Abstract
Purpose
To determine whether diabetes mellitus is protective for nuclear sclerotic cataract at baseline and 6 and 12 months after vitrectomy surgery.
Design
Prospective interventional cohort study
Methods
Phakic diabetic and non-diabetic patients undergoing vitrectomy surgery for a variety of retinal conditions underwent Scheimpflug lens photography in the operated and fellow eye at baseline and 6 and 12 months following vitrectomy surgery.
Results
Of 52 eyes included in the analysis, 23 eyes were from diabetic patients, 14 of which had surgery for ischemic retinopathy. At baseline, eyes with ischemic diabetic retinopathy had less nuclear sclerotic cataract than non-ischemic diabetic and non-diabetic eyes. This was true for eyes undergoing vitrectomy surgery (p=0.0001) and for fellow eyes (p=0.003). After vitrectomy surgery, non-ischemic diabetic eyes and non-diabetic eyes developed nuclear sclerotic cataract at the same rate. Diabetic eyes with ischemic retinopathy showed no significant progression of nuclear opacification and, therefore, had significantly less post-vitrectomy nuclear cataract at 6 months (p<1×10−6) and at 12 months (p<0.001) than non-diabetic or non-ischemic diabetic eyes. Normalizing to baseline opacity and adjusting for age and other co-morbidities did not alter this result.
Conclusions
Ischemic diabetic retinopathy, not just systemic diabetes mellitus, protected against nuclear sclerotic cataract at baseline and following vitrectomy surgery. These findings are consistent with the hypothesis that increased exposure to oxygen is responsible for nuclear cataract formation.
INTRODUCTION
With increasing age, the nucleus of the crystalline lens gradually increases in opacity and hardness in all individuals.1–3 When nuclear opacities become visually significant they are called nuclear sclerotic cataracts. In most western populations, nuclear sclerotic cataracts are the most common reason for cataract surgery. In the United States, cataract surgery is the most often performed surgery at a total direct cost of more than 3 billion dollars per year.4
Epidemiologic studies in populations around the world have identified recurring risk factors for nuclear cataracts including age, smoking, poorer nutrition, and living in a warmer climate.5 Studies that measured the incidence of specific types of cataract between monozygotic and dizygotic twins, showed that approximately one-third of the risk of nuclear cataracts can be attributed to heredity.6, 7 Surprisingly, the biochemical links that connect environmental and hereditary risk factors to nuclear cataracts are not well understood.
While much effort has been applied toward identifying risk factors for nuclear cataract, new information is emerging about possible protective factors. The most provocative of these is the presence of systemic diabetes mellitus. Two large epidemiologic studies revealed that diabetic patients may have a lower risk of nuclear cataract. The Age- Related Eye Disease Study, which examined 4477 individuals 60 to 80 years of age, found that moderate nuclear opacities were less common in persons with diabetes mellitus (odds ratio, 0.68; 95% CI, 0.47–0.98).8 The five-year follow-up results from the Reykjavik Eye Study confirmed that the incidence of nuclear sclerotic cataract was significantly lower in diabetic than in non-diabetic individuals (relative risk, 0.32; 95% CI, 0.12–0.75; P < .01) (Sasaki K, et. al. IOVS 2006;47:ARVO E-Abstract 4135.) A recent study suggested that systemic diabetes mellitus may protect against post-vitrectomy nuclear sclerotic cataract. Smiddy and Feuer found that incident cataract surgery for post vitrectomy nuclear cataract was lower in diabetic than in non-diabetic individuals.9 The case-control study used cataract extraction as a primary end point in patients who had undergone vitrectomy for diabetic retinopathy, epiretinal membrane, or macular hole. The cumulative cataract extraction rates for the three groups two years after vitrectomy were 15%, 66%, and 53%, respectively. After adjusting for age, the difference between diabetic patients and the other two groups combined was highly significant (P < .006). A significant weakness of this small, retrospective study was the use of cataract surgery as a surrogate for visually significant cataract, since diabetic patients may have reason to delay or avoid additional surgical procedures.
Nuclear sclerotic cataract is caused by oxidation of the proteins in the lens nucleus.10 In a study of donor eyes, degeneration of the vitreous body was also associated with nuclear cataract, independent of age.11 Vitrectomy surgery leads to nuclear cataract within two years in most patients over 50. Since vitrectomy and vitreous degeneration increase fluid circulation in the eye, it was suggested that oxidative damage in nuclear cataract is caused by molecular oxygen from the retinal vasculature reaching the lens due to fluid circulation within the vitreous cavity.12 In 2006, we reported that vitreous oxygen tension was significantly lower in diabetic than in non-diabetic patients.13 We postulated that lower oxygen in the vitreous might be responsible for the lower prevalence and incidence of nuclear sclerotic cataract and a decreased incidence of surgery for post-vitrectomy nuclear sclerotic cataract in diabetic patients.8,9 The lower oxygen tension found in the vitreous of diabetic patients may be a result of ischemic retinopathy and associated small vessel disease within the vascularized tissues of the eye. Quantification of the rate of cataract progression after vitrectomy in diabetic and non-diabetic patients could help to test this possibility. Herein we used quantitative Scheimpflug photography to prospectively document progression of nuclear sclerotic cataract from baseline to 6 and 12 months after vitrectomy surgery in patients with ischemic diabetic retinopathy, diabetes with no retinopathy, and in non-diabetic patients.
METHODS
In a single academic-based retina practice, patients who were undergoing vitreous surgery for a variety of retinal conditions were asked to participate in the research study. Inclusion criteria were age 51 to 80 years, ability to provide informed consent, better than hand motion vision in the fellow eye, and willingness to be followed for 1 year. Exclusion criteria were prior cataract surgery, history of traumatic cataract, and prior vitrectomy surgery. Informed consent was obtained by the surgeon (N.M.H.).
