Abstract
Objective
To determine the prevalence of retrodots in the lens, the association of these lesions to age-related cataract, and to assess their association with visual impairment and contrast sensitivity.
Design
Longitudinal epidemiologic study.
Participants
The Beaver Dam Eye Study cohort.
Methods
Eye examinations including grading of standard photographs of the lens and measures of visual function.
Main Outcome Measures
Statistical associations of retrodots with nuclear, cortical and posterior subcapsular cataracts and with incident visual impairment and impairment of contrast sensitivity.
Results
The prevalence of retrodots at the baseline examination increased with age from 1.68% in those 43–54 years of age to 31.20% in those 75 years of age and older. After controlling for age, there was no significant difference in the prevalence of retrodots between men and women. The odds ratio (OR) for visual impairment associated with retrodots was 2.22, 95% Confidence Interval (CI) = 1.80, 2.75 after controlling for age-related cataracts and other associated characteristics. The association with impaired contrast sensitivity was not significant. Retrodots were not significantly associated with incidence of any type of age-related cataract. The 15-year cumulative incidence of retrodots in right eyes increased with age from 9.3% in those 43–54 years of age to 21.1% in those 75 years of age and older. In addition to age, incidence of retrodots was independently associated with smoking (OR 1.27, 95% CI 1.04, 1.56 for ever vs. never smoking).
Conclusions
Retrodots are frequently occurring age-related lens opacities that are associated with decreased vision independent of the presence of age-related cataract and their incidence is associated with smoking. Further research to determine the underlying process leading to retrodots will be necessary before efforts to develop preventions are undertaken.
There are many different lesions that can be identified by slit lamp examination1 in the lenses of adults, but there are three that are most commonly cited by cataract surgeons and epidemiologists.2,3 These are nuclear, cortical, and posterior subcapsular cataracts. However, Frost and colleagues found in a cross sectional study that retrodots4 were associated with decreased visual acuity, independent of other lens opacities. We had the opportunity to investigate the epidemiology of retrodots in cross sectional and longitudinal data in a general population. We also examined the relationship of this lesion to the common age-related cataracts, visual acuity, and to its possible risk factors.
METHODS
Population
Methods used to identify the population and descriptions of the population have appeared in previous reports.5,6 In brief, a private census of the population of Beaver Dam, Wisconsin, was performed from September 15, 1987 to May 4, 1988. Eligibility requirements for study participation included living in the city or township of Beaver Dam and being 43 to 84 years of age at the time of the census. There were 5,925 eligible individuals, institutionalized and non-institutionalized; 4,926 participated in the examination phase between March 1, 1988 and September 14, 1990. Ninety-nine percent of the population was white. All participants were invited for a 5-year (3,684 examined), a 10-year (2,764 examined), and a 15-year (2,119 examined) follow-up examination. The primary reason for nonparticipation was death. Comparisons between participants and non-participants at the baseline and at the 5-, 10-, and 15-year follow-up examinations have appeared in previous publications.5,7–9 In general, non-participants were older, had higher blood pressures, and were more likely to have had cataract and age-related macular degeneration at baseline. The mean and median times between the baseline and 15-year follow-up examinations were 14.9 years (standard deviation was 0.5 years) and 14.8 years, respectively.
Procedures
The work reported hereupon was HIPAA compliant and conformed to the tenets of the Declaration of Helsinki. Approval was granted by the Institutional Review Board at the University of Wisconsin, and informed consent was obtained from each participant at the beginning of each examination. The same procedures were followed at each examination. A history was obtained. Visual acuity was measured with a modification of the Early Treatment Diabetic Retinopathy Study (ETDRS) protocol. Best-corrected distance visual acuity was measured after refraction. Refraction was obtained using the Humphrey 530 refractor (Allergan Humphrey, San Leandro, CA) for each eye. The correction was placed in a trial lens frame, and the best-corrected visual acuity was measured using the ETDRS chart R, which had been modified for a 2.0 meter distance.5,10 If the best-corrected visual acuity was 20/40 or worse, a complete ETDRS refraction was performed, and the visual acuity was measured. When no letters could be correctly identified at 2.0 meters, further testing was performed according to the following protocol. An ETDRS chart magnified by 2 replaced the usual chart. Testing was then performed at 1 meter.
