same session together with TI and structural MRI. Given the findings from previous PET/SPECT as well as DTI studies, we hypothesized that veterans with PTSD are characterized by patterns of altered cortical rCBF and diminished subcortical microstructures in a network that comprises areas in the frontal lobe and the limbic system.
METHODS
Participants
The subjects in this study completely overlap with those in the previous MRI studies on hippocampal subfields in PTSD (Wang et al., 2010). Briefly, subjects were recruited from the outpatient mental health clinic of the San Francisco Veterans Affairs Medical Center and by advertising from the community. After a complete description of the study was given to the participants, written informed consent was obtained. All procedures were approved by the Committees of Human Research at the University of California and the Veterans Affairs Medical center and were compliant with HIPAA regulations.
The study design required recruitment of two groups: Veterans with current PTSD and healthy veterans without PTSD. Veterans participating in the study were evaluated by a clinical interviewer using the Structured Clinical Interview for DSM-IV Diagnosis (SCID) (First et al., 1994) and the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995). Subjects were then classified belonging either to a PTSD negative or positive group. An interview version of the Life Stressor Checklist-Revised (LSC-R) (Wolfe et al., 1996) was used to determine exposure to traumatic events. The LSC-R assesses 21 stressful life events (e.g., experiencing or witnessing serious accidents, illnesses, sudden death, physical and sexual assault). The SCID was used to rule out individuals with a lifetime history of psychotic or bipolar disorders, alcohol abuse or dependence with in the previous 12 months, and drug abuse or dependence within the previous six months. Veterans with past but not current PTSD or current subsyndromal PTSD were also excluded. Other exclusion criteria were neurological illness, head trauma with loss of consciousness greater than 10 minutes, any head injury with history of post-concussive symptoms, medical disorders affecting brain function, and conditions ineligible for MRI. Note, veterans with a history of blast exposure were not excluded.
Twenty participants with PTSD and 20 negative for PTSD received MRI. All subjects with PTSD suffered from traumatic exposure related to combat. The trauma histories of those negative for PTSD included 14 individuals who served in combat, five without a traumatic life event, and one who experienced a military accident. Three subjects with PTSD and five without PTSD had to be excluded from the analysis of rCBF because their ASL imaging data showed substantial artifacts, mainly due to movement. For DTI, only one subject with and another without PTSD had to be excluded because of movement artifacts. The higher number of exclusions for ASL imaging compared to DTI is due to the higher sensitivity of ASL to motion, because ASL relies on accurate subtraction of two sequential MRI scans to extract a rCBF related signal. Thus, the final analysis included 17 PTSD and 15 control subjects for ASL imaging and 19 PTSD and 19 control subjects for DTI. The sample of subjects with combat related PTSD (N=19 for DTI) are a heterogeneous group whose warzone experience ranged from the Vietnam War (N=4), the conflict in Beirut (N=1), Persian Gulf War (N=4), and the current wars in Iraq (N=8) and Afghanistan (N=2). Similarly, the combat exposed control subjects were veterans with service in the Vietnam War (N=2), Persian Gulf War (N=1), the wars in Iraq (N=9), Afghanistan (N=1), and a military accident (N=1) during the Iraq war. The demographics and clinical characteristics of the participants are summarized in Table 1, separately for the ASL and DTI studies.
Table 1.