The following information was recorded for all patients: age, medical history, presence of diabetes mellitus, surgical eye, diagnosis, presence or absence of ischemic diabetic retinopathy, procedure performed, presence of panretinal photocoagulation (PRP) before vitrectomy, use of intraocular gas tamponade, any intraoperative adverse events, such as lens damage, and any deviations from the study protocol. A 5% dextrose solution was added to the vitrectomy infusion fluid for all diabetic eyes. Otherwise, there were no differences in surgical technique for diabetic or non-diabetic patients. For purposes of this study, “ischemic diabetic retinopathy” was defined as eyes with proliferative diabetic retinopathy, diabetic vitreous hemorrhage, or diabetic traction retinal detachment requiring vitrectomy surgery.
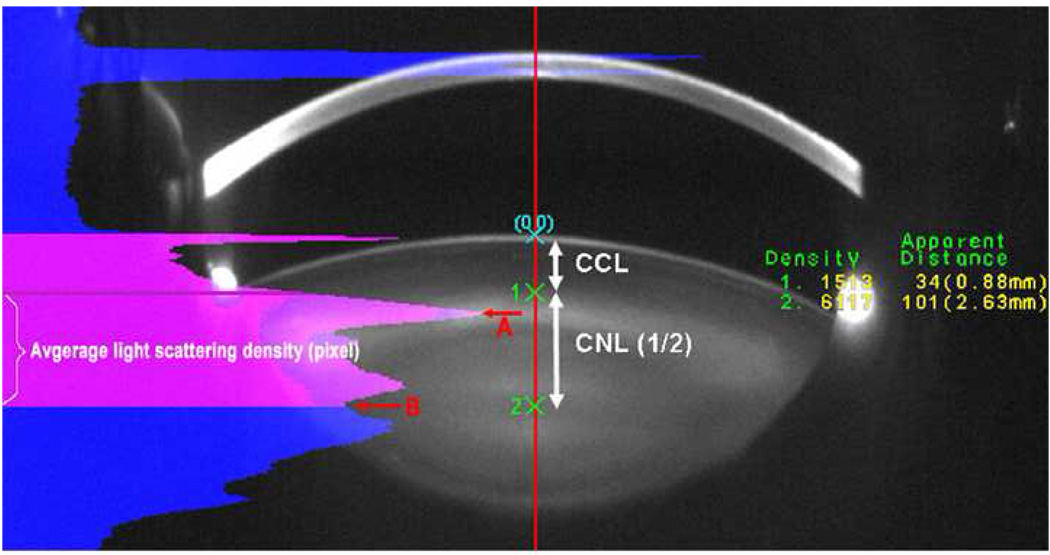
Prior to vitrectomy surgery, each patient underwent digital Scheimpflug lens photography. While Scheimpflug technology has the ability to image cortical cataract, posterior subcapsular cataract, and nuclear cataract, no patients in this study had significant cortical or posterior subcapsular cataract at baseline or during follow-up. Only nuclear opacities progress after vitrectomy surgery. Therefore, the primary endpoint of the study was nuclear sclerotic cataract progression as measured by Scheimpflug photography at 6 and 12 months following vitrectomy surgery. Scheimpflug photographs quantify the density of nuclear light scattering in pixel intensity units. Lens images for both eyes were taken by the EAS-1000 anterior eye segment analysis system (NIDEK EAS-1000, Japan). Lens images were taken at the same settings: slit length 10 mm, flash power 150ws, and at 0 and 180 degrees. Images were acquired and analyzed with the EAS-1000 analysis software (EAS_Win_Ver 123J). Nuclear opacity was quantified using average light scattering per pixel on the central line between the anterior surface of adult nucleus and central clear zone (a comparatively clear area at the center of the lens). (Figure 1)
Figure 1.
A Scheimpflug image showing the location at which nuclear opacity was measured. CCL: central cortical line; CNL: central nuclear line; A: Peak of light scattering at the anterior border of the adult nucleus; B: Central clear zone. EAS analysis method: the software automatically sets up the central line on the slit image; point “0” is the anterior lens capsule. Points 1 and 2 are manually selected using the intensity plot. The average light scattering intensity per pixel along CNL was used to quantify lens nuclear changes.
Statistical analysis was performed using Student’s t test adjusted for multiple comparisons (Bonferroni; GraphPad Software Inc). Multiple regression analysis was performed using Rweb1.03 on the server at bayes.math.montana.edu and SPSS 14.0 (SigmaPlot). P values less than 0.05 were considered significant.
RESULTS
Fifty-two patients were enrolled. Three patients were excluded from the analysis due to lens trauma during surgery, poor cooperation with lens photography, or vasculopathy not related to diabetes. For 46 patients, one eye was included in the study. For three patients both eyes were included in the study. Therefore, 52 eyes were included in the final analysis. Table 1 shows the retinal diagnoses for the eyes that were included in the study. There were 23 eyes from diabetic patients. Among them, 14 eyes had surgery for proliferative diabetic retinopathy. Nine eyes from diabetic patients had surgery for retinal diseases not related to ischemic diabetic retinopathy. Table 2 shows the number and age distribution of the patients in the study. Patients undergoing vitrectomy surgery for complications of ischemic diabetic retinopathy were significantly younger than the non-ischemic patients (non-diabetic patients and non-ischemic diabetic patients) requiring vitrectomy (p=0.005).
Table 1.