The Pelli-Robson letter charts were used to measure contrast sensitivity11 for each eye. The scores were assigned based on triplets. Participants used the refraction which gave them the best correction for distance. The chart was placed 1 meter in front of the participant. Scores were calculated as the logarithm of the contrast sensitivity and ranged from 0 to 1.95.11,12 Those eyes judged to have low risk of angle closure had their pupils dilated pharmacologically. A photograph was taken of the lens of each eye using a Topcon SL5 Photoslit Lamp camera (Paramus, NJ). The camera had been specially modified for this study so that uniform photographs could be obtained for each participant.13 Retroillumination photographs of the lens were taken using a Neitz CR-T camera (Torrance, CA). This camera was also modified specifically for this study to obtain uniform photographs.13 Kodak Ektachrome 200 ASA film was used.
Definitions
Age was defined as the age at the time of the baseline examination.
Smoking was classified as ever smoked (>100 cigarettes/lifetime).
Diabetes was defined as 1) a history of diabetes mellitus, treated with either insulin, oral hypoglycemic agents, and/or diet or 2) history of diabetes mellitus and either random blood sugar >200 mg/dL or elevated glycosylated hemoglobin or 3) elevated glycosylated hemoglobin in persons with no previous medical history of diabetes mellitus. An elevated glycosylated hemoglobin value is a value greater than two standard deviations above the mean for a given age-sex group (43–54 years of age, men >9.5% and women >9.6%; 55–64 years of age, men >9.4% and women >10.0%; 65–74 years of age, men >9.6% and women >9.6%; and 75 years of age or older, men >9.5% and women >9.6%). Persons with history of diabetes mellitus but not treated with diet, insulin or other hypoglycemic agents and with “normal” blood sugars and glycosylated hemoglobins were excluded from analyses (N=52).
Participants were asked about residential history and based on this information, a measure of the average annual ambient ultraviolet light B (UVB) exposure for each individual using an adaptation of the technique employed by the Maryland Watermen Study14,15 was calculated. One Wisconsin sun year (WISY) is equivalent to the total ambient UVB irradiance of a horizontal surface in Wisconsin over one year. The cumulative ambient UVB exposure was computed and divided by age to compute the average annual ambient UVB exposure for an individual. Because most participants had spent most of their lives in Wisconsin, this variable was categorized into two groups (less than 1.01 WISY and 1.01 or more WISY).16
Impaired contrast sensitivity was defined as <1.55 dB.
Visual impairment was defined as 20/40 or worse (40 or fewer letters correct).9
Slit lamp photographs were judged for severity of nuclear sclerosis by comparing them with a set of four standard photographs of increasingly severe nuclear sclerosis resulting in five levels of severity. Severity greater than standard three was considered to be nuclear cataract (NSC). Retro-illumination photographs were used to grade cortical and posterior subcapsular opacities and retrodots.
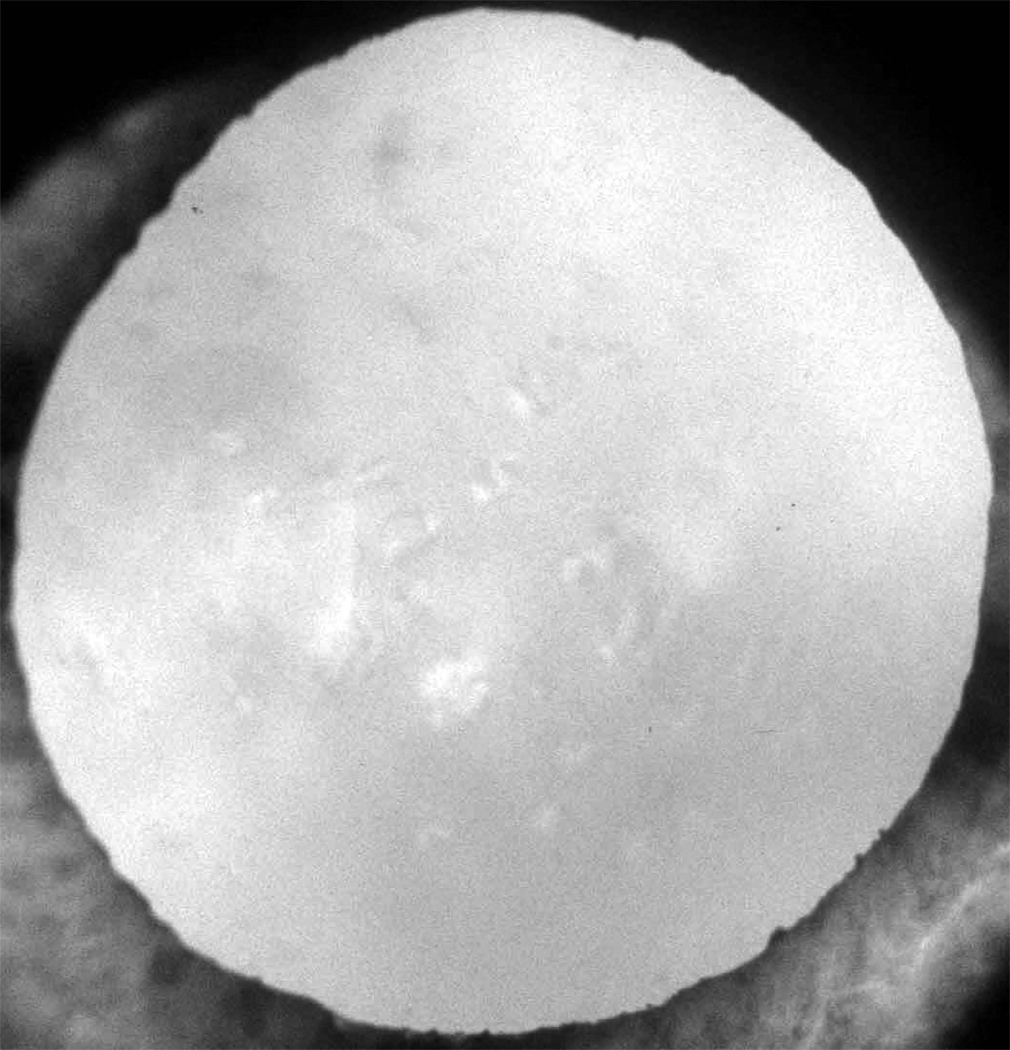