Demographic data
| PTSD | Control | p-value | |
|---|---|---|---|
| CBF Study N | 17 | 15 | |
| Trauma exposed | 17 | 12 | p = 0.2 * |
| Age [years] | 45 ± 14 | 37 ± 13 | p = 0.3 |
| CAPS (1) | 59 ± 13 | 7 ± 7 | p < 0.001 |
| DTI Study N | 19 | 19 | |
| Trauma exposed | 19 | 14 | p = 0.6 * |
| Age [years] | 46 ± 12 | 40 ± 15 | p = 0.4 |
| CAPS (1) | 62 ± 13 | 6 ± 7 | p < 0.001 |
using a Chi-square test, all other comparisons use t-tests;
CAPS: Clinician administered PTSD scale, ranging from zero to 136 with higher scores indicating greater PTSD severity (reference (Blake et al., 1995));
MRI acquisitions
MRI scans were performed at the Center for Imaging of Neurodegenerative Diseases (CIND) at the San Francisco VA Medical Center using a Bruker/Siemens MedSpec 4T MRI system equipped with an eight channel array receiver coil. The scan protocol for structural MRI consisted of a volumetric T1-weighted magnetization prepared gradient echo (MPRAGE) sequence and a T2-weighted turbospin-echo sequence, which were used respectively to correct ASL and DTI data for partial volume effects and geometrical distortions. The parameters of MPRAGE were TR/TI/TE = 2300/950/4 ms, 7° degree excitation pulses, 1 × 1 × 1 mm3 resolution. The parameters of the T2-weighted turbospin echo sequence were TR/TE 3000/356 ms, 109 echoes per k-space segment with variable flip angles, 1 mm × 1 mm × 2 mm nominal resolution, 120 slices, acquisition time 3.40 min.
In addition to structural MRI, ASL perfusion images were acquired using a continuous arterial spin labeling (cASL) sequence (Detre et al., 1992) with a single-shot echo-planar imaging (EPI) part to map the perfusion signal. The sequence consisted of 16 slices, each with 5 mm thick with 1.2 mm inter-slice gap and 3.75 × 3.75 mm2 in-plane resolution. Arterial spin labeling was accomplished with 2 seconds long labeling pulses and a one second long post labeling delay before the signal was mapped by EPI with TR/TE = 5200/9 ms timing. DTI maps were acquired using a dual spin-echo EPI sequence, augmented by diffusion weighting gradients (b=800 s/mm2) along six collinear directions and the acquisition of a reference image (b=0). Other parameters of DTI were: TR/TE = 6000/77ms, a matrix size of 128 × 112 across 256 × 224cm field-of-view, yielding 2 × 2 mm2 in-plane resolution across 40 slices, each 3mm thick. Twofold parallel imaging acceleration was used for DTI to reduce geometrical distortions (Griswold et al., 2002) and four scans were averaged to boost the signal.
MRI processing
The key image processing steps for the computation of quantitative maps of ASL perfusion and DTI followed largely published procedures by the authors and included the following (Jahng et al., 2007; Zhang et al., 2009): For ASL, processing started with pairwise subtraction of co-registered labeled from unlabeled ASL images, yielding perfusion-weighted images. This followed normalization of the ALS signal to the unlabeled ALS image, elimination of spurious signal contributions of very high intensity from arterial vessels, affine alignment of EPI to the corresponding T2-weighted structural image, followed by fluid-flow warping based nonlinear geometry distortion corrections to establish anatomical correspondence between ASL perfusion and structural MRI data, and finally fusion of ASL perfusion and tissue segmented structural MRI data to achieve anatomical correspondence and to compute partial volume corrected rCBF maps of gray matter. Tissue segmentation was performed using the parametric expectation maximization segmentation (EMS) tool available with the Statistical Parametric Mapping (SPM) software (http://www.fil.ion.ucl.ac.uk/spm/). Partial volume correction of rCBF was performed by rescaling rCBF in each voxel proportionately to the gray and white matter content, assuming that perfusion of white matter is only 25% of that of gray matter. Finally, the partial-volume-corrected rCBF maps were spatially normalized to the brain template standard of the Montreal Neurological Institute (MNI) by applying to rCBF maps the same transformations than the one used for normalization of the corresponding structural MRI maps. Note, rCBF was expressed in institutional rather than in absolute units of ml/100mg/min, because transit time of the ASL signal as well as T1 relaxation, which both impact the magnitude of the signal, could not be determined experimentally due to prohibitively long scan times. Nonetheless, rCBF in institutional unit will not diminish a group effect because differences in transit time and T1 relaxation between the groups are not expected.