Ischemic Diabetic Retinopathy May Protect Against Nuclear Sclerotic Cataract: Patient Characteristics
| Reason for surgery | Patients | Surgical eyes | DM | Ischemia |
|---|---|---|---|---|
| Proliferative diabetic retinopathy | 14 | 14 | 14 | Yes |
| Macular hole | 17 | 20 | 5 | No |
| Retinal detachment | 3 | 3 | 0 | No |
| Premacular fibrosis | 12 | 12 | 3 | No |
| Vitreous opacity | 3 | 3 | 1 | No |
| Total | 49 | 52 | 23 |
DM=diabetes mellitus
Table 2.
Baseline and follow up information for ischemic and non-ischemic groups of patients. Numbers in parentheses at 6 and 12 months follow up are the number with cataract surgery (IOL).
| Age (Years) |
Number of Cases | |||
|---|---|---|---|---|
| Baseline | 6M (IOL) | 12M (IOL) | ||
| Ischemic | 59 ± 6 * | 14 | 13 (0) | 8 (2) |
| Non-ischemic | 65 ± 7 * | 38 | 28 (2) | 26 (14) |
| Total Patients | 52 | 41 | 34 | |
p=0.005
M=months
IOL=intraocular lens
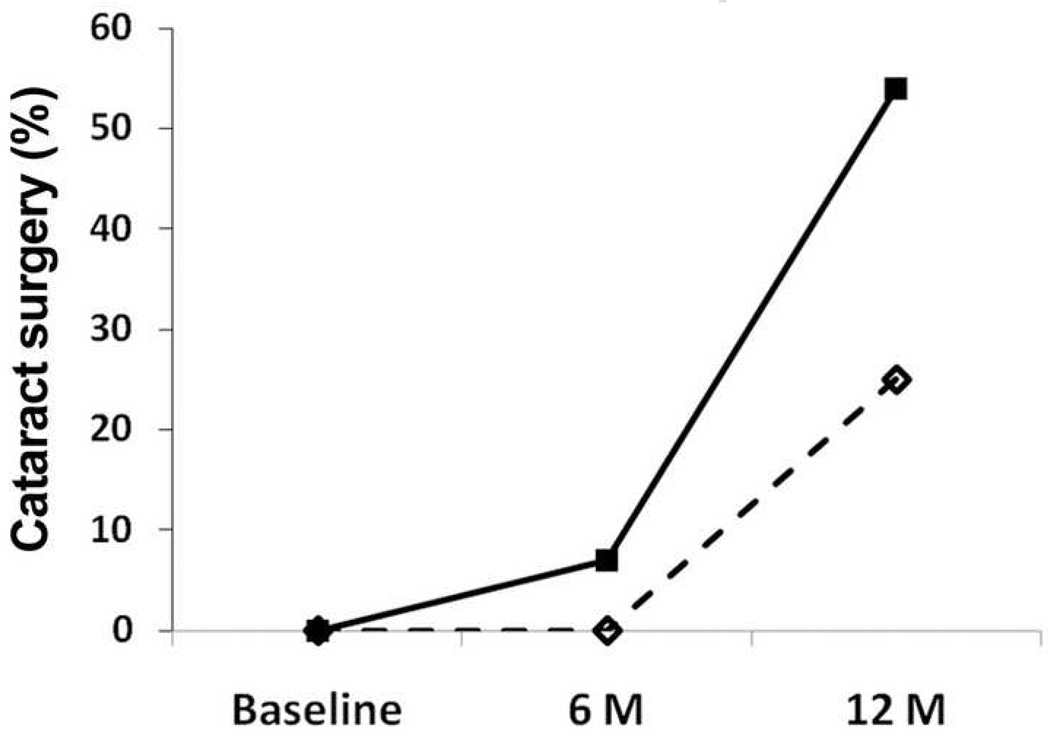
Of 14 eyes with ischemic retinopathy, 13 patients had Scheimpflug photographs taken at 6 months. One patient was lost to follow-up and no eyes had cataract surgery. For this group of eyes, 8 patients returned for follow up at 12 months. Two had cataract surgery and 6 had Scheimpflug photographs. Four patients in the ischemic group were lost to follow-up and 2 were awaiting the 12 month time point. Therefore, of the 8 eyes for which follow-up was available at 12 months, 2 of 8 (25%) had cataract surgery (Fig. 2). Of the 38 eyes with non-ischemic retinopathy, 26 eyes had Scheimpflug photographs taken at 6 months, 2 had cataract surgery and 10 were lost to follow up. For the non-ischemic eyes at 12 months, 12 patients had Scheimpflug photographs, 12 were lost to follow up and 14 had cataract surgery (Fig. 2). Therefore, of the 26 eyes for which follow-up was available at 12 months, 14 of 26 (54%) had cataract surgery (Fig. 2). Therefore, a smaller percentage of ischemic eyes had cataract surgery at 6 and 12 months than non-ischemic eyes (Fig. 2). Due to the small sample size, this difference was not statistically significant.
Figure 2.
The percentage of patients having cataract surgery at 6 and 12 months after vitrectomy. The solid line represents non-ischemic patients and the dotted line patients with ischemic diabetic retinopathy.