When grading opacities from retroilluminated photographs of the lens, a circular grid was used to define nine areas of possible involvement. For cortical and posterior subcapsular opacities, the grader estimated the percent of involvement in each segment and a weighted average based on segment size was used to calculate the total “area” of the lens (considering the lens as a plane surface) resulting in a continuous measure of severity from zero to 100. Cortical cataract (CC) was defined as involvement of more than 5% of the lens area. Posterior subcapsular cataract (PSC) was defined as 5% or more of any lens segment.13 For retrodots, the grader indicated the presence or absence of lesions in each grid segment (Figure 1).17 For analyses using estimated severity of retrodots, the number of segments as defined by the grading grid, with retrodots present is used as ordered categories. Reproducibility of grading between graders range from 78% to 92% for exact agreement and from 88% to 97% for agreement including one step difference. We refer to the dichotomies of nuclear, cortical, posterior subcapsular cataracts, and retrodots as absence/presence of the lesion in the lens.
Figure 1.
Retrodots in a digital image.
Persons (right eyes) included in incidence analyses in this paper are based on those persons who were seen at the baseline examination, did not have the types of the specific age-related lens change or cataract surgery, and had follow-up information for this lesion with and without each of the other lesions.
Steroid use included current use of oral steroids. Multivitamin use included current use of multivitamin preparations.
All covariate information applies to status at the beginning of each 5 year interval.
Analyses
Cumulative incidence rates were computed using the competing risk approach, an adaptation of the Kaplan-Meier product limit method, where death and cataract surgery were the competing outcomes.18 Odds ratios for prevalence associations were estimated using logistic regression. Odds ratios for incidence associations were estimated using discrete linear logistic regression (a proportional hazard model approach). Results are presented for the right eyes as there were no systematic differences between eyes.
SAS version 9 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
Prevalence
Associations with other lens lesions
At baseline, there were 4565 persons with lens photographs of the right eye gradable for retrodots and 4575 persons with lens photographs of the left eye gradable for retrodots. Of these, 436 (9.6%) right eyes had retrodots and 401 (8.8%) left eyes had retrodots. Retrodots were bilateral in 4.5% of 4469 persons gradable in both eyes. Description of relations to cataract and other ocular characteristics are limited to right eyes but similar relations were found in left eyes.
Retrodots increased in prevalence with age (Table 1). They were slightly more common in women than in men but the difference was not statistically significant after adjusting for age. The number of subfields involved varied with age from 0.3% in those 43–54 years of age to 16.5% in persons 75 years of age and older having 4 or more subfields with this lesion.
Table 1.