Processing of DTI data involved the tract-based spatial statistics (TBSS) technique (Smith et al., 2006) for quantification and spatial normalization of spatially invariant DTI measures, such as fractional anisotropy (FA) and mean diffusivity (MD) in following steps: (i) a representative subject in the control group was selected as the “target” to which the volumetric FA maps of all subjects were aligned by using a 12 degrees of freedom affine transformation, followed by a nonlinear registration procedure in TBSS; (ii) the aligned FA maps were interpolated to 1×1×1 mm resolution and spatially normalized to a T1-weighted template in Talairach space; and (iii) for each subject, the FA map was spatially normalized through the transformation pipeline estimated in steps (i) and (ii). Voxel-based DTI analysis was applied within a WM mask obtained by applying a threshold FA > 0.2 onto the mean FA map of all subjects in the normalized space.
Statistics
Differences in rCBF between subjects with and without PTSD were tested voxelwise using a general linear model with group as main contrast and global mean perfusion as covariates. To reduce local variations due to image registrations and overall improve sensitivity, the rCBF images were smoothed using a 12-mm full width half height Gaussian kernel. The analysis of rCBF data was performed within the framework of SPM version 2. The statistical significance for differences in rCBF tested initially at the voxel level was set to an uncorrected p value of 0.001. For an analysis of group differences in the DTI data, a 3-D multivariate linear regression program (3dRegAna) from the Analysis of Functional NeuroImage (AFNI) group (Cox, 1996) was applied. A voxelwise DTI analysis was performed using non-parametric permutation t-tests. The statistical significance for FA differences tested initially at the voxel level was set to an uncorrected p value of 0.001. To reduce the risk for type I errors (false discoveries), adjustments for multiple comparisons were performed on a cluster level and the concept of false discovery rate (FDR) applied (Benjamini and Hochberg, 1995). For rCBF, where the number of significant clusters was relatively small, we set the FDR threshold to 20% equivalent to accepting one false discovery. For DTI, which does not involve spatial smoothing and where more clusters were found, we used a FDR threshold of 5%. We consider the level of control for type I errors reasonable since the cost of avoiding any type I error is extremely high for exploratory investigations. We also tested for correspondences between regional variations in DTI and CBF using the non-parametric Spearman rank correlation test.
RESULTS
Demographics and clinical information of the two groups are summarized in Table 1, separately for the analysis of ASL perfusion and DTI data. In each instance, the two groups were comparable in age (p > 0.2 by t-test) and proportion of trauma exposed subjects (p > 0.8, by Chi-square test). As expected PTSD subjects had significantly higher CAPS scores than PTSD negative subjects (p < 0.001, t-test) and their duration of PTSD symptoms ranged from 2 to 38 years (mean=14, SD=14).
Pattern of rCBF
Figure 1 depicts representative rCBF maps (after fusion with structural MRI and partial volume correction) of a subject with PTSD (right) and one without (left). The maps depict rCBF alterations in PTSD in the right parietal lobule, a region which showed systematically elevated rCBF values in PTSD than in nonPTSD subjects. Figure 2 shows statistical parametric maps (SPM) of group differences in rCBF, superimposed on the surface rendered MNI brain template. Subjects with PTSD had higher rCBF values than those without PTSD in the right parietal cortex (Brodmann Area (BA) 40, t>5.31), right superior temporal cortex (BA 13, t>3.65), right inferior frontal gyrus (BA10, t>3.99) and right superior frontal gyrus (BA 10, t>3.48). In contrast, findings of lower rCBF values in PTSD were not significant. The significant rCBF findings are summarized in Table 2. Within the PTSD group, no significant associations were found between rCBF alterations and duration of symptoms.
Figure 1.

Representative maps of regional cerebral blood flow (rCBF - after fusion with structural MRI and partial volume correction) from two trauma exposed veterans, one diagnosed with PTSD (PTSD+, age =34 years, CAPS = 80) and another without PTSD (PTSD-, age = 22 years, CAPS = 13). The arrows in the rCBF maps point to the inferior posterior lobule, which showed a systematic increase in rCBF in PTSD.
Figure 2.