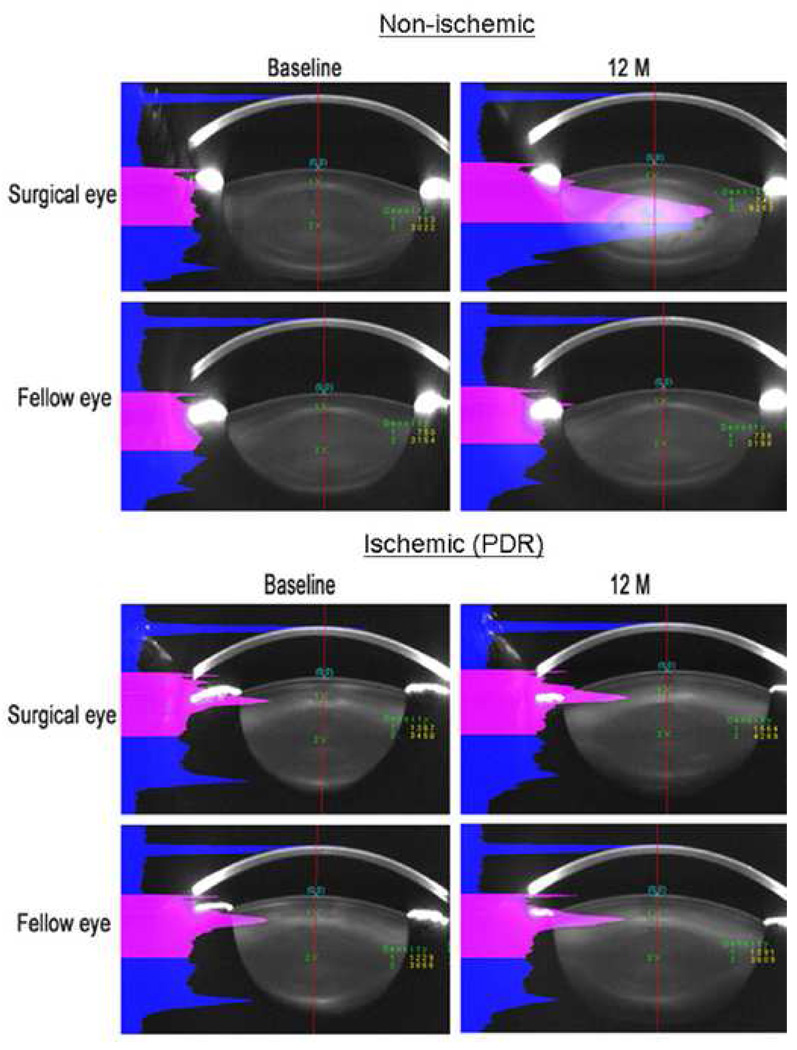
Scheimpflug photography was used to quantify nuclear opacity before and at 6 and 12 months following vitrectomy. Figure 3 shows Scheimpflug images from the operated and un-operated eyes of a non-diabetic patient, age 51, and from a diabetic patient with ischemic retinopathy, age 56. In the non-diabetic patient, the eye undergoing vitrectomy surgery developed significantly more nuclear light scatter than in the lens of the un-operated fellow eye. By contrast, in the patient with ischemic diabetic retinopathy, the eye undergoing vitrectomy surgery showed very little nuclear cataract progression compared to the lens in the fellow eye.
Figure 3.
Scheimpflug lens photos showing typical changes in nuclear opacity before and after vitrectomy in non-ischemic eyes and eyes with ischemic diabetic retinopathy. The upper group of images is from the eyes of a non-diabetic patient, age 51. The lower group of images is from a diabetic patient with ischemic retinopathy, age 56. Left images are baseline photos taken before vitrectomy. Right images were photos taken 12 months after vitrectomy. Pink areas indicate the light scattering intensity along the anterior half of the central line.
Non-ischemic eyes had similar progression of nuclear cataract after vitrectomy. Table 3 shows the average nuclear light scattering units as measured by Scheimpflug photography before and after vitrectomy in the non-diabetic eyes and the non-ischemic diabetic eyes undergoing surgery for non-diabetes related conditions such as macular hole and macular pucker. There was no statistically significant difference between these two groups. Therefore, for the purpose of the analysis, non-diabetic and non-ischemic diabetic eyes were collectively grouped as “non-ischemic” eyes for comparison to eyes with ischemic diabetic retinopathy.
Table 3.
Results of Scheimpflug lens photography for non-diabetic eyes and nonischemic diabetic eyes undergoing surgery for non-diabetes related conditions, such as macular hole and macular pucker. Baseline age and average nuclear light scattering (per pixel intensity) at baseline and 6 and 12 months after vitrectomy are shown.