Age and Gender Distribution for Prevalence of Retrodots in Right Eyes of Beaver Dam Eye Study Participants.
| Female | Male | All | ||||
|---|---|---|---|---|---|---|
| Age (years) | N | % | N | % | N | % |
| 43–54 | 785 | 1.66 | 706 | 1.70 | 1491 | 1.68 |
| 55–64 | 679 | 2.80 | 591 | 5.58 | 1270 | 4.09 |
| 65–74 | 692 | 13.29 | 487 | 14.78 | 1179 | 13.91 |
| 75+ | 397 | 32.24 | 228 | 29.39 | 625 | 31.20 |
| ALL | 2553 | 9.87 | 2012 | 9.15 | 4565 | 9.55 |
Retrodots often occurred in the presence of cataract in the same eye (Table 2). While controlling for sex and age, the odds ratio (OR) if NSC was present was 2.08, if CC was present it was 1.14, and it was 2.29 if PSC was present.
Table 2.
Odds of Having Retrodots in Right Eyes When Cataract of Specific Types Are Present at Baseline*
| Cataracts | Retrodots | |||||
|---|---|---|---|---|---|---|
| Type | Status | N | Present (%) | OR | 95% CI | P-value |
| NSC† | Absent | 3935 | 6.5 | 1.00 | - | - |
| Present | 575 | 29.9 | 2.08 | 1.61, 2.68 | <.001 | |
| CC† | Absent | 3997 | 7.9 | 1.00 | - | - |
| Present | 520 | 21.0 | 1.14 | 0.86, 1.51 | 0.36 | |
| PSC† | Absent | 4369 | 8.9 | 1.00 | - | - |
| Present | 155 | 28.4 | 2.29 | 1.54, 3.41 | <.001 | |
Adjusted for age and sex
NSC = nuclear cataract, CC = cortical cataract, PSC = posterior subcapsular cataract
Abbreviations: OR = odds ratio; CI = confidence interval
Visual function
Retrodots were associated with visual impairment such that 6.7% of persons without retrodots but 31.7% of those with retrodots were so impaired. Retrodots remained significantly associated with visual impairment even in the presence of any of the types of age related cataract (Table 3). Visual impairment was most common in those with 4 or more subfields involved (vs 0 subfields OR 3.66, 95% Confidence Interval [CI] = 2.52, 5.32) controlling for age, sex, and presence of cataract. Retrodots were not as strongly associated with impaired contrast sensitivity (OR 1.66, 95% CI = 0.99, 2.78) after adjusting for age, sex and presence of cataract by type (data not shown).
Table 3.
Odds Ratios For Visual Impairment in Presence of Retrodots in the Beaver Dam Eye Study.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Sex | 0.77 | 0.63, 0.94 | .009 |
| Age (per 5 years) | 1.44 | 1.35, 1.53 | <.001 |
| Nuclear sclerotic cataract | 2.91 | 2.37, 3.57 | <.001 |
| Cortical cataract | 1.70 | 1.38, 2.09 | <.001 |
| Posterior subcapsular cataract | 3.07 | 2.25, 4.21 | <.001 |
| Retrodots | 2.22 | 1.80, 2.75 | <.001 |
Abbreviations: OR = odds ratio; CI = confidence interval
Incidence
Association with other lens lesions
There were 500 persons (18.3%) with incident retrodots in their right eyes and 460 persons (16.7%) with incident retrodots in their left eyes over the fifteen year interval.
The 15-year cumulative incidence increased with age through age 74 and then decreased in those 75 or more years of age (Table 4, P < .001 for time dependent covariate model adjusting for sex). While women had higher overall incidence, after age adjustment there was no difference between men and women (P = .89). Those with NSC or PSC were more likely to develop retrodots than persons without any cataract, after adjusting for age and sex in time varying covariates models. Those with cortical cataract did not have increased incidence of retrodots. Because of low frequencies for some combinations of cataract types, we could not determine whether specific combinations were associated with incident retrodots.
Table 4.
Cumulative 15-Year Incidence of Retrodots in Right Eyes and Competing Risks in the Beaver Dam Eye Study.
| Retrodots | Death | Cataract Surgery | ||
|---|---|---|---|---|
| Age at Baseline (years) | N | (%) | (%) | (%) |
| 43–54 | 1195 | 9.3 | 6.3 | 3.8 |
| 55–64 | 990 | 21.5 | 15.7 | 12.8 |
| 65–74 | 821 | 28.3 | 28.9 | 20.7 |
| 75+ | 279 | 21.1 | 61.1 | 12.4 |
| ALL | 3285 | 18.3 | 18.5 | 11.1 |
Retrodots were not significantly associated with incidence of any cataract type, while controlling for age and sex. Retrodots were associated with incident cataract surgery while controlling for age, sex and other cataract types (in time dependent model OR 2.03, 95% CI = 1.52, 2.72).