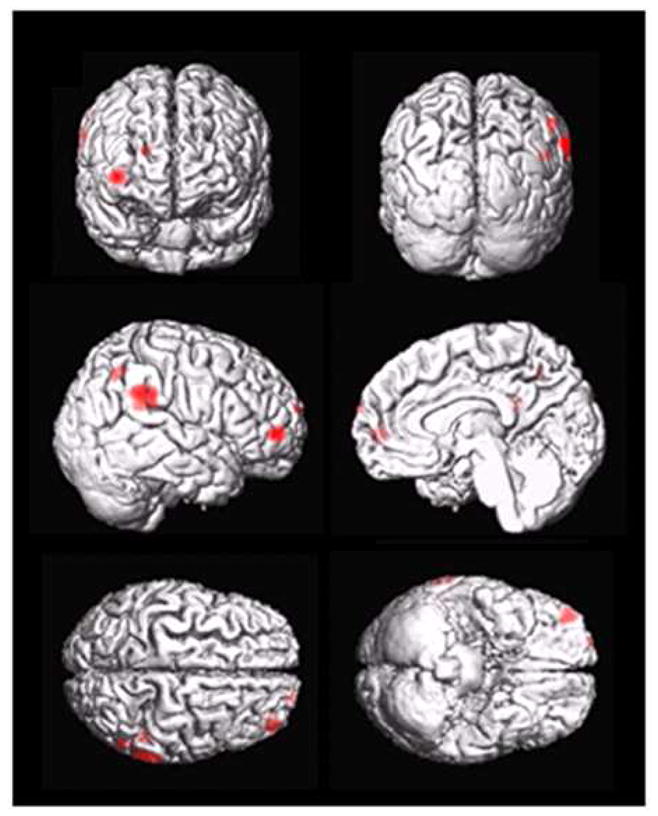
Statistical parametric maps of significantly increased regional cerebral blood flow (rCBF) in PTSD relative to controls.
Table 2.
Regions of significantly increased regional cortical blood flow (rCBF) between subjects with and without PTSD
| Brain Regions | Brodmann Area | Cluster Size | T-score | FDR(1) P-value | MNI(2) x, y, z |
|---|---|---|---|---|---|
| Right inferior parietal lobule | 40 | 212 | 5.31 | 0.15 | 66, −40, 28 |
| Right inferior frontal gyrus | 10 | 107 | 3.99 | 0.16 | 40, 52, 0 |
| Right superior temporal gyrus | 13 | 46 | 3.65 | 0.17 | 50, −44, 20 |
| Right superior frontal gyrus | 10 | 19 | 3.48 | 0.20 | 20, 66, 18 |
False discovery rate (Benjamini and Hochberg, 1995)
Cluster coordinates in the frame of the Montreal Neurological Institute (MNI) brain template.
Pattern of DTI
DTI results of FA differences between the groups are shown in Figure 3. Significant regional differences are depicted in color and superimposed on the average FA maps of the study population. Subjects with PTSD had - compared to subjects without PTSD - reduced FA primarily in white matter regions of the prefrontal lobe, including areas near the anterior cingulate cortex (ACC), prefrontal cortex (PFC) and precentral gyrus (PCG), as well as in the posterior internal capsule (p.IC) and the posterior angular gyrus (AG). Note, no brain region in subjects with PTSD had a significantly higher FA value than brain regions in subjects without PTSD. Within the PTSD group, no significant associations were found between DTI alterations and duration of symptoms, similar to the rCBF findings. Furthermore, no significant correspondences were found between regional alterations in DTI and rCBF in PTSD.
Figure 3.