| Age | Surgical eyes | Fellow eyes | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6m | 12m | Baseline | 6m | 12m | ||
| Non-diabetic | 63.9 ± 6.8 | 72.6 | 102.1 | 107.1 | 68.1 | 67.7 | 70.0 |
|
Non-ischemic diabetic |
68.9 ± 7.2 | 69.4 | 102.6 | 107.4 | 68.8 | 70.7 | 79.1 |
| p-value | 0.09 | 0.50 | 0.96 | 0.98 | 0.90 | 0.70 | 0.65 |
m=months
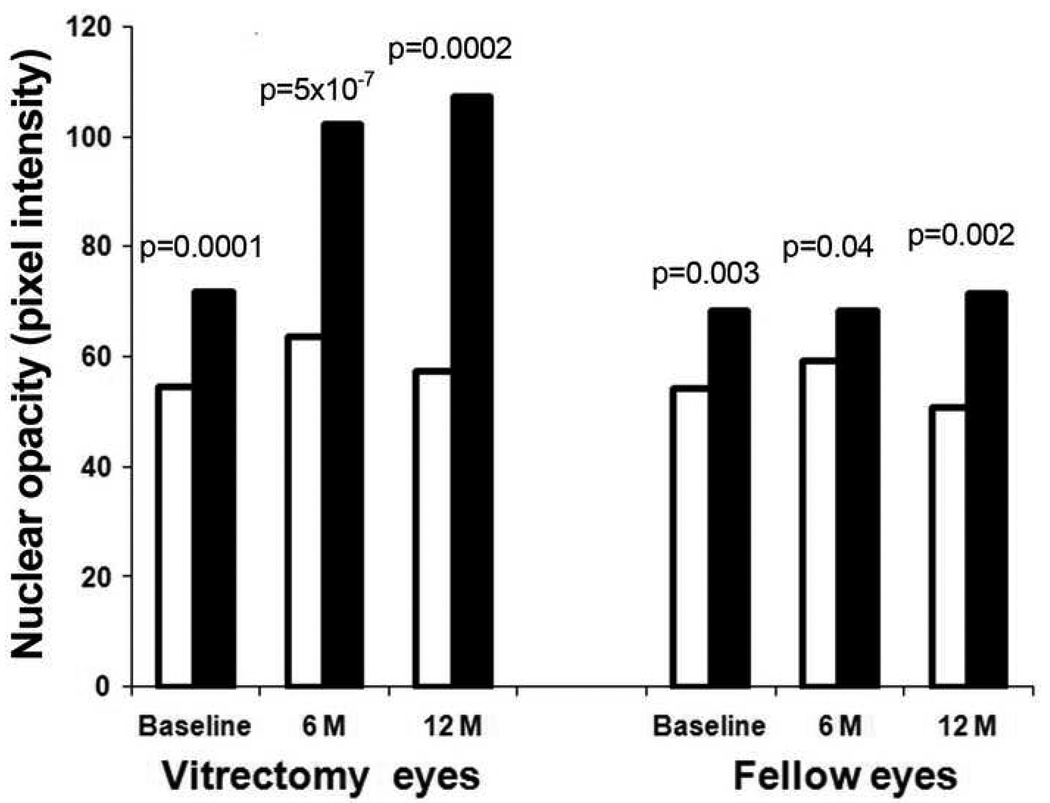
Ischemic and non-ischemic eyes had different rates of cataract progression after vitrectomy. Figure 4 shows the average nuclear light scattering before and after vitrectomy in the ischemic eyes with diabetic retinopathy compared to the non-ischemic eyes. The fellow eyes are also represented. At baseline (before vitrectomy), eyes with ischemic diabetic retinopathy had less nuclear light scattering than non-ischemic eyes. This was true for eyes scheduled for vitrectomy surgery (p=0.0001) as well as fellow eyes (p=0.003). At 6 and at 12 months after vitrectomy surgery, eyes with ischemic retinopathy continued to have significantly lower nuclear opacity than non-ischemic eyes (p<0.001 for both). In the fellow, un-operated eye the significant difference in nuclear opacity remained at 6 and 12 months.
Figure 4.
Results of Scheimpflug photography: Average nuclear opacity measured in pixel intensity in the ischemic DM and non-ischemic (diabetic and non-diabetic) eyes at baseline and at 6 and 12 months after vitrectomy.
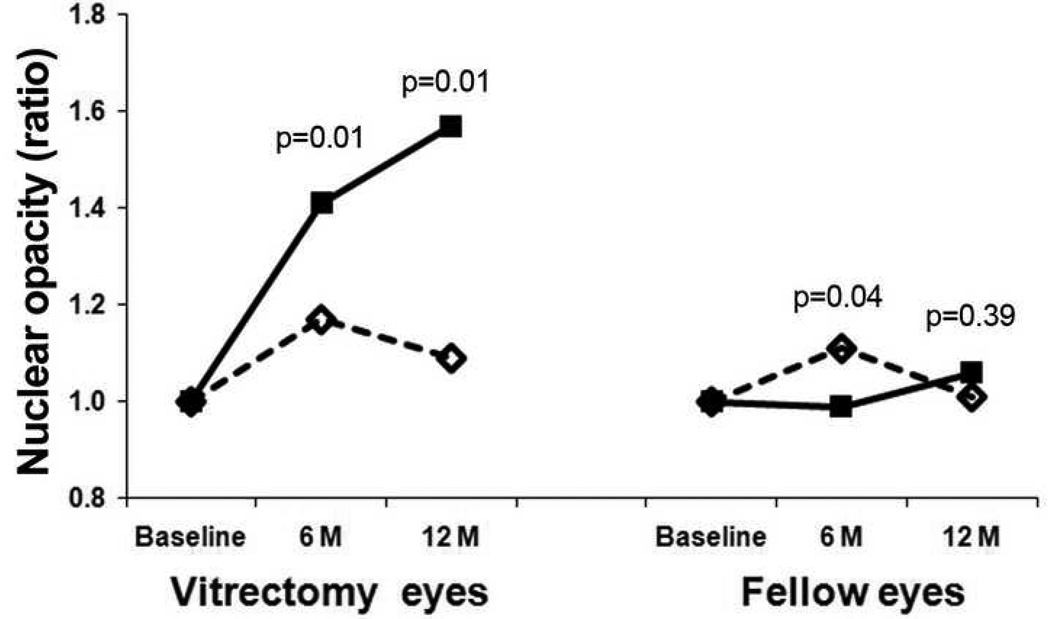
Patients entered the study with differing degrees of nuclear opacity. To minimize the impact of baseline nuclear opacity on post-vitrectomy nuclear cataract formation and to better represent the change in lens opacity, baseline light scattering intensity values for each lens were normalized to a value of 1.0 (Fig. 5). For the eyes undergoing vitrectomy surgery, the increase in nuclear light scattering was significantly greater in non-ischemic eyes than eyes with ischemic diabetic retinopathy at 6 and 12 months (p=0.01 for both). Eyes with ischemic diabetic retinopathy had slightly increased nuclear opacity at 6 months after vitrectomy, although this difference was not present at the 12-month follow up. In this way, the lenses from ischemic eyes that had vitrectomy closely resembled those from un-operated fellow eyes over the 12 month period of this study.