Visual Function
Retrodots were significantly associated with incident visual impairment even while controlling for age, sex, and other cataract types (Table 5). There is no significant association of retrodots with incidence of impaired contrast sensitivity.
Table 5.
Odds Ratios for Incidence of Visual Impairment in Right Eyes with Retrodots in Time-Varying Model in the Beaver Dam Eye Study.
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Sex | 0.77 | 0.61, 0.96 | .021 |
| Age (per 5 years) | 1.77 | 1.64, 1.91 | <.001 |
| Nuclear sclerotic cataract | 1.78 | 1.36, 2.31 | <.001 |
| Cortical cataract | 1.07 | 0.79, 1.43 | .674 |
| Posterior subcapsular cataract | 2.43 | 1.55, 3.81 | <.001 |
| Retrodots | 1.43 | 1.07, 1.92 | .017 |
Abbreviations: OR = odds ratio; CI = confidence interval
Risk Factors
We examined exposures that we have found to be associated with the three types of age-related cataract to determine whether they were associated with increased incidence of retrodots (Table 6). We found that ever smoking was associated with increased incidence. The odds for other exposures were slightly attenuated when cataract type was also included in the model. However, no other exposure was significantly associated with increased risk of incident retrodots.
Table 6.
Odds of Incident Retrodots for Potentially Related Exposures in Right Eyes (Time Dependent Models), Adjusted for Age and Sex
| OR | 95% CI | P-value | |
|---|---|---|---|
| History of ever smoking | 1.27 | 1.04, 1.56 | .02 |
| Ever heavy drinking | 1.17 | 0.89, 1.54 | .27 |
| Diabetes | 1.05 | 0.73, 1.51 | .78 |
| Sun exposure* | 1.17 | 0.94, 1.46 | .16 |
| Multivitamin use | 0.89 | 0.72, 1.10 | .85 |
| Oral steroids | 1.14 | 0.67, 1.94 | .27 |
Calculated as Wisconsin Sun Years (WISY)
Abbreviations: OR = odds ratio; CI = confidence interval
DISCUSSION
We examined the epidemiology of retrodots of the crystalline lens and found that these lesions are common. They are associated with age and visual impairment. They commonly occur in the presence of age-related NSC and PSC. Shun-Shin and colleagues reported an association of retrodots with nuclear scatter, a characteristic of nuclear cataract.19 The association of retrodots with decreased vision has been reported by Frost and colleagues.4 Like Frost et al., we find that the decrease in vision is independent of the presence of NSC. In addition, retrodots preceded cataract extraction independent of the presence of any other cataract type in our study. Thus, in describing lens features that are related to decreased vision and impose health care needs, a fourth lesion should be included in the list with nuclear, cortical, and posterior subcapsular cataracts. This is not to imply that there may not be other concomitant age-related conditions that can occur in the presence of retrodots or other lens lesions that could be associated with decreased vision. However, most retinal lesions are not associated with lens lesions after accounting for age and so are unlikely to confound this relationship in a meaningful way.
The only risk factor association aside from age and sex for retrodots in our data is a history of ever smoking. These are the same risk factors for NSC that we have found in our data. It is possible that retrodots, seen as entities distinct from NSC on film imaging, are lesions that are closely linked to NSC in distribution in the population and in risk factor associations. This may be due to similar genetic determinants with different environmental triggers. Alternatively, similar environmental determinants may give rise to two distinguishable lesions that may be morphologically distinct and genetically different. Biochemical and genetic analyses might help to sort this out. Steroid use and diabetes, important risk factors for PSC, were not associated with cumulative incidence of retrodots. That may be related to the relative rarity of PSC. It may be that retrodots and PSC have common causes but at the moment we cannot attribute this to common vulnerability to any risk factors known to us.
Adults’ lenses may manifest lesions other than those discussed in this paper such as vacuoles and water clefts,1 and these can be seen at the slit lamp. We have found that lens vacuoles were not related to increasing age or other risk factors in our population, nor were they associated with visual acuity (Klein BEK et al., unpublished data). Our estimates of prevalence, incidence, and risk factor relationships are based on lesions that were imaged by our standardized photographic techniques. Water clefts were not visible in the retroillumination images we graded. Thus, we cannot address their distributions in the population nor can we estimate any risk factor relationships for them.