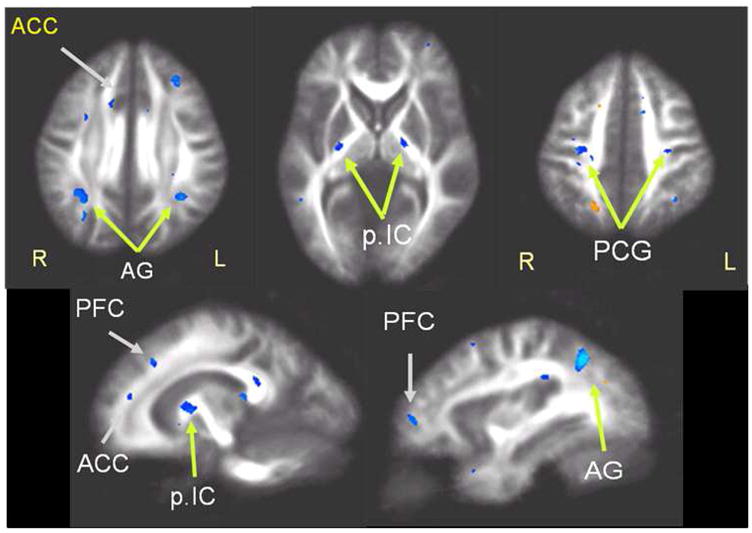
Statistical maps of significant reduction in fractional anisotropy (FA, shown in blue) in PTSD relative to control subjects (p = 0.001, uncorrected). Areas of significant FA reduction include regions near the anterior cingulate cortex (ACC), prefrontal cortex (PFC), posterior central gyrus (PCG) and angular gyrus (AG). FA reductions in PTSD are also seen in the posterior internal capsule (pIC). FA increase (orange) is seen at a single location in an unspecific anatomical region (far right image in the top row).
DISCUSSION
We have two major findings: First, PTSD was associated with a systematic pattern of elevated rCBF values in right parietal and temporal brain regions. These findings are consistent with several PET and SPECT studies in PTSD. Second, PTSD was also associated with a systematic pattern of FA reduction in white matter tracts that involved prefrontal areas and the anterior cingulate cortex. Moreover, the rCBF and FA alterations were seen in addition to findings of smaller dentate gyrus/CA3 subfields in the same PTSD subjects, as previously reported (Wang et al., 2010). Taken together, the findings suggest that PTSD is associated with physiological and microstructural abnormalities of specific brain circuits that include frontal cortex and the limbic system in addition to structural damage in specific hippocampal subfields.
Our finding of increased rCBF in right parietal and temporal brain regions in PTSD is consistent with several resting state rCBF studies using SPECT (Bonne et al., 2003; Sachinvala et al., 2000). Our finding cannot simply be discredited as artifact of underlying structural differences between PTSD and control subjects because we corrected rCBF for variations in partial gray and white matter volumes. In addition, the right hemispheric dominance of rCBF increase in PTSD has been observed with SPECT and PET (Bonne et al., 2003; Rauch et al., 1996; Sachinvala et al., 2000). Moreover, brain activation studies using electroencephalography also found a unique pattern of tonically high right parietal and low left frontal activation in PTSD (Metzger et al., 2004) and typically more right than left activity during anxious arousal (Nitschke et al., 1999). It is generally accepted that the right hemisphere plays a major role in subjective experience and expression of emotion (Geschwind and Galaburda, 1985). The lateral hyperactivity implied by rCBF increase may therefore reflect pathophysiology of PTSD. It is interesting to note that while we found increased rCBF in the inferior parietal lobule in PTSD, several functional studies reported decreased rCBF in this region when PTSD patients were confronted with provocative trauma stimuli (Bremner et al., 2004; Shin et al., 1999). The findings are not necessarily conflicting because functional studies have shown that stress stimulators can result in de-activation of brain networks in PTSD (Bremner et al., 2004). In particular, alterations in the inferior parietal lobule are thought to be related to increased attention or fear response seen in PTSD patients when under stress (Bremner et al., 2004).
The observation that ASL detects similar patterns of rCBF alterations in patients with PTSD than PET and SPECT studies encourages the use of this MRI technique in broader studies of PTSD, especially since MRI is more widely available than PET and SPECT and also is more compliant with health safety issues as no injections of radio-isotopes are needed. However, PET and SPECT have an advantage for veterans, who have shrapnel and retained metal fragments in their bodies and therefore are excluded from MRI.