Figure 5.
The graph depicts the change in nuclear opacity from baseline to 6 and 12 months. To eliminate the differences in NS cataract at baseline and to represent the amount of change in the lens, all lens values at the baseline were set to 1.0.
In this prospective study, individual eyes were followed with Scheimpflug lens photographs from baseline through 12 months after surgery. This allowed for a longitudinal assessment of the progression of post-vitrectomy nuclear sclerotic cataracts (Table 4) For the 6 eyes with ischemic retinopathy, there was no significant increase in light scattering intensity over the 12 month period. The lenses of the 12 non-ischemic eyes, developed significantly increased average light scattering intensity 6 months and 12 months after vitrectomy (p<1×10−5).. Therefore, there was significant post-vitrectomy cataract progression in eyes with non-ischemic retinopathy. It is important to consider that, due to cataract surgery, opacity measurements were not available for two eyes in the ischemic group and 14 eyes in the non-ischemic group at the 12-month time point. Because cataract surgery would remove from analysis the most severely affected eyes, the post-vitrectomy increase in nuclear opacity would have been greater in both groups, but certainly more pronounced in the non-ischemic eyes.
Table 4.
Results of Scheimpflug lens photography for eyes with ischemic and nonischemic retinal disease. Baseline age and average nuclear light scattering (per pixel intensity) at baseline and 6 and 12 months after vitrectomy are shown.
| Ischemic | Non-ischemic | |||||
|---|---|---|---|---|---|---|
| Time points | Baseline | 6 Months | 12 Months | Baseline | 6 Months | 12 Months |
| Mean pixel intensity ± SE |
54.4 ± 3.0 | 63.6 ± 3.4 | 57.4 ± 5.3 | 71.9 ± 2.4 | 102.2 ± 5.3 | 107.2 ± 8.7 |
| p value | 6M vs. Baseline: p=0.05 12M vs. Baseline: p=0.60 |
6M vs. Baseline: p=2.49E-07 12M vs. Baseline: p=1.30E-06 |
||||
Age is an important risk factor for nuclear cataract. Because the patients undergoing vitrectomy surgery for ischemic diabetic retinopathy were significantly younger than patients undergoing vitrectomy surgery for other diagnoses, multiple regression analysis was used to correct for age and to identify other potential confounders. Table 5 shows that, after adjusting for age, there was still a significant association between ischemic diabetic retinopathy and lower nuclear opacity at baseline (p<0.01). Ischemia accounted for a mean, age-adjusted decrease in 11 pixel intensity units at baseline and was associated with significant age-adjusted protection against post-vitrectomy cataract at 6 and 12 months after surgery. At 12 months, this difference amounted to an average of 47 pixel intensity units after adjusting for age. Presumably, the difference in opacity between ischemic and non-ischemic eyes would have been greater, had the most severely-affected eyes not had cataract surgery. Gender, race, surgical eye, gauge of surgery (i.e. 20, 23, 25 gauge vitrectomy), or use of intraocular gas tamponade were not significantly associated with nuclear opacity after vitrectomy (not shown).
Table 5.
Multiple regression analysis. Both age and ischemia were related to nuclear pixel intensity at baseline. Only ischemia was related to nuclear pixel intensity at 6 and 12 months after vitrectomy.
| Baseline | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Correlation coefficient |
p-value | Correlation coefficient |
p-value | Correlation coefficient |
p-value | |
| Age | 1.06 | 5.41×10−5 | 0.50 | 0.38 | 0.65 | 0.46 |
| Ischemia | −11.00 | 0.007 | −35.60 | 0.0002 | −46.99 | 0.004 |
DISCUSSION
It is well established that vitrectomy surgery causes an acceleration of nuclear sclerotic cataract formation. In fact, within 2 years of vitrectomy surgery 60 to 98 percent of eyes will develop post-vitrectomy nuclear sclerosis requiring cataract surgery.14–18 It has been suggested that the presence of systemic diabetes mellitus may protect against the development of post-vitrectomy nuclear cataract.9 The present study suggests that, by itself, systemic diabetes mellitus does not protect against post-vitrectomy nuclear cataract. Post-vitrectomy nuclear cataracts progressed at the same rate in the eyes of patients with diabetes mellitus undergoing vitrectomy surgery for non-ischemic retinal conditions, such as macular hole or macular pucker, as the eyes of patients without diabetes mellitus undergoing vitrectomy surgery for similar conditions. Importantly, this study indicates that ischemic retinopathy is the key factor that protects against post-vitrectomy nuclear cataract in diabetics. Eyes of patients undergoing vitrectomy surgery for ischemic diabetic retinopathy showed no significant change in nuclear opacity after vitrectomy. This was reflected in the smaller percentage of ischemic eyes that had cataract surgery. Differences in the nuclear opacity between these groups were highly statistically significant at 6 and 12 months after vitrectomy surgery.
Another finding from this study is that ischemic diabetic retinopathy was associated with lower nuclear sclerotic cataract at baseline. This finding was true for eyes undergoing vitrectomy surgery and for fellow eyes.
Age is an important determinant of the rate of post-vitrectomy nuclear cataract formation. In one study, the two-year incidence of post-vitrectomy nuclear sclerotic cataract for patients under 50 years of age was 7%, compared to 79% for patients over 50 years of age.14 In the present study, patients with ischemic diabetic retinopathy undergoing vitrectomy surgery were significantly younger than patients without ischemic retinopathy undergoing vitrectomy surgery. However, after adjusting for age, the differences between ischemic and non-ischemic eye remained statistically significant: ischemic diabetic retinopathy protected against nuclear cataract at baseline and after vitrectomy surgery.