An apparent inconsistency in our data is that the incidence of retrodots increased with age through age 74 but then decreased in those 75 years of age and older. We suspect that this apparent truncation of the age effect is related to cataract surgery and mortality in this age category and not to a real inverse trend in age specific incidence. This effect is inherent in longitudinal studies of many age related findings but does not invalidate the analyses we did as our data reflects the real situation in survivors (or surviving eyes).
Limitations of our study include the possibility that the Beaver Dam cohort, being composed largely of persons of northern European ancestry, may have prevalence and incidence of lens lesions that differ from those of other ethnic groups. There is also the possibility that the use of other imaging techniques may result in estimates that differ from ours.
In summary, we found high prevalence and incidence of retrodots, a lesion that is associated with age-related decrease in vision. Further research to determine the underlying process leading to overt retrodots will be necessary before efforts to develop preventions are undertaken.
Acknowledgment
The authors wish to thank John Sparrow, D Phil, FRCS, FRCOphth, a Consultant Ophthalmologist at Bristol Eye Hospital and Honorary Senior Lecturer at London School of Hygiene and Tropical Medicine in the United Kingdom. Dr. Sparrow reviewed a portion of our images and provided guidance as to classification of specific lesion types.
Financial Support: This study was supported by National Institutes of Health grant # EY06594 (BEK Klein and R Klein) and, in part, by the Research to Prevent Blindness (R. Klein and BEK Klein Senior Scientific Investigator Awards), New York, NY. The National Eye Institute provided funding for entire study including collection and analyses and of data; RPB provided further additional support for data analyses. Neither funding organization had a role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Parts of this study were presented in abstract form at the Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, Fort Lauderdale, FL, 3’7 May 2009.
References
- 1.Brown NA, Bron AJ, Ayliffe W, et al. The objective assessment of cataract. Eye (Lond) 1987;1:234–246. doi: 10.1038/eye.1987.43. [DOI] [PubMed] [Google Scholar]
- 2.Frost NA, Sparrow JM. The assessment of lens opacities in clinical practice: results of a national survey. Br J Ophthalmol. 2001;85:319–321. doi: 10.1136/bjo.85.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston PM, Carson CA, Taylor HR. The epidemiology of cataract: a review of the literature. Ophthalmic Epidemiol. 1995;2:151–164. doi: 10.3109/09286589509057097. [DOI] [PubMed] [Google Scholar]
- 4.Frost NA, Sparrow JM, Moore L. Associations of human crystalline lens retrodots and waterclefts with visual impairment: an observational study. Invest Ophthalmol Vis Sci. 2002;43:2105–2109. [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 6.Linton KL, Klein BE, Klein R. The validity of self-reported and surrogate-reported cataract and age-related macular degeneration in the Beaver Dam Eye Study. Am J Epidemiol. 1991;134:1438–1446. doi: 10.1093/oxfordjournals.aje.a116049. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- 11.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci. 1988;2:187–199. [Google Scholar]
- 12.Klein BE, Klein R, Lee KE, Cruickshanks KJ. Associations of performance-based and self-reported measures of visual function. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1999;6:49–60. doi: 10.1076/opep.6.1.49.1569. [DOI] [PubMed] [Google Scholar]
- 13.Klein BE, Klein R, Linton KL, et al. Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology. 1990;97:1428–1433. doi: 10.1016/s0161-6420(90)32391-6. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal FS, West SK, Munoz B, et al. Ocular and facial skin exposure to ultraviolet radiation in sunlight: a personal exposure model with application to a worker population. Health Phys. 1991;61:77–86. doi: 10.1097/00004032-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Taylor HR, West SK, Rosenthal FS, et al. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988;319:1429–1433. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- 16.Cruickshanks KJ, Klein BE, Klein R. Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health. 1992;82:1658–1662. doi: 10.2105/ajph.82.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population. The Beaver Dam Eye Study. Ophthalmology. 1992;99:546–552. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Shun-Shin GA, Bron AJ, Brown NP, Sparrow JM. The relationship between central nuclear scatter and perinuclear retrodots in the human crystalline lens. Eye (Lond) 1992;6:407–410. doi: 10.1038/eye.1992.84. [DOI] [PubMed] [Google Scholar]