Another major finding is decreased FA values in frontal lobe regions in subjects with PTSD. FA decrease of the anterior cingulate white matter tract in PTSD subjects has been reported before (Abe et al., 2006; Kim et al., 2006). Moreover, the finding of FA decrease in anterior cingulate and prefrontal areas is in line with numerous functional studies of compromised function of these regions in PTSD (for a review see (Lanius et al., 2006)) as well as with MRI findings of reduced size (Woodward et al., 2006; Yamasue et al., 2003) and metabolism (Schuff et al., 2008) of the anterior cingulate cortex in PTSD. The correspondent findings from all these imaging indices of an involvement of the anterior cingulate in PTSD raise speculations about the common mechanism that might underpin the structural compromise, which at a minimum implicates both white and gray matter. One potential etiological avenue is that chronic stress is the basis for structural abnormalities in PTSD by means of diminished neuronal plasticity, including reduced dendritic branching, in response to glucocorticoids, which may have directly and indirectly have neurotoxic effects. Studies on rats showed that stress can lead to a decrease in the number of dendritic length and branch points in the anterior cingulate (Radley et al., 2006). In addition to the effects of glucocorticoids, numerous studies on animals showed that repeated stress activates the glutamate neurotransmission in the anterior cingulate that leads in turn to persistent activation of extracellular-regulated kinase, causing neuronal damage by destabilizing the cytoskeletal (for a review see (Moghaddam, 2002)). The possibility of stress-induced structural aberrations in the anterior cingulate also comes from human studies of monozygotic twins discordant for PTSD that showed the combat-exposed PTSD twins had lower gray matter density than their own combat-unexposed cotwins (Gilbertson et al., 2002). However, another avenue is that abnormal structural integrity of the anterior cingulate pre-exists and increases vulnerability for PTSD. A diverse literature suggests that anterior cingulate function is central to emotional self-control, focused problem solving and adaptive response to changing conditions (Hamner et al., 1999). Similarly, convergent data implicate that the prefrontal cortex plays a key role in the recall and expression of extinction memory (Hull, 2002). It is thought that disruption of anterior cingulate and prefrontal cortex function is integral to many symptoms of PTSD due to impaired extinction of fear conditioning. Our DTI findings may reflect compromised integrity of white matter fiber connections across these regions in PTSD.
Our finding of FA reduction in areas near the angular gyrus is consistent with SPECT findings of decreased rCBF levels in this region in PTSD (Chung et al., 2006). The angular gyrus plays an important role in organizing language and thoughts (Mazoyer et al., 2001). People with lesions in the angular gyrus have difficulties in creating abstractions. Diminished microstructural integrity of the angular gyrus, as indicated by FA reduction, may therefore contribute to symptoms of altered phenomenology of traumatic memories in PTSD patients. However, we acknowledge that the FA alterations of the angular gyrus in PTSD – and for that matter also the FA alterations in the insular and dorsocentral brain regions - are an unusual finding without strong neuropsychiatric corroboration and therefore requiring replication.
One interesting aspect of our observations with rCBF and FA in PTSD is the pattern of impacted brain regions of the frontal cortex and limbic system. It is consistent with a functional neuroanatomic model of PTSD which posits hyperactivation of the amygdala and insula in response to external stressors together with hypoactivation of anterior structures particularly the anterior cingulate cortex and medial prefrontal cortex (Etkin and Wager, 2007). In summary, this model suggests insufficient cognition regulation of emotion such as anxiety and fear resulting in excessive threat appraisal, enhanced fear conditioning, and impaired extinction. Another important consideration is that previous studies of monozygotic twin pairs discordant for PTSD implicated brain abnormalities may precede PTSD and increase vulnerability (Gilbertson et al., 2002). However, another PTSD study in twin pairs implies that a smaller anterior cingulate cortex can be stress-induced, highlighting differences between the roles of the hippocampus and anterior cingulate in PTSD (Kasai et al., 2008). Whether our findings of abnormal rCBF and white matter degradation in this study are the result of PTSD or predisposing for the condition needs to be determined.