There may be other confounding factors in the development of nuclear cataract. For example, myopia is known to have a higher prevalence of nuclear cataract. Interestingly, it is known to also have a lower prevalence of diabetic retinopathy. While the mechanism by which myopia increases the risk of nuclear cataract is not known with certainty, we have suggested that it is related to the early vitreous degeneration that accompanies axial elongation.19 Similarly, reduced severity of diabetic retinopathy is associated with PVD, which is also associated with myopia. Both of these effects are related to the state of the vitreous. In our studies, the vitreous was removed. The role of myopia in post-vitrectomy cataracts has not been studied; it could be of some interest, but this would be unlikely to be important if the effect of myopia is primarily due to the vitreous.
The findings of this study are consistent with our current understanding of post-vitrectomy nuclear cataract formation and with the development of nuclear cataract in general. Nuclear sclerotic cataract is caused by oxidation of the nuclear proteins at the center of the crystalline lens.10 The source of oxidative damage is most likely molecular oxygen arising from the retinal vasculature. In the normal, un-operated eye, molecular oxygen diffuses into the vitreous gel from the retinal vasculature. Much of this oxygen is metabolized by the adjacent retinal tissue20 and some is consumed by reacting with ascorbate in the vitreous gel.12 This assures that the crystalline lens remains in a low-oxygen environment, which is thought to be essential for lens clarity.21 If the vitreous gel liquefies with age or is removed by vitrectomy surgery, the vitreous fluid can circulate, delivering more oxygen to the lens.11 The liquefied vitreous fluid also has a diminished capacity to consume oxygen.12 The net result is that more oxygen is able to reach the lens, leading to increased oxidation of nuclear proteins and the increased light scattering recognized clinically as nuclear sclerotic cataract.
Long-term systemic diabetes mellitus, when not adequately controlled, leads to diffuse small vessel disease, often accounting for complications such as retinopathy, nephropathy, and neuropathy. Therefore, ischemic diabetic retinopathy is likely to be an indicator or marker for substantial, diffuse microvascular disease leading to lower perfusion and hypo-oxygenation of ocular tissues. Supporting evidence for this hypothesis comes from a recent study in which eyes of diabetic patients undergoing vitrectomy surgery for complications of diabetic retinopathy were found to have significantly lower oxygen levels in the vitreous than non-diabetic eyes undergoing vitrectomy surgery for other reasons.13 It remains possible that some other aspect of ischemic diabetic retinopathy protects the lens from nuclear cataract. However, lower oxygen in the vitreous of these patients is the explanation that is most consistent with our understanding of the etiology of nuclear sclerotic cataract.
The strengths of this study are its prospective design and the use of digital Scheimpflug lens photography to quantify the intensity of nuclear light scattering. The weaknesses of this study include the relatively small number of patients involved and the substantial numbers of patients lost to follow-up at 6 and 12 months. Many patients with non-ischemic retinopathy were lost to follow-up because of incident cataract surgery. It was not deemed ethical to withhold routine patient care (i.e. surgery for visually significant cataract) for the purpose of this study.
In conclusion, ischemic diabetic retinopathy, not just the presence of systemic diabetes mellitus, was associated with decreased nuclear opacity in general and much slower progression of nuclear sclerotic cataract following vitrectomy surgery. The findings are likely due to the significant microvascular disease and subsequent relative hypoxia of vascularized ocular tissues that can result from poorly controlled diabetes mellitus and the resulting decrease in oxygen in the vitreous. The findings of this study help to explain the results of two large epidemiologic studies that found diabetes mellitus to be protective against nuclear cataract and the study in which diabetic patients required less frequent cataract surgery following vitrectomy surgery. These results are consistent with our current understanding of the cause of nuclear sclerotic cataract. Molecular oxygen from the vascularized tissues of the eye, if not adequately metabolized by the vitreous gel or protective mechanisms within the lens itself, will lead to oxidative damage to the lens nucleus, an increase in light scattering, and the clinical scenario recognized as nuclear sclerotic cataract. Finally, this study in which patients with ischemic retinopathy developed less post-vitrectomy nuclear cataract suggests that reducing the amount of molecular oxygen reaching the crystalline lens may be a successful strategy to prevent or delay nuclear sclerotic cataract formation.
ACKNOWLEDGEMENTS
Funding: Research was supported by NIH Grant EY015863, an unrestricted research grant and a Senior Scientist award from Research to Prevent Blindness (DCB), the Department of Ophthalmology and Visual Sciences and the Barnes Retina Institute Retina Research and Development Fund.
Financial Disclosure: None.
-
Contributions of Authors:
Design and Conduct of Study: (NMH, FB, YS, AA, DCB).
Collection:(NMH, FB, YS)
Management: (NMH, FB, YS, DCB).
Analysis and Interpretation of Data(NMH, FB, YS, AA, DCB)
Review and Approval of Manuscript: (NMH, FB, YS, AA, DCB)
Conformity with Author Information: Approval was obtained from the Human Studies Committee and the Institutional Review Board of the Washington University School of Medicine, St. Louis, MO.
Other Acknowledgements: None.