Several limitations of this study ought to be mentioned: First, the low spatial resolution of both ASL and DTI precludes the precise localization of compromised brain function and microstructural alterations. In addition, the nature of the association between the MRI findings and PTSD pathophysiology as well as causality cannot be determined from this study. Alterations in brain activity and compromised microstructural integrity may precede PTSD and increase vulnerability or could be a result of PTSD. Another limitation is that we did not contrast PTSD subjects with and without MTBI and therefore the extent to which the abnormal rCBF and FA patterns are distinct features of PTSD or relate also to MTBI remains unclear. We did exclude subjects who self reported onset of post-concussive symptoms following any type of head injury. However, symptoms of post-concussive syndrome, a separate entity from MTBI, partially overlap with symptoms of PTSD and are difficult to control for separately in an analysis of PTSD. Additional studies involving not only PTSD subjects with and without MTBI but also subjects with a range of post-concussive symptoms are warranted to elucidate the differential patterns of brain alterations across these conditions. A technical limitation is that the inferior temporal lobe is difficult to cover with ASL. It is therefore possible that we missed important physiological alterations in this region. Lastly, PTSD patients may perceive imaging more stressful than healthy subject and the assumption in rCBF imaging of a comparable ‘resting state” between subjects with and without PTSD may be problematic.
In summary, we observed a systematic pattern of rCBF alterations in PTSD using ASL MRI that was largely in agreement with functional studies using SPECT and PET. In addition, we found a systematic pattern of FA reduction, implying compromised integrity of neural pathways that involve prefrontal and anterior cingulate regions. These findings are in addition to previous observations in this PTSD cohort of smaller dentate gyrus/CA3 subfields. Taken together, the findings suggest PTSD is associated with neuropathological abnormalities of specific brain circuits. The abnormal patterns that MRI detects could yield a potential marker for PTSD and also may aid a differential diagnosis between PTSD and MTBI.
Acknowledgments
This research was supported in part by grants from the Mental Illness Research and Education Clinical Center (MIRECC) of the US Veterans Health Administration, Office of Research and Development and the Department of Defense and the National Center for Research Resource of NIH (RR23953). This material is the result of work supported with resources and the use of facilities at the Veterans Administration Medical Center, San Francisco California. The authors thank all the participants in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, Ohtani T, Masutani Y, Kato N, Ohtomo K. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. 2006;146:231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bonne O, Gilboa A, Louzoun Y, Brandes D, Yona I, Lester H, Barkai G, Freedman N, Chisin R, Shalev AY. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol Psychiatry. 2003;54:1077–1086. doi: 10.1016/s0006-3223(03)00525-0. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Chung YA, Kim SH, Chung SK, Chae JH, Yang DW, Sohn HS, Jeong J. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Detre J, Alsop D, Vives L, Maccotta L, Teener J, Raps E. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998;50:633–641. doi: 10.1212/wnl.50.3.633. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magnetic Resonance in Medicine. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Hamner MB, Lorberbaum JP, George MS. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety. 1999;9:1–14. [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hull AM. Neuroimaging findings in post-traumatic stress disorder. Systematic review. Br J Psychiatry. 2002;181:102–110. [PubMed] [Google Scholar]
- Jahng GH, Weiner MW, Schuff N. Improved arterial spin labeling method: applications for measurements of cerebral blood flow in human brain at high magnetic field MRI. Med Phys. 2007;34:4519–4525. doi: 10.1118/1.2795675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jeong DU, Sim ME, Bae SC, Chung A, Kim MJ, Chang KH, Ryu J, Renshaw PF, Lyoo IK. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pitman RK, Orr SP. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. J Abnorm Psychol. 2004;113:324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Subfield atrophy pattern in temporal lobe epilepsy with and without mesial sclerosis detected by high resolution MRI at 4 Tesla: Preliminary Results. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02010.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Sachinvala N, Kling A, Suffin S, Lake R, Cohen M. Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Military Medicine. 2000;165:473–479. [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Fox-Bosetti S, Lenoci M, Samuelson KW, Studholme C, Kornak J, Marmar CR, Weiner MW. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Research. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tanielian TL, Jaycox LH. In: Invisible wounds of war: psychological and cognitive injuries, their consequences and services to assist recovery. Tanielian TL, Jaycox LH, editors. The RAND Corporation; Santa Monica, CA: 2008. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, Weiner MW, Schuff N. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JW, Kimerling R, Brown PJ, Chrestman KR, Levin K. Psychometric review of The Life Stressor Checklist-Revised. In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Sidran Press; Lutherville, MD: 1996. pp. 198–201. [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009 doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]