Biography

Bio for Nancy M. Holekamp, MD
Nancy Melberg Holekamp, MD is a Professor of Clinical Ophthalmology and Visual Sciences at the Washington University School of Medicine in St. Louis, Missouri. She is also a partner in the Barnes Retina Institute. Dr. Holekamp is actively involved in clinical research, having been principal investigator or sub-investigator in more than 15 national clinical trials dealing with age-related macular degeneration, retinal vascular occlusion, and diabetic retinopathy. Her efforts in research have resulted in more than 55 peer-reviewed publications, more than 15 book chapters, and numerous speaking invitations both nationally and internationally. She is a member of the major subspecialty societies, including the Retina Society and the Macula Society. She acts as a reviewer for all the major ophthalmology journals and as a consultant to two ophthalmic pharmaceutical companies. After 6 years on the American Academy of Ophthalmology Ethics Committee, she has developed an interested in medical ethics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cook CA, Koretz JF, Pfahnl A, Hyun J, Kaufman PL. Aging of the human crystalline lens and anterior segment. Vision Res. 1994;34(22):2945–2954. doi: 10.1016/0042-6989(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 2.Heys KR, Cram SL, Truscott RJ. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004;10:956–963. [PubMed] [Google Scholar]
- 3.Tabandeh H, Wilkins M, Thompson G, Nassiri D, Karim A. Hardness and ultrasonic characteristics of the human crystalline lens. J Cat & Ref Surg. 2000;26(6):838–841. doi: 10.1016/s0886-3350(00)00305-9. [DOI] [PubMed] [Google Scholar]
- 4.Salm M, Belsky D, Sloan FA. Trends in Cost of Major Eye Diseases to Medicare, 1991 to 2000. Am J of Ophthalmol. 2006;142(6):976–982. doi: 10.1016/j.ajo.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 5.West S. Epidemiology of Cataract: Accomplishments over 25 years and Future Directions. Ophthalmic Epidemiol. 2007;14(4):173–178. doi: 10.1080/09286580701423151. [DOI] [PubMed] [Google Scholar]
- 6.Hammond CJ, Snieder H, Spector TD, Gilbert CE. Genetic and environmental factors in age-related nuclear cataracts in monozygotic and dizygotic twins. N Engl J Med. 2000;342(24):1786–1790. doi: 10.1056/NEJM200006153422404. [DOI] [PubMed] [Google Scholar]
- 7.Heiba IM, Elston RC, Klein BE, Klein R. Genetic etiology of nuclear cataract: evidence for a major gene. Am J Med Genet. 1993;47(8):1208–1214. doi: 10.1002/ajmg.1320470816. [DOI] [PubMed] [Google Scholar]
- 8.AREDS. Risk factors associated with age-related nuclear and cortical cataract: a case-control study in the Age-Related Eye Disease Study, AREDS Report No. 5. Ophthalmology. 2001;108(8):1400–1408. doi: 10.1016/s0161-6420(01)00626-1. [DOI] [PMC free article] [PubMed]
- 9.Smiddy WE, Feuer W. Incidence of Cataract Extraction after Diabetic Vitrectomy. Retina. 2004;24(4):574–581. doi: 10.1097/00006982-200408000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Truscott RJW. Age-related nuclear cataract--oxidation is the key. Exp Eye Res. 2004;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Harocopos GJ, Shui Y-B, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of Vitreous Liquefaction in Age-Related Cataract. Invest Ophthalmol Vis Sci. 2004;45(1):77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 12.Shui Y-B, Holekamp NM, Kramer BC, et al. The Gel State of the Vitreous and Ascorbate-dependent Oxygen Consumption: Relationship to the Etiology of Nuclear Cataracts. Arch Ophthalmol. 2009;127(4):475–482. doi: 10.1001/archophthalmol.2008.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holekamp NM, Shui Y-B, Beebe D. Lower Intraocular Oxygen Tension in Diabetic Patients: Possible Contribution to Decreased Incidence of Nuclear Sclerotic Cataract. Am J Ophthalmol. 2006;141(6):1027–1032. doi: 10.1016/j.ajo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 1995;102(10):1466–1471. doi: 10.1016/s0161-6420(95)30844-5. [DOI] [PubMed] [Google Scholar]
- 15.Cherfan GM, Michels RG, de Bustros S, Enger C, Glaser BM. Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am J Ophthalmol. 1991;111(4):434–438. doi: 10.1016/s0002-9394(14)72377-3. [DOI] [PubMed] [Google Scholar]
- 16.de Bustros S, Thompson JT, Michels RG, Enger C, Rice TA, Glaser BM. Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol. 1988;105(2):160–164. doi: 10.1016/0002-9394(88)90180-8. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JT, Glaser BM, Sjaarda RN, Murphy RP. Progression of nuclear sclerosis and long-term visual results of vitrectomy with transforming growth factor beta-2 for macular holes. Am J Ophthalmol. 1995;119(1):48–54. doi: 10.1016/s0002-9394(14)73812-7. [DOI] [PubMed] [Google Scholar]
- 18.Van Effenterre G, Ameline B, Campinchi F, Quesnot S, Le Mer Y, Haut J. Is vitrectomy cataractogenic? Study of changes of the crystalline lens after surgery of retinal detachment. J Fr Ophtalmol. 1992;15(8–9):449–454. [PubMed] [Google Scholar]
- 19.Holekamp NM, Harocopos GJ, Shui YB, Beebe DC. Myopia and axial length contribute to vitreous liquefaction and nuclear cataract. Arch Ophthalmol. 2008;126(5):744. doi: 10.1001/archopht.126.5.744-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alder VA, Niemeyer G, Cringle SJ, Brown MJ. Vitreal oxygen tension gradients in the isolated perfused cat eye. Curr Eye Res. 1986;5(4):249–256. doi: 10.3109/02713688609020050. [DOI] [PubMed] [Google Scholar]
- 21.Eaton JW. Is the lens canned? Free Radic Biol Med. 1991;11(2):207–213. doi: 10.1016/0891-5849(91)90173-z. [DOI] [PubMed] [Google Scholar